Abstract
Oxidative C–H/N–H cross-coupling is one of the most atom-economical methods for the construction of C–N bonds. However, traditional oxidative C–H/N–H cross-coupling either required the use of strong oxidants or high reaction temperature, which makes it difficult to tolerate redox active functional groups. Herein we describe an external chemical oxidant-free electrooxidative C–H/N–H cross-coupling between electron-rich arenes and diarylamine derivatives. Under undivided electrolytic conditions, a series of triarylamine derivatives are produced from electron-rich arenes and diarylamine derivatives with high functional group tolerance. Both of the coupling partners are redox active in oxidative C–H/N–H cross-coupling, which enables high regioselectivity in C–N bond formation. Exclusive para-selectivity is observed for the coupling with anilines.
Oxidative C–H/N–H cross-coupling is a convenient method to construct C–N bonds, however this is rarely achieved in a sustainable manner. Here, the authors show an oxidant-free electrochemical C–H/N–H cross-coupling between electron-rich arenes and diarylamines to afford triarylamines with exclusive para-selectivity.
Introduction
Aryl C–N bond formation has long been considered to be important since C(sp2)–N bonds widely exist in pharmaceuticals, agrochemicals, and materials1,2. Normal ways to access C–N bonds are transition metal catalyzed amination of aryl halides or aryl boronic acids3–6. Recent achievements have revealed that oxidative C–H/N–H cross-coupling can serve as an atom-economical way to construct C–N bonds since it avoids substrate prefuncationalization steps7–9. However, research on oxidative aryl C–N bond formation remains elusive in its early stages when compared with the development of oxidative cross-coupling for C–C bond formation. The developed oxidative aryl C–N bond formation either required the use of strong oxidants10–17 or high reaction temperature18–22. As a result, redox active functional groups such as amino, hydroxyl, sulfur, and alkynyl are hard to be tolerated under these oxidative conditions.
An ideal way to solve this problem is to achieve oxidative C–H/N–H cross-coupling without the use of external chemical oxidants under mild conditions23–26. In 2016, our group developed an oxidative aryl C–H amination of arenes with azoles by utilizing synergistic cooperation of a photocatalyst and cobalt oxime complex27. No chemical oxidants were required and hydrogen gas was the only byproduct in that transformation. However, the amination source was limited to redox inactive azoles. The C–N bond was proposed to be formed through the nucleophilic addition of azoles to the in situ generated arene cation radical28. Electrochemistry provides new possibilities for green organic synthesis, which has attracted increasing interests in the area of cross-coupling reactions29–42. As early as 2013, Yoshida and co-workers reported analogous mechanism for aryl C–H amination of arenes by utilizing electrochemical oxidation (Fig. 1a)43,44. Similarly, the amination sources were limited to redox inactive pyridine and imidazoles. More recently, our group45 and Ackermann group46 independently studied the cobalt-catalyzed electrooxidative C–H amination of arenes with secondary amines (Fig. 1b). Due to the effect of directing group, only ortho-selective C–H amination products were obtained. In this transformation, none of the substrates were directly oxidized by anode. Up to now, there is no precedent on the electrooxidative aryl C–H amination with both redox active arenes and amination sources. Last year, our group have reported an electrooxidative C–H sulfenylation of electron-rich arenes with aromatic thiols47. Both of the coupling partners were redox active under the utilized electrolytic conditions. The high regioselectivity for C–S bond formation was believed to be a result of the radical/radical cross-coupling between the two coupling partners. As for electrooxidative C–H amination, we reported an oxidative C–H amination of phenols with phenothiazines recently48. Phenols were not oxidized due to relatively higher oxidation potentials. The amination source was limited to phenothiazines and both the ortho and para-amination products were observed. Aniline and its derivatives have been recognized as redox active substrates by electroanalytical techniques49. In 1992, Yang and Bard discovered that a para-selective homo-coupling product, p-amino diphenylamine, was formed as the dominate intermediate at the initial stage of electropolymerization of aniline in acidic aqueous solution (Fig. 1c)50. We envisioned that oxidative amination between two redox active coupling partners such as aniline derivatives might enable different regioselectivity and broaden the compatibility of functional groups in oxidative C–H amination.
