Abstract
Bats and birds are key providers of ecosystem services in forests. How climate and habitat jointly shape their communities is well studied, but whether biotic predictors from other trophic levels may improve bird and bat diversity models is less known, especially across large bioclimatic gradients. Here, we achieved multi-taxa surveys in 209 mature forests replicated in six European countries from Spain to Finland, to investigate the importance of biotic predictors (i.e. the abundance or activity of defoliating insects, spiders, earthworms and wild ungulates) for bat and bird taxonomic and functional diversity. We found that nine out of 12 bird and bat diversity metrics were best explained when biotic factors were added to models including climate and habitat variables, with a mean gain in explained variance of 38% for birds and 15% for bats. Tree functional diversity was the most important habitat predictor for birds, while bats responded more to understorey structure. The best biotic predictors for birds were spider abundance and defoliating insect activity, while only bat functional evenness responded positively to insect herbivory. Accounting for potential biotic interactions between bats, birds and other taxa of lower trophic levels will help to understand how environmental changes along large biogeographical gradients affect higher-level predator diversity in forest ecosystems.
Keywords: defoliating insects, earthworms, functional diversity, spiders, trophic interactions, ungulate browsing
1. Introduction
Biodiversity is a key driver of many ecosystem functions and services [1,2], particularly through the maintenance of functional trait diversity [3]. Despite the long history of studies examining the local, regional and global drivers of biodiversity, it remains challenging to disentangle the relative importance of climate, habitat and biotic factors [4–6]. An increasing number of studies is questioning the role of multiple biotic interactions across various trophic levels in shaping ecological communities. However, they usually focus only on local scales, while the influence of these interactions on biodiversity across larger geographical extents has rarely been explored [7–9]. Thus, incorporating multispecies interactions in biodiversity response models is still challenging, although it would improve our understanding of large-scale biodiversity patterns when compared with classical studies focusing on single taxa [7–11]. The benefits of considering multi-taxa interactions may be particularly useful for species at higher trophic levels such as birds and bats, which are affected by both direct effects of climate and habitat changes, and their cascading effects across trophic levels [9,12,13].
Importantly, the consequences of changes in bird and bat communities for ecosystem functioning cannot be fully understood by focusing only on changes in taxonomic diversity [14–16]. The use of functional traits not only allows the monitoring of changes in biodiversity response to land use changes [14] but also the clarification of respective importance of multiple assembly processes in shaping species communities along large environmental gradients, e.g. abiotic filtering, competition or facilitation [15,17,18]. For example, functional diversity can increase at opposite ends of resource availability gradients, depending on whether the traits involved are more related to abiotic filtering or to competitive interactions [17]. Similarly, higher functional dispersion at low productivity levels suggests increased competitive exclusion with the loss of functionally redundant species [15].
Taxonomic and functional diversity also tend to show distinct responses to global change [19]. Functional trait diversity and composition, rather than species richness, are often the most important biodiversity-related drivers of ecosystem functioning [12,16]. The number and diversity of species with any particular functional traits in a given community has direct effects on ecosystem-level processes. Functional diversity is, therefore, an indicator of resource use complementarity and community responses to disturbance [20,21]. A more efficient resource use by species in a given ecosystem can be inferred from higher functional evenness (FEve), while strong niche differentiation and low resource competition within species assemblages lead to higher functional dispersion [21–24]. Functional traits related to habitat and resource use are particularly efficient at accounting for changes in ecosystem-level processes such as productivity or trophic interactions. While body mass is a relevant surrogate for bird responses to environmental changes [25], the trophic niche of bird and bat species allows prediction of their responses to local habitat changes as well as energy input and food availability along large biogeographical gradients [4,5,26,27].
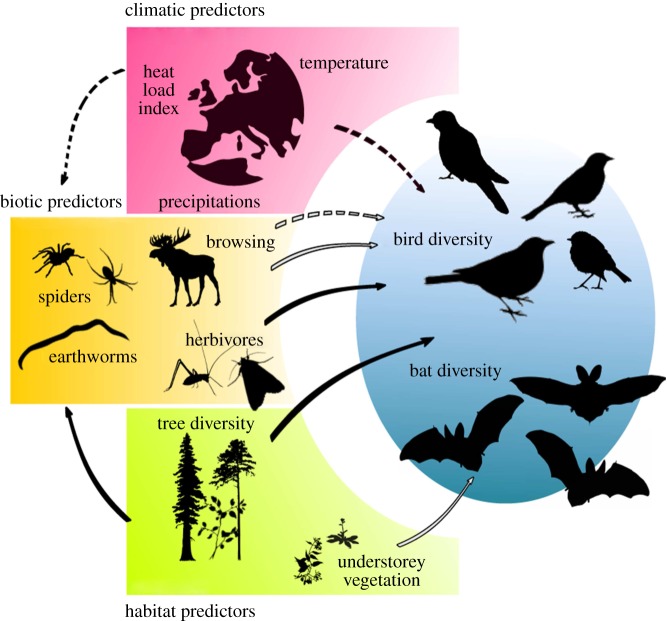
Here, we hypothesize that biotic drivers (i.e. abundance and activity of taxa from lower trophic levels) can complement response models of bird and bat diversity, along a continental-scale gradient (figure 1 and table 1). We first tested if: (i) including a set of biotic predictors contribute to explain patterns of bird and bat taxonomic and functional diversity, once accounted for climate (temperature, heat load index and precipitations) and habitat variables (forest composition and structure). We further hypothesized (table 1) that (ii) insect and spider abundance would positively affect bird and bat abundance and diversity, as key food resources; (iii) earthworm abundance would increase bird and bat diversity either directly or by improving forest soil structure and favouring high soil arthropod abundance; (iv) ungulate browsing would negatively affect bird and bat diversity, by reducing food resources and foraging niches provided by understorey cover; and (v) bird and bat abundance would be positively correlated in mature forest habitats across Europe, as they partly respond to the same biotic drivers (i.e. food resources) at such a large scale.
Figure 1.
Conceptual figure of hypothetical direct and indirect effects of climate (red panel), habitat (green panel) and biotic (yellow panel) predictors on bird and bat diversity metrics. Black and white arrows indicate positive and negative effects, respectively; full and dotted arrows indicate direct and indirect effects, respectively. (Online version in colour.)
Table 1.
