Abstract
Cervical cancer is one of the most common gynecological tumors, and the majority of early-stage cervical cancer patients achieve good recovery through surgical treatment and concurrent chemoradiotherapy (CCRT). However, for patients with recurrent, persistent, metastatic cervical cancer, effective treatment is rare, except for bevacizumab combined with chemotherapy. Programmed cell death-1/programmed cell death-ligand 1 (PD-1/PD-L1) inhibitors might be a novel choice to improve the clinical outcomes of these patients. Thus far, some pivotal trials, including Keynote 028, Keynote 158 and Checkmate 358, have indicated established clinical benefit of PD-1/PD-L1 inhibitors in cervical cancer. In light of these data, the FDA has approved pembrolizumab for patients with recurrent or metastatic cervical cancer with disease progression during or after chemotherapy. There are also some ongoing studies that may provide more evidence for the PD-1/PD-L1 pathway as a therapeutic target in cervical cancer. In this review, we have summarized the status and application of PD-1/PD-L1 inhibitors in clinical trials for the treatment of cervical cancer and suggested some future directions in this field.
Keywords: cervical cancer, programmed cell death-1/programmed cell death-ligand 1 (PD-1/PD-L1), immune checkpoint inhibitors, immunotherapy, human papillomavirus (HPV)
Introduction
Cervical cancer is one of the most common gynecological tumors. More than 569,847 women are diagnosed with cervical cancer annually worldwide, resulting in over 311,365 deaths (Bray et al., 2018). Although the incidence of cervical cancer has been greatly reduced by the use of HPV vaccines and cervical cancer screening (Goodman, 2015), cervical cancer is second in terms of morbidity among gynecological tumors in developing countries (Sahasrabuddhe et al., 2012). Over 70% of cervical cancer cases diagnosed in developing countries are locally invasive or metastatic, contributing to the high mortality rate of cervical cancer. The 5-year OS rate of local cervical cancer can achieve approximately 75–85% through effective treatments such as surgery CCRT, etc. (Chen et al., 2015). Nevertheless, the 5-year OS of recurrent, persistent, metastatic cervical cancer is only approximately 15%. The poor prognosis is mainly due to limited therapeutic options (Guitarte et al., 2014). The majority of these patients can only be treated with palliative chemotherapy (Boussios et al., 2016), in which platinum-based chemotherapies were the prior choice (Monk et al., 2009). In 2014, the GOG 240 trial indicated that when bevacizumab was added to the chemotherapy, the ORR was improved from 36 to 48% (Tewari et al., 2014), and the OS could be prolonged from 13 to 17 months for recurrent, persistent, metastatic cervical cancer, thus laying the foundation for the first-line choice of combining bevacizumab with chemotherapy for this population (Tewari et al., 2017). However, for those who progress during the first-line treatment, the lack of effective second-line treatment remains to be the main reason for the high mortality rate (Minion and Tewari, 2018). Currently, immune checkpoint inhibitors (Schumacher and Schreiber, 2015), especially PD-1/PD-L1 inhibitors (Constantinidou et al., 2018), have achieved favorable efficacy in treating multiple solid tumors (Gettinger et al., 2018), including cervical cancer (Borcoman and Le Tourneau, 2017). Accumulating evidence has demonstrated that PD-1/PD-L1 inhibitors may be a promising approach for cervical cancer treatment.
Immune Checkpoint Inhibitors
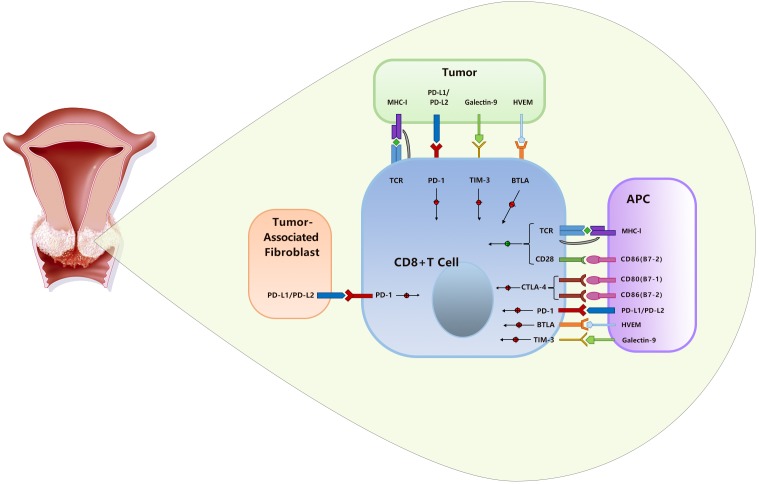
Numerous immunomodulatory therapies are being investigated in clinical trials with diverse potential targets, including PD-1/PD-L1, CTLA-4, Tim-3, ICOS, 4-1BB, and OX-40. Among these novel targets, ICOS (Amatore et al., 2018), 4-1BB (Compte et al., 2018), and OX-40 (Polesso et al., 2018) are costimulatory receptors, while PD-1/PD-L1 (Raedler, 2015), CTLA-4 (Lheureux et al., 2018), and Tim-3 (Gorris et al., 2018) are negative immune regulators of T cells. Currently, only CTLA-4 inhibitors (Hodi et al., 2010) and PD-1/PD-L1 inhibitors (Bagcchi, 2014) have been approved by the FDA. CTLA-4 integrates with the costimulatory molecules CD80 (B7-1) and CD86 (B7-2) that express on the surfaces of APCs (Fife and Bluestone, 2008), while PD-L1 is expressed on a wide variety of cell types, including tumor-associated fibroblasts, tumor cells, APCs, etc. (Boussiotis, 2016). As a result, CTLA-4 inhibits T cell activation within secondary lymphoid organs (Kurup et al., 2017), but PD-1/PD-L1 chiefly regulates T cell function within peripheral tissues and the tumor microenvironment (Pardoll, 2012). Therefore, PD-1/PD-L1 signaling is more specific to tumor than CTLA-4 signaling, and inhibitors of PD-1/PD-L1 may cause less damage to healthy tissue (Boussiotis, 2016; Minion and Tewari, 2018) (Figure 1).