Fig. 1.
Electrooxidative intermolecular aryl C–H amination. a Electrooxidative aryl C–H amination of arenes with redox inactive pyridine and imidazoles. b Cobalt-catalyzed electrooxidative ortho-selective aryl C–H amination of arenes with secondary amines. c Electrooxidative homo-coupling of aniline in fast scan cyclic voltammetry. d Catalyst-free electrooxidative para-selective aryl C–H amination of anilines with diarylamine derivatives
Triarylamine derivatives have already demonstrated highly important applications as redox mediators32 and photocatalysts51–54. Meanwhile, triarylamine-based molecules have dramatically promoted the development of optoelectronic materials during the past decade55–59. Conventional approaches toward the installation of triarylamines largely rely on the cross-coupling of diarylamines with prefunctionalized aryl halides60–63. Here we report an electrooxidative C–H/N–H cross-coupling between electron-rich arenes and diarylamine derivatives under catalyst- and external chemical oxidant-free conditions. This method provides an atom-economical and easy-to-handle way to access a series of triarylamine derivatives with potential electronic and optical properties64–66. Interestingly, exclusive para-selective C–H amination of anilines is observed in this transformation (Fig. 1d). Since both of the substrates are redox active, C–N bonds are proposed to be formed through the radical/radical cross-coupling between in situ generated aniline radical cations and nitrogen radicals.
Results
Investigation of reaction conditions and substrate scope
Direct mixing N,N-dimethylaniline (1a) with phenothiazine (2a) under 7 mA constant current for 2 h in a simple undivided cell could furnish a selective para-selective oxidative C(sp2)–H/N–H cross-coupling product 3aa in 71% yield (Fig. 2). Effect of reaction parameters was shown in Supplementary Information (Supplementary Table 1). The major side reaction is the dimerization and decomposition of 2a21. At the same time, the homo-coupling product of 1a could also be observed. In the next step, different amine substrates were applied to react with 1a (Fig. 2). Phenothiazines bearing electron-withdrawing and donating substituents were all able to furnish the desired products in good to high yields (3ab-3ae). Phenoxazine could also achieve good reaction efficiency in this oxidative C–H amination reaction (3af). Besides phenothiazine derivatives, diarylamines were also tested under the standard conditions. The reaction between 1a and 3-methyl-N-(p-tolyl)aniline (2g) only afforded corresponding triphenylamine in 20% yield (3ag). The major side reaction is the decomposition of 3-methyl-N-(p-tolyl)aniline under the standard conditions. By using hexafluoro-2-propanol (HFIP) instead of methanol, the yield of 3ag could be increased to 55% after certain degree of optimization. Interestingly, the para-selective homo-coupling product of 1a could be isolated in 21% yield. Other diphenylamines with free para C–H bond were also able to couple with 1a in acceptable yields (3ah-3aj). The reaction between 1a and di-p-tolylamine afforded corresponding triphenylamine in 54% yield (3ak). Highly functionalized diarylamine with four continuous chiral centers could afford the desired triphenylamine in 43% yield (3al).
Fig. 2.
para-Selective C–H amination of N,N-dimethylaniline with different diarylamine derivatives. Reaction conditions: graphite rod anode (ϕ 6 mm), platinum plate cathode (15 mm × 15 mm × 0.3 mm), constant current = 7 mA (Janode ≈ 7.8 mA cm−2), 1 (0.30 mmol), 2 (0.20 mmol), nBu4NBF4 (0.15 mmol), MeCN/MeOH (7.0 mL/3.0 mL), room temperature, N2, 2.0 h (2.6 F). Isolated yields were shown. aConstant current = 12 mA (Janode ≈ 13.3 mA cm−2), HFIP/MeCN (5.0 mL/5.0 mL)
In the next step, different anilines were applied as substrates in this transformation (Fig. 3). N,N-dimethylanilines bearing electron-donating methyl and methoxyl groups at the meta-position could afford the amination product in increased reaction yields (3ba, 3ca). Halides including bromide and iodide were well tolerated in this process, which furnished the desired para-amination product with good reaction yields (3dd, 3ed). Notably, 3-ethynyl-N,N-dimethylaniline was also suitable in this electrooxidative C–H amination reaction and afforded the selective para-amination product in 90% yield (3fd). N,N-dimethylanilines bearing two methyl or two methoxyl groups at the meta-position showed excellent reactivity in the amination reaction (3ga, 3ha). As for the reaction with 2,3-disubstituted-N,N-dimethylaniline, N,N-dimethyl-5,6,7,8-tetrahydronaphthalen-1-amine could afford the para-amination product in 63% yield (3ia). Moreover, N,N-dimethylnaphthalen was compatible in this transformation as well, which afforded the desired amination product at C-4 position in high selectivity (3ja). Anilines with cyclic N-alkyl substituents also demonstrated good reactivity in this C(sp2)–N bond formation reaction (3ka, 3la, and 3md). It is worthy of noting that N,N-dimethylanilines bearing para substituents were unreactive in this transformation.
