Abstract
The fossilized traces of burrowing worms have taken on a considerable importance in studies of the Cambrian explosion, partly because of their use in defining the base of the Cambrian. Foremost among these are the treptichnids, a group of relatively large open probing burrows that have sometimes been assigned to the activities of priapulid scalidophoran worms. Nevertheless, most Cambrian burrows have an uncertain progenitor. Here we report a suite of exceptionally preserved trace and body fossils from sandstones of the lower Cambrian (Stage 4) File Haidar Formation of southern Sweden that can unequivocally be assigned to a scalidophoran producer. We further present the first burrow casts produced via actualistic experiments on living priapulids, and demonstrate the remarkable morphological parallels between these modern and Cambrian fossil equivalents. In addition, co-occurrence of scalidophoran-derived cuticular remains permits a unique synthesis of evidence from trace fossil, body and organic remains. Comparative analysis of these exceptionally preserved fossils supports a scalidophoran producer for treptichnids and by extension suggests a latest Ediacaran origin of the ecdysozoan clade.
Keywords: priapulid, trace fossil, Cambrian evolutionary radiation, Treptichnus, Ecdysozoa
1. Introduction
The origin of animals close to the Ediacaran–Cambrian boundary permanently transformed ecology and earth systems more generally [1–4]. One of the most important components of this revolution was the transformation of marine substrates by sediment churning bilaterians (e.g. [5]). Indeed, the Global Boundary Stratotype Section and Point (GSSP) marking the base of the Cambrian at Fortune Head, Newfoundland, is defined by the appearance of complex trace fossils, notably the canonical Treptichnus pedum [6,7]. In common with most other trace fossils, frustratingly little is known about the affinities of their producers. Despite this uncertainty, however, there have been consistent suggestions that some of the earliest large burrows, such as T. pedum, were made by priapulid-like scalidophoran worms [8,9]. Treptichnids, however, pre-date the oldest body fossil records of scalidophorans (e.g. [10–13]).
Well-preserved Cambrian body fossil assemblages show that scalidophoran worms were both common and diverse faunal constituents of level bottom communities [14]. They are abundant in Cambrian Konservat-Lagerstätten (e.g. [15,16]) and as minute phosphatized larval forms [17,18]. Complete scalidophoran worms are rarely known outside the Cambrian Lagerstätten, typically being inferred from the presence of characteristic sclerites such as Hadimopanella and Milaculum (e.g. [19]) or from the burgeoning small carbonaceous fossil (SCF) biota (e.g. [20–22]).
With few exceptions [23–25], Cambrian scalidophorans were priapulid-like, even if their precise affinities within total-group Cycloneuralia remain contested [14,26–28]. Extant priapulids are surprisingly widespread in modern marine settings, in terms of water depth, salinity and substrate (e.g. [29]), despite a persistent view in the palaeobiological literature that they have been marginalized to low-oxygen environments, perhaps through increased competition with annelids (e.g. [15]).
In addition to scattered scalidophoran body fossil sources, trace fossils are a primary source of information, especially in otherwise poorly fossiliferous facies. Here, then, we present trace and body fossils from shallow marine sandstones of the lower Cambrian of Baltica that were clearly made by priapulid-like scalidophorans, and we further discuss their relevance to the Ediacaran–Cambrian treptichnids.
2. Exceptional trace/body fossil preservation
One strength of the trace fossil record in general is its unexceptional nature, in terms of its almost universal distribution across most sedimentary facies (including a continuous record in the otherwise poorly fossiliferous early Cambrian [30]). Nevertheless, some trace fossil assemblages may reasonably be regarded as ‘trace fossil Lagerstätten’ for their diversity and extraordinary fidelity of preservation, often at sub-millimetric levels (e.g. [31]). One such example is the lower Cambrian Mickwitzia Sandstone Member (MSM) of the File Haidar Formation (figure 1) of southern Sweden [32]. The MSM is an approximately 10 m-thick heterolithic unit characterized by thin-bedded siltstones and fine-grained sandstones containing mudstone interbeds. It is typically considered to be of late early Cambrian age (Stage 4) but in principle could be somewhat older. Sedimentary structures point towards deposition being largely in the offshore to lower shoreface, influenced by storms and possibly tidally driven wave action [32]. Certain sandstone bed surfaces of the MSM preserve an extraordinary range of trace fossils, including trilobite hunting traces [33], constructional stages of Cruziana [34] and well-preserved examples of treptichnids.
Figure 1.
Map and stratigraphic position of the Mickwitzia Sandstone Member (MSM), File Haidar Formation (Cambrian Series 2 Stage 4). Fossil material in this study was collected from Hjälmsäter, Västergötland, south-central Sweden. Coordinates 58.579696, 13.329519.