FIGURE 1.
The CTLA-4 and PD-1/PD-L1 pathways in cervical cancer.
Based on the above mechanism, ipilimumab (monoclonal anti-CTLA-4), the first immune checkpoint inhibitor, approved for melanoma, had little clinical benefit until the emergence of pembrolizumab, and the combination of the two drugs further improved treatment efficacy in malignant melanoma (Wang et al., 2017). To date, another mAb for CTLA-4, tremelimumab, has not been approved for the treatment in any type of cancer. However, mAbs targeting PD-1 [pembrolizumab (Paz-Ares et al., 2018), nivolumab (Long et al., 2018), and cemiplimab (Sidaway, 2018)] and PD-L1 [atezolizumab (Hsu et al., 2018), durvalumab (Siu et al., 2018), and avelumab (Le Tourneau et al., 2018)] have presented clinical advantages in malignant melanoma, advanced NSCLC, urothelial cancer (Zhang and Li, 2018) and other tumors (Lim et al., 2018) (Table 1). In addition, extensive research has been carried out on gynecological tumors, such as ovarian cancer (Liu and Zamarin, 2018) and breast cancer (Julia et al., 2018), and clinical researches on cervical cancer are ongoing. At present, some initial results have been achieved.
Table 1.
The characteristics of the clinical application of monoclonal antibodies (mAbs) of immune checkpoint inhibitors in cervical cancer.
| Target | Drug (trade name) | Antibody type | Formerly name | Manufacturer | Time to market (FDA) | Indications |
|---|---|---|---|---|---|---|
| CTLA-4 | Ipilumumab (Yervoy) | IgG1 | – | BMS | March, 2011 | Melanoma, colorectal cancer, renal cell carcinoma |
| Tremelimumab | IgG2 | Ticilimumb, CP-675,206 | Pfizer | – | Undergoing human trials has not attained approval for any | |
| PD-1 | Pembrolizumab (Keytruda) | IgG4 | MK-3475 Lambrolizumab | MSD | September, 2014 | Advanced melanoma, non-small cell lung cancer, Hodgkin’s lymphoma, and head and neck SCC1 |
| Nivolumab (Opdivo) | IgG4 | BMS-9365580 NO-4538 | BMS | December, 2014 | Metastatic melanoma, squamous non-small cell lung cancer, renal cell carcinoma | |
| Cemiplimab (REGN2810) | IgG4 | – | Sanofi | September, 2018 (EMA2) | squamous cell skin cancer (EMPOWER-CSCC 1) | |
| PD-L1 | Durvalumab (Imfiniz) | IgGlK | – | AstraZeneca | May, 2017 | Bladder cancer, NSCLC3 |
| Atezolizumab (Tecentriq) | IgGl | – | Roche | April, 2016 | Lung cancer, bladder cancer, advanced triple negative breast cancer | |
1SCC, squamous cell cancers; 2EMA, European Medicines Agency; 3NSCLC, non-small cell lung cancer.
Theoretical Basis for PD-1/PD-L1 Inhibitors in Cervical Cancer
The PD-1/PD-L1 axis is one of the most well-known immune-checkpoint pathways with a mechanism of immune evasion for cancer cells and thus inhibiting the immune response in various kinds of solid tumors, including cervical cancer Cancer Genome Atlas Research Network et al. (2017). In brief, PD-L1 expresses on the surface of cervical tumor cells, APCs and TILs, while the PD-1-positive cells were mostly identified as T cells in the stroma of cervical tumors. For the expression of PD-1 in the tumor stroma of cervical cancer, Meng et al. (2018) reported that 60.82% (59/97) of the patients exhibited PD-1 expression, while another study showed PD-1 expression in 46.97% (31/66) of the patients (Feng et al., 2018).
To date, numerous studies have investigated the expression of PD-L1 in cervical cancer (Yang et al., 2013; Chen et al., 2016). The expression of PD-L1 has been reported in 34.4–96% of cervical carcinoma tissues, while expression of PD-L1 in histologically normal cervical tissues was rarely found (Enwere et al., 2017). Opal Reddy et al. (2017) showed that PD-L1 expression was positive in 32 of 93 (34.4%) cervical carcinoma samples, subcategorically in 28 of 74 (37.8%) SCCs, 2 of 7 (28.6%) adenosquamous carcinomas, and 2 of 12 (16.7%) endocervical adenocarcinomas. In another study, PD-L1 expression was found in 96% of the samples (Enwere et al., 2017). Specifically, for cervical SCC, PD-L1 expression was found in 80% (56/70) cases (Mezache et al., 2015). In the TCGA database for cervical SCCs, the amplification or gain of PD-L1 was found in 28 of 129 (22%) cases (Dijkstra et al., 2016). In addition, PD-L1 can also be expressed on TILs, which plays a role in antitumor response inhibition. A study found that for cervical SCCs samples, the expression rates of PD-L1 on cancer cells and TILs were 59.1 and 47.0%, respectively (Feng et al., 2018). Collectively, these data suggest that both PD-L1 and PD-1 are widely expressed in cervical cancer tumor cells and stroma, providing potential therapeutic targets for PD-1/PD-L1 inhibitors.
Notoriously, persistent HPV infection is involved in the pathogenesis of cervical cancer and is related to its prognosis. Several teams have interrogated whether HPV infection could affect PD-L1 expression in cervical cancer and found that HPV positivity was positively correlated with increased PD-L1 expression (Mezache et al., 2015; Liu et al., 2017).