Main hypotheses tested regarding the role of biotic predictors for bat and bird diversity metrics. (Based on available data and previous works compiled from the literature, we focused on the following four biotic predictors: (i) defoliating insect activity measured through canopy leaf herbivory rates; (ii) spider abundance sampled by foliage-beating of selected trees and shrubs; (iii) earthworm abundance sampled by standard litter and soil extraction; and (iv) wild ungulate browsing estimated through biomass removal on understorey vegetation (see the electronic supplementary material, S7 for sampling methods).)
| defoliating insect activity | spider abundance | earthworm abundance | ungulate browsing | |
|---|---|---|---|---|
| birds | ||||
| abundance | increase | increase | increase | no effect |
| species div. | increase | increase | no effect | no effect |
| funct. rich. | increase | increase | increase | decrease |
| funct. div. | increase | increase | no effect | decrease |
| body mass | increase | no effect | increase | decrease |
| main references | [24,25,28,29] | [30–32] | [33,34] | [35–37] |
| bats | ||||
| abundance | increase | increase | increase | no effect |
| species div. | increase | no effect | no effect | no effect |
| funct. rich. | increase | increase | no effect | decrease |
| funct. div. | increase | increase | no effect | decrease |
| body mass | increase | no effect | no effect | decrease |
| main references | [38–41] | [38,42] | [38,41] | [35–37] |
For each biotic predictor, we tested specific hypotheses for the positive, negative or null response of each bird and bat diversity metrics, according to the literature (table 1). We expected an increase in all bird and bat community metrics, including mean body mass, with higher insect herbivore activity owing to increased food availability [24–29,38–41]; an increase in bird taxonomic and functional diversity with spider abundance, but not in body mass [30–32]; an increase in bat abundance and functional diversity with spider abundance (increased specialized food resources), but neither in species diversity nor body mass [42]; an increase in bird abundance, functional richness (FRic) and body mass with earthworm abundance as increased resources for large bird specialists [33,34]; no effect of earthworm abundance on bats except on overall bat activity [38]; a decrease on both bat and bird functional diversity and body mass with ungulate browsing owing to reduction in understorey cover and changes in shrub composition, but no effect on abundance nor species diversity [35–37].
2. Material and methods
(a). Study sites
A network of 209 mature forest plots was established in 2011 across a latitudinal gradient in Europe ranging from 40° N to 63° N [28], in the framework of the FP7-FunDiv EUROPE project (www.fundiveurope.eu). The network covered six regions within Mediterranean, temperate and boreal forest biomes (Spain, Italy, Germany, Romania, Poland and Finland; electronic supplementary material, figure S1). The aim of this exploratory platform was to quantify the effects of tree species richness on multiple forest ecosystem functions. Within each region, plots were selected along a gradient of tree species richness ranging from one to five tree species per plot. Each region had a pool of three to five target tree species (for a total of 16 target species across Europe) that are regionally common and economically important. Each sampled forest plot covered an area of 900 m² and was surrounded by a 20 m buffer to avoid edge effects. To maximize their comparability, all plots within a country had similar ages, management and abiotic conditions [43].
(b). Bird and bat sampling
We surveyed breeding bird communities using standardized point counts performed by trained observers within a limited distance of 80 m around the observer in April–June 2012 (Italy, Germany and Finland) and April–June 2013 (Spain, Romania and Poland). We recorded all birds, except flyovers, which were heard or seen in 15 min, during the first 4 h after sunrise on days without strong wind, snow or rain. We carefully mapped the location of every recorded individual bird on circular plot fieldsheets to avoid double counting the same individuals. The total number of bird individuals recorded per species in each plot was used as an estimate of bird species abundance. Species detectability was considered to be comparable across the six regions because of similar age and structure in all sampled forest habitat types [43].
Bat communities were sampled by passive acoustic monitoring in April–June 2012 (Italy, Germany and Finland) and May–July 2013 (Spain, Romania and Poland) with an automatic ultrasound recorder (Sound Meter SM2BAT, Wildlife Acoustics) located at the centre of each plot. Recorders were calibrated to record all bat calls from 1 h before sunset to 1 h after sunrise, during one night per plot. Recordings were performed only when the ambient temperature was greater than 10°C, when there was no rain and wind speed was less than 30 km h−1. Bat echolocation calls were identified to species level by a trained operator using dedicated software [44]. Several groups of closely related species difficult to separate based on their calls were merged for data analyses when co-occurring in some countries: Myotis mystacinus/alcathoe/brandtii; Myotis myotis/blythii; Plecotus auritus/austriacus; and Pipistrellus kuhlii/nathusii. Bat activity was calculated as the total number of 5 s sequences with two or more calls per species, as a proxy for species abundance [45].
(c). Bird and bat taxonomic and functional diversity
We recorded a total of 76 bird species in the six regions after excluding raptors and flyovers, and a total of 27 bat species, i.e. 72% and 79% of the total pool of forest-dwelling bird and bat species in Europe, respectively [44]. We first calculated the Shannon index of taxonomic species diversity per forest plot. Then, we performed rarefaction and extrapolation curves for Shannon species diversity using the Hill number of order 1, to quantify species diversity patterns that were independent from total community abundance across the six studied regions (electronic supplementary material, S2). Furthermore, we compiled 10 species life traits related to habitat and resource use to compute functional diversity metrics for both birds and bats (electronic supplementary material, S3) [24,44]. For birds, these 10 traits were foraging guild, adult diet, nest site location, migration strategy, mean laying date, home range size, clutch size, body mass, species habitat specialization (SSI) and species thermal index (STI, in°C) [19,46]. For bats, the 10 traits were selected to be as similar as possible to those used for birds, i.e. foraging guild, diet specialization, nursery site, migration strategy, mean birth date, home range size, female fecundity, body mass, STI and SSI. The latter four traits were continuous for both taxa, while all others were categorical (electronic supplementary material, S3). Bird and bat STIs were calculated from European distribution maps as the average temperature experienced by a species across its geographical range during the breeding season (see the electronic supplementary material, S4). Bird and bat SSIs were calculated as the coefficient of variation of species abundance across all habitats in the European breeding bird survey [47], and in an independent dataset provided by the French national bat monitoring scheme [45], respectively (see the electronic supplementary material, S5).