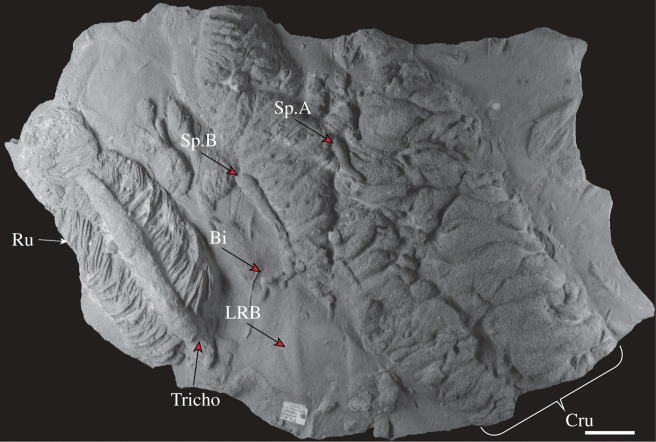
The fossils we document here occur in positive semirelief on the base of a sandstone bed (Sp.A and Sp.B in figure 2). They are associated with a large, partly washed-out Cruziana and Rusophycus (figure 2). Specimen A is a discrete cylindrical structure of a few centimetres in length and of relatively constant width (0.5–0.8 cm) with a gentle curve. It displays a series of well-defined transverse annulations, each approximately 0.4 mm wide, together with fainter longitudinal striations (figure 3a). Specimen B is a cumulative structure that represents several distinct probing attempts by the maker, in total consisting of approximately 16 cm burrow, with two smaller bulb-like downward-pointing branches emerging approximately at 6 cm along the main burrow (figures 3b and 4a). The bulb-like excursions show 20 symmetrically radiating striations which bifurcate distally from a small featureless anterior depression (figure 4b–d). Following these excursions, the main burrow continues at considerably lower relief.
Figure 2.
Sandstone slab from the MSM preserving a large partially washed-out Cruziana (Cru), within which are Specimens A and B that we attribute to priapulids. The Cruziana is flanked by a Rusophycus (Ru) intersecting a Trichophycus (Tricho). Note that the prominent burrow comprising Specimen B continues as a faintly preserved low relief burrow (LRB) after a series of bifurcations (Bi). Slab specimen number SMN X172. Scale bar 4 cm.
Figure 3.
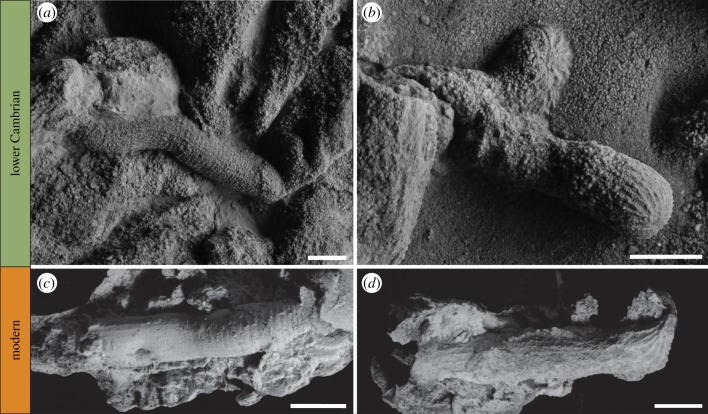
(a) Specimen A, discrete cylindrical structure interpreted as a mouldic priapulid body fossil. (b) Specimen B, elongated burrow, with bulb-shaped aborted probes emanating from the main burrow. (c,d) Plaster of Paris casts of extant Priapulus caudatus burrows exhibiting strikingly similar wall ornamentation to that of Specimens A, B. Scale bars: 5 mm (a,b), 10 mm (c), 8 mm (d).
Figure 4.
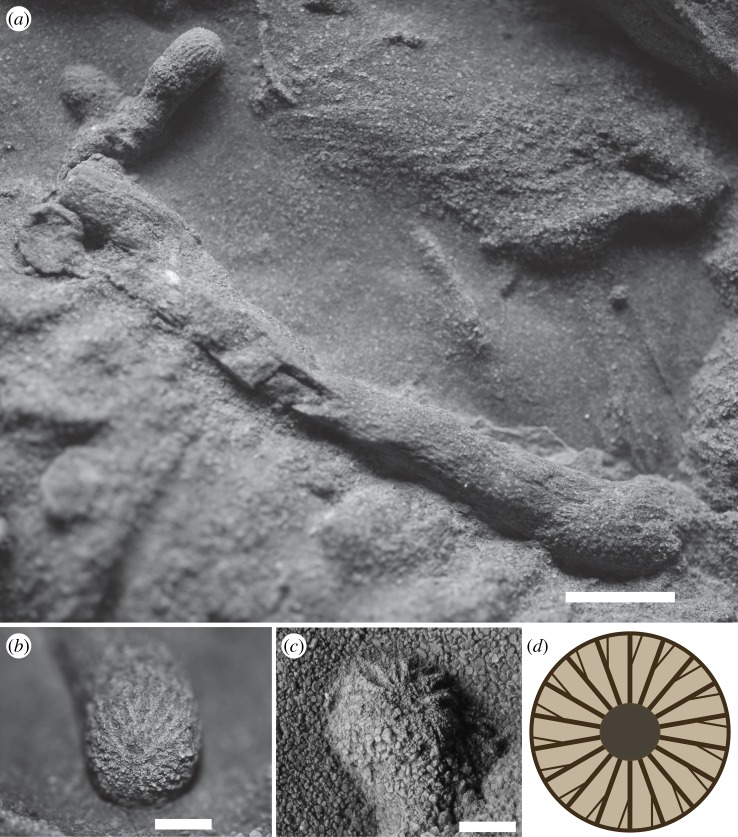
(a) Close-up of Specimen B detailing lateral bulbous protrusions (aborted probes). (b,c) Anterior details of bulb-like terminations in Specimen B, illustrating 20 radiating longitudinal striations. Note that striations bifurcate distally, indicating rotation of the worm's prosoma during excavation. (d) Schematic of morphology observed in (b,c) Scale bars: 5 mm (a), 2 mm (b,c).