Considerable effort has been made to dissect the underlying mechanism of the association between HPV status and PD-L1 expression in HPV-related solid tumors, mainly HNSCC and cervical cancer. In HPV-HNSCCs, membranous expression of PD-L1 and significant increased levels of mRNA of IFN-γ were found in the tonsillar crypts, As tonsillar crypts witnesses the initial HPV infection, and IFN-γ induces PD-L1 expression, this evidence might support the role of the PD-1/PD-L1 interaction in creating an “immune-privileged” site for initial viral infection and subsequent adaptive immune resistance (Franzen et al., 2018). In another study, DNA methylation of PD-L1 was inversely correlated with PD-L1 mRNA expression (p ≤ 0.002) and was further significantly associated with HPV infection in the TCGA cohort, indicating that DNA methylation of PD-L1 is associated with transcriptional silencing and HPV infection in HNSCCs (Balermpas et al., 2017). In cervical cancer, Qin et al. (2017) indicated that HPV-induced somatic mutations and a multitude of neoantigens, which played a crucial role in the inhibitory tumor microenvironment and could lead to notable alterations among checkpoint-related genes such as CTLA-4, PD-1, and PD-L1. Specifically, PD-L1 showed a positive correlation with ENO1, PRDM1, OVOL1, and MNT, all of which are related master regulators of HPV16 E6 and E7 (Qin et al., 2017). Of note, a single-arm, phase II study investigated durvalumab in patients with recurrent/metastatic HNSCCs (n = 112) and found that HPV-positive patients had a higher response rate and better survival than that of the HPV-negative patients (Zandberg et al., 2018). Nevertheless, for cervical cancer, the association of HPV status and the efficacy of PD-1/PD-L1 inhibitors is not yet certain due to the paucity of available data.
Several studies have probed the role of PD-L1 expression in the prognosis and therapeutic efficacy of cervical cancer. These results separately proved that an increase in PD-L1 expression was positively associated with tumor metastasis (Yang et al., 2017), tumor progression (Hsu et al., 2018) and poor prognosis in cervical cancer (Heeren et al., 2016). In this regard, the negative relationship between HPV infection and the clinical outcomes of cervical cancer may be partially attributed to the PD-L1 expression induced by HPV infection (Yang et al., 2017). For patients with locally advanced cervical adenocarcinoma and adenosquamous carcinoma treated with CRT, the underexpression of PD-L1 was a prognostic factor for tumor relapse (p = 0.041), indicating that PD-L1 expression might be a novel biomarker for CRT outcome (Lai et al., 2017).
Clinical Research Outcomes of PD-1/PD-L1 Inhibitors in Cervical Cancer
Since 2015, multiple clinical trials have been conducted to explore the application of PD-1/PD-L1 antibodies in cervical cancer. To date, four studies have yielded preliminary results (Table 2). Keynote 028 (a phase Ib study) and Keynote 158 (a phase II study) evaluated pembrolizumab at the dose of 10 mg/kg and 200 mg/kg, respectively, in recurrent, metastatic cervical cancer. In Keynote 028 (Frenel et al., 2017), 24 patients were enrolled, and the overall response rate (RECIST v1.1) was 17% (95% CI: 5 to 37%). In terms of toxicity, 5 patients experienced grade 3 AEs (NCI-CTCAE 3.0), while no grade 4 AEs was observed. In Keynote 158 (Schellens et al., 2017), 98 patients with recurrent or metastatic cervical cancer were enrolled. With a median follow-up time of 11.7 months, the ORR in 77 patients was 14.3% (95% CI: 7.4 to 24.1%), including 2.6% of the patients with CRs and 11.7% of patients with PRs, whereas no response was observed in patients without PD-L1 expression in tumor cells. The most frequent serious adverse reactions included anemia (7%), fistula (4.1%), hemorrhage (4.1%), and infection (4.1%). Based on Keynote 158, the FDA approved pembrolizumab on June 12, 2018, for advanced cervical cancer with disease progression during or after chemotherapy1. Checkmate 358 (Hollebecque et al., 2017) (phases I–II studies) adopted nivolumab (200 mg/kg q2w) for the treatment of recurrent, metastatic cervical cancer and resulted in an ORR of 26.3%. The disease control rate was 70.8%. The related grades 3–4 toxic effects included hyponatremia, syncope, diarrhea, and hepatocellular injury. From these three studies, pembrolizumab and nivolumab showed promising antitumor effects and were well-tolerated in patients with recurrent or metastatic cervical cancer. However, due to a limited follow-up time, PFS and OS were not reported. Additionally, the REGN2810 study (Papadopoulos et al., 2016), a phase I multicenter study, assessed REGN2810 (a PD-1 mAb) as a monotherapy and in combination with hfRT, in combination with cyclophosphamide (CTX) or with CTX + hfRT in patients with advanced solid tumors, including cervical cancer. This study adopted a dose escalation design, and as of February 2016, no dose-limiting toxicity (DLT) was observed. The most common treatment-related AEs were fatigue (n = 14, 24.1%), arthralgia (n = 7, 12.1%), and nausea (n = 6, 10.3%). Additionally, 4 patients experienced grade ≥ 3 AEs. For 9/22 (40.9%) patients who received REGN2810 + hfRT and 2/21 (9.5%) patients who received REGN2810 monotherapy, they were determined to have partial/uPRs, suggesting that the treatment response was augmented by the addition of hfRT.
Table 2.