Fig. 3.
para-Selective C–H amination of different anilines. Reaction conditions: graphite rod anode (ϕ 6 mm), platinum plate cathode (15 mm × 15 mm × 0.3 mm), constant current = 7 mA (Janode ≈ 7.8 mA cm−2), nBu4NBF4 (0.15 mmol), 1 (0.30 mmol), 2 (0.20 mmol), MeCN/MeOH (7.0 mL/3.0 mL), room temperature, N2, 2.0 h (2.6 F). Isolated yields were shown. aConstant current = 10 mA (Janode ≈ 11 mA cm−2)
Diarylamine derivatives with free para C–H bonds were also able to react with phenothiazine-2-carbonitrile (Fig. 3). 3-Methyl-N-(p-tolyl)aniline showed a good reactivity in the synthesis of a para C–H amination product 4a. As for the reaction with N-(4-halogenated phenyl)-3-methylanilines, the amination reaction showed an exclusive selectivity toward the para C–H position of the 3-methylaniline moiety and halides including chloride, bromide, and iodide were remained (4b–4d). Free hydroxyl group was also well tolerated (4e). Substrates bearing electron-donating substituents could still afford the desired product in good to high yields (4f and 4g) while decreased reaction efficiency was observed for substrates bearing electron-withdrawing trifluoromethyl group (4h). 9,9-Dimethyl-N-(m-tolyl)-9H-fluoren-2-amine was also able to give a para amination product at the 3-methylaniline moiety (4i). A mono para C–H amination product could be observed for di-m-tolylamine in 66% yield under the standard conditions (4j). Notably, the reaction with N-phenylnaphthalen-2-amine demonstrated exclusive selectivity toward the N-naphthalen moiety with an excellent reaction efficiency (4k). Diphenylamines without free para C–H bonds such as di-p-tolylamine were unreactive in this electrolytic transformation.
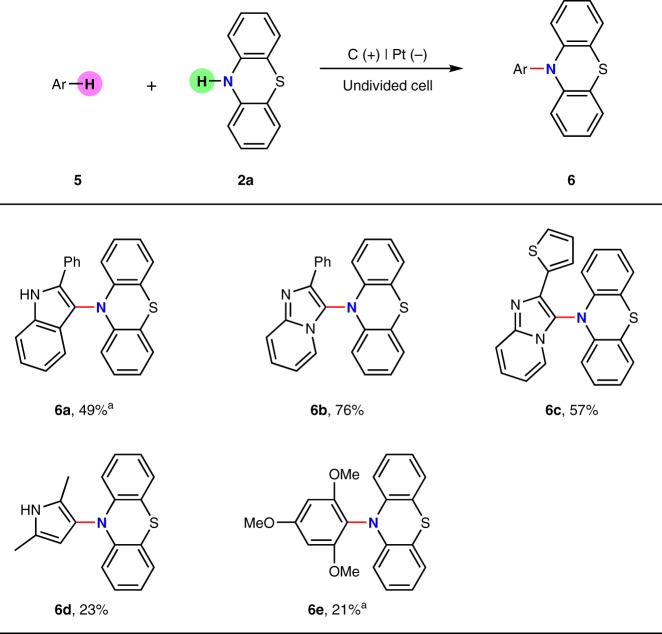
Besides anilines, the reactivity of other electron-rich arenes were also tested in this electrooxidative C–H amination reactions with phenothiazine (Fig. 4). 2-Phenylindole could afford a C-3 amination product in 49% yield while 2-phenylimidazo[1,2-a]pyridine furnished the desired amination product in 76% yield (6a, 6b). The reaction between 2-(thiophen-2-yl)imidazo[1,2-a]pyridine and phenothiazine demonstrated exclusive reactivity at the imidazo[1,2-a]pyridine ring with moderate yield (6c). 2,5-dimethylpyrrole and 1,3,5-trimethoxybenzene showed decreased reactivity in this transformation (6d, 6e).