3. Anatomy of the producer
The fossils reveal unusual and exceptionally fine details about the external anatomy of early Cambrian worms. Indeed, because Specimen A (figure 3a) appears to be a cast of the organism's external surface, it seems reasonable to consider it as a body rather than trace fossil.
The division of Specimen B (figure 4a) into a bulbous protrusion with longitudinal striations and a longer annulated posterior portion is strikingly reminiscent of a modern priapulid such as Priapulus caudatus with its introvert extended (figure 5). This posture occurs during the normal burrowing cycle of priapulids [9,35]. Extant adult priapulids possess rings of scalids organized into 20 (e.g. in Tubiluchus [36]) or 25 longitudinal rows (most other genera), consistent with the number of anterior longitudinal striations in these fossils.
Figure 5.
Cycle of activity of Priapulus caudatus. Invagination of the prosoma precedes its forceful expulsion and penetration of the sediment where scalids, later acting as anchoring hooks, enable the forward movement of the organism.
In order to further understand the relationship between trace and body fossils and the anatomy of priapulids, P. caudatus burrows were experimentally produced, cast and photographed (figure 3c,d). Muddy sediment was introduced into small (20 cm by 30 cm) plastic tanks and allowed to settle for about one month until it reached a final thickness of about 20 cm. The sediment was then covered with a thin layer of fine sand (approx. 3 cm). Specimens of P. caudatus were allowed to burrow for between 1 and 4 days. The plastic tanks were removed and the sediment gently opened so as to examine burrow morphologies formed within the mud. The most frequently observed feature of the burrow margin are longitudinal narrow grooves. These features were observed only in either the terminal parts of a burrow or aborted probes. This suggests that the subsequent passage of the body largely obliterates details of anatomical imprints. Some of the open burrows were subsequently cast with plaster of Paris. In tanks where the mud was covered by a layer of sand, this readily sifted into the open burrow, and eventually filled the burrow to a depth of at least 10 cm. This suggests that casting of the type of anatomical detail reproduced above by plaster of Paris should readily occur under natural conditions.
The experimentally produced casts reveal a suite of features also seen in the fossils we describe. The burrow surface of P. caudatus bears longitudinal grooves, which in casts show as ridges (figure 3c,d). These ridges are spaced at approximately 1 mm. Their position corresponds to longitudinal rows of conical sensory papillae on the proboscis (scalids), which also have the mechanical function of increasing friction during the burrowing cycle and so reduce back-slip. Under each row of scalids, there are longitudinal muscles separated into distinct bands distal to the circular muscles [16,37,38]. The grooves may therefore be formed by either the scalids or a combination of scalids and muscle bands, and appear identical in their formation to the longitudinal ridges observed in the Cambrian material (compare figure 3a,b with figure 3c,d, respectively). Longitudinal ridges or striations are often ascribed to an arthropod producer (e.g. [39]), but the above shows that scalidophorans can form such bioprints.
A scalidophoran affinity for these trace/body fossils is further supported by the coeval presence of scalidophoran cuticular fragments (scalids and teeth) preserved as SCFs in muddy intervals of the File Haidar Formation (electronic supplementary material, figure S1). Overall, then, our data suggest that priapulid-like scalidophorans are present in both sandy and muddy facies of the early Cambrian File Haidar Formation.