Clinical research outcomes on PD-1/PD-L1 inhibitors in cervical cancer.
| Study | Author | Study population (n) | Phase | Treatment arm(s) | Principal results | Toxicity | Significance |
|---|---|---|---|---|---|---|---|
| REGN2810 | Papadopoulos et al., 2016 | Advanced solid tumors | I | Cemiplimab | 62.8% patients had disease control | No dose-limiting toxicities | Higher response rate when combined with radiation suggesting abscopal responses |
| Keynote 028 | Frenel et al., 2017 | Recurrent cervical cancer with PD-L1 positive tumors (24) | Ib | Pembrolizumab 10 mg/kg q2w | ORR1 17% (95% CI: 5–37%) | Grade=3 AE2 including rash and proteinuria | Well-tolerated and active in cervical cancer |
| Keynote 158 | Schellens et al., 2017 | Recurrent cervical cancer with progression or intolerance to standard therapy (82) | II | Pembrolizumab 200 mg/kg q2w | Preliminary results: ORR1 17% (95% CI: 8–31%); patients with >27 weeks of follow up, ORR 27% (95% CI: 8–55%) | Grades 3–4 AE2 included AST/ALT3 elevation and pyrexia | Demonstrates activity in cervical cancer and increasing response with a longer duration of follow-up |
| Checkmate 358 | Hollebecque et al., 2017 | Recurrent or metastatic HPV4-related cancers (19) | I–II | Nivolumab 240 mg q2w | Preliminary results: ORR1 26% (95% CI: 9.1–51.2%) in cervical cancer patients | Grade 3–4 AE2 included hyponatremia, syncope, diarrhea and hepatocellular injury | Durable responses demonstrated in cervical cancer patients, with at least 6 months duration |
1ORR, objective response rate; 2AE, adverse event; 3AST/ALT, aspartate transaminase/alanine transaminase; 4HPV, human papillomavirus.
Ongoing Clinical Research on PD-1/PD-L1 in Cervical Cancer
As of September 2018, 11 clinical trials have been conducted, mainly in patients with persistent, recurrent, or metastatic cervical cancer, with only three studies on patients with locally advanced cervical cancer. Twenty to thirty cases were intended to be included in the majority of these studies, while there were only three studies (Keynote 826, GOG 3016/ENGOT-cx9, and NCT03556839) in which more than 200 cases were intended to be included. Except for the two studies (IMMUVIX, GHR002) aimed at exploring the immune status of PD-1/PD-L1 in patients with locally advanced cervical cancer, the remaining 12 studies all looked into the applicability of PD-1/PD-L1 inhibitors in cervical cancer. Of these 12 studies, there are 2 studies on nivolumab, 2 on pembrolizumab, 4 on durvalumab, 2 on atezolizumab, 1 on cemiplimab (REGN2810) and 1 on AGEN2034. For PD-1 inhibitors, the difference between the 2 studies on nivolumab is the study population. NRG-GYO-02 was conducted in patients with persistent, recurrent, or metastatic cervical cancer, while the NiCOL study enrolled more patients with locally advanced cervical cancer. The main difference between the two studies on pembrolizumab is that KEYNOTE-826 adopted pembrolizumab in combination with chemotherapy versus placebo, while PAPAYA mainly adopted pembrolizumab in combination with platinum and radiotherapy. The GOG 3016/ENGOT-cx9 (EMPOWER-Cervical) study is an important phase III clinical study to advance the clinical application of cemiplimab (REGN2810) in advanced cervical cancer. NCT03104699 is a phase I/II clinical study on AGEN2034, another PD-1 inhibitor, in advanced solid tumors that includes 75 cases of cervical cancer. In terms of treatment combinations, tremelimumab (a fully human mAb against CTLA-4), Vigil vaccine for cervical cancer, bevacizumab, and chemotherapy were paired with PD-1/PD-L1 inhibitors throughout these studies (Table 3).
Table 3.
Ongoing clinical research on PD-1/PD-L1 in cervical cancer.
| Clinical trial code | Study | Study population (n) | Phase | Treatment arm(s) | Primary outcome measures | Secondary outcome measures |
|---|---|---|---|---|---|---|
| NCT02257528 | Nivolumab in Treating Patients with Persistent, Recurrent, or Metastatic Cervical Cancer (NRG-GYO-02) | Recurrent or metastatic cervical cancer (25) | II | Nivolumab | ORR1 [5 y]; AE2 [100 d] | PFS3 [5 y], OS4 [5 y] |
| NCT03298893 | Nivolumab in Association with Radiotherapy and Cisplatin in Locally Advanced Cervical Cancers Followed by Adjuvant Nivolumab for up to 6 Months (NiCOL) | Locally advanced cervical cancer (21) | III | Nivolumab | DLT5 [11 w] | ORR1 [2 m], PFS3 [2 y], DFS6 [2 y], SAE7 [100 d], AE2 [100 d], etc. |
| NCT03257267 | Study of REGN2810 in Adults with Cervical Cancer (GOG 3016/ENGOT-cx9) (EMPOWER-Cervical) | Recurrent or metastatic platinum-refractory cervical cancer (436) | III | Cemiplimab (REGN2810) | OS4 [32 m] | PFS3 [32 m], ORR1 [32 m], DOR8 [32 m], Quality of life (QOL) [100 w] |
| NCT03104699 | Phase 1/2 Study of AGEN2034 in Advanced Tumors and Cervical Cancer | Advanced cervical cancer (75) | I–II | AGEN2034 | DLTs5 [3 w], MTD9 [1 y], BOR10 [1 y] | Cmax11 [1 y], AUC12 [1 y], PFS3 [1 y], DOR8 [1 y], OS4 [1 y] |
| NCT03518606 | Metronomic Oral Vinorelbine Plus Anti-PD-L1/Anti-CTLA4 ImmunothErapy in Patients with Advanced Solid Tumors (MOVIE) | Advanced solid tumors (150) including cervical cancer | I–II | Durvalumab+Tremelimumab+metronomic Vinorelbine | Phase I: MTD9 and RP2D13 [9 m] Phase II: CBR14 [24 m] | None |
| NCT03556839 | Platinum Chemotherapy Plus Paclitaxel with Bevacizumab and Atezolizumab in Metastatic Carcinoma of the Cervix | Carcinoma of the cervix, stage IVB (404) | III | Atezolizumab | OS4 [48 m] | PFS3 [48 m], ORR1 [48 m], DOR8 [48 m], AE2 [48 m], etc. |