Fig. 4.
C–H amination of electron-rich arenes with phenothiazine. Reaction conditions: graphite rod anode (ϕ 6 mm), platinum plate cathode (15 mm × 15 mm × 0.3 mm), constant current = 12 mA (Janode ≈ 13.3 mA cm−2), nBu4NBF4 (0.15 mmol), 5 (0.24 mmol), 2a (0.20 mmol), MeCN/CF3CH2OH (7.0 mL/2.0 mL), room temperature, N2, 1.5 h (3.3 F). Isolated yields were shown. aMeCN/AcOH (9.0 mL/1.0 mL)
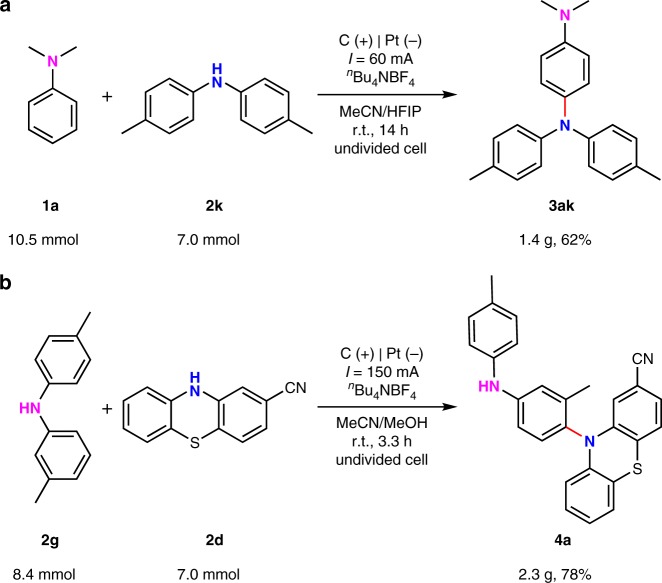
The scalability of this para-selective electrooxidative aryl C–H amination was then evaluated by performing 7.0 mmol scale reactions in a simple beaker equiped with graphite rod as the anode and platinum plate as the cathode. Under 60 mA constant current, the gram-scale reaction between 1a and 2k could give corresponding triphenylamine 3ak in 62% yield (Fig. 5a). Under 150 mA constant current, the gram-scale reaction between 2g and 2d afforded 4a in high selectivity with 78% yield (Fig. 5b).
Fig. 5.
Large scale synthesis. a Gram-scale synthesis of 3ak. b Gram-scale synthesis of 4a
Discussion
Since the method has been established, efforts were then paid to understand the mechanism for this selective oxidative C–H/N–H cross-coupling. First, cyclic voltammetry (CV) experiments were conducted to study the redox potential of the substrates. N,N-Dimethylaniline (1a) could be oxidized when the oxidation potential exceed 0.65 V (Fig. 6a, red line). Phenothiazine (2a) started to get oxidized from 0.50 V (Fig. 6a, black line) while 3-methyl-N-(p-tolyl)aniline (2g) could be oxidized from 0.70 V (Fig. 6a, blue line). Under the standard conditions in MeCN/MeOH, the voltage for whole electrolytic cell ranged from 3.10 to 3.42 V while the oxidation potential of anode (vs Ag/AgCl) ranged from 1.19 to 0.96 V. The two coupling partners could both be oxidized since the operating oxidation potential is higher than 0.96 V. This hypothesis could be supported by the fact that homo-coupling of both coupling partners were observed under the electrolytic conditions. Furthermore, experiments using potential controlled electrolysis were conducted to study the oxidation of the substrates. First, we tried to do the reaction between 1a and 2a at a controlled potential of 0.55 V where only 2a could be oxidized. Only 14% yield of 3aa could be obtained until complete consumption of the substrates. By contrast, 66% yield of 3aa could be obtained when the reaction was conducted at a controlled potential of 0.75 V where both 1a and 2a could be oxidized (Fig. 6b). In the electrolytic reaction between 1a and 2g, only 6% yield of 3ag could be obtained at a controlled potential of 0.66 V while 20% yield could be obtained at a controlled potential of 0.80 V (Fig. 6c). According to these results, we knew that best results were obtained when both of the substrates could be oxidized. Though with low yields, the reaction could also happen when only one of the substrates could be oxidized. Since the operating potentials under the standard reaction conditions were higher than the oxidation potential of both substrates, the cross-coupling of an arene radical cation and a nitrogen radical was likely to be the major reaction pathway.