4. Ichnotaxonomy
While the detailed morphology of Specimen B cannot be exactly compared to known ichnotaxa, it may nevertheless be relevant to discussions about the producers of treptichnids. Treptichnids such as T. rectangularis (Orłowski and Żylińska [40]; = Manykodes rectangularis of Dzik [8]) show both a similar morphology, and longitudinal striations along the burrows, that Dzik [8] estimates to number 15–20, compatible with the number of introvert scalid rows estimated for our material. Both T. pedum and T. bifurcus occur in the MSM (see fig. 62 in Jensen [32]; (electronic supplementary material, figure S2)) and it is possible that the material we describe here has the same producer (most probably the same as T. bifurcus based on the similar terminal probe morphology). In particular, the two probing traces with scalidophoran features in Specimen B (figure 4b,c) appear to be associated with the elongated burrow. These larger burrows are typically assigned to Treptichnus or Trichophycus depending on their internal morphology—the evidence here suggests at least some of these burrows could also have been made by priapulids. Indeed, the type species, Trichophycus lanosum [41,42], possesses a button-like termination with radiating striae strikingly reminiscent of those in Specimen B. Trichophycus-like specimens with multiple probing terminations are also known from the MSM (electronic supplementary material, figure S3).
5. Behavioural implications
On the basis of actualistic experiments, Vannier et al. [9] argued that extant priapulids could produce treptichnid-like traces. However, by placing P. caudatus in a shallow tray of mud, they confined movement to the horizontal plane, a restriction which does not occur in natural conditions. Although the burrowing mechanism of priapulids, in particular P. caudatus, has been well studied, and consists of the alternate formation of penetration and terminal anchors typical of many burrowing animals [35,43,44], very little is known about the types of structures they produce, or any burrow ornamentation. Priapulus caudatus typically burrows in soupy muddy sediments that immediately collapse behind the animal after passage [16,35,45]. Conversely, Leckenby [46] and Lang [47] reported P. caudatus maintaining a vertical orientation within sediments with the proboscis tip at, or just above, the sediment–water interface. We also observed such stationary behaviour in our experiments (figure 6), suggesting an activity likely to have high preservation potential. However, it is not known how representative this is of P. caudatus behaviour, or what the cumulative burrow configuration would be. The smaller priapulid Halicryptus spinulosus has been shown to generate open burrow systems [48], presumably with high fossilization potential, though no information is available on any burrow surface sculpture.
Figure 6.
Aquarium burrowing experiments with extant Priapulus caudatus. (a) Initial lateral burrowing continuous into vertical burrowing. (b) Lateral burrowing of P. caudatus. Posture with the caudal appendage (white arrow) at the sediment–water interface probably has a respiratory function. Note the displacement of sediment at the anterior of P. caudatus as the animal propagates its burrow through the forceful expulsion of the prosoma (red-headed arrow). Burrowing experiments were confined to the vertical plane within a 2 cm-wide sand-filled compartment. Burrowing medium consisted of fine sand sieved and washed with artificial salt water made to the specifications of seawater at the collection depth of P. caudatus specimens at Gulmarsfjord, Lysekil, Sweden. After the suspended sediment was allowed to settle, P. caudatus were introduced and photographed at regular intervals.
Although the burrowing behaviour of priapulids in the wild is less well known, our own observations and the older literature (e.g. [29,35,47,48]) suggest that priapulids can and do extensively burrow vertically as well as horizontally. When placed in a tank of fine sand with restricted vertical rather than horizontal movement, P. caudatus tend to burrow directly downwards, though they also rest in burrows with their introvert or caudal appendage at the sediment–water interface (figure 6). However, given the probable relationship between priapulid-like scalidophorans and many treptichnid traces, the question of why Cambrian scalidophoran-like traces predominantly consist of horizontal burrows arises. Either these organisms behaved in a markedly different manner to extant P. caudatus, or they were restricted in some way. Indeed, the ethology of Lower Palaeozoic Treptichnus pedum remains open to question: generally interpreted as open, passively filled, burrows made by deposit feeders, but also as surface detritus feeders, surface scavengers or undermat miners [9,32,49]. One possibility is that such worms were burrowing in mud covered with a layer of sand. These trace fossils would thus represent examples of ‘barrier prospecting traces' with worms exploring the sand-mud boundary, perhaps due to resource concentration there (cf. [50,51]), or the presence of a redox discontinuity surface [52]. Conversely, this boundary setting may preferentially preserve trace fossils.
6. Early trace fossils and scalidophorans
The bilaterian trace fossil record extends back as far as ca 560 Ma, into the latest Ediacaran [7,53]. The oldest such trace fossils are morphologically simple and formed near the sediment surface; but just prior to the Cambrian, trace fossils started to develop three dimensionality (e.g. [7]), recording the widening behavioural repertoire of their makers [54]. Examples include treptichnids and Streptichnus from Namibia [11,55]. Other than treptichnids, few trace fossils have been attributed to cycloneuralians (but see [8,56]). Książkiewicz [57, p. 59] suggested priapulids as possible producers of Fucusopsis (a genus now generally attributed to Palaeophycus or Halopoa) from the Late Cretaceous of the Polish Carpathians. These have irregular longitudinal wrinkles and step-like breaks, and some specimens exhibit branching resembling that of treptichnids. The longitudinal ornamentation in these trace fossils is coarser than that in the MSM specimens and it is unclear if they are analogues. Pemberton & Frey [58] assigned some of the material described by Książkiewicz [57] to Palaeophycus alternatus, an ichnospecies characterized by alternating sections of striae and annuli. Cycloneuralian body fossils in direct association with their supposed trace fossils have been reported from lower Cambrian strata of southern China [59,60]. These are cylindrical or sac-shaped structures with no preserved surface detail, though such detail is hardly to be expected in the relatively homogeneous sediments in which they are found. However, Martin et al. [61] noted the great variety of postures in this material, offering the alternate interpretation of failed escape attempts by animals in a sedimentary obrution event.