Based on the functional traits, we computed abundance-weighted functional diversity metrics, using log-transformed bat species activity as a measure of bat abundance per plot, and the number of bird individuals recorded as a measure of bird abundance per plot. We calculated FRic, FEve and functional entropy (Rao's Q) as three complementary measures of the multivariate functional trait space using the ‘FD’ R-package [48]. FRic measures the convex hull volume of the functional trait space, while FEve measures the regularity of trait abundance distribution within this functional space, and Rao's Q the dispersion of species in functional trait space [21,48]. We further performed null models to quantify functional diversity metrics corrected for species richness levels in order to disentangle the drivers of trait diversity per se, independent from those of taxonomic diversity. To this end, we reshuffled trait sets among species (i.e. by random permutations of the rows of the species-trait table) without replacement. We then recalculated FD metrics for artificial communities that were equally species-rich as the observed communities and with the same species compositions, but with random sets of traits. We repeated this procedure 1000 times, and calculated the standardized deviation of FD (FDdev) values as:
where FDobs is the observed FD value, is the average of the 1000 randomized (i.e. expected) FD values and is the standard deviation of the 1000 randomized (i.e. expected) FD values. Thus, FDdev is independent from species richness, and if values are greater than 0, observed FD value was higher than expected based on the taxonomical species richness, whereas values below 0 indicate the opposite (see the electronic supplementary material, S6).
(d). Climate, habitat and biotic predictors
Using the WorldClim database (http://www.worldclim.org/) and the geographical coordinates of plots, we derived climatic variables for each forest plot (i.e. mean annual temperature and precipitation at a 30 s resolution) and calculated a unitless heat load index based on equations correcting for aspect [49]. According to preliminary analyses [44], the variation of forest habitat structure and composition across all sampled plots could be summarized by a limited number of vegetation attributes, including deciduous tree proportion, tree functional diversity (Rao's Q), understorey species richness and vertical stratification index. In addition, we selected other key taxa sampled in the same plots to build the set of biotic predictors (see detailed hypotheses in table 1 and sampling methods in the electronic supplementary material, S7). We sampled spider abundance and monitored insect herbivory as a proxy for defoliating insect abundance, to assess overall availability of bird and bat preferred prey in tree canopies, i.e. caterpillars, moths and spiders [28,29,38–40]. We further sampled earthworm abundance as food resources for some specialist ground-probing birds, as well as ecosystem engineers having potential bottom-up effects on both bat and bird communities [33,34]. Finally, ungulate browsing was also quantified, as it can negatively affect both forest birds and bats, whose foraging habitat and behaviour might be affected by any browsing-induced changes in understorey density [35,36].
(e). Data analysis
The importance of biotic drivers for bird and bat diversity metrics was evaluated using a hierarchical model fitting framework, comparing a set of competing models with an information-theoretic approach (Akaike's information criterion (AIC)-based model selection). For each of the 12 metrics tested (bat and bird abundance, species diversity, three indices of functional diversity and mean body mass; table 2), we increased model complexity in three steps (figure 1) by successively including: (i) three climate predictors (mean annual temperature, mean annual precipitation and heat load index); (ii) four habitat predictors (deciduous tree proportion, forest stratification index, understorey species richness and tree functional diversity); and (iii) four biotic predictors from multiple trophic levels (defoliating insects, spiders, earthworms and ungulate browsing). At each step, the best set of predictors (those in the model with the lowest AIC value) was selected before the predictors of the next step were added to the model. We checked for the absence of multicollinearity among climate, habitat and biotic variables using variance inflation factor correlation diagnostic tests to exclude potentially collinear predictors. We selected significant predictors at each modelling step using the function ‘drop1’ in ‘lme4’ R-package, which allows for a comparison of models based on AIC weights [50]. We performed a χ² test on ΔAICc to test for significant decrease in AICc when a given set of predictors was included (table 2). We did not include interaction terms to avoid model inflation and fitted linear mixed models (LMMs) for all 12 bird and bat diversity metrics, except for total bird abundance, which was fitted with a generalized linear mixed model (GLMM) with a Poisson error distribution and a log-link function. We used a logit transformation for FEve because it is constrained between 0 and 1 [48].
Table 2.
Respective performance of climate-only (CLI), climate-habitat (CLI-HAB) and climate-habitat-biotic (CLI-HAB-BIO) mixed models to predict bird and bat taxonomic and functional community metrics. (Best final models are indicated in italics. R²m = marginal Nakagawa's R² for fixed effects; R²c = conditional Nakagawa's R² for both random and fixed effects; FEve and Rao are logit-transformed and log-transformed bat activity was used as a proxy for abundance; n.s., not significant. χ² tests indicates significance level of final model according to ΔAICc. Significance levels for tests and individual predictors are as follows: *p < 0.05; **p < 0.01; ***p < 0.001. Codes for response variables and predictors as follows: SDI, Shannon diversity index; FRic, functional richness; FEve, functional evenness; Rao, functional entropy; CWM mass, community weighted mean body mass; Temp, mean annual temperature; Precip, mean annual precipitation; HLI, heat load index; Decid, deciduous tree proportion; Undric, understorey plant species richness; Stratif, understorey stratification index; Treerao, tree functional entropy; Brows, ungulate browsing; Earth, earthworm abundance; Insect, defoliating insect abundance; Spider, spider abundance.)
| CLI |
CLI-HAB |
CLI-HAB-BIO |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R²m | R²c | R²m | R²c | R²m | R²c | χ² | significant predictors | |||
| birds | climate | + habitat | + biotic | |||||||
| abundance | n.s. | n.s. | 0.024 | 0.294 | 0.085 | 0.344 | 0.004** | — | + Treerao** | + Earth*+ Insect** |
| SDI | 0.024 | 0.389 | 0.066 | 0.397 | 0.098 | 0.453 | 0.04* | + HLI** | + Treerao** | + Insect*+ Spider* |
| FRic | 0.090 | 0.293 | n.s. | n.s. | 0.121 | 0.308 | 0.009** | + Temp* | − | + Insect*+ Spider* |
| FEve | 0.083 | 0.093 | n.s. | n.s. | n.s. | n.s. | n.s. | − Prec** | — | − |
| Rao | 0.136 | 0.270 | n.s. | n.s. | 0.205 | 0.329 | 0.01* | − Prec** | — | + Spider*+ Brows* |
| CWM mass | 0.154 | 0.264 | n.s. | n.s. | 0.180 | 0.302 | 0.04* | + Temp*- Prec* | — | + Spider* |
| bats | ||||||||||
| abundance | n.s. | n.s. | 0.094 | 0.283 | 0.106 | 0.309 | 0.04* | — | + Decid**- Stratif** | + Spider* |
| SDI | n.s. | n.s. | 0.079 | 0.242 | 0.088 | 0.219 | 0.04* | — | − Stratif***+ Undric* | + Earth* |
| FRic | n.s. | n.s. | 0.036 | 0.248 | n.s. | n.s. | n.s. | — | − Stratif* | — |
| FEve | n.s. | n.s. | 0.103 | 0.366 | 0.111 | 0.286 | 0.02* | — | + Undric* | + Insect* |
| Rao | n.s. | n.s. | 0.054 | 0.262 | n.s. | n.s. | n.s. | — | − Stratif** | − |
| CWM mass | n.s. | n.s. | 0.035 | 0.153 | 0.052 | 0.153 | 0.05* | — | − Decid* | + Brows* |
To account for pseudoreplication owing to spatial autocorrelation and clustering of forest plots per region, region identity was added as a random factor to the models. We further controlled for differences in common target tree species identity across regions with an additional random effect for target species composition, since not all tree combinations occur in all regions [43]. Bat activity was log-transformed before modelling, and all model predictors were scaled and centred to allow a comparison of their relative effects on bird and bat community metrics. We assessed model performance by reporting marginal (for fixed effects) and conditional (for both fixed and random effects) R² at each modelling step [51]. We also tested for the direction of individual effects by modelling univariate relationships between biotic factors and bird and bat community metrics using a set of LMMs with the same random effect structure as above. We systematically checked all model residuals for normality and homoscedasticity in LMMs and overdispersion in GLMMs.
