Abstract
Diets must satisfy the everyday metabolic requirements of organisms and can also serve as medicines to combat disease. Currently, the medicinal role of diets is much better understood in terrestrial than in aquatic ecosystems. This is surprising because phytoplankton species synthesize secondary metabolites with known antimicrobial properties. Here, we investigated the medicinal properties of phytoplankton (including toxin-producing cyanobacteria) against parasites of the dominant freshwater herbivore, Daphnia. We fed Daphnia dentifera on green algae and toxic cyanobacteria diets known to vary in their nutritional quality and toxin production, and an additional diet of Microcystis with added pure microcystin-LR. We then exposed Daphnia to fungal and bacterial parasites. Anabaena, Microcystis and Chlorella diets prevented infection of Daphnia by the fungal parasite Metschnikowia, while Nodularia toxins increased offspring production by infected hosts. In contrast to their medicinal effects against Metschnikowia, toxic phytoplankton generally decreased the fitness of Daphnia infected with the bacterial parasite, Pasteuria. We also measured the amount of toxin produced by phytoplankton over time. Concentrations of anatoxin-a produced by Anabaena increased in the presence of Metschnikowia, suggesting parasite-induced toxin production. Our research illustrates that phytoplankton can serve as toxins or medicines for their consumers, depending upon the identity of their parasites.
Keywords: phytoplankton, Daphnia, microcystin, anatoxin, parasite–host interactions, medicines
1. Introduction
Primary producers synthesize a dazzling array of secondary metabolites that can either increase or decrease levels of consumption, and thereby the storage and cycling of nutrients in ecosystems [1–3]. In addition to their pervasive direct effects on herbivores [4–6], secondary metabolites can also influence the susceptibility of herbivores to their natural enemies by making herbivores less palatable or by decreasing herbivore fitness [7–9]. Moreover, some herbivores gain protection from their parasites and pathogens by using the toxins in primary producers as sources of medicine [10]. In terrestrial systems, impacts of plant diet on host–pathogen interactions have been reported in diverse animal taxa from primates to insects [11]. For example, baboons consume anthelmintic bitter plants as prophylactics [12], while toxic milkweeds protect monarch butterflies from protozoan parasites [13]. To date, there are far fewer examples of primary producers in aquatic systems conferring medicinal effects on hosts [10,14]. This is surprising given the well-known capability of diverse phytoplankton to produce secondary metabolites, many of which have anti-bacterial and anti-fungal properties [15–17].
While some secondary metabolites produced by phytoplankton may serve as defensive compounds against herbivores [2,18], many zooplankton grazers appear largely unaffected by certain phycotoxins. For example, Daphnia individuals and populations exhibit variable feeding and growth responses to the toxin microcystin, which is produced by the common bloom-forming cyanobacterium Microcystis and other cyanobacterial taxa. The growth and performance of some strains and species of Daphnia decline substantially in the presence of microcystins [19–21], while other strains and species are much less susceptible [19,20,22,23]. Notably, microcystin synthesis probably predates the evolution of the Metazoa [24], suggesting that it evolved originally for purposes other than defence against herbivory; the same holds true for the toxin nodularin, produced by cyanobacteria in the genus Nodularia [24]. One possibility is that these toxins evolved as a result of competition with other microbes. In support of this idea, a variety of secondary metabolites produced by Microcystis aeruginosa are allelopathic against macro- and micro-algae and other bacteria [15,25]. Likewise, extracts from Aphanizomenon flos-aquae and Nodularia harveyana also exhibit antibacterial and antifungal properties [16]. If phytoplankton secondary metabolites are generally antimicrobial, they might also be active against microbial parasites of consumers.
In combination, nutritional and defensive traits of host diets influence the ability of those hosts to defend themselves against parasites, as well as mediate variation in parasite fitness [26,27]. Potential dietary effects cut both ways: nutritious resources can be good for the host, allowing for investment in defences, but also provide more resources for the parasite to exploit. The impacts of toxins can also cut both ways: toxins may stress the host, increasing susceptibility to parasites, but also might serve as medications that prevent infection or mitigate its effects [28,29]. We know from terrestrial systems that this can lead to complex diet–host–parasite interactions [6,9,30–32]. Surprisingly, we know relatively little about how diets serve as sources of medicines in aquatic systems [14]. This is problematic because global environmental change is driving changes in phytoplankton composition [33–35], especially increases in cyanobacterial blooms [36,37], which are of low nutritional value for grazers and which often produce toxins [38–40]. This means that environmental change might alter disease outbreaks in aquatic systems, with potential consequences for entire food webs [10,41].
In this study, we exposed Daphnia dentifera, a common herbivore in North American lakes, to two common parasites: the fungal parasite Metschnikowia bicuspidata and the bacterial parasite Pasteuria ramosa. We cultured Daphnia on eight phytoplankton species that differed in nutritional value and toxin production (electronic supplementary material, table S1). We used five different species of green algae and three species of cyanobacteria, with green algae being a better quality diet compared to cyanobacterial diets [20,42,43]. All three of the cyanobacteria we used produce compounds known to be toxic to Daphnia species as well as to vertebrates including humans (electronic supplementary material, table S1) [19,20,44]. We also generated a ninth diet treatment by adding pure microcystin to some cultures of Microcystis; during many cyanobacterial blooms, concentrations of extracellular toxins in water become high enough to cause significant impacts on other organisms [45,46]. Our additional treatment tests whether extracellular microcystin may have impacts on Daphnia–parasite interactions. We recorded the survival, reproduction and growth of individual Daphnia over 30 days to test the hypotheses that phytoplankton diets influence host and parasite fitness and that phycotoxins have medicinal effects against the parasites of Daphnia. Specifically, we asked the following questions in our study: (i) Does phytoplankton quality influence parasitism in Daphnia? (ii) What are the fitness impacts of consuming chemically defended phytoplankton for infected hosts and parasites? (iii) Can phycotoxins act as anti-bacterial and/or anti-fungal compounds for Daphnia?
2. Material and methods
(a). Parasite–host system
Daphnia dentifera is a common zooplankton of stratified lakes in temperate North America [47]. For our experiments, we used the Midland 37 (Mid37) genotype, which was isolated from Midland Lake in Greene County, Indiana and is commonly used in disease experiments [48,49]. The parasites chosen for this study were the fungal parasite Metschnikowia bicuspidata (isolated from Baker Lake in Barry County, Michigan), and the bacterium Pasteuria ramosa (isolate (G/18) from Midland Lake in Greene County, Indiana) [48,49]. While the size of disease outbreaks varies across populations, both of these parasites are relatively common, and single lakes sometimes show outbreaks of both pathogens in a single season (M. A. Duffy et al. 2013–2018, unpublished data). Daphnia encounter these parasites in nature while grazing for phytoplankton food and become infected by consuming transmission spores that have been released in the water from dead host individuals. Additionally, individual daphniids can be found infected with more than one parasite species (co-infection [50]). Both parasites infect via the gut wall after being consumed by Daphnia. The two parasites vary in their effects on the host but both parasites are obligate killers, with spores only released after the host's death [48]. Metschnikowia kills Daphnia at a younger age while Pasteuria greatly reduces fecundity. The parasites can co-infect individual Daphnia, with Metschnikowia outperforming Pasteuria in within-host competition [51]. We predicted that, even if diets were shown to have medicinal properties against the parasites individually, they would be unable to mitigate the deleterious impacts of infection simultaneously for both parasites.