| NCT01975831 | A Phase 1 Study to Evaluate MEDI4736 in Combination with Tremelimumab | Solid tumors (106) including cervical cancer | I | MEDI4736 (Durvalumab)+Trem elimumab | AE2 [1 y] | AUC12, Cmax11 [15 m], PFS3 [15 m], OS4 [15 m], etc. |
| NCT02914470 | Pilot Study of Durvalumab and Vigil in Advanced Women’s Cancers (PROLOG) | Solid tumors (12) including cervical cancer | I | Durvalumab and Vigil | Toxicity [30 d] | ORR1 [120 m] |
| NCT02725489 | Pilot Study of Durvalumab and Vigil in Advanced Women’s Cancers | Solid tumors (15) including cervical cancer | II | Vigil+durvalumab | AEs2 [90 d] | ORR1 [12 m], Disease status [12 m], IFNγ-ELISPOT conversion rate [12 w] |
| NCT02921269 | Atezolizumab and Bevacizumab in Treating Patients with Recurrent, Persistent, or Metastatic Cervical Cancer | Recurrent, persistent, or metastatic cervical cancer (22) | II | Atezolizumab+Bevac izumab | ORR1 [2 y] | PFS3 [2 y], OS4 [2 y] AE2 [30 d], PD-L1, etc. |
| NCT03635567 | Efficacy and Safety Study of First-line Treatment with Pembrolizumab (MK-3475) Plus Chemotherapy Versus Placebo Plus Chemotherapy in Women with Persistent, Recurrent, or Metastati Cervical Cancer (MK-3475-826/KEYNOTE-826) | Cervical cancer (600) c | I–II | Pembrolizumab | PFS3 [2y] OS4 [2 y] | ORR1 [2 y], DOR8 [2 y], etc. |
| NCT03144466 | A Study of Pembrolizumab And Platinum with Radiotherapy in Cervix Cancer (PAPAYA) | Cervical cancer (26) | I | Pembrolizum | MTD9 [2 y] ab Efficacy [2 y] | OS4 [2 y], PFS3 [2 y], etc. |
| NCT03255252 | Assessment Study to Evaluate Specific Immune Response in Locally Advanced Cervix Cancer After Radio-chemotherapy (IMMUVIX) | Cervical cancer (100) | II | Cisplatin | Expression of CD8+CD39+PD1+ | Effect on 1-year DFS6 of other putative biomarkers (CD73, CD39, PD1 and Tim3) |
| NCT03559803 | A Prospective Study of Monitoring Immune Response in Locally Advanced Cervix Cancer(GHR002) | Cervical cancer(50) | Not appli cable | Cisplatin | PD-L1 [3w, 2 m] | PD1+CD4+T [3w, 2 m], PD1+CD8+T [3w, 2 m], TCR[3w, 2 m] |
1ORR, objective response rate; 2AE, adverse event; 3PFS, progression-free survival; 4OS, overall survival rate; 5DLT, dose limiting toxicity; 6DFS, disease-free survival; 7SAE, serious adverse event; 8DOR, duration of response; 9MTD, maximum tolerated dose; 10BOR, best overall response; 11Cmax, maximum plasma concentration; 12AUC, area under curve; 13RP2D, phase II recommended dose; 14CBR, clinical benefit response.
Conclusion
Although there are a few studies suggesting the potential feasibility of PD-1/PD-L1 inhibitors for the treatment of cervical cancer, a consideration should be made for the clinical application of PD-1/PD-L1 inhibitors. The inadequate number of cases included and the insufficient follow-up time are the main defects of all the studies, leading to the unavailability of data regarding OS, PFS, AEs, drug resistance and the treatment mechanism as well. These data are very pivotal not only for obtaining a more convincing result, but also for guiding physicians to select the appropriate patients for PD-1/PD-L1 inhibitors.
Currently, most of these studies, including ongoing studies, are mostly limited to recurrent, persistent, metastatic cervical cancer, which accounts for only a minor portion of patients with cervical cancer. There are several future directions that can be given more attention. First, the latest evidence suggests a clinical benefit of PD-1/PD-L1 inhibitors as neoadjuvant therapy in lung cancer (Lommatzsch et al., 2018). For patients with early-stage cervical cancer, studies in a small sample size can be conducted to investigate PD-1/PD-L1 inhibitors with attempted surgical treatment or to prevent post-operative recurrence. Second, for patients with locally advanced cervical cancer who are not sensitive to CCRT or who relapse in the short term after initial treatment, PD-1/PD-L1 inhibitors may be a useful treatment, and we are looking forward to the research targeting this population. Third, for locally advanced cervical cancer patients, whether PD-1/PD-L1 inhibitors can achieve better therapeutic efficacy in tumors with higher PD-L1 expression before CCRT begins will provide a better understanding of the effects of these inhibitors. Finally, since PD-L1 expression is correlated with HPV status, more studies are warranted to provide further insights into the association of HPV status and the efficacy of PD-1/PD-L1 inhibitors in patients with cervical cancer. Combining the level of HPV DNA with the expression of PD-L1 may also provide a novel predictive biomarker of the efficacy of PD-1/PD-L1 inhibitors and the prognosis of patients with cervical cancer.
Author Contributions
JX and YoL conceived the review. YuL and LYi searched the literature. YuL, LYi, LW, FY, ML, LY, and RT critically appraised the literature and wrote and all authors approved the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
- AE
adverse event
- APCs
antigen-presenting cells
- CCRT
concurrent chemoradiotherapy
- CRs
complete responses
- CRT
chemoradiotherapy
- CTLA-4
cytotoxic T-lymphocyte-associated protein-4
- hfRT
hyperfraction radiotherapy
- HNSCC
head and neck squamous cell carcinoma
- HPV
human papillomavirus
- mAb
monoclonal antibody
- NSCLC
non-small cell lung cancer
- ORR
objective response rate
- OS
overall survival rate
- PD-1/PD-L1
programmed cell death-1/programmed cell death-ligand 1
- PFS
progression-free survival
- PRs
partial responses
- SCCs
squamous cell cancers
- TILs
tumor infiltrating lymphocytes
- uPRs
unconfirmed partial responses
Funding. This work was supported by National Natural Science Foundation of China (grants 81672982 and 81602670) and Sichuan Provincial Research Foundation for Basic Research (No. 18YYJC1284).