There has been a consistent trend in the literature to associate treptichnids or other Cambrian burrows with priapulids (e.g. [8,9,33,40,62]). This assignment has, however, not hitherto rested on a particularly firm basis. The MSM material described here is clearly of at least scalidophoran affinity, adding credibility to claims of priapulids as producers of some important early Cambrian trace fossils. A stem and crown group perspective invites caution in a more precise taxonomic assignment, as the morphology of the basal priapulids and scalidophorans remains difficult to distinguish (e.g. [14,28,63,64]). Indeed, the presence of scalidophoran-like features in stem-group arthropods such as Pambdelurion [63,65] hints that even stem-group ecdysozoans may have also broadly resembled modern priapulids. While treptichnids and other burrows cannot be assumed to have all had the same maker, the evidence here indirectly associates a scalidophoran maker with a Treptichnus bifurcus and perhaps a Trichophycus trace fossil morphology (electronic supplementary material, figures S2 and S3). Given that treptichnid trace fossil morphologies extend back into the terminal Ediacaran, this would imply that priapulid-like scalidophorans, or at least stem-group ecdysozoans that resembled priapulids, also did.
Supplementary Material
Acknowledgements
We thank Christian Skovsted and Jonas Hagström of the Natural History Museum of Stockholm for assistance with the collections, Tom Claybourn for assistance with experiments, Ralf Janssen for providing laboratory space, and the two referees.
Data accessibility
This article has no additional data.
Authors' contributions
S.J. carried out burrowing and casting experiments on P. caudatus at the University Marine Biological Station, Millport, Scotland in 1999. G.K. and B.J.S. carried out actualistic experiments at Uppsala University. All authors (G.K., B.J.S., S.J. and G.E.B.) made significant contributions to the conception and design of the study, and contributed to the drafting of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by the Marie Curie ITN ‘NEPTUNE’ grant (no. 317172), under FP7 of the European Commission, Sören Jensen acknowledges funding from project CGL 2017–87631-P from Spanish MINECO. The experimental work at University Marine Biological Station, Millport, Scotland, was funded by NERC grant GR3/10713 to Simon Conway Morris.
References
- 1.Zhuravlev AI, Riding R (eds). 2000. The ecology of the Cambrian radiation. New York, NY: Columbia University Press. [Google Scholar]
- 2.Bottjer DJ, Hagadorn JW, Dornbos SQ. 2000. The Cambrian substrate revolution. GSA Today 10, 1–7. [Google Scholar]
- 3.Butterfield NJ. 2007. Macroevolution and macroecology through deep time. Palaeontology 50, 41–55. ( 10.1111/j.1475-4983.2006.00613.x) [DOI] [Google Scholar]
- 4.Budd GE, Jensen S. 2017. The origin of the animals and a ‘savannah’ hypothesis for early bilaterian evolution. Biol. Rev. 92, 446–473. ( 10.1111/brv.12239) [DOI] [PubMed] [Google Scholar]
- 5.Mángano MG, Buatois LA. 2017. The Cambrian revolutions: trace-fossil record, timing, links and geobiological impact. Earth-Sci. Rev. 173, 96–108. ( 10.1016/j.earscirev.2017.08.009) [DOI] [Google Scholar]
- 6.Narbonne GM, Myrow PM, Landing E, Anderson MM. 1987. A candidate stratotype for the Precambrian–Cambrian boundary, Fortune Head, Burin Peninsula, southeastern Newfoundland. Can. J. Earth Sci. 24, 1277–1293. ( 10.1139/e87-124) [DOI] [Google Scholar]