(b). Cultivation of phytoplankton food
We cultivated eight species of phytoplankton (five green algae and three cyanobacteria) for the experiment. We obtained Anabaena flos-aquae (CYA 139), Chlamydomonas reinhardtii (CHL 159), Chlorella sp. (CHL 61), and Microcystis aeruginosa (CYA 160/1) from the Norwegian Institute for Water Research (NIVA); Nodularia spumigena (UTEX B2091) and Ulothrix sp. (UTEX 420) from the Culture Collection of Algae at the University of Texas at Austin, and Scenedesmus acutus from the Canadian Phycological Culture Centre (CPCC 10). The Ankistrodesmus falcatus culture is originally from the Academy of Natural Science of Philadelphia and has been in culture since at least the 1980s [52]; its exact origin is unknown. Anabaena, Microcystis and Nodularia are well known cyanobacteria that commonly form harmful cyanobacterial blooms; the remaining phytoplankton are green algae. We grew cultures in chemostats using appropriate media (electronic supplementary material, table S1) at room temperature under 24 h light with the exception of Chlorella which was grown on a 12 : 12 light : dark cycle. However, as with all phytoplankton cultures in chemostats, we recognize that there may be undetected contamination by microbes that contributed to our observations. The phytoplankton selected for this study cover a broad range of nutritional quality (including different composition of essential lipids) for Daphnia and vary in their production of toxic compounds (electronic supplementary material, table S1).
(c). Experimental design
Our experiment was a factorial design of nine diet treatments crossed with four types of parasite inoculation (including a zero inoculation control) for a total of 36 treatment combinations. Each combination was replicated 10 times, for a total of 360 observational units. Specifically, our nine diets were comprised of the eight phytoplankton species (above), plus a 9th diet comprised of Microcystis with an addition of 15 µg l−1 of microcystin LR added to experimental beakers daily. We chose to add this additional diet treatment because preliminary analysis indicated that our strain produced relatively low levels of the toxin, and we wanted to ensure that extracellular concentrations of microcystin reached those commonly observed in water during Microcystis blooms. Dissolved microcystin levels in water increase markedly during Microcystis blooms [53], so this is a realistic means of exposing both Daphnia and their parasites to microcystin. The concentration that we added (15 µg l−1) is well within the range observed during natural Microcystis blooms [54–56], and represents one half of the LC50 reported in previous Daphnia toxicology experiments [19]; we therefore expected that 15 µg l−1 of microcystin-LR would provide a realistic stress to Daphnia as well as the opportunity to observe any antimicrobial effects on the parasites.
Our four parasite inoculation treatments were (i) 2000 spores ml−1 of the bacterium, (ii) 500 spores ml−1 of the fungus, (iii) 1000 spores ml−1 of the bacterium and 250 spores ml−1 of the fungus, or (iv) no spores as a control, with 10 replicates on each of the diet types. The spore values were chosen based on prior studies, which indicate that they provide moderate infection levels when Daphnia are fed green algae [49] (M. A. Duffy 2016, unpublished data). We maintain stock cultures of these parasites (seperately) in the laboratory, and obtained the spores by grinding up infected animals from those cultures.
Experimental Daphnia were reared on their assigned diets under a 16 : 8 L : D photoperiod at 20°C. From our laboratory culture, we collected neonate (less than 24 h old) Daphnia and placed them in 250 ml beakers with 200 ml of filtered lake water (10 individuals per beaker). Juveniles were reared for 3 days on 2 mg C/L Ankistrodesmus per day. Then, we placed four-to-five-day-old Daphnia juveniles in 50 ml beakers (one animal per beaker) with 30 ml of filtered lake water. Each individual was fed 2.0 mg C/L of one of the nine diet treatments: Ankistrodesmus, Anabaena, Chlamydomonas, Chlorella, Microcystis, Microcystis with 15 µg l−1 of microcystin-LR (Mic+), Nodularia, Scenedesmus and Ulothrix.
We exposed five-to-six-day-old Daphnia to parasite spores (or left them unexposed in the control treatment) for 24 h. Daphnia were fed 1 mg C/L of their treatment diet during the spore exposure period. We used less food during inoculation to minimize the potential for feeding inhibition by phycotoxins, which could indirectly prevent consumption of parasite spores. Lower food levels have also been found to increase infection levels in earlier studies using Ankistrodesmus as food. After 24 h, we transferred each replicate Daphnia individually into new 50 ml beakers with fresh filtered lake water. Animals that died (approx. 1%) during parasite exposure or within 3 days of exposure were not included in the analyses. From then on, each Daphnia was fed 2.0 mg C/L per day of the appropriate phytoplankton for the 30 days of the experimental trial. Twice each week, we transferred Daphnia into new beakers with fresh filtered lake water prior to feeding. Each diet by infection treatment consisted of 10 replicates, which we performed in two temporal blocks (five replicates per diet by infection treatment per block). The first block took place in June 2016 and the second in December 2016. We checked the mortality of individuals daily and counted and removed their offspring twice per week. We collected subsamples of the water from the beakers in all cyanobacteria treatments on the day prior to, the day of, and the day after exposure to parasite spores (and at the equivalent times in our control treatments), as well as on days 15 and 30 post exposure, and analysed them for phycotoxins using Abraxis Cyanotoxin kits. The ELISA kits measured all microcystin and nodularin variants, and anatoxin-a concentrations. Adults that died during the experiment were placed in 1.5 ml tubes with 100 µl of nanopure water and preserved for spore counts. At the end of 30 days, we measured the length of all surviving individuals using a dissecting microscope. After measurement, we ground the Daphnia to release spores and counted the spores using a haemocytometer.
(d). Statistical analyses
Wherever possible, we analysed data using generalized linear mixed models, with temporal block as a random effect [57]. Where missing cells (e.g. zero parasite infection on certain phytoplankton diets) prevented mixed model convergence, we incorporated block as a fixed effect in a generalized linear model [57]. In no case was there a significant block effect in our analyses. All analyses were conducted using SAS v. 9.4. In most models that included infection treatment as a fixed effect, we included only those hosts that had become infected after exposure to parasites. The exception was those models in which we explicitly explored disease prevalence (infection present or absent), in which we included those individuals that were exposed to parasites yet remained uninfected.
We analysed the effects of diet on disease prevalence (infection present or absent) with a binomial distribution and a logit link function using two approaches. Our first model compared disease prevalence in the single infection treatments, and included parasite infection treatment, phytoplankton diet, and their interaction as fixed effects. Our second model evaluated differences in disease prevalence when Daphnia were exposed to a single infection with the prevalence of individuals exposed to both parasites simultaneously (co-infection); the models included phytoplankton diet, infection treatment (single or co-infection) and their interaction as fixed effects. For this second model, data from bacterial and fungal infections were analysed separately.
We analysed the effects of phytoplankton diet, infection treatment and their interaction on parasite fitness (spore yield per infected individual) using a negative binomial distribution with a log link function [57]. Because of strong interaction effects, we also analysed each parasite treatment separately to evaluate differential effects of phytoplankton diets on bacterial and fungal spore yields.
We analysed the effects of parasite infection on host fitness in the following ways. First, in a full model, we compared lifetime offspring production by Daphnia across all four infection treatments (bacteria only, fungus only, co-infection, uninfected controls; Poisson distribution and log link function). Second, because we observed strong interactions between parasite treatment and diet treatment on host reproduction, we compared lifetime offspring production of hosts separately for each parasite; this analysis included data from fungus-only or bacteria-only treatments, with the uninfected treatment serving as the control in both analyses. Third, to establish whether the effects of diet on host fitness differed between parasites, we compared host offspring production between bacterial and fungal infection treatments, without including the uninfected controls or individuals from the coinfection treatment. We repeated the above three analyses using an additional measure of host fitness, specifically whether individuals survived to 30 days (a binary outcome of ‘yes’ or ‘no’) and individual growth (length at the end of 30 days or at the day of death). In the survival analyses, we used a binomial distribution and logit link function.
To determine if individuals who failed to get infected paid a fitness cost for resistance, we compared offspring production of individual Daphnia in the control treatment (no parasites) to offspring production of all individuals that were challenged with parasites but did not become infected during the experiment. Our model included infection treatment, diet and their interactions as fixed effects, using the Poisson distribution and log link function.
To determine if cyanobacterial toxin production varied among treatments, we analysed variation in toxin concentrations using general linear mixed models, with repeated measures from individual beakers treated as within-subject random effects, temporal block as a random effect, and infection treatment as a fixed effect [57]. Finally, we correlated the average toxin concentration within each beaker with parasite spore yield and number of Daphnia offspring.