Fig. 6.
Study of the oxidation potential during electrolysis. a Cyclic voltammogram on a glassy carbon electrode (ϕ 3 mm) at 0.1 V s−1 under nitrogen. Red line, N,N-dimethylaniline (1a); black line, phenothiazine (2a); and blue line, 3-Methyl-N-(p-tolyl)aniline (2g). b Potential controlled electrolysis between 1a and 2a. c Potential controlled electrolysis between 1a and 2g
To confirm the oxidation of the two coupling partners in Figs. 2-4, electron paramagnetic resonance (EPR) experiment (X band, 9.4 GHz, room temperature) was carried out to determine the oxidation species of 1a, 2a, and 2g during the electrolysis. First, each substrate was separately electrolyzed under the standard conditions for 15 min. No obvious signals could be observed for 1a (Fig. 7a, black line). Notably, a clear radical signal could be observed for 2a (Fig. 7a, red line, g = 2.0058). The formation of a nitrogen radical was suggested (see Supplementary Fig. 1). However, only a very weak radical signal could be observed for 2g (Fig. 7a, blue line, g = 2.0038). It has been reported that HFIP could stabilize radical cations67,68. When HFIP was used instead of MeOH in the EPR detection, clear radical signals could be observed for 1a (Fig. 7b, red line, g = 2.0035), 2a (Fig. 7b, black line, g = 2.0057), and 2g (Fig. 7b, blue line, g = 2.0037). These results proved that dialkylamine, phenothiazine and diarylamine could all generate radical species through single-electron-transfer (SET) oxidation by the carbon anode under the electrolytic conditions.
Fig. 7.
Electron paramagnetic resonance (EPR) spectra. a After electrolysis in MeCN/MeOH (7.0 mL/3.0 mL) for 15 min. b After electrolysis in MeCN/HFIP (5.0 mL/5.0 mL) for 15 min
Based on the above experimental results, a plausible reaction mechanism between 1a and 2a was shown in Fig. 8. In the first step, 1a could be oxidized at carbon anode to generate a radical cation I. Homo-coupling of radical cation I could lead to the formation of 5a. At the same time, 2a might also be oxidized by the carbon anode to generate a nitrogen radical II. C–N bond was likely to be formed from the radical/radical cross-coupling between radical cation I and nitrogen radical II. This unique reaction pathway might explain the exclusive para-selectivity for this oxidative C–H/N–H cross-coupling. Subsequent deprotonation of intermediate III could afford the final para-amination product. Concomitant cathodic reduction could release hydrogen gas during the reaction process. According to the results in Fig. 6, the cross-coupling of an arene radical cation and a nitrogen radical was likely to be the major reaction pathway. However, addition of nitrogen radical to aniline or nucleophilic addition of diarylamine derivatives to aniline radical cation were also possible to be involved as minor reaction pathways.
Fig. 8.