- 7.Jensen S. 2003. The Proterozoic and earliest Cambrian trace fossil record: patterns, problems and perspectives. Integr. Comp. Biol. 43, 219–228. ( 10.1093/icb/43.1.219) [DOI] [PubMed] [Google Scholar]
- 8.Dzik J. 2005. Behavioral and anatomical unity of the earliest burrowing animals and the cause of the ‘Cambrian explosion’. Paleobiology 31, 503–521. ( 10.1666/0094-8373(2005)031[0503:BAAUOT]2.0.CO;2) [DOI] [Google Scholar]
- 9.Vannier J, Calandra I, Gaillard C, Żylińska A. 2010. Priapulid worms: pioneer horizontal burrowers at the Precambrian–Cambrian boundary. Geology 38, 711–714. ( 10.1130/G30829.1) [DOI] [Google Scholar]
- 10.Gehling JG, Jensen S, Droser ML, Myrow PM, Narbonne GM. 2001. Burrowing below the basal Cambrian GSSP, Fortune Head, Newfoundland. Geol. Mag. 138, 213–218. ( 10.1017/S001675680100509X) [DOI] [Google Scholar]
- 11.Jensen S, Runnegar BN. 2005. A complex trace fossil from the Spitskop Member (terminal Ediacaran-? Lower Cambrian) of southern Namibia. Geol. Mag. 142, 561–569. ( 10.1017/S0016756805000853) [DOI] [Google Scholar]
- 12.Liu Y, Xiao S, Shao T, Broce J, Zhang H. 2014. The oldest known priapulid-like scalidophoran animal and its implications for the early evolution of cycloneuralians and ecdysozoans. Evol. Dev. 16, 155–165. ( 10.1111/ede.12076) [DOI] [PubMed] [Google Scholar]
- 13.Shao TQ, Liu YH, Wang Q, Zhang HQ, Tang HH, Li Y. 2016. New material of the oldest known scalidophoran animal Eopriapulites sphinx. Palaeoworld 25, 1–11. ( 10.1016/j.palwor.2015.07.003) [DOI] [Google Scholar]
- 14.Wills MA, Gerber S, Ruta M, Hughes M. 2012. The disparity of priapulid, archaeopriapulid and palaeoscolecid worms in the light of new data. J. Evol. Biol. 25, 2056–2076. ( 10.1111/j.1420-9101.2012.02586.x) [DOI] [PubMed] [Google Scholar]
- 15.Conway Morris S. 1977. Fossil priapulid worms. Spec. Pap. Palaeontol. 20, 1–95. [Google Scholar]
- 16.Vannier J, Martin EL. 2017. Worm-lobopodian assemblages from the Early Cambrian Chengjiang biota: Insight into the ‘pre-arthropodan ecology’? Palaeogeogr. Palaeoclimatol. Palaeoecol. 468, 373–387. ( 10.1016/j.palaeo.2016.12.002) [DOI] [Google Scholar]
- 17.Dong XP, Donoghue PC, Cunningham JA, Liu JB, Cheng H. 2005. The anatomy, affinity, and phylogenetic significance of Markuelia. Evol. Dev. 7, 468–482. ( 10.1111/j.1525-142X.2005.05050.x) [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Qin J, Wang Q, Maas A, Duan B, Zhang Y, Zhang H, Shao T, Zhang H. In press. New armoured scalidophorans (Ecdysozoa, Cycloneuralia) from the Cambrian Fortunian Zhangjiagou Lagerstätte, South China. Pap. Palaeontol. ( 10.1002/spp2.1239) [DOI] [Google Scholar]
- 19.Hinz I, Kraft P, Mergl M, Müller KJ. 1990. The problematic Hadimopanella, Kaimenella, Milaculum and Utahphospha identified as sclerites of Palaeoscolecida. Lethaia 23, 217–221. ( 10.1111/j.1502-3931.1990.tb01362.x) [DOI] [Google Scholar]
- 20.Smith MR, Harvey THP, Butterfield NJ. 2015. The macro-and microfossil record of the Cambrian priapulid Ottoia. Palaeontology 58, 705–721. ( 10.1111/pala.12168) [DOI] [Google Scholar]
- 21.Slater BJ, Harvey THP, Guilbaud R, Butterfield NJ. 2017. A cryptic record of Burgess Shale-type diversity from the early Cambrian of Baltica. Palaeontology 60, 117–140. ( 10.1111/pala.12273) [DOI] [Google Scholar]
- 22.Slater BJ, Harvey THP, Butterfield NJ. 2018. Small carbonaceous fossils (SCFs) from the Terreneuvian (lower Cambrian) of Baltica. Palaeontology 61, 417–439. ( 10.1111/pala.12350) [DOI] [Google Scholar]