3. Results
(a). Infection prevalence
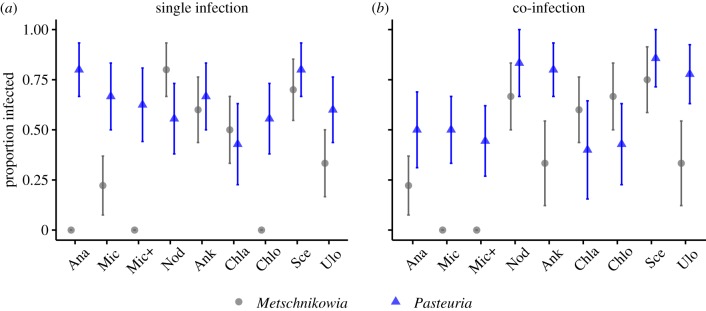
Phytoplankton diet had striking effects on infection prevalence of the fungal parasite, Metschnikowia (figure 1a; electronic supplementary material, table S2). Specifically, two of the cyanobacterial treatments (Anabaena and the Microcystis+ treatment, which contained Microcystis with additional microcystin toxin) completely prevented Metschnikowia fungal infections and a third (Microcystis without additional microcystin) resulted in very low infection prevalence. Metschnikowia fungal infections were also prevented when Daphnia fed on the green alga Chlorella, but fungal infection levels were high on the other green algae. In contrast, there was much less variation in infection prevalence among diet treatments when Daphnia were exposed to the bacterial parasite, Pasteuria (figure 1a; electronic supplementary material, table S2). Daphnia became infected with bacteria on all diet treatments, which strongly suggests that the lack of fungal infection on some phytoplankton diets was not driven by feeding inhibition on toxic diets.
Figure 1.
Proportion of Daphnia infected with the fungal parasite Metschnikowia bicuspidata, and the bacterial parasite Pasteuria ramosa, on nine phytoplankton diets. Daphnia were exposed either in single parasite treatments (a) or co-infection treatments (b). Prevalence was calculated as the proportion (means ± s.e.) of infected Daphnia out of all exposed individuals. Note that not all individuals in the co-infection treatments (b) became infected with both parasite species. Abbreviations for phytoplankton names: Ana—Anabaena, Mic—Microcystis, Mic+—Microcystis spiked with additional microcystin, Nod—Nodularia, Ank—Ankistrodesmus, Chla—Chlamydomonas, Chlo—Chlorella, Sce—Scenedesmus, Ulo—Ulothrix. For single Metschnikowia infection n = (10, 9, 9, 10, 10, 10, 8, 10, 9), for single Pasteuria infection n = (10, 9, 8, 9, 9, 7, 9, 10, 10), and for co-infection n = (9, 10, 9, 10, 10, 10, 10, 10, 10) all in order of diet. See electronic supplementary material, table S2 for a summary of the statistical analyses. (Online version in colour.)
The impact of diet on infection prevalence changed when hosts were exposed to both parasites at once. Exposure to both parasites simultaneously increased prevalence of the fungus Metschnikowia in two of the three diet treatments that had conferred complete resistance to the fungus when exposed to it alone (figure 1b). In contrast, prevalence of the bacterium Pasteuria was similar when individuals were exposed alone or in combination with the fungus (figure 1b; electronic supplementary material, table S2).
(b). Spore yield
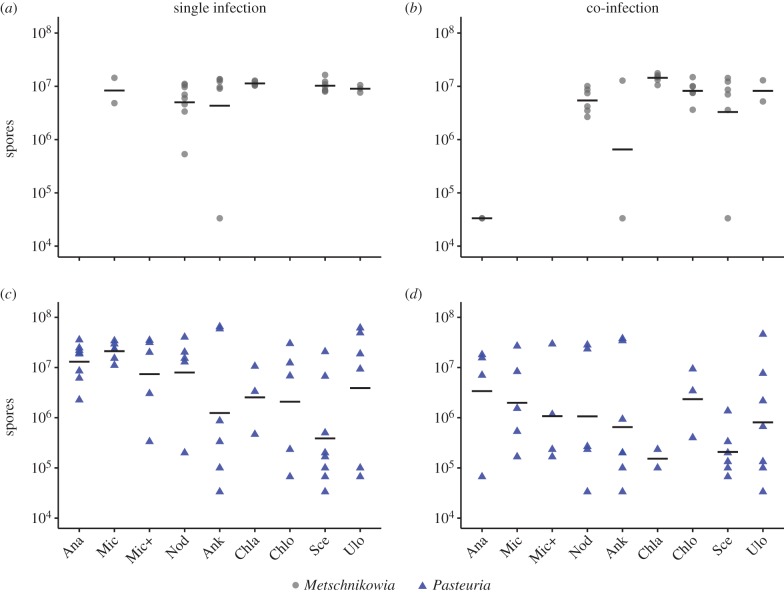
While the major effect of phytoplankton diet on the fungal parasite Metschnikowia was to influence the probability of Daphnia becoming infected (above), the principal effect of phytoplankton diet on the bacterial parasite Pasteuria was to influence post-infection spore yield from Daphnia (figure 2; electronic supplementary material, table S2). Specifically, the lower-quality cyanobacteria diets tended to yield more spores of the bacterium Pasteuria than did the green algae diets (figure 2c). This suggests that high-quality non-toxic diets may help Daphnia suppress Pasteuria replication. In contrast to bacterial spore yields, spore yields of the fungus Metschnikowia did not differ across diets (figure 2a) suggesting that, once infection has taken place, diet quality does not impact the number of Metschnikowia spores produced.
Figure 2.
Spore yields from Daphnia infected with (a,b) the fungal parasite Metschnikowia bicuspidata and (c,d) the bacterial parasite Pasteuria ramosa on nine phytoplankton diets. Treatments were either single-parasite infections (a,c) or co-infections with both parasites (b,d). Spore yields are lacking for those treatments in which Daphnia were protected from infection by phytoplankton diet (figure 1). Abbreviations for algal names: Ana—Anabaena, Mic—Microcystis, Mic+—spiked Microcystis, Nod—Nodularia, Ank—Ankistrodesmus, Chla—Chlamydomonas, Chlo—Chlorella, Sce—Scenedesmus, Ulo—Ulothrix. Points are individual replicates and black lines represent means.
Spore yield for a given diet was influenced by whether a host was exposed to both parasites simultaneously. Spore yield was lower for both parasites (but especially for the bacterium, Pasteuria) in co-infection treatments compared to single infections (electronic supplementary material, table S2; figure 2c,d). This may be due to within-host competition [51], but may also be influenced by the lower initial doses per parasite provided to Daphnia in the co-infection treatment.