Proposed mechanism for the reaction between 1a and 2a. Tentative reaction mechanism involves anodic oxidation of aniline to generate aniline cation radical and diphenylamine to generate nitrogen radical, cross-coupling of aniline cation radical with nitrogen radical and deprotonation to furnish the final product
In summary, we have developed an electrooxidative C–H/N–H cross-coupling between electron-rich arenes and diarylamine derivatives under catalyst- and external oxidant-free conditions. Under undivided electrolytic conditions, exclusive para-selectivity are observed in the aryl C–H amination of anilines. This method provides a simple and efficient way to access triarylamine derivatives with high reaction efficiency. Highly active functional groups such as amino, hydroxyl, sulfur and even ethinyl could be well reserved after electrolysis. CV and EPR experiments suggest that C–N bonds are likely to be formed through the radical/radical cross-coupling between in situ generated aniline radical cations and nitrogen radicals.
Methods
General procedure for the electrooxidative C–H amination of N,N-dialkylanilines
In an oven-dried undivided three-necked bottle (25 mL) equipped with a stir bar, phenothiazine (0.20 mmol) and nBu4NBF4 (0.15 mmol) was added. The bottle was equipped with graphite rod (ϕ 6 mm, about 15 mm immersion depth in solution) as the anode and platinum plate (15 mm × 15 mm × 0.3 mm) as the cathode and charged with nitrogen. Subsequently, N,N-dialkylanilines (0.30 mmol) and CH3CN/MeOH (7.0 mL/3.0 mL) were added. Then the electrolysis system was stirred at a constant current of 7 mA under room temperature for 2 h. When the reaction was finished, the reaction mixture was washed with water and extracted with diethyl ether (10 mL × 3). The organic layers were combined, dried over Na2SO4, and concentrated. The pure product was obtained by flash column chromatography on silica gel (petroleum ether:ethyl acetate = 50:1). With diarylamines as the NH sources, diarylamines (0.20 mmol) and nBu4NBF4 (49.4 mg, 0.15 mmol) were added. The bottle was equipped with graphite rod (ϕ 6 mm, about 15 mm immersion depth in solution) as the anode and platinum plate (15 mm × 15 mm × 0.3 mm) as the cathode and then charged with nitrogen. Subsequently, N,N-dialkylanilines (0.30 mmol) and CH3CN/HFIP (5.0 mL/5.0 mL) were added. The reaction mixture was stirred and electrolyzed at a constant current of 12 mA under room temperature for 2 h. When the reaction was finished, the reaction mixture was washed with water and extracted with diethyl ether (10 mL × 3). The organic layers were combined, dried over Na2SO4, and concentrated. The pure product was obtained by flash column chromatography on silica gel (petroleum ether:ethyl acetate = 150:1). Full experimental details and characterization of the compounds are given in the Supplementary Information.
General procedure for the electrooxidative C–H amination of diarylamines
In an oven-dried undivided three-necked bottle (25 mL) equipped with a stir bar, diarylamine (0.24 mmol), phenothiazine-2-carbonitrile (0.20 mmol), and nBu4NBF4 (0.15 mmol) were combined. The bottle was equipped with graphite rod (ϕ 6 mm, about 15 mm immersion depth in solution) as the anode and platinum plate (15 mm × 15 mm × 0.3 mm) as the cathode and was charged with nitrogen. Then CH3CN/MeOH (6.0 mL/4.0 mL) was added. The reaction mixture was stirred and electrolyzed at a constant current of 7 mA under room temperature for 2 h. When the reaction was finished, the reaction mixture was washed with water and extracted with diethyl ether (10 mL × 3). The organic layers were combined, dried over Na2SO4, and concentrated. The pure product was obtained by flash column chromatography on silica gel (petroleum ether:ethyl acetate = 50:1). Full experimental details and characterization of the compounds are given in the Supplementary Information.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21390402, 21520102003) and the Hubei Province Natural Science Foundation of China (2017CFA010). The Program of Introducing Talents of Discipline to Universities of China (111 Program) is also appreciated.
Author contributions
K.L. and S.T. contributed equally to this work. A.L. and S.T. contributed to the conception and design of the experiments. K.L., T.W. and M.Z. performed the electrochemical synthesis. K.L. and S.W. performed the EPR experiments. H.C. resolved the X-ray structure of 4k. S.T., K.L. and A.L. co-wrote the manuscript and contributed to data analysis and scientific discussion.