3. Results
Including biotic predictors significantly improved nine out of 12 final models of bird and bat community metrics compared to those only including climate and/or habitat (table 2). The mean gain in variance explained by fixed effects (R²m) when biotic factors were added to fixed climate and habitat predictors was 38.0% for birds and 15.4% for bats (range 14.4–71.8% for birds and 7.2–32.7% for bats). Tree functional diversity was more influential for bird abundance and species diversity while bat diversity responded more to understorey structure (table 2).
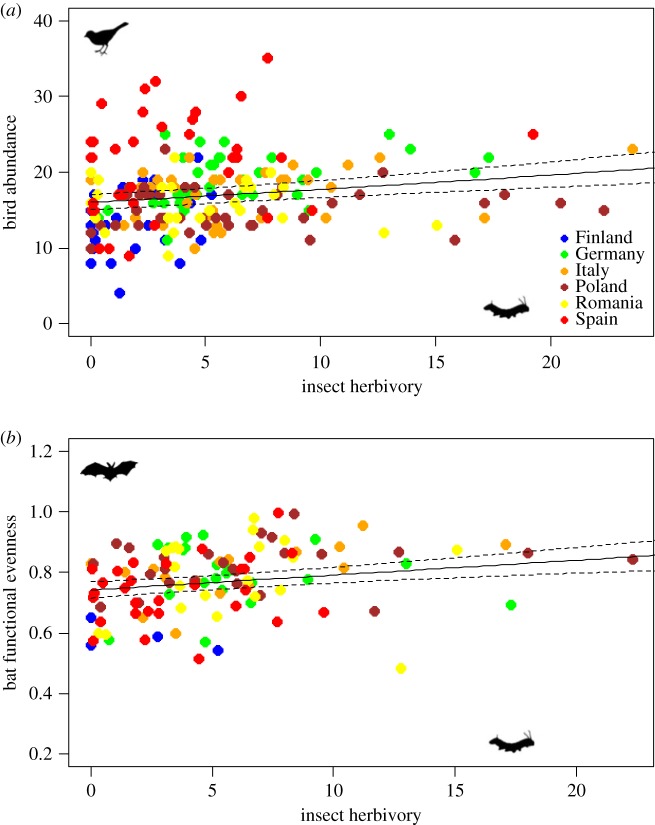
All bird community metrics except FEve were positively correlated to either spider abundance or insect herbivory (see results from univariate LMMs in table 3 and figure 2a). Bird abundance also significantly increased with earthworm abundance (table 3). By contrast, only bat FEve increased with insect herbivory (figure 2b). However, bat mean body mass significantly increased with ungulate browsing, while earthworm abundance had a positive effect on bat species diversity and a negative effect on bat body mass (table 3). Moreover, bird FRic and mean body mass, but not bird abundance, were positively correlated with bat activity (LMMs with t = 1.96, p < 0.05; t = 2.30, p < 0.02, respectively).
Table 3.
Effects of biotic variables on bird and bat community metrics (univariate linear mixed models). (Significant models are indicated in italics. All predictors were scaled and centered before modelling. See table 2 for codes of bird and bat diversity metrics.)
| defoliating insects |
spiders |
earthworms |
ungulates |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| estimates | R2m | p-values | estimates | R2m | p-values | estimates | R2m | p-values | estimates | R2m | p-values | |
| birds | ||||||||||||
| abundance | 0.045 | 0.025 | 0.01 | 0.030 | — | n.s. | 0.046 | 0.025 | 0.05 | −0.0004 | — | n.s. |
| SDI | 0.027 | — | n.s. | 0.047 | 0.022 | 0.02 | 0.037 | — | n.s. | 0.0004 | — | n.s. |
| FRic | 0.006 | 0.014 | 0.05 | 0.007 | 0.018 | 0.05 | 0.006 | — | n.s. | 0.001 | — | n.s. |
| FEvea | −0.010 | — | n.s. | −0.005 | — | n.s. | −0.030 | — | n.s. | 0.019 | — | n.s. |
| Raoa | −0.009 | — | n.s. | 0.021 | 0.016 | 0.05 | 0.009 | — | n.s. | 0.017 | — | n.s. |
| CWM mass | −1.799 | — | n.s. | 5.135 | 0.020 | 0.05 | 3.185 | — | n.s. | −0.618 | — | n.s. |
| bats | ||||||||||||
| abundanceb | 0.189 | — | n.s. | — | n.s. | — | 0.247 | — | n.s. | −0.168 | — | n.s. |
| SDI | −0.0005 | — | n.s. | — | n.s. | — | 0.076 | 0.024 | 0.05 | 0.029 | — | n.s. |
| FRic | −0.001 | — | n.s. | — | n.s. | — | −0.003 | — | n.s. | 0.001 | — | n.s. |
| FEvea | 0.230 | 0.066 | 0.005 | — | n.s. | — | 0.142 | — | n.s. | −0.005 | — | n.s. |
| Raoa | 0.002 | — | n.s. | — | n.s. | — | 0.0001 | — | n.s. | 0.002 | — | n.s. |
| CWM mass | −0.162 | — | n.s. | — | n.s. | — | −0.540 | 0.034 | 0.04 | 0.465 | 0.026 | 0.03 |
aLogit-transformation was used for response variable.
bLog-transformed bat activity was used as a proxy for abundance.