(c). Host fitness
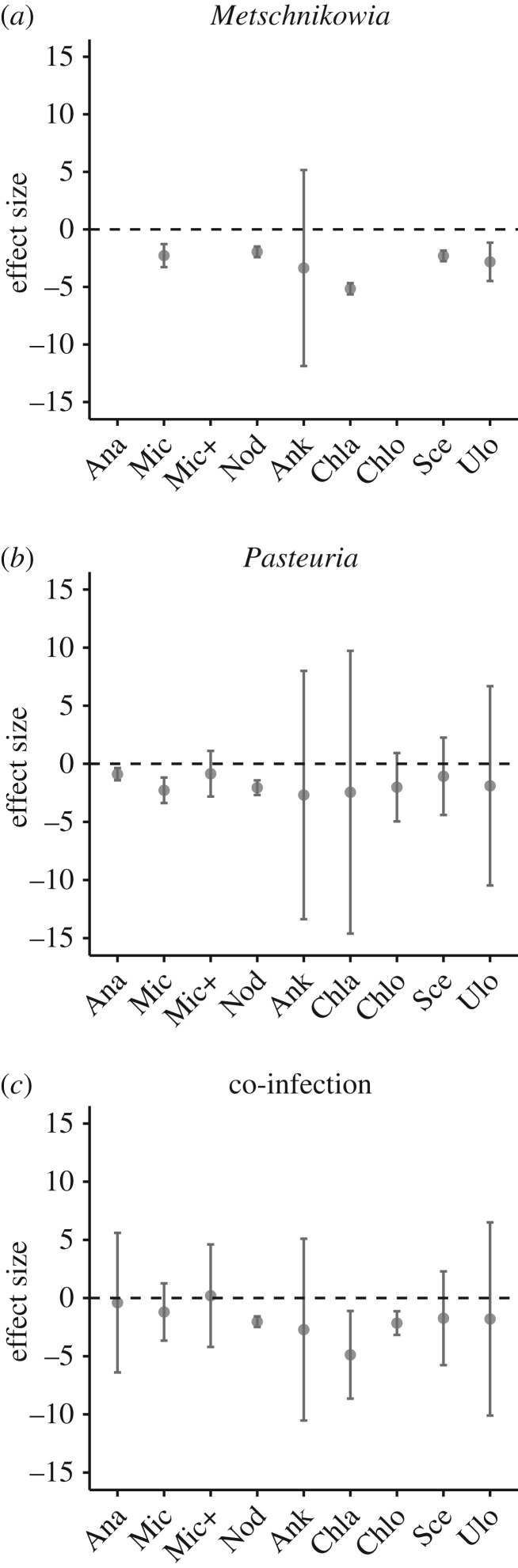
Parasite infection reduced host fitness (total offspring production over 30 days, figure 3; electronic supplementary material, figure S1), but the magnitude of the reduction depended on phytoplankton diet and parasite identity (as indicated by strong interaction effects; electronic supplementary material, table S2). Offspring production was especially low in those individuals infected with the fungus Metschnikowia, regardless of phytoplankton diet (figure 3a; electronic supplementary material, figure S1a). This probably reflects the short lifespan of Daphnia when infected with this fungus (electronic supplementary material, table S2). Most Daphnia infected with the fungal parasite died before day 30, although around 20% survived when feeding on Ankistrodesmus (electronic supplementary material, figure S8). Daphnia infected with the bacterial parasite, Pasteuria, produced fewer offspring (figure 3b; electronic supplementary material, figure S1b) while feeding on cyanobacteria diets but lived longer than did uninfected Daphnia on cyanobacteria diets (electronic supplementary material, table S2 and figure S8). These life-history differences perhaps reflect a cost in longevity of higher reproduction by controls. Uninfected Daphnia consuming green algae produced two- to three-fold more offspring than did those consuming cyanobacteria (electronic supplementary material, figure S1d), confirming the higher nutritional quality of our green algal diets. In the absence of parasites, cyanobacterial diets did not vary in their impacts on host fitness (electronic supplementary material, figure S1d).
Figure 3.
Effect sizes for lifetime offspring production of individual Daphnia infected with (a) fungal and (b) bacterial parasites, and (c) the co-infection treatment. Each point represents Glass's Δ calculated from the mean number of offspring produced by individuals in an infection treatment compared to the mean number of offspring produced by controls. Error bars represent standard error of mean. Note that there are no data for Metschnikowia infections for Daphnia reared on Anabaena, spiked Microcystis or Chlorella diets, because no individuals became infected. Not all individuals exposed to both parasites in the co-infection treatment became co-infected. (Abbreviations for algal names: Ana—Anabaena, Mic—Microcystis, Mic+—spiked Microcystis, Nod—Nodularia, Ank—Ankistrodesmus, Chla—Chlamydomonas, Chlo—Chlorella, Sce—Scenedesmus, Ulo—Ulothrix).
Similarly, growth was reduced by parasite infection and the extent of this decrease was dependent on diet also (electronic supplementary material, table S2). Daphnia infected with the fungus were small, irrespective of diet (electronic supplementary material, figure S3a). As we mentioned above for offspring production, this is a reflection of the short lifespan of individuals when infected by the fungal parasite. Animals infected with the bacterium varied in size among diets (electronic supplementary material, figure S3b). Infected animals consuming Anabaena, Ankistrodesmus, Chlorella and Scenedesmus did not differ in size compared to controls, while infected animals consuming Microcystis, Nodularia, Chlamydomonas and Ulothrix were smaller compared to control individuals eating the same diet (electronic supplementary material, table S2). The only instance in which treatment animals were larger than controls were in the microcystin added diet treatment, however this increase in size does not result in more spores being produced by the parasite or more offspring produced by the host. This increase in growth is probably caused by the parasite which is known to cause gigantism and/or castration, with castration duration dependent on diet quality [50,58].
(d). Cost of resistance
We estimated potential fitness costs of parasite resistance by comparing the fecundity and mortality of uninfected control Daphnia with uninfected parasite-exposed Daphnia. Individuals that were exposed to the bacterial parasite Pasteuria but that did not become infected had lower offspring production (all diets) and higher mortality (most diets) than did unchallenged controls (electronic supplementary material, table S2 and figures S6 and S7), suggesting that there is a cost of resisting infection.
(e). Toxin production
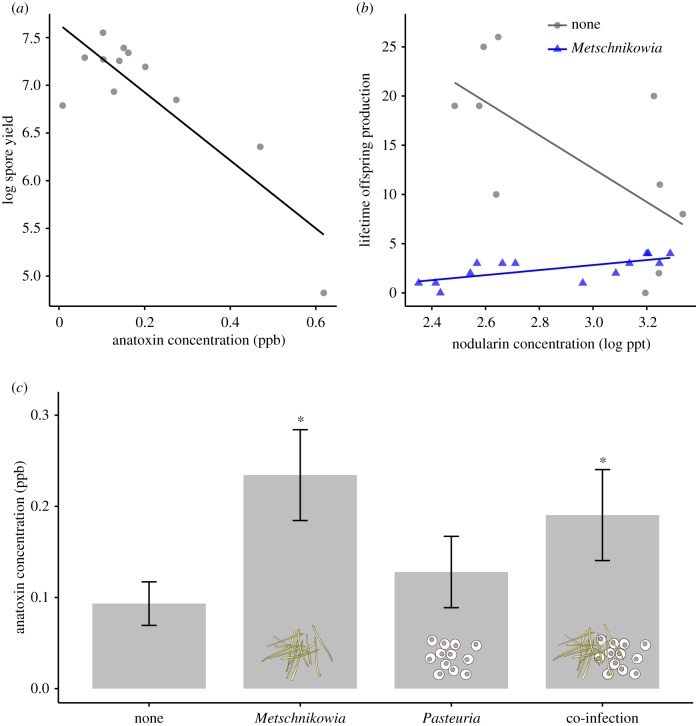
From subsamples of water collected from each replicate, we confirmed (using ELISA) that all cyanobacterial diets were producing their expected phycotoxins: anatoxin-a (Anabaena treatment), microcystins (Microcystis and Mic+ treatments) or nodularin (Nodularia treatment) (electronic supplementary material, table S1). Interestingly, concentrations of anatoxin-a in water were 2.3-fold higher in the presence of Metschnikowia (F3,33 = 2.98, p = 0.0456; figure 4c), suggesting that this fungal parasite may induce phycotoxin production, or cause cell lysis, in Anabaena. Notably, Daphnia resisted Metschnikowia infection when they fed on Anabaena (figure 1a), suggesting that Daphnia may benefit from anatoxin-a release when the fungus is present. Moreover, bacterial spore yield declined with anatoxin-a concentration (F1,10 = 26.37, p = 0.0004, R² = 0.725, figure 4a), suggesting that the presence of the fungus Metschnikowia may decrease fitness of the bacterium Pasteuria via phycotoxin production. This could result from two mechanisms: first, higher toxin concentrations may have direct antibacterial effects on Pasteuria once infection has taken place. Second, the presence of anatoxin-a may decrease feeding rates of Daphnia, thereby decreasing infection levels or Daphnia growth rates. We favour the former explanation because there was no significant correlation between concentrations of anatoxin-a and Daphnia growth (F1,8 = 0.98, p = 0.351; electronic supplementary material, figure S9).
Figure 4.
Medicinal effects of phytoplankton toxins on (a) spore yield of the bacterial parasite Pasteuria, and (b) offspring production by Metschnikowia-infected and uninfected control Daphnia. The algal toxin anatoxin-a (means ± s.e.) appears to be induced (c) by the presence of the fungal pathogen, Metschnikowia, in water. Statistical differences (p < 0.05) in (c) are indicated with an asterisk.