Data availability
The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number CCDC 1554125 (4k). The data can be obtained free of charge from The Cambridge Crystallographic Data Centre [http://www.ccdc.cam.ac.uk/data_request/cif]. The data supporting the findings of this study are available within the article and its Supplementary Information files. Any further relevant data are available from the authors on request.
Competing interests
The authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Kun Liu, Shan Tang.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-019-08414-8.
References
- 1.Hili R, Yudin AK. Making carbon-nitrogen bonds in biological and chemical synthesis. Nat. Chem. Biol. 2006;2:284–287. doi: 10.1038/nchembio0606-284. [DOI] [PubMed] [Google Scholar]
- 2.Ricci, A. Amino Group Chemistry: from Synthesis to the Life Sciences. (John Wiley & Sons, Inc, 2008).
- 3.Surry DS, Buchwald SL. Biaryl phosphane ligands in palladium-catalyzed amination. Angew. Chem. Int. Ed. 2008;47:6338–6361. doi: 10.1002/anie.200800497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartwig JF. Evolution of a fourth generation catalyst for the amination and thioetherification of aryl halides. Acc. Chem. Res. 2008;41:1534–1544. doi: 10.1021/ar800098p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bariwal J, Van der Eycken E. C-N bond forming cross-coupling reactions: an overview. Chem. Soc. Rev. 2013;42:9283–9303. doi: 10.1039/c3cs60228a. [DOI] [PubMed] [Google Scholar]
- 6.Sambiagio C, Marsden SP, Blacker AJ, McGowan PC. Copper catalysed Ullmann type chemistry: from mechanistic aspects to modern development. Chem. Soc. Rev. 2014;43:3525–3550. doi: 10.1039/C3CS60289C. [DOI] [PubMed] [Google Scholar]
- 7.Louillat ML, Patureau FW. Oxidative C-H amination reactions. Chem. Soc. Rev. 2014;43:901–910. doi: 10.1039/C3CS60318K. [DOI] [PubMed] [Google Scholar]
- 8.Jiao J, Murakami K, Itami K. Catalytic methods for aromatic C–H Amination: an ideal strategy for nitrogen-based functional molecules. ACS Catal. 2016;6:610–633. doi: 10.1021/acscatal.5b02417. [DOI] [Google Scholar]
- 9.Kim H, Chang S. Transition-metal-mediated direct C–H amination of hydrocarbons with amine reactants: the most desirable but challenging C–N bond-formation approach. ACS Catal. 2016;6:2341–2351. doi: 10.1021/acscatal.6b00293. [DOI] [Google Scholar]
- 10.Kantak AA, Potavathri S, Barham RA, Romano KM, DeBoef B. Metal-free intermolecular oxidative C–N bond formation via tandem C–H and N–H bond functionalization. J. Am. Chem. Soc. 2011;133:19960–19965. doi: 10.1021/ja2087085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HJ, Kim J, Cho SH, Chang S. Intermolecular oxidative C–N bond formation under metal-free conditions: control of chemoselectivity between Aryl sp2 and Benzylic sp3 C–H bond imidation. J. Am. Chem. Soc. 2011;133:16382–16385. doi: 10.1021/ja207296y. [DOI] [PubMed] [Google Scholar]
- 12.Xiao B, Gong TJ, Xu J, Liu ZJ, Liu L. Palladium-catalyzed intermolecular directed C−H amidation of aromatic ketones. J. Am. Chem. Soc. 2011;133:1466–1474. doi: 10.1021/ja108450m. [DOI] [PubMed] [Google Scholar]
- 13.Manna S, Matcha K, Antonchick AP. Metal-free annulation of arenes with 2-aminopyridine derivatives: the methyl group as a traceless non-chelating directing group. Angew. Chem. Int. Ed. 2014;53:8163–8166. doi: 10.1002/anie.201403712. [DOI] [PubMed] [Google Scholar]