Figure 2.
Univariate linear mixed models in response to insect herbivory for (a) bird abundance and (b) bat functional evenness. See table 3 for model coefficients, R2m values and p-values. (Online version in colour.)
The results of the null model analysis for functional metrics (FRic, FEve, Rao's Q and community weighted mean (CWM) body mass) are presented in the electronic supplementary material, S6. We found consistencies in model predictor selection for bird Rao's Q, bat FEve and bird and bat mean body mass. By contrast, bird and bat simulated FRic differed in model selection from the observed values, as well as bird FEve and bat Rao's Q (electronic supplementary material, table S6).
4. Discussion
In the present study, we confirm the importance of different trophic groups, especially arthropod prey such as spiders and defoliating insects, as important determinants for forest bird and bat communities along crossed bioclimatic and habitat gradients. Our results are thus in accordance with several recent studies pointing out that local abundance of bird and bat foraging guilds were best predicted when accounting for interactions between vegetation structure and actual prey abundance [9,26,27,52]. We also found a positive relationship between bat activity and bird FRic, rather than taxonomic diversity, across European forests. This suggests that a large range of bird functional types are able to coexist in forests with high levels of bat foraging activity, probably linked to higher food availability [37,53].
While several metrics of bird and, to a lesser extent, of bat functional diversity were related to high abundances of lower trophic levels, some of these relationships were driven by taxonomic diversity patterns, rather than by trait composition or diversity per se. This was supported by the use of null models, in which we calculated the deviation of observed functional diversity patterns from simulated communities that differed in their trait composition, but not in their taxonomic composition, from observed communities. In particular, the significant predictors in the response models based on simulated values for bird and bat FRic differed from the ones based on observed values, because FRic is generally correlated to taxonomic richness [21]. On the other hand, we found consistencies in the selection of biotic predictors in observed and simulated models for four functional diversity metrics, namely bird Rao's Q, bat FEve and bird and bat mean body mass.
In the present work, spider, defoliating insect and earthworm abundances were positively correlated with either bird abundance or functional diversity, as expected from our initial hypotheses. This is consistent with the recognized importance of defoliating caterpillars, spiders and earthworms as preferred food items for forest birds [28–33,38,39,42]. Defoliating Lepidoptera larvae are key prey items for forest birds during the breeding season, which usually matches the peak in caterpillar abundance [25,29]. Caterpillars and moths are also major food resources for forest bats in temperate forests [26,38–40]. However, contrary to expectations, we found that insect herbivory was not affecting all bird and bat community metrics equally. For bats, only FEve responded positively to herbivory, as expected [44], while it was not the case for birds, although we expected the strongest response to insect abundance for this particular metric generally indicating an efficient resource use by the predator community [21,24]. Spider abundance had a widespread positive effect on bird taxonomic and functional diversity, as well as mean body mass, while it had little effect on bats, because only specialist gleaning bats feed on spiders [30–32,38,42].
In contrast with our initial hypotheses, earthworm abundance had no effect on bird FRic or body mass, but as expected, earthworm abundance correlated positively with bird abundance [33]. Earthworms also had additional effects on bat species diversity and body mass, possibly through cascading effects across trophic levels from forest soils to these higher-level predators. The relationships of bird and bat diversity with taxa from other trophic levels can thus partly be explained by foraging niches and diet specialization of particular species or genera. Large ground-foraging birds such as thrushes (Turdus spp.) and waders (woodcock Scolopax rusticola, common snipe Gallinago gallinago and sandpipers Tringa spp) specialize on earthworms during the breeding season [33], while spiders are preferred prey items for bark-foraging specialists such as treecreepers Certhia spp. [30]. By contrast, only a few European forest bats, including some Myotis spp., can specialize on arachnids but bats do not feed directly on earthworms [38,42]. However, earthworms increase soil biogeochemical heterogeneity and organic matter turnover, so that their activity might lead to higher insect prey abundance ultimately available for both birds and bats [34].
Contrary to our initial hypotheses, we did not detect any negative effects of wild ungulate browsing on birds, but browsing intensity was associated with a decrease in the dominance of small-bodied bat species, as expected. Although these effects were not detectable on birds along the sampled bioclimatic gradient, this suggests that the negative effect of large herbivores previously observed on many taxa also extend to smaller-sized forest insectivorous bats [36]. Such a potentially negative effect of browsing on bats is probably owing to indirect changes in resource quality and availability provided by understorey vegetation rather than a direct effect of wild ungulate disturbance [35]. However, how precisely bat species respond to increased ungulate densities in European forests remains to be investigated and should be highly guild-dependent [37]. Smaller foliage-gleaning specialist bats might be particularly sensitive to changes in understorey density and associated food resources following increase in browsing intensity from wild large ungulates, while larger aerial foragers would be favoured by clearer forest understorey created by increased browsing [26]. Most bats actually forage in the forest gaps and only few specialists can use multi-layered forests (e.g. Myotis nattereri or M. bechsteinii) [42], but species such as M. myotis also need a low grass layer to forage on carabid beetles. The observed increase in mean bat body mass with ungulate browsing might also be an indication for more free space that can be used by larger bat species in heavily browsed shrub understoreys.
Beyond the direct effects of food resources, these significant biotic factors may thus not always imply a mechanistic interaction, but can also serve as surrogates for mechanisms underlying diversity patterns in bat and bird communities [6,9,13]. In line with our initial hypotheses, the abundance and activity of several lower trophic levels were, across geographical scales, correlated with higher abundances and diversity of birds and bats. On the other hand, some expected relationships were not supported, or were relatively weak compared to similar relationships documented at more local scales. We therefore suggest that, while the effects of abundance and activity of lower trophic levels are often strong enough to improve models explaining bird and bat diversity at a continental scale, in some cases relationships were weaker or non-significant, meaning that climate and habitat variables were informative enough to model bat and bird responses at the large spatial extents studied here. Moreover, the use of multi-trait functional diversity metrics can somewhat obscure the relationships between individual traits and environmental gradients, which need further investigation to better infer the exact mechanisms linking the abundance of taxa from distinct trophic levels in diverse forest ecosystems [17,54].