We also found strong evidence of a medicinal effect of consuming Nodularia for Daphnia infected with the fungus, Metschnikowia. In uninfected Daphnia from control treatments, offspring production declined as nodularin concentration increased ( d.f. = 1, p < 0.0001; figure 4b), illustrating the highly toxic nature of nodularin. In marked contrast, for Daphnia infected with Metschnikowia, offspring production actually increased as nodularin concentration increased ( d.f. = 1, p = 0.0362; figure 4b). Such opposing effects of secondary metabolites on hosts in the presence or absence of parasites are a key signature of medicinal activity [11].
4. Discussion
While medicinal effects of primary-producer secondary metabolites are increasingly recognized in terrestrial ecosystems [11], they have remained understudied in aquatic systems [10,14]. Here, we report that toxin-producing cyanobacteria have differential effects on the fitness of two common parasites of Daphnia. When exposed to the fungus Metschnikowia, toxic phytoplankton conferred resistance to Daphnia hosts, largely preventing infection. Intriguingly, anatoxin-a concentrations were higher when Daphnia were exposed to this fungal parasite; this has potential implications for drinking water and human health, as anatoxin is a potent neurotoxin [59]. This would mean that in natural lakes where both Metschnikowia and Anabaena are present, there exists the potential of higher than expected anatoxin-a levels in the water during a disease outbreak, even in cases where there are no signs of an actual bloom. We also found that bacterial spore yield declined as anatoxin-a concentration increased. Thus, while Anabaena is a low-quality food for uninfected Daphnia [40], it confers substantial benefits to the host in the presence of parasites. Notably, the impact of diet on host fitness depended strongly on parasite identity in most cases. Diets that were medicinal for hosts exposed to Metschnikowia (the fungal parasite) tended to result in higher spore yields in hosts exposed to Pasteuria (the bacterium). This contradicts the prevailing wisdom [60–64] that diets that are good for Daphnia are also good for their parasites.
Previous studies in the Daphnia system suggest that high quality phytoplankton resources that are beneficial for the host are often beneficial for the parasite too, while low quality diets that impose a fitness cost on the host also impose a cost on the parasite [60–64]. However, there is some evidence that diets of low nutritional quality may also have medicinal properties. For example, Coopman et al. [62] demonstrated that low levels of a non-microcystin producing strain of Microcystis aeruginosa ameliorated the effects of white fat cell disease in Daphnia magna. At 20% levels of Microcystis in a background diet of the nutritious alga Scenedesmus obliquus, host fitness was higher for infected than uninfected individuals. Fitness of infected hosts was lower when their diet did not contain Microcystis, and fitness of all hosts (infected and uninfected) was low when their diet contained ≥50% Microcystis [62]. While the Coopman et al. [62] study illustrates the potential for cyanobacterial diets to mitigate parasite infection, a recent study of a different Daphnia–parasite system found the opposite pattern [65], emphasizing the need for additional work in this area. In combination with the existing literature on how resource quality influences Daphnia performance, our data illustrate that the net impact of the nutritional quality, medicinal effectiveness, and toxicity of phytoplankton diets depends on whether or not the host is exposed to a parasite as well as on the identity of the parasite to which it is exposed (electronic supplementary material, figure S10). Additional work will be required to understand the outcomes generated by particular Daphnia–phytoplankton–parasite combinations (figure 1).
Our results also provide strong evidence for anti-fungal properties of cyanobacterial toxins. First, when we supplemented Microcystis diets with an ecologically relevant concentration of microcystin-LR, we further increased the inhibition of infection by our fungal parasite (figure 1a). Second, the increase of anatoxin-a in Metschnikowia treatments (figure 4c) was associated with complete inhibition of fungal infection (figure 1a). Third, rather than preventing fungal infection, increasing concentrations of nodularin were correlated positively with the fecundity of infected Daphnia, but negatively with the fecundity of uninfected Daphnia (figure 4b). This last result is typical of medicines that operate after infection, in which there are often significant costs of consuming medicines in the absence of disease [11,66]. These antimicrobial effects of cyanobacterial toxins are consistent with an earlier study that found reduced bacterial growth when Microcystis was plated with Escherichia coli [62]. Additionally, another study found induction of oligopeptides, including microcystin, in a Planktothrix strain helped the cyanobacteria resist infection by a chytrid parasite [67].
We also observed apparent anti-fungal activity in the green alga, Chlorella. There has been relatively little research on the secondary metabolites produced by this species; however, chlorellin (a mixture of fatty acids and hydrocarbons; [68,69]) was reported to have detrimental effects on bacteria, fungi, and Chlorella itself [70–72]. However, we cannot discount the possible role of nutrients in mediating immune system responses rendering hosts less susceptible to infection. In two separate studies looking at Pasteuria and Pseudomonas parasites, Daphnia magna feeding on the higher quality Nannochloropsis limnetica diet were less susceptible to infection by these parasites than were animals feeding on Scenedesmus. The authors did not find allelopathic effects of Nannochloropsis on Pseudomonas in separate agar assays, suggesting that reduced susceptibility may be the product of an immune system response by the host [58,73]. We plan to conduct further research on the anti-microbial properties of microcystin-LR, anatoxin-a and chlorellin. Future studies that coat these toxins onto particles of nutritious food would help disentangle the effects of nutrition and toxins on host–parasite interactions.
Notably, the influence of phytoplankton diet on parasite fitness (spore yield) differed between the two parasites. Post-infection, diet did not appear to influence fitness of the fungus Metschnikowia; in contrast, the bacterium Pasteuria performed better when Daphnia were fed cyanobacteria diets. In contrast to our results, Schlotz et al. [58] found that parasite spore production was higher on higher quality diets (diets that contained long-chain PUFAs or were supplemented with eicosapentaenoic acid) but the risk of infection on these high-quality diets was actually low compared to diets lacking these essential PUFAs [58]. These conflicting results highlight the importance of nutrient and secondary metabolite composition for the ability of daphniids to resist parasites. Thus, it is not surprising that previous studies have found conflicting results, with some finding increased infection and/or spore yield with higher food quality or quantity [58,63,64] while others have found decreased infection and/or spore yield [62,74–76]. Likewise, there have been opposing results regarding whether cyanobacteria increase or decrease disease in Daphnia [62–65]. We propose that studies that jointly examine nutritional value, toxicity and medicinal value of diets will help to disentangle these varied responses.
While our results indicate strong medicinal effects of some phytoplankton diets, we do not yet know if Daphnia can self-medicate. Self-medication would require that Daphnia feed selectively and alter relative phytoplankton consumption based on disease risk [11]. Numerous studies provide direct or indirect evidence for selective feeding by Daphnia [74–79]. While dietary preferences of Daphnia remain unclear, studies suggest that phytoplankton size, digestibility and nutrient quality influence selectivity [77,78]. To our knowledge, selective foraging in the presence and absence of parasites has not yet been investigated in Daphnia. In terrestrial systems, many animals (including invertebrates) choose lower quality and/or toxic plants only when confronted with disease agents [11]. Given the range of secondary metabolites produced by phytoplankton, our study highlights the importance of studying selective grazing by Daphnia under variable parasite threat, as well as the importance of studying the potential for self-medication in aquatic ecosystems.
Global environmental change continues to influence aquatic ecosystems [33–35,80]. Harmful algal blooms are expected to increase in frequency and toxicity [80] and may have differential effects on parasites and their hosts [33,34,37]. Most importantly, our research suggests that increases in cyanobacterial blooms might decrease the frequency of Daphnia infection by the fungal parasite, while increasing spore production in the bacterial parasite. Therefore, cyanobacterial blooms would be expected to decrease the prevalence of the fungal parasite but might increase that of the bacterium. This could have important consequences for lake food webs, as Daphnia are key grazers and disease outbreaks within Daphnia populations can influence ecosystem-level processes [41].