References
- Amatore F., Gorvel L., Olive D. (2018). Inducible co-stimulator (ICOS) as a potential therapeutic target for anti-cancer therapy. Expert. Opin. Ther. Targets 22 343–351. 10.1080/14728222.2018.1444753 [DOI] [PubMed] [Google Scholar]
- Bagcchi S. (2014). Pembrolizumab for treatment of refractory melanoma. Lancet Oncol. 15:e419 10.1016/S1470-2045(14)70348-1 [DOI] [PubMed] [Google Scholar]
- Balermpas P., Martin D., Wieland U., Rave-Frank M., Strebhardt K., Rodel C., et al. (2017). Human papilloma virus load and PD-1/PD-L1, CD8(+) and FOXP3 in anal cancer patients treated with chemoradiotherapy: rationale for immunotherapy. Oncoimmunology 6:e1288331. 10.1080/2162402X.2017.1288331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borcoman E., Le Tourneau C. (2017). Pembrolizumab in cervical cancer: latest evidence and clinical usefulness. Ther. Adv. Med. Oncol. 9 431–439. 10.1177/1758834017708742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussios S., Seraj E., Zarkavelis G., Petrakis D., Kollas A., Kafantari A., et al. (2016). Management of patients with recurrent/advanced cervical cancer beyond first line platinum regimens: where do we stand? A literature review. Crit. Rev. Oncol. Hematol. 108 164–174. 10.1016/j.critrevonc.2016.11.006 [DOI] [PubMed] [Google Scholar]
- Boussiotis V. A. (2016). Molecular and biochemical aspects of the PD-1 checkpoint pathway. N. Engl. J. Med. 375 1767–1778. 10.1056/NEJMra1514296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A. (2018). Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68 394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network, Albert Einstein College of Medicine, Analytical Biological Services, Barretos Cancer Hospital, Baylor College of Medicine, Beckman Research Institute of City of Hope, et al. (2017). Integrated genomic and molecular characterization of cervical cancer. Nature 543 378–384. 10.1038/nature21386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Gu W., Yang L., Chen C., Shao R., Xu K., et al. (2015). Nanotechnology in the management of cervical cancer. Rev. Med. Virol. 25(Suppl. 1), 72–83. 10.1002/rmv.1825 [DOI] [PubMed] [Google Scholar]
- Chen Z., Pang N., Du R., Zhu Y., Fan L., Cai D., et al. (2016). Elevated expression of programmed death-1 and programmed death ligand-1 negatively regulates immune response against cervical cancer cells. Med. Inflamm. 2016:6891482. 10.1155/2016/6891482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compte M., Harwood S. L., Munoz I. G., Navarro R., Zonca M., Perez-Chacon G., et al. (2018). A tumor-targeted trimeric 4-1BB-agonistic antibody induces potent anti-tumor immunity without systemic toxicity. Nat. Commun. 9:4809. 10.1038/s41467-018-07195-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidou A., Alifieris C., Trafalis D. T. (2018). Targeting programmed cell death -1 (PD-1) and ligand (PD-L1): a new era in cancer active immunotherapy. Pharmacol. Ther. 18 30173–30176. 10.1016/j.pharmthera.2018.09.008 [DOI] [PubMed] [Google Scholar]
- Dijkstra K. K., Voabil P., Schumacher T. N., Voest E. E. (2016). Genomics- and transcriptomics-based patient selection for cancer treatment with immune checkpoint inhibitors: a review. JAMA Oncol. 2 1490–1495. 10.1001/jamaoncol.2016.2214 [DOI] [PubMed] [Google Scholar]
- Enwere E. K., Kornaga E. N., Dean M., Koulis T. A., Phan T., Kalantarian M., et al. (2017). Expression of PD-L1 and presence of CD8-positive T cells in pre-treatment specimens of locally advanced cervical cancer. Mod. Pathol. 30 577–586. 10.1038/modpathol.2016.221 [DOI] [PubMed] [Google Scholar]
- Feng Y. C., Ji W. L., Yue N., Huang Y. C., Ma X. M. (2018). The relationship between the PD-1/PD-L1 pathway and DNA mismatch repair in cervical cancer and its clinical significance. Cancer Manag. Res. 10 105–113. 10.2147/CMAR.S152232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife B. T., Bluestone J. A. (2008). Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev. 224 166–182. 10.1111/j.1600-065X.2008.00662.x [DOI] [PubMed] [Google Scholar]
- Franzen A., Vogt T. J., Muller T., Dietrich J., Schrock A., Golletz C., et al. (2018). PD-L1 (CD274) and PD-L2 (PDCD1LG2) promoter methylation is associated with HPV infection and transcriptional repression in head and neck squamous cell carcinomas. Oncotarget 9 641–650. 10.18632/oncotarget.23080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenel J. S., Le Tourneau C., O’neil B., Ott P. A., Piha-Paul S. A., Gomez-Roca C., et al. (2017). Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase Ib KEYNOTE-028 trial. J. Clin. Oncol. 35 4035–4041. 10.1200/JCO.2017.74.5471 [DOI] [PubMed] [Google Scholar]
- Gettinger S., Horn L., Jackman D., Spigel D., Antonia S., Hellmann M., et al. (2018). Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: results from the CA209-003 study. J. Clin. Oncol. 36 1675–1684. 10.1200/JCO.2017.77.0412 [DOI] [PubMed] [Google Scholar]
- Goodman A. (2015). HPV testing as a screen for cervical cancer. BMJ 350:h2372. 10.1136/bmj.h2372 [DOI] [PubMed] [Google Scholar]
- Gorris M. A. J., Halilovic A., Rabold K. (2018). Eight-color multiplex immunohistochemistry for simultaneous detection of multiple immune checkpoint molecules within the tumor microenvironment. J. Immunol. 200 347–354. 10.4049/jimmunol.1701262 [DOI] [PubMed] [Google Scholar]