The effects of forest structure on bat communities were mediated by understorey richness and stratification, and appeared largely negative. Bats use more specialized foraging techniques (i.e. echolocation) than forest birds, which makes them particularly sensitive to understorey vertical structure [26,52]. Overall, we found that forest composition, especially tree functional diversity and the proportion of deciduous trees, was more influential for both bird and bat communities than forest structure. However, the effects of forest composition and structure were not independent, e.g. an increase in the proportion of deciduous trees will also have an effect on structure, e.g. canopy architecture. This is consistent with the hypothesis that increasing forest habitat heterogeneity through higher functional diversity of tree species should increase the abundance of taxa from higher trophic levels such as insectivorous birds and bats [4,27,37]. The mechanism behind this positive effect of tree species diversity on bats and birds is generally related to increased food and roost/nest availability, but defoliating insect activity could be a key factor underlying the effect of tree diversity, at least partly reflecting overall prey availability for insectivorous vertebrates in mixed forests [29,37,39]. In addition, the buffering effect of deciduous forests on climate-sensitive, cold-dwelling birds is more and more acknowledged at both local and macro-scales [46]. Together with a direct microclimatic buffering during the breeding season, such an effect could also be linked to more abundant and predictable food resources in deciduous forests compared to conifer trees for forest-dwelling bats and birds [40,55].
5. Conclusion
Biodiversity loss is known to cascade across trophic levels in complex ecosystems, with declines in some species affecting the abundance and diversity of other, dependent trophic groups [12,13]. Modelling bat and bird diversity across large biogeographical scales thus requires taking into account not only climate and habitat variables but also direct and indirect multi-trophic interactions [7–10]. Our findings confirm that we need to consider biodiversity changes at multiple trophic levels and large spatial scales to predict the future dynamics of biodiversity conservation and ecosystem functioning under global change [56]. In such a context, upper trophic levels are at a higher risk of decline, thus questioning the resilience of ecosystems to global change [13,57]. There is, therefore, a critical need to better understand and monitor biotic drivers, especially those involving trophic interactions between bats, birds and their prey to predict how climate and land use changes might affect the diversity of these key predators in forest ecosystems.
Supplementary Material
Acknowledgements
We thank L. Baeten, J.Y. Barnagaud, J. Bauhus, A. Coppi, V. Devictor, P. Gaüzère, C. Grossiord, S. Hättenschwiler, F.X. Joly, S. Mueller, J. Nezan, F. Selvi, J. Van Keer, F. Vetillard and the site managers O. Bouriaud, H. Bruelheide, F. Bussotti, L. Finér, B. Jaroszewicz, F. Valladares and their field teams for their help. We thank K.R. Burgio and S. Fritz for their constructive comments that helped improve the former draft versions.
Data accessibility
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.t48p8c0 [58]. Data tables for site variables, bird species per sites, bat species per sites and bird and bat species traits.
Authors' contributions
L.B., E.A., E.A., B.C., Y.C., H.D.W., H.J., J.K., B.M., K.V. and F.v.d.P. conceived and designed the study and M.S.L. coordinated the project. L.B., E.A, B.C., Y.C., H.D.W., H.T.M. and M.C. carried out the fieldwork and E.A., E.A., H.d.W., C.K., H.T.M., A.V., M.C., M.D., P.D.S., H.J., J.K., I.L.V., B.M., K.V. and F.v.d.P. contributed to building the dataset. L.B., E.A., E.A., B.C., H.D.W., H.J. and F.v.d.P. designed the analyses and L.B., F.v.d.P. and B.C. performed the analyses and designed the figures. L.B. led the writing and all authors contributed to writing and editing the manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
The present research was funded by the European Commission's Seventh Framework Program (FP7/2007-2013) under the Grant Agreement no. 265171.
References
- 1.Soliveres S, et al. 2016. Biodiversity at multiple trophic levels is needed for ecosystem multifunctionality. Nature 536, 456–459. ( 10.1038/nature19092) [DOI] [PubMed] [Google Scholar]
- 2.Brockerhoff EG, et al. 2017. Forest biodiversity, ecosystem functioning and the provision of ecosystem services. Biodivers. Conserv. 26, 3005–3035. ( 10.1007/s10531-017-1453-2) [DOI] [Google Scholar]
- 3.Allan E, et al. 2015. Land use intensification alters ecosystem multifunctionality via loss of biodiversity and changes to functional composition. Ecol. Lett. 18, 834–843. ( 10.1111/ele.12469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kissling WD, Sekercioglu CH, Jetz W. 2012. Bird dietary guild richness across latitudes, environments and biogeographic regions. Global Ecol. Biogeogr. 21, 328–340. ( 10.1111/j.1466-8238.2011.00679.x) [DOI] [Google Scholar]
- 5.Cisneros LM, Fagan ME, Willig MR. 2015. Season-specific and guild-specific effects of anthropogenic landscape modification on metacommunity structure of tropical bats. J. Anim. Ecol. 84, 373–385. ( 10.1111/1365-2656.12299) [DOI] [PubMed] [Google Scholar]
- 6.Vollstädt MGR, Ferger SW, Hemp A, Howell KM, Töpfer T, Böhning-Gaese K, Schleuning M. 2017. Direct and indirect effects of climate, human disturbance and plant traits on avian functional diversity. Global Ecol. Biogeogr. 26, 963–972. ( 10.1111/geb.12606) [DOI] [Google Scholar]
- 7.Kissling WD, Schleuning M. 2015. Multispecies interactions across trophic levels at macroscales: retrospective and future directions. Ecography 38, 346–357. ( 10.1111/ecog.00819) [DOI] [Google Scholar]
- 8.Mönkkönen M, Devictor V, Forsman JT, Lehikoinen A, Elo M. 2017. Linking species interactions with phylogenetic and functional distance in European bird assemblages at broad spatial scales. Global Ecol. Biogeogr. 26, 952–962. ( 10.1111/geb.12605) [DOI] [Google Scholar]