Our research highlights the impact of phytoplankton toxins on disease in zooplankton. The observation of context-dependent effects of phytoplankton quality on host and parasite fitness helps reconcile previous contradictory reports of phytoplankton–Daphnia–parasite interactions. This research also emphasizes the need to study additional aspects of phytoplankton diets as well as the behavioural and immune responses of aquatic hosts to their parasites. Our evidence of non-conventional roles for phytoplankton toxins in aquatic ecosystems has relevance not only for Daphnia, but for phytoplankton consumers more generally.
Supplementary Material
Acknowledgements
We thank T. Duda for his comments on this manuscript, K. Hunsberger, C. Shaw and H. Streit for their laboratory assistance, J. Morris and T. Ong for their assistance on data visualization and J. Megahan for illustrations in figure 4 and electronic supplementary material, figure S10. We also thank four reviewers for their feedback on this manuscript.
Ethics
No humans or vertebrate organisms were used in this study.
Data accessibility
Data are available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.31b9h5m [81].
Authors' contributions
All authors certify that they have participated in the work and take responsibility for the content of this manuscript. K.F.S. performed experiments and analysis of samples, analysed and interpreted data, wrote the manuscript and acts as corresponding author. N.H. performed experiments and analysis of samples, helped in data interpretation and manuscript evaluation. M.A.D. and M.D.H. supervised the development of work, analysed and interpreted data, and helped write and edit the manuscript.
Competing interests
We declare no competing interests.
Funding
This work was supported by grants from the Department of Ecology and Evolutionary Biology and the Frontiers Master's program at the University of Michigan, and by the US National Science Foundation (DEB-1353806 to M.A.D. and DEB-1256115 to M.D.H.).
References
- 1.Vining LC. 1990. Functions of secondary structural complexity of secondary metabolites. Annu. Rev. Microbiol. 44, 395–427. ( 10.1146/annurev.mi.44.100190.002143) [DOI] [PubMed] [Google Scholar]
- 2.Leflaive J, Ten-Hage L. 2007. Algal and cyanobacterial secondary metabolites in freshwaters: a comparison of allelopathic compounds and toxins. Freshw. Biol. 52, 199–214. ( 10.1111/j.1365-2427.2006.01689.x) [DOI] [Google Scholar]
- 3.Hunter MD. 2016. The phytochemical landscape. Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Paul VJ, Van Alstyne KL.. 1992. Activation of chemical defenses in the tropical green algae Halimeda spp. J. Exp. Mar. Biol. Ecol. 160, 191–203. ( 10.1016/0022-0981(92)90237-5) [DOI] [Google Scholar]
- 5.Hay ME, Quaker KE, Fenical W. 1994. Synergisms in plant defenses against herbivores: interactions of chemistry, calcification, and plant quality. Ecology 75, 1714–1726. ( 10.2307/1939631) [DOI] [Google Scholar]
- 6.Agrawal AA, Petschenka G, Bingham RA, Weber MG, Rasmann S. 2012. Toxic cardenolides: chemical ecology and coevolution of specialized plant-herbivore interactions. New Phytol. 194, 28–45. ( 10.1111/j.1469-8137.2011.04049.x) [DOI] [PubMed] [Google Scholar]
- 7.Hay ME, Duffy JE, Pfister CA, Fenical W. 1987. Chemical defense against different marine herbivores: are amphipods insect equivalents? Ecology 68, 1567–1580. ( 10.2307/1939849) [DOI] [PubMed] [Google Scholar]
- 8.Hay ME, Pawlik JR, Duffy JE, Fenical W. 1989. Seaweed-herbivore-predator interactions: host-plant specialization reduces predation on small herbivores. Oecologia 81, 418–427. ( 10.1007/BF00377093) [DOI] [PubMed] [Google Scholar]
- 9.Gowler CD, Leon KE, Hunter MD, De Roode JC.. 2015. Secondary defense chemicals in milkweed reduce parasite infection in monarch butterflies, Danaus plexippus. J. Chem. Ecol. 41, 520–523. ( 10.1007/s10886-015-0586-6) [DOI] [PubMed] [Google Scholar]
- 10.Sorensen FJ, Dearing MD, Gross EM, Orians CM, Sotka EE, Foley WJ. 2013. A pharm-ecological perspective of terrestrial and aquatic plant-herbivore interactions. J. Chem. Ecol. 39, 465–480. ( 10.1007/s10886-013-0267-2) [DOI] [PubMed] [Google Scholar]
- 11.de Roode JC, Lefèvre T, Hunter MD.. 2013. Self-medication in animals. Science 340, 150–151. ( 10.1126/science.1235824) [DOI] [PubMed] [Google Scholar]
- 12.Huffman MA. 2003. Animal self-medication and ethno-medicine: exploration and exploitation of the medicinal properties of plants. Proc. Nutr. Soc. 62, 371–381. ( 10.1079/PNS2003257) [DOI] [PubMed] [Google Scholar]
- 13.Lefèvre T, Oliver L, Hunter MD, De Roode JC.. 2010. Evidence for trans-generational medication in nature. Ecol. Lett. 13, 1485–1493. ( 10.1111/j.1461-0248.2010.01537.x) [DOI] [PubMed] [Google Scholar]
- 14.Sotka EE, Forbey J, Horn M, Poore AGB, Raubenheimer D, Whalen KE. 2009. The emerging role of pharmacology in understanding consumer-prey interactions in marine and freshwater systems. Integr. Comp. Biol. 49, 291–313. ( 10.1093/icb/icp049) [DOI] [PubMed] [Google Scholar]
- 15.Borowitzka MA. 1995. Microalgae as sources of pharmaceuticals and other biologically active compounds. J. Appl. Phycol. 7, 3–15. ( 10.1007/BF00003544) [DOI] [Google Scholar]
- 16.Volk RB, Furkert FH. 2006. Antialgal, antibacterial and antifungal activity of two metabolites produced and excreted by cyanobacteria during growth. Microbiol. Res. 161, 180–186. ( 10.1016/j.micres.2005.08.005) [DOI] [PubMed] [Google Scholar]
- 17.Lane AL, et al. 2009. Desorption electrospray ionization mass spectrometry reveals surface-mediated antifungal chemical defense of a tropical seaweed. Proc. Natl Acad. Sci. USA 106, 7314–7319. ( 10.1073/pnas.0812020106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evangelista V, Barsanti L, Frassanito A. 2008. Algal toxins: nature, occurrence, effect and detection. Dordrecht, Netherlands: Springer. [Google Scholar]
- 19.DeMott WR, Zhang Q, Carmichael WW. 1991. Effects of toxic cyanobacteria and purified toxins on the survival and feeding of a copepod and three species of Daphnia. Limnol. Oceanogr. 36, 1346–1357. ( 10.4319/lo.1991.36.7.1346) [DOI] [Google Scholar]
- 20.Ferrao-Filho A, Azevedo SM, Demott WR. 2000. Effects of toxic and non-toxic cyanobacteria on the life history of tropical and temperate cladocerans. Freshw. Biol. 45, 1–19. ( 10.1046/j.1365-2427.2000.00613.x) [DOI] [Google Scholar]
- 21.Schwarzenberger A, et al. 2014. Deciphering the genetic basis of microcystin tolerance. BMC Genomics15, 776.