- 23.Peel JS. 2010. A corset-like fossil from the Cambrian Sirius Passet Lagerstätte of North Greenland and its implications for cycloneuralian evolution. J. Paleontol. 84, 332–340. ( 10.1666/09-102R.1) [DOI] [Google Scholar]
- 24.Zhang H, Xiao S, Liu Y, Yuan X, Wan B, Muscente A, Shao T, Gong H, Cao G. 2015. Armored kinorhynch-like scalidophoran animals from the early Cambrian. Sci. Rep. 5, 16521 ( 10.1038/srep16521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey TH, Butterfield NJ. 2017. Exceptionally preserved Cambrian loriciferans and the early animal invasion of the meiobenthos. Nat. Ecol. Evol. 1, 0022 ( 10.1038/s41559-016-0022) [DOI] [PubMed] [Google Scholar]
- 26.Budd GE. 2003. The Cambrian fossil record and the origin of the phyla. Integr. Comp. Biol. 43, 157–165. ( 10.1093/icb/43.1.157) [DOI] [PubMed] [Google Scholar]
- 27.Conway Morris S, Peel JS. 2009. New palaeoscolecidan worms from the lower Cambrian: Sirius Passet, Latham Shale, and Kinzers Shale. Acta Palaeontol. Pol. 55, 141–156. ( 10.4202/app.2009.0058) [DOI] [Google Scholar]
- 28.Harvey TH, Dong X, Donoghue PC. 2010. Are palaeoscolecids ancestral ecdysozoans? Evol. Dev. 12, 177–200. ( 10.1111/j.1525-142X.2010.00403.x) [DOI] [PubMed] [Google Scholar]
- 29.Van der Land J. 1970. Systematics, zoogeography, and ecology of the Priapulida. Zool. Verh. 112, 1–118. [Google Scholar]
- 30.Mángano MG, Buatois LA. 2014. Decoupling of body-plan diversification and ecological structuring during the Ediacaran–Cambrian transition: evolutionary and geobiological feedbacks. Proc. R. Soc. B 281, 20140038 ( 10.1098/rspb.2014.0038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savrda CE. 2007. Taphonomy of trace fossils. In Trace fossils (ed. Miller W.), pp. 92–109. Amsterdam, The Netherlands: Elsevier Science. [Google Scholar]
- 32.Jensen S. 1997. Trace fossils from the Lower Cambrian Mickwitzia sandstone, south-central Sweden. Fossils Strata 42, 1–110. [Google Scholar]
- 33.Jensen S. 1990. Predation by early Cambrian trilobites on infaunal worms - evidence from the Swedish Mickwitzia Sandstone. Lethaia 23, 29–42. ( 10.1111/j.1502-3931.1990.tb01779.x) [DOI] [Google Scholar]
- 34.Kesidis G, Budd GE, Jensen S. 2019. An intermittent mode of formation for the trace fossil Cruziana as a serial repetition of Rusophycus, the case of Cruziana tenella (Linnarsson, 1871). Lethaia 52, 133–148. ( 10.1111/let.12303) [DOI] [Google Scholar]
- 35.Hammond RA. 1970. The burrowing of Priapulus caudatus. J. Zool. 162, 469–480. ( 10.1111/j.1469-7998.1970.tb01281.x) [DOI] [Google Scholar]
- 36.Van der Land J. 1985. 2 new species of Tubiluchus (Priapulida) from the Pacific Ocean. Proc. K. Ned. Akad. Wet. C 88, 371–377. [Google Scholar]
- 37.Théel H. 1911. Priapulids and sipunculids dredged by the Swedish Antarctic Expedition 1901–1903 and the phenomenon of antitropicality. Kungl Svenska. Vetenskapsakakademiens Handlingar. 47, 1–36. [Google Scholar]
- 38.Hyman LH. 1951. The invertebrates: Platyhelminthes and Rhynchocoela. The acoelomate Bilateria 2. New York, NY: McGraw-Hill Book Co. [Google Scholar]
- 39.Rindsberg AK, Kopaska-Merkel DC. 2005. Treptichnus and Arenicolites from the Steven C. Minkin Paleozoic footprint site (Langsettian, Alabama, USA) Pennsylvanian Footprints in the Black Warrior Basin of Alabama. Alabama Paleontological Society Monograph, pp. 121–141. [Google Scholar]
- 40.Orłowski S, Żylińska A. 1996. Non-arthropod burrows from the middle and late Cambrian of the Holy Cross Mountains, Poland. Acta Palaentol. Pol. 41, 385–409. [Google Scholar]
- 41.Miller SA, Dyer C. 1878. Contributions to paleontology, no. 2. Cincinnati, OH: James Barclay.