- 9.Zhang J, Qian H, Girardello M, Pellissier V, Nielsen SE, Svenning J-C. 2018. Trophic interactions among vertebrate guilds and plants shape global patterns in species diversity. Proc. R. Soc. B 285, 20180949 ( 10.1098/rspb.2018.0949) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heikkinen RK, Luoto M, Virkkala R, Pearson RG, Körber JH. 2007. Biotic interactions improve prediction of boreal bird distributions at macro-scales. Global Ecol. Biogeogr. 16, 754–763. ( 10.1111/j.1466-8238.2007.00345.x) [DOI] [Google Scholar]
- 11.Dorazio RM, Connor EF, Askins RA. 2015. Estimating the effects of habitat and biological interactions in an avian community. PLoS ONE 10, e0135987 ( 10.1371/journal.pone.0135987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bregman TP, Lees AC, MacGregor HEA, Darski B, de Moura NG, Aleixo A, Barlow J, Tobias JA.. 2016. Using avian functional traits to assess the impact of land-cover change on ecosystem processes linked to resilience in tropical forests. Proc. R. Soc. B 283, 20161289 ( 10.1098/rspb.2016.1289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnes AD, et al. 2017. Direct and cascading impacts of tropical land-use change on multi-trophic biodiversity. Nat. Ecol. Evol. 1, 1511–1519. ( 10.1038/s41559-017-0275-7) [DOI] [PubMed] [Google Scholar]
- 14.Vandewalle M, et al. 2010. Functional traits as indicators of biodiversity response to land use changes across ecosystems and organisms. Biodiver. Conserv. 19, 2921–2947. ( 10.1007/s1053101097989) [DOI] [Google Scholar]
- 15.Cisneros LM, Burgio KR, Dreiss LM, Klingbeil BT, Patterson BD, Presley SJ, Willig MR.. 2014. Multiple dimensions of bat biodiversity along an extensive tropical elevational gradient. J. Anim. Ecol. 83, 1124–1136. ( 10.1111/1365-2656.12201) [DOI] [PubMed] [Google Scholar]
- 16.Gagic V, et al. 2015. Functional identity and diversity of animals predict ecosystem functioning better than species-based indices. Proc. R. Soc. B 282, 20142620 ( 10.1098/rspb.2014.2620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spasojevic MK, Suding KN. 2012. Inferring community assembly mechanisms from functional diversity patterns: the importance of multiple assembly processes. J. Ecol. 100, 652–661. ( 10.1111/j.1365-2745.2011.01945.x) [DOI] [Google Scholar]
- 18.Lamanna C, et al. 2014. Functional trait space and the latitudinal diversity gradient. Proc. Natl Acad. Sci. USA 111, 13 745–13 750. ( 10.1073/pnas.1317722111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaüzère P, Jiguet F, Devictor V. 2015. Rapid adjustment of bird community compositions to local climatic variations and its functional consequences. Glob. Change Biol. 21, 3367–3378. ( 10.1111/gcb.12917) [DOI] [PubMed] [Google Scholar]
- 20.Petchey OL, Gaston KJ. 2006. Functional diversity: back to basics and looking forward. Ecol. Lett. 9, 741–758. ( 10.1111/j.1461-0248.2006.00924.x) [DOI] [PubMed] [Google Scholar]
- 21.Mouillot D, Graham NAJ, Villéger S, Mason NWH, Bellwood DR. 2013. A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 28, 167–177. ( 10.1016/j.tree.2012.10.004) [DOI] [PubMed] [Google Scholar]
- 22.Thompson PL, Davies TJ, Gonzalez A. 2015. Ecosystem functions across trophic levels are linked to functional and phylogenetic diversity. PLoS ONE 10, e0117595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gravel D, Albouy C, Thuiller W. 2016. The meaning of functional trait composition of food webs for ecosystem functioning. Phil. Trans. R. Soc. B 371, 20150268 ( 10.1098/rstb.2015.0268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbaro L, Rusch A, Muiruri EW, Gravellier B, Thiery D, Castagneyrol B. 2017. Avian pest control in vineyards is driven by interactions between bird functional diversity and landscape heterogeneity. J. Appl. Ecol. 54, 500–508. ( 10.1111/1365-2664.12740) [DOI] [Google Scholar]
- 25.Rioux PS, Pelletier F, Garant D, Belisle M. 2014. Severe recent decrease of adult body mass in a declining insectivorous bird population. Proc. R. Soc. B 281, 20140649 ( 10.1098/rspb.2014.0649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Müller J, Mehr M, Bässler C, Fenton MB, Hothorn T, Pretzsch H, Klemmt H-J, Brandl R. 2012. Aggregative response in bats: prey abundance versus habitat. Oecologia 169, 673–684. ( 10.1007/s00442-011-2247-y) [DOI] [PubMed] [Google Scholar]
- 27.Ferger SW, Schleuning M, Hemp A, Howell KM, Böhning-Gaese K. 2014. Food resources and vegetation structure mediate climatic effects on species richness of birds: climate and bird species richness. Global Ecol. Biogeogr. 23, 541–549. ( 10.1111/geb.12151) [DOI] [Google Scholar]
- 28.Kristin A, Patocka J. 1997. Birds as predators of Lepidoptera: selected examples. Biologia 52, 319–326. [Google Scholar]
- 29.Smith KW, et al. 2011. Large-scale variation in the temporal patterns of the frass fall of defoliating caterpillars in oak woodlands in Britain: implications for nesting woodland birds. Bird Study 58, 506–511. ( 10.1080/00063657.2011.616186) [DOI] [Google Scholar]
- 30.Jäntti A, Aho T, Hakkarainen H, Kuitunen M, Suhonen J. 2001. Prey depletion by the foraging of the Eurasian treecreeper, Certhia familiaris, on tree-trunk arthropods. Oecologia 128, 488–491. ( 10.1007/s004420100677) [DOI] [PubMed] [Google Scholar]
- 31.Gunnarsson B. 2007. Bird predation on spiders: ecological mechanisms and evolutionary consequences. J. Arachnol. 35, 509–529. ( 10.1636/RT07-64.1) [DOI] [Google Scholar]
- 32.Kozlov MV, Stańska M, Hajdamowicz I, Zverev V, Zvereva EL. 2015. Factors shaping latitudinal patterns in communities of arboreal spiders in northern Europe. Ecography 38, 1026–1035. ( 10.1111/ecog.01401) [DOI] [Google Scholar]
- 33.Granval P, Muys B. 1995. Predation on earthworms by terrestrial vertebrates. In Proceedings of the international union of game biologists XXII congress (eds Golovatch S, Penev L), pp. 480–491. Sofia, Bulgaria: Pensoft. [Google Scholar]