- 22.Wilson AE, Sarnelle O, Tillmanns AR. 2006. Effects of cyanobacterial toxicity and morphology on the population growth of freshwater zooplankton: meta-analyses of laboratory experiments. Limnol. Oceanogr. 51, 1915–1924. ( 10.4319/lo.2006.51.4.1915) [DOI] [Google Scholar]
- 23.Asselman J, Hochmuth JD, De Schamphelaere KAC. 2014. A comparison of the sensitivities of Daphnia magna and Daphnia pulex to six different cyanobacteria. Harmful Algae 39, 1–7. ( 10.1016/j.hal.2014.06.008) [DOI] [Google Scholar]
- 24.Rantala A, Fewer DP, Hisbergues M, Rouhiainen L, Vaitomaa J, Borner T, Sivonen K. 2004. Phylogenetic evidence for the early evolution of microcystin synthesis. Proc. Natl Acad. Sci. USA 101, 568–573. ( 10.1073/pnas.0304489101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berry JP, Gantar M, Perez MH, Berry G, Noriega FG. 2008. Cyanobacterial toxins as allelochemicals with potential applications as algaecides, herbicides and insecticides. Mar. Drugs 6, 117–146. ( 10.3390/md6020117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raubenheimer D, Simpson SJ. 2009. Nutritional PharmEcology: doses, nutrients, toxins, and medicines. Integr. Comp. Biol. 49, 329–337. ( 10.1093/icb/icp050) [DOI] [PubMed] [Google Scholar]
- 27.Povey S, Cotter SC, Simpson SJ, Wilson K. 2014. Dynamics of macronutrient self-medication and illness-induced anorexia in virally infected insects. J. Anim. Ecol. 83, 245–255. ( 10.1111/1365-2656.12127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smilanich AM, Dyer LA, Chambers JQ, Bowers MD. 2009. Immunological cost of chemical defence and the evolution of herbivore diet breadth. Ecol. Lett. 12, 612–621. ( 10.1111/j.1461-0248.2009.01309.x) [DOI] [PubMed] [Google Scholar]
- 29.Singer MS, Mason PA, Smilanich AM. 2014. Ecological immunology mediated by diet in herbivorous insects. Integr. Comp. Biol. 54, 913–921. ( 10.1093/icb/icu089) [DOI] [PubMed] [Google Scholar]
- 30.de Roode JC, Pedersen AB, Hunter MD, Altizer S.. 2008. Host plant species affects virulence in monarch butterfly parasites. J. Anim. Ecol. 77, 120–126. ( 10.1111/j.1365-2656.2007.01305.x) [DOI] [PubMed] [Google Scholar]
- 31.Sternberg ED, Li J, De Castillejo CLF, Li H, Hunter MD, De Roode JC. 2011. Food plant-derived disease tolerance and resistance in a natural butterfly-plant-parasite interactions. Evolution 66, 3367–3376. ( 10.1111/j.1558-5646.2012.01693.x) [DOI] [PubMed] [Google Scholar]
- 32.Tao L, Berns AR, Hunter MD. 2014. Why does a good thing become too much? Interactions between foliar nutrients and toxins determine performance of an insect herbivore. Funct. Ecol. 28, 190–196. ( 10.1111/1365-2435.12163) [DOI] [Google Scholar]
- 33.Montes-Hugo M, Doney SC, Ducklow HW, Fraser W, Martinson D, Stammerjohn SE, Schofield O. 2009. Recent changes in phytoplankton communities associated with rapid regional climate change along the western Antarctic Peninsula. Science 323, 1470–1473. ( 10.1126/science.1164533) [DOI] [PubMed] [Google Scholar]
- 34.Hallegraeff GM. 2010. Ocean climate change, phytoplankton community responses, and harmful algal blooms: a formidable predictive challenge. J. Phycol. 46, 220–235. ( 10.1111/j.1529-8817.2010.00815.x) [DOI] [Google Scholar]
- 35.Hoegh-Guldberg O, Bruno J. 2010. The impact of climate change on the world's marine ecosystems. Science 328, 1523–1528. ( 10.1126/science.1189930) [DOI] [PubMed] [Google Scholar]
- 36.Paul VJ. 2008. Global warming and cyanobacterial harmful algal blooms. Adv. Exp. Med. Biol. 619, 239–257. ( 10.1007/978-0-387-75865-7_11) [DOI] [PubMed] [Google Scholar]
- 37.Paerl HW, Paul VJ. 2012. Climate change: links to global expansion of harmful cyanobacteria. Water Res. 46, 1349–1363. ( 10.1016/j.watres.2011.08.002) [DOI] [PubMed] [Google Scholar]
- 38.Muller-Solger AB, Jassby AD, Muller-Navarra DC. 2002. Nutritional quality of food resources for zooplankton (Daphnia) in a tidal freshwater system (Sacramento, San Joaquin River Delta). Limnol. Oceanogr. 47, 1468–1476. ( 10.4319/lo.2002.47.5.1468) [DOI] [Google Scholar]
- 39.Carmichael WW, Boyer GL. 2016. Health impacts from cyanobacteria harmful algae blooms: implications for the North American Great Lakes. Harmful Algae 54, 194–212. ( 10.1016/j.hal.2016.02.002) [DOI] [PubMed] [Google Scholar]
- 40.Martin-Creuzburg D, Elert E, Hoffmann KH. 2008. Nutritional constraints at the cyanobacteria–Daphnia magna interface: the role of sterols. Limnol. Oceanogr. 53, 456–468. ( 10.4319/lo.2008.53.2.0456) [DOI] [Google Scholar]
- 41.Duffy MA. 2007. Selective predation, parasitism, and trophic cascades in a bluegill-Daphnia-parasite system. Oecologia 153, 453–460. ( 10.1007/s00442-007-0742-y) [DOI] [PubMed] [Google Scholar]
- 42.Ahlgren G, Lundstedt L, Brett M, Forsberg C. 1990. Lipid composition and food quality of some freshwater phytoplankton for cladoceran zooplankters. J. Plankton Res. 12, 809–818. ( 10.1093/plankt/12.4.809) [DOI] [Google Scholar]
- 43.Ahlgren G, Gustafsson IB, Boberg M. 1992. Fatty-acid content and chemical-composition of fresh-water microalgae. J. Phycol. 28, 37–50. ( 10.1111/j.0022-3646.1992.00037.x) [DOI] [Google Scholar]
- 44.Rohrlack T, Dittmann E, Henning M, Börner T, Kohl JG. 1999. Role of microcystins in poisoning and food ingestion inhibition of Daphnia galeata caused by the cyanobacterium Microcystis aeruginosa. Appl. Environ. Microbiol. 65, 737–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cembella AD. 2003. Chemical ecology of eukaryotic microalgae in marine ecosystems. Phycologia 42, 420–447. ( 10.2216/i0031-8884-42-4-420.1) [DOI] [Google Scholar]
- 46.Pohnert G, Steinke M, Tollrian R. 2007. Chemical cues, defence metabolites and the shaping of pelagic interspecific interactions. Trends Ecol. Evol. 22, 198–204. ( 10.1016/j.tree.2007.01.005) [DOI] [PubMed] [Google Scholar]
- 47.Tessier AJ, Woodruff P. 2017. Cryptic trophic cascade along a gradient of lake size. Ecology 83, 1263–1270. ( 10.1890/0012-9658(2002)083[1263:CTCAAG]2.0.CO;2) [DOI] [Google Scholar]
- 48.Auld SKJR, Hall SR, Duffy MA. 2012. Epidemiology of a Daphnia-multiparasite system and its implications for the Red Queen. PLoS ONE 7, 1–6. ( 10.1371/journal.pone.0039564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Auld SKJR, Hall SR, Ochs JH, Sebastian M, Duffy MA. 2014. Predators and patterns of within-host growth can mediate both among-host competition and evolution of transmission potential of parasites. Am. Nat. 184, S77–S90. ( 10.1086/676927) [DOI] [PubMed] [Google Scholar]
- 50.Ebert D. 2005. Ecology, epidemiology, and evolution of parasitism in Daphnia. Bethesda, MD: National Center for Biotechnology Information; See https://www.ncbi.nlm.nih.gov/books/NBK2036/. [Google Scholar]
- 51.Clay PA, Dhir KL, Rudolf VHW, Duffy MA. 2018. Within-host priority effects systematically alter pathogen coexistence. Am. Nat. 193, 1–58. [DOI] [PubMed] [Google Scholar]
- 52.Tessier AJ, Henry LL, Goulden CE, Durand MW. 1983. Starvation in Daphnia: energy reserves and reproductive allocation. Limnol. Oceanogr. 28, 667–676. ( 10.4319/lo.1983.28.4.0667) [DOI] [Google Scholar]
- 53.Francy DS, et al. 2015. Water quality, cyanobacteria, and environmental factors and their relations to microcystin concentrations for use in predictive models at Ohio Lake Erie and Inland Lake recreational sites, 2013–14. Reston, VA: US Geological Survey.