- Guitarte C., Alagkiozidis I., Mize B., Stevens E., Salame G., Lee Y. C. (2014). Glassy cell carcinoma of the cervix: a systematic review and meta-analysis. Gynecol. Oncol. 133 186–191. 10.1016/j.ygyno.2014.01.048 [DOI] [PubMed] [Google Scholar]
- Heeren A. M., Punt S., Bleeker M. C., Gaarenstroom K. N., Van Der Velden J., Kenter G. G., et al. (2016). Prognostic effect of different PD-L1 expression patterns in squamous cell carcinoma and adenocarcinoma of the cervix. Mod. Pathol. 29 753–763. 10.1038/modpathol.2016.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi F. S., O’day S. J., Mcdermott D. F., Weber R. W., Sosman J. A., Haanen J. B., et al. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363 711–723. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollebecque A., Meyer T., Moore K. N., Machiels J.-P. H., De Greve J., López-Picazo J. M., et al. (2017). An open-label, multicohort, phase I/II study of nivolumab in patients with virus-associated tumors (CheckMate 358): efficacy and safety in recurrent or metastatic (R/M) cervical, vaginal, and vulvar cancers. J. Clin. Oncol. 35:5504 10.1200/JCO.2017.35.15_suppl.5504 [DOI] [Google Scholar]
- Hsu P. C., Li S. H., Yang C. T. (2018). Recurrent pneumonitis induced by atezolizumab (anti-programmed death ligand 1) in NSCLC patients who previously experienced anti-programmed death 1 immunotherapy-related pneumonitis. J. Thorac. Oncol. 13 e227–e230. 10.1016/j.jtho.2018.06.022 [DOI] [PubMed] [Google Scholar]
- Julia E. P., Amante A., Pampena M. B., Mordoh J., Levy E. M. (2018). Avelumab, an IgG1 anti-PD-L1 immune checkpoint inhibitor, triggers NK cell-mediated cytotoxicity and cytokine production against triple negative breast cancer cells. Front. Immunol. 9:2140. 10.3389/fimmu.2018.02140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurup S. P., Obeng-Adjei N., Anthony S. M., Traore B., Doumbo O. K., Butler N. S., et al. (2017). Regulatory T cells impede acute and long-term immunity to blood-stage malaria through CTLA-4. Nat. Med. 23 1220–1225. 10.1038/nm.4395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y. L., Chen L. C., Huang C. Y., Chiang S. F., Liang J. A., Chao K. S. C., et al. (2017). PD-L1 as the prognostic immune biomarker for predicting the relapse of locally advanced cervical adenocarcinoma and adenosquamous carcinoma treated with definitive chemoradiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 99 E299–E300. 10.1016/j.ijrobp.2017.06.1318 [DOI] [Google Scholar]
- Le Tourneau C., Hoimes C., Zarwan C., Wong D. J., Bauer S., Claus R., et al. (2018). Avelumab in patients with previously treated metastatic adrenocortical carcinoma: phase 1b results from the JAVELIN solid tumor trial. J. Immunother. Cancer 6:111. 10.1186/s40425-018-0424-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lheureux S., Butler M. O., Clarke B., Cristea M. C., Martin L. P., Tonkin K., et al. (2018). Association of ipilimumab with safety and antitumor activity in women with metastatic or recurrent human papillomavirus-related cervical carcinoma. JAMA Oncol. 4:e173776. 10.1001/jamaoncol.2017.3776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim M., Xia Y., Bettegowda C., Weller M. (2018). Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 15 422–442. 10.1038/s41571-018-0003-5 [DOI] [PubMed] [Google Scholar]
- Liu C., Lu J., Tian H., Du W., Zhao L., Feng J., et al. (2017). Increased expression of PDL1 by the human papillomavirus 16 E7 oncoprotein inhibits anticancer immunity. Mol. Med. Rep. 15 1063–1070. 10.3892/mmr.2017.6102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. L., Zamarin D. (2018). Combination immune checkpoint blockade strategies to maximize immune response in gynecological cancers. Curr. Oncol. Rep. 20:94. 10.1007/s11912-018-0740-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommatzsch M., Bratke K., Stoll P. (2018). Neoadjuvant PD-1 blockade in resectable lung cancer. N. Engl. J. Med. 379:e14. 10.1056/NEJMc1808251 [DOI] [PubMed] [Google Scholar]
- Long G. V., Tykodi S. S., Schneider J. G., Garbe C., Gravis G., Rashford M., et al. (2018). Assessment of nivolumab exposure and clinical safety of 480 mg every 4 weeks flat-dosing schedule in patients with cancer. Ann. Oncol. 29 2208–2213. 10.1093/annonc/mdy408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y., Liang H., Hu J., Liu S., Hao X., Wong M. S. K., et al. (2018). PD-L1 Expression correlates with tumor infiltrating lymphocytes and response to neoadjuvant chemotherapy in cervical cancer. J. Cancer 9 2938–2945. 10.7150/jca.22532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezache L., Paniccia B., Nyinawabera A., Nuovo G. J. (2015). Enhanced expression of PD L1 in cervical intraepithelial neoplasia and cervical cancers. Mod. Pathol. 28 1594–1602. 10.1038/modpathol.2015.108 [DOI] [PubMed] [Google Scholar]