- 34.Ausden M, Sutherland WJ, James R. 2001. The effects of flooding lowland wetland grassland on soil macroinvertebrate prey of breeding waders. J. Appl. Ecol. 38, 320–338. ( 10.1046/j.1365-2664.2001.00600.x) [DOI] [Google Scholar]
- 35.Nuttle T, Yerger EH, Stoleson SH, Ristau TE. 2011. Legacy of top-down herbivore pressure ricochets back up multiple trophic levels in forest canopies over 30 years. Ecosphere 2, art4 ( 10.1890/ES10-00108.1) [DOI] [Google Scholar]
- 36.Foster CN, Barton PS, Lindenmayer DB. 2014. Effects of large native herbivores on other animals. J. Appl. Ecol. 51, 929–938. ( 10.1111/1365-2664.12268) [DOI] [Google Scholar]
- 37.Renner SC, Suarez-Rubio M, Kaiser S, Nieschulze J, Kalko EKV, Tschapka M, Jung K. 2018. Divergent response to forest structure of two mobile vertebrate groups. Forest Ecol. Manag. 415–416, 129–138. ( 10.1016/j.foreco.2018.02.028) [DOI] [Google Scholar]
- 38.Vaughan N. 1997. The diets of British bats (Chiroptera). Mam. Rev. 27, 77–94. ( 10.1111/j.1365-2907.1997.tb00373.x) [DOI] [Google Scholar]
- 39.Wilson JM, Barclay RM. 2006. Consumption of caterpillars by bats during an outbreak of western spruce budworm. Am. Midland Nat. 155, 244–249 ( 10.1674/0003-0031(2006)155[0244:COCBBD]2.0.CO;2) [DOI] [Google Scholar]
- 40.Charbonnier Y, Barbaro L, Theillout A, Jactel H. 2014. Numerical and functional responses of forest bats to a major insect pest in pine plantations. PLoS ONE 9, e109488 ( 10.1371/journal.pone.0109488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Penone C, et al. 2018. Body size information in large-scale acoustic bat databases. PeerJ 6, e5370 ( 10.7717/peerj.5370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siemers BM, Schnitzler HU. 2000. Natterer's bat (Myotis nattereri Kuhl, 1818) hawks for prey close to vegetation using echolocation signals of very broad bandwidth. Behav. Ecol. Sociobiol. 47, 400–412. ( 10.1007/s002650050683) [DOI] [Google Scholar]
- 43.Baeten L, et al. 2013. A novel comparative research platform designed to determine the functional significance of tree species diversity in European forests . Perspect. Plant Ecol. Evol. Syst. 15, 281–291. ( 10.1016/j.ppees.2013.07.002) [DOI] [Google Scholar]
- 44.Charbonnier YM, Barbaro L, Barnagaud JY, Ampoorter E, Nezan J, Verheyen K, Jactel H. 2016. Bat and bird diversity along independent gradients of latitude and tree composition in European forests. Oecologia 182, 529–537. ( 10.1007/s00442-016-3671-9) [DOI] [PubMed] [Google Scholar]
- 45.Azam C, Le Viol I, Julien J-F, Bas Y, Kerbiriou C.. 2016. Disentangling the relative effect of light pollution, impervious surfaces and intensive agriculture on bat activity with a national-scale monitoring program. Landscape Ecol. 31, 2471–2483. ( 10.1007/s10980-016-0417-3) [DOI] [Google Scholar]
- 46.Barnagaud JY, Barbaro L, Hampe A, Jiguet F, Archaux F. 2013. Species' thermal preferences affect forest bird communities along landscape and local scale habitat gradients. Ecography 36, 1218–1226. ( 10.1111/j.1600-0587.2012.00227.x) [DOI] [Google Scholar]
- 47.Le Viol I, Jiguet F, Brotons L, Herrando S, Lindstrom A, Pearce-Higgins JW, Reif J, Van Turnhout C, Devictor V.. 2012. More and more generalists: two decades of changes in the European avifauna. Biol. Lett. 8, 780–782. ( 10.1098/rsbl.2012.0496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laliberté E, Legendre P, Shipley B, Laliberté ME. 2015. Package ‘FD’. Measuring functional diversity from multiple traits, and other tools for functional ecology. R-package version 1.0-12. See http://cran.r-project.org/web/packages/FD/FD.pdf .
- 49.McCune B, Keon D. 2002. Equations for potential annual direct incident radiation and heat load. J. Vegetation Sci. 13, 603–606. ( 10.1111/j.1654-1103.2002.tb02087.x) [DOI] [Google Scholar]
- 50.Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, Dai B, Grothendieck G. 2016. Package ‘lme4’. R package version 1.1-10. See http://cran.r-project.org/web/packages/lme4/lme4.pdf.
- 51.Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R 2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. ( 10.1111/j.2041-210x.2012.00261.x) [DOI] [Google Scholar]
- 52.Blakey RV, Law BS, Kingsford RT, Stoklosa J, Tap P, Williamson K. 2016. Bat communities respond positively to large-scale thinning of forest regrowth. J. Appl. Ecol. 53, 1694–1703. ( 10.1111/1365-2664.12691) [DOI] [Google Scholar]
- 53.Speakman JR, Rydell J, Webb PI, Hayes JP, Hays GC, Hulbert IAR, McDevitt RM. 2000. Activity patterns of insectivorous bats and birds in northern Scandinavia (69N), during continuous midsummer daylight. Oikos 88, 75–86. ( 10.1034/j.1600-0706.2000.880109.x) [DOI] [Google Scholar]
- 54.Lopez B, Burgio K, Carlucci M, Palmquist K, Parada A, Weinberger V, Hurlbert A. 2016. A new framework for inferring community assembly processes using phylogenetic information, relevant traits and environmental gradients. One Ecosyst. 1, e9501 ( 10.3897/oneeco.1.e9501) [DOI] [Google Scholar]
- 55.Dubos N, et al. 2018. Disentangling the effects of spring anomalies in climate and net primary production on body size of temperate songbirds. Ecography 41, 1319–1330 ( 10.1111/ecog.03413) [DOI] [Google Scholar]
- 56.van der Plas F, et al. 2016. Biotic homogenization can decrease landscape-scale forest multifunctionality. Proc. Natl. Acad Sci. USA 113, 3557–3562. ( 10.1073/pnas.1517903113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Classen A, et al. 2014. Complementary ecosystem services provided by pest predators and pollinators increase quantity and quality of coffee yields. Proc. R. Soc. B 281, 20133148 ( 10.1098/rspb.2013.3148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barbaro L, et al. 2019. Data from: Biotic predictors complement models of bat and bird responses to climate and tree diversity in European forests. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Barbaro L, et al. 2019. Data from: Biotic predictors complement models of bat and bird responses to climate and tree diversity in European forests. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.t48p8c0 [58]. Data tables for site variables, bird species per sites, bat species per sites and bird and bat species traits.