- 42.Osgood RG., Jr 1970. Trace fossils of the Cincinnati area. Palaeontogr. Am. 41, 281–444. [Google Scholar]
- 43.Hunter RD, Moss VA, Elder HY. 1983. Image analysis of the burrowing mechanisms of Polyphysia crassa (Annelida: Polychaeta) and Priapulus caudatus (Priapulida). J. Zool. 199, 305–323. ( 10.1111/j.1469-7998.1983.tb02099.x) [DOI] [Google Scholar]
- 44.Hunter RD, Elder HY. 1989. Burrowing dynamics and energy cost of transport in the soft-bodied marine invertebrates Polyphysia crassa and Priapulus caudatus. J. Zool. 218, 209–222. ( 10.1111/j.1469-7998.1989.tb02533.x) [DOI] [Google Scholar]
- 45.Schäfer W. 1972. Ecology and palaeoecology of marine environments. Chicago, IL: University of Chicago Press. [Google Scholar]
- 46.Leckenby J. 1855. On the structure and habits of Priapulus caudatus; illustrated by drawings and the exhibition of living examples obtained at Scarborough. Annu. Rep. Scarborough Philos. Archaeol. Soc. 1855, 17–25. [Google Scholar]
- 47.Lang K. 1948. Contribution to the ecology of Priapulus caudatus Lam. Arkiv för Zoologi 41A, 1–12. [Google Scholar]
- 48.Powilleit M, Kitlar J, Graf G. 1994. Particle and fluid bioturbation caused by the priapulid worm Halicryptus spinulosus (v. Seibold). Sarsia 79, 109–117. ( 10.1080/00364827.1994.10413551) [DOI] [Google Scholar]
- 49.Seilacher A. 2007. Trace fossil analysis. Amsterdam, The Netherlands: Springer Science & Business Media. [Google Scholar]
- 50.Llobet-Brossa E, Rosselló-Mora R, Amann R. 1998. Microbial community composition of Wadden Sea sediments as revealed by fluorescence in situ hybridization. Appl. Environ. Microbiol. 64, 2691–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jensen S, Atkinson R. 2001. Experimental production of animal trace fossils, with a discussion of allochthonous trace fossil producers. Neues Jahrb. Geol. Paläontol. 10, 594–606. [Google Scholar]
- 52.Buatois LA, Mángano MG. 2011. Ichnology: organism–substrate interactions in space and time. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 53.Martin MW, Grazhdankin DV, Bowring SA, Evans DAD, Fedonkin M, Kirschvink J. 2000. Age of Neoproterozoic bilatarian [sic] body and trace fossils, White Sea, Russia. Implications for metazoan evolution. Science 288, 841–845. ( 10.1126/science.288.5467.841) [DOI] [PubMed] [Google Scholar]
- 54.Conway Morris S. 1987. The search for the Precambrian–Cambrian boundary. Amer. Sci. 75, 156–167. [Google Scholar]
- 55.Jensen S, Saylor BZ, Gehling JG, Germs GJB. 2000. Complex trace fossils from the terminal Proterozoic of Namibia. Geology 28, 143–146. () [DOI] [Google Scholar]
- 56.Dzik J. 2007. The Verdun Syndrome: simultaneous origin of protective armour and infaunal shelters at the Precambrian–Cambrian transition. Geol. Soc. Spec. Pub. 286, 405–414. ( 10.1144/SP286.30) [DOI] [Google Scholar]
- 57.Książkiewicz M. 1977. Trace fossils in the flysch of the Polish Carpathians. Palaeontol. Polon. 36, 1–208. [Google Scholar]
- 58.Pemberton SG, Frey RW. 1982. Trace fossil nomenclature and the Planolites-Palaeophycus dilemma. J. Paleontol. 56, 843–881. [Google Scholar]
- 59.Zhang X, Hou X, Bergström J. 2006. Early Cambrian priapulid worms buried with their lined burrows. Geol. Mag. 143, 743–748. ( 10.1017/S0016756806002445) [DOI] [Google Scholar]
- 60.Huang D, Chen J, Zhu M, Zhao F. 2014. The burrow dwelling behavior and locomotion of palaeoscolecidian worms: new fossil evidence from the Cambrian Chengjiang fauna. Palaeogeogr. Palaeoclimatol. Palaeoecol. 398, 154–164. ( 10.1016/j.palaeo.2013.11.004) [DOI] [Google Scholar]
- 61.Martin ELO, Lerosey-Aubril R, Vannier J. 2016. Palaeoscolecid worms from the Lower Ordovician Fezouata Lagerstätte, Morocco palaeoecological and palaeogeographical implications. Palaeogeogr. Palaeoclimatol. Palaeoecol. 460, 130–141. ( 10.1016/j.palaeo.2016.04.009) [DOI] [Google Scholar]
- 62.Lerosey-Aubril R, Peel JS. 2018. Gut evolution in early Cambrian trilobites and the origin of predation on infaunal macroinvertebrates: evidence from muscle scars in Mesolenellus . Palaeontology 61 747–750. ( 10.1111/pala.12365) [DOI] [Google Scholar]
- 63.Budd GE. 1998. Arthropod body-plan evolution in the Cambrian with an example from anomalocaridid muscle. Lethaia 31, 197–210. ( 10.1111/j.1502-3931.1998.tb00508.x) [DOI] [Google Scholar]
- 64.Budd GE. 2001. Tardigrades as ‘stem-group arthropods': the evidence from the Cambrian fauna. Zool. Anz. 240, 265–279. ( 10.1078/0044-5231-00034) [DOI] [Google Scholar]
- 65.Vinther J, Porras L, Young FJ, Budd GE, Edgecombe GD. 2016. The mouth apparatus of the Cambrian gilled lobopodian Pambdelurion whittingtoni. Palaeontology 59, 841–849. ( 10.1111/pala.12256) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.