- Minion L. E., Tewari K. S. (2018). Cervical cancer - state of the science: from angiogenesis blockade to checkpoint inhibition. Gynecol. Oncol. 148 609–621. 10.1016/j.ygyno.2018.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk B. J., Sill M. W., Mcmeekin D. S., Cohn D. E., Ramondetta L. M., Boardman C. H., et al. (2009). Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a gynecologic oncology group study. J. Clin. Oncol. 27 4649–4655. 10.1200/JCO.2009.21.8909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos K. P., Crittenden M. R., Johnson M. L., Lockhart A. C., Moore K. N., Falchook G. S. (2016). A first-in-human study of REGN2810, a monoclonal, fully human antibody to programmed death-1 (PD-1), in combination with immunomodulators including hypofractionaed radiotherapy (hfRT). J. Clin. Oncol. 34:3024 10.1200/JCO.2016.34.15_suppl.3024 [DOI] [Google Scholar]
- Pardoll D. M. (2012). The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12 252–264. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Ares L., Luft A., Vicente D., Tafreshi A., Gumus M., Mazieres J., et al. (2018). Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Engl. J. Med. 379 2040–2051. 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- Polesso F., Weinberg A., Moran A. E. (2018). Late stage tumor regression after PD-L1 blockade with a concurrent OX40 agonist. Cancer Immunol. Res. 10.1158/2326-6066.CIR-18-0222 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Qin Y., Ekmekcioglu S., Forget M. A., Szekvolgyi L., Hwu P., Grimm E. A., et al. (2017). Cervical cancer neoantigen landscape and immune activity is associated with human papillomavirus master regulators. Front. Immunol. 8:689. 10.3389/fimmu.2017.00689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raedler L. A. (2015). Opdivo (Nivolumab): second PD-1 inhibitor receives FDA approval for unresectable or metastatic melanoma. Am. Health Drug Benefits 8 180–183. [PMC free article] [PubMed] [Google Scholar]
- Reddy O. L., Shintaku P. I., Moatamed N. A. (2017). Programmed death-ligand 1 (PD-L1) is expressed in a significant number of the uterine cervical carcinomas. Diagn. Pathol. 12:45. 10.1186/s13000-017-0631-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahasrabuddhe V. V., Parham G. P., Mwanahamuntu M. H., Vermund S. H. (2012). Cervical cancer prevention in low- and middle-income countries: feasible, affordable, essential. Cancer Prevent. Res. 5 11–17. 10.1158/1940-6207.CAPR-11-0540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellens J. H. M., Marabelle A., Zeigenfuss S., Ding J., Pruitt S. K., Chung H. C. (2017). Pembrolizumab for previously treated advanced cervical squamous cell cancer:preliminary results from the phase 2 KEYNOTE-158 study. J. Clin. Oncol. 35:5514 10.1200/JCO.2017.35.15_suppl.5514 [DOI] [Google Scholar]
- Schumacher T. N., Schreiber R. D. (2015). Neoantigens in cancer immunotherapy. Science 348 69–74. 10.1126/science.aaa4971 [DOI] [PubMed] [Google Scholar]
- Sidaway P. (2018). Cemiplimab effective in cutaneous SCC. Nat. Rev. Clin. Oncol. 15:472. 10.1038/s41571-018-0056-5 [DOI] [PubMed] [Google Scholar]
- Siu L. L., Even C., Mesia R., Remenar E., Daste A., Delord J. P., et al. (2018). Safety and efficacy of durvalumab with or without tremelimumab in patients with PD-L1-low/negative recurrent or metastatic HNSCC: the phase 2 CONDOR randomized clinical trial. JAMA Oncol. 10.1001/jamaoncol.2018.4628 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari K. S., Sill M. W., Long H. J., III, Penson R. T., Huang H., Ramondetta L. M., et al. (2014). Improved survival with bevacizumab in advanced cervical cancer. N. Engl. J. Med. 370 734–743. 10.1056/NEJMoa1309748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari K. S., Sill M. W., Penson R. T., Huang H., Ramondetta L. M., Landrum L. M., et al. (2017). Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (gynecologic oncology group 240). Lancet 390 1654–1663. 10.1016/S0140-6736(17)31607-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Chmielowski B., Pellissier J., Xu R., Stevinson K., Liu F. X. (2017). Cost-Effectiveness of pembrolizumab versus ipilimumab in ipilimumab-naive patients with advanced melanoma in the United States. J. Manag. Care Spec. Pharm. 23 184–194. 10.18553/jmcp.2017.23.2.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Lu Y. P., Yang Y. Z., Kang J. R., Jin Y. D., Wang H. W. (2017). Expressions of programmed death (PD)-1 and PD-1 ligand (PD-L1) in cervical intraepithelial neoplasia and cervical squamous cell carcinomas are of prognostic value and associated with human papillomavirus status. J. Obstet. Gynaecol. Res. 43 1602–1612. 10.1111/jog.13411 [DOI] [PubMed] [Google Scholar]
- Yang W., Song Y., Lu Y. L., Sun J. Z., Wang H. W. (2013). Increased expression of programmed death (PD)-1 and its ligand PD-L1 correlates with impaired cell-mediated immunity in high-risk human papillomavirus-related cervical intraepithelial neoplasia. Immunology 139 513–522. 10.1111/imm.12101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandberg D. P., Algazi A. P., Jimeno A., Good J. S., Fayette J., Bouganim N., et al. (2018). Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: results from a single-arm, phase II study in patients with > / = 25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur. J. Cancer 107 142–152. 10.1016/j.ejca.2018.11.015 [DOI] [PubMed] [Google Scholar]
- Zhang S., Li W. (2018). The effort in exploration of a definitive predictive factor from PD-1/PD-L1 blockade in advanced or metastatic urothelial cancer. J. Clin. Oncol. 36 3056–3057. 10.1200/JCO.2018.79.1400 [DOI] [PubMed] [Google Scholar]