- 54.Jones GJ, Orr PT. 1994. Release and degradation of microcystin following algicide treatment of a Microcystis aeruginosa bloom in a recreational lake, as determined by HPLC and protein phosphatase inhibition assay. Water Res. 28, 871–876. ( 10.1016/0043-1354(94)90093-0) [DOI] [Google Scholar]
- 55.Ibelings BW, Bruning K, De Jonge J, Wolfstein K, Pires LMD, Postma J, Burger T. 2005. Distribution of microcystins in a lake foodweb: no evidence for biomagnification. Microb. Ecol. 49, 487–500. ( 10.1007/s00248-004-0014-x) [DOI] [PubMed] [Google Scholar]
- 56.Ha JH, Hidaka T, Tsuno H. 2009. Quantification of toxic microcystis and evaluation of its dominance ratio in blooms using real-time PCR. Environ. Sci. Technol. 43, 812–818. ( 10.1021/es801265f) [DOI] [PubMed] [Google Scholar]
- 57.Littell RC, Stroup WW, Freund RJ. 2002. SAS for linear models, 4th edn Cary, NC: SAS Institute. [Google Scholar]
- 58.Schlotz N, Ebert D, Martin-creuzburg D. 2013. Dietary supply with polyunsaturated fatty acids and resulting maternal effects influence host–parasite interactions. BMC Ecol. 13, 41 ( 10.1186/1472-6785-13-41) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.U.S. EPA (United States Environmental Protection Agency). 2015. Health Effects Support Document for the Cyanobacterial Toxin Anatoxin-a. EPA 820R15104, Washington, DC; June, 2015. Available from http://water.epa.gov/drink/standards/hascience.cfm. Washington, DC: USEPA.
- 60.Hall SR, Simonis JL, Nisbet RM, Tessier AJ, Cáceres CE. 2009. Resource ecology of virulence in a planktonic host-parasite system: an explanation using dynamic energy budgets. Am. Nat. 174, 149–162. ( 10.1086/600086) [DOI] [PubMed] [Google Scholar]
- 61.Hall SR, Becker CR, Duffy MA, Cáceres CE. 2012. A power-efficiency trade-off in resource use alters epidemiological relationships. Ecology 93, 645–656. ( 10.1890/11-0984.1) [DOI] [PubMed] [Google Scholar]
- 62.Coopman M, Muylaert K, Lange B, Reyserhove L, Decaestecker E. 2014. Context dependency of infectious disease: the cyanobacterium Microcystis aeruginosa decreases white bacterial disease in Daphnia magna. Fresw. Biol. 59, 714–723. ( 10.1111/fwb.12298) [DOI] [Google Scholar]
- 63.Lange B, Reuter M, Ebert D, Muylaert K, Decaestecker E. 2014. Diet quality determines interspecific parasite interactions in host populations. Ecol. Evol. 4, 3093–3102. ( 10.1002/ece3.1167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Penczykowski RM, Lemanski BCP, Sieg RD, Hall SR, Housley Ochs J, Kubanek J, Duffy MA. 2014. Poor resource quality lowers transmission potential by changing foraging behaviour. Funct. Ecol. 28, 1245–1255. ( 10.1111/1365-2435.12238) [DOI] [Google Scholar]
- 65.Tellenbach C, Tardent N, Pomati F, Keller B, Hairston NG, Wolinska J, Spaak P. 2016. Cyanobacteria facilitate parasite epidemics in Daphnia. Ecology 97, 3422–3432. ( 10.1002/ecy.1576) [DOI] [PubMed] [Google Scholar]
- 66.Singer MS, Mace KC, Bernays EA. 2009. Self-medication as adaptive plasticity: increased ingestion of plant toxins by parasitized caterpillars. PLoS ONE 4, e4796 ( 10.1371/journal.pone.0004796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rohrlack T, Christiansen G, Kurmayer R. 2013. Putative antiparasite defensive system involving ribosomal and nonribosomal oligopeptides in cyanobacteria of the genus Planktothrix. Appl. Environ. Microbiol. 79, 2642–2647. ( 10.1128/AEM.03499-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spoehr HA, Smith JHC, Strain HH, Milner HW, Hardin GJ. 1949. Fatty acid antibacterials from plants. Washington, DC: Carnegie Institution of Washington Publication. [Google Scholar]
- 69.DellaGreca M, Zarrelli A, Fergola P, Cerasuolo M, Pollio A, Pinto G. 2010. Fatty acids released by Chlorella vulgaris and their role in interference with Pseudokirchneriella subcapitata: experiments and modelling. J. Chem. Ecol. 36, 339–349. ( 10.1007/s10886-010-9753-y) [DOI] [PubMed] [Google Scholar]
- 70.Pratt R, et al. 1944. Chlorellin, an antibacterial substance from Chlorella. Science 99, 351–352. ( 10.1126/science.99.2574.351) [DOI] [PubMed] [Google Scholar]
- 71.Hayes LE. 1947. Survey of higher plants for presence of antibacterial substances. Bot. Gaz. 108, 408–414. ( 10.1086/335424) [DOI] [Google Scholar]
- 72.Scutt JE. 1964. Autoinhibitor production by Chlorella vulgaris. Am. J. Bot. 51, 581–584. ( 10.1002/j.1537-2197.1964.tb06674.x) [DOI] [Google Scholar]
- 73.Schlotz N, Pester M, Freese HM, Martin-creuzburg D. 2014. A dietary polyunsaturated fatty acid improves consumer performance during challenge with an opportunistic bacterial pathogen. FEMS Microbiol. Ecol. 90, 467–477. ( 10.1111/1574-6941.12407) [DOI] [PubMed] [Google Scholar]
- 74.Hall SR, Sivars-Becker L, Becker C, Duffy MA, Tessier AJ. 2007. Eating yourself sick: transmission of disease as a function of foraging ecology. Ecol. Lett. 10, 207–218. ( 10.1111/j.1461-0248.2007.01011.x) [DOI] [PubMed] [Google Scholar]
- 75.Frost PC, Ebert D, Smith VH. 2008. Responses of a bacterial pathogen to phosphorus limitation of its aquatic invertebrate host. Ecology 89, 313–318. ( 10.1890/07-0389.1) [DOI] [PubMed] [Google Scholar]
- 76.Schoebel CN, Wolinska J, Spaak P. 2010. Higher parasite resistance in Daphnia populations with recent epidemics. J. Evol. Biol. 23, 2370–2376. ( 10.1111/j.1420-9101.2010.02097.x) [DOI] [PubMed] [Google Scholar]
- 77.Porter KG. 1973. Selective grazing and differential digestion of algae by zooplankton. Nature 244, 179–180. ( 10.1038/244179a0) [DOI] [Google Scholar]
- 78.Yin XW, Liu PF, Zhu SS, Chen XX. 2010. Food selectivity of the herbivore Daphnia magna (Cladocera) and its impact on competition outcome between two freshwater green algae. Hydrobiologia 655, 15–23. ( 10.1007/s10750-010-0399-0) [DOI] [Google Scholar]
- 79.Rakowski C, Cardinale BJ. 2016. Herbivores control effects of algal species richness on community biomass and stability in a laboratory microcosm experiment. Oikos 125, 1626–1635. ( 10.1111/oik.03105) [DOI] [Google Scholar]
- 80.Michalak AM, et al. 2013. Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proc. Natl Acad. Sci. USA 110, 6448–6452. ( 10.1073/pnas.1216006110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sánchez KF, Huntley N, Duffy MA, Hunter MD. 2019. Data from: Toxins or medicines? Phytoplankton diets mediate host and parasite fitness in a freshwater system Dryad Digital Repository. ( 10.5061/dryad.31b9h5m) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Sánchez KF, Huntley N, Duffy MA, Hunter MD. 2019. Data from: Toxins or medicines? Phytoplankton diets mediate host and parasite fitness in a freshwater system Dryad Digital Repository. ( 10.5061/dryad.31b9h5m) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.31b9h5m [81].