- 14.Manna S, Serebrennikova PO, Utepova IA, Antonchick AP, Chupakhin ON. Hypervalent iodine(III) in direct oxidative amination of arenes with heteroaromatic amines. Org. Lett. 2015;17:4588–4591. doi: 10.1021/acs.orglett.5b02320. [DOI] [PubMed] [Google Scholar]
- 15.Jin R, Patureau FW. Mild, periodate-mediated, dehydrogenative C–N bond formation with phenothiazines and phenols. Org. Lett. 2016;18:4491–4493. doi: 10.1021/acs.orglett.6b02223. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Huang B, Yang C, Xia W. Visible-light-promoted direct amination of phenols via oxidative cross-dehydrogenative coupling reaction. Org. Lett. 2016;18:3326–3329. doi: 10.1021/acs.orglett.6b01371. [DOI] [PubMed] [Google Scholar]
- 17.He G, Lu G, Guo Z, Liu P, Chen G. Benzazetidine synthesis via palladium-catalysed intramolecular C−H amination. Nat. Chem. 2016;8:1131–1136. doi: 10.1038/nchem.2585. [DOI] [Google Scholar]
- 18.Tsang WCP, Munday RH, Brasche G, Zheng N, Buchwald SL. Palladium-catalyzed method for the synthesis of carbazoles via tandem C−H functionalization and C−N bond formation. J. Org. Chem. 2008;73:7603–7610. doi: 10.1021/jo801273q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran LD, Roane J, Daugulis O. Directed amination of non-acidic arene C–H bonds by a copper–silver catalytic system. Angew. Chem. Int. Ed. 2013;52:6043–6046. doi: 10.1002/anie.201300135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H, Qiao X, Yang S, Shen Z. Cu-catalyzed direct amidation of aromatic C–H bonds: an access to arylamines. J. Org. Chem. 2014;79:4414–4422. doi: 10.1021/jo5003592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louillat-Habermeyer ML, Jin R, Patureau FW. O2-mediated dehydrogenative amination of phenols. Angew. Chem. Int. Ed. 2015;54:4102–4104. doi: 10.1002/anie.201500089. [DOI] [PubMed] [Google Scholar]
- 22.Roane J, Daugulis O. A general method for aminoquinoline-directed, copper-catalyzed sp2 C–H bond amination. J. Am. Chem. Soc. 2016;138:4601–4607. doi: 10.1021/jacs.6b01117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang S, Zeng L, Lei A. Oxidative R1–H/R2–H cross-coupling with hydrogen evolution. J. Am. Chem. Soc. 2018 doi: 10.1021/jacs.1028b07327. [DOI] [PubMed] [Google Scholar]
- 24.Gong M, Huang JM. Electrochemical oxidative C−H/N−H coupling between γ-lactams and anilines. Chem. Eur. J. 2016;22:14293–14296. doi: 10.1002/chem.201602454. [DOI] [PubMed] [Google Scholar]
- 25.Wu J, Zhou Y, Zhou Y, Chiang CW, Lei A. Electro-oxidative C (sp3)–H amination of azoles via intermolecular oxidative C(sp3)–H/N–H cross-coupling. ACS Catal. 2017;7:8320–8323. doi: 10.1021/acscatal.7b03551. [DOI] [Google Scholar]
- 26.Zhao HB, et al. Amidinyl radical formation through anodic N−H Bond cleavage and its application in aromatic C−H bond functionalization. Angew. Chem. Int. Ed. 2017;56:587–590. doi: 10.1002/anie.201610715. [DOI] [PubMed] [Google Scholar]
- 27.Niu L, et al. Photo-induced oxidant-free oxidative C–H/N–H cross-coupling between arenes and azoles. Nat. Commun. 2017;8:14226. doi: 10.1038/ncomms14226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romero NA, Margrey KA, Tay NE, Nicewicz DA. Site-selective arene C-H amination via photoredox catalysis. Science. 2015;349:1326–1330. doi: 10.1126/science.aac9895. [DOI] [PubMed] [Google Scholar]
- 29.Sperry JB, Wright DL. The application of cathodic reductions and anodic oxidations in the synthesis of complex molecules. Chem. Soc. Rev. 2006;35:605–621. doi: 10.1039/b512308a. [DOI] [PubMed] [Google Scholar]
- 30.Jutand A. Contribution of electrochemistry to organometallic catalysis. Chem. Rev. 2008;108:2300–2347. doi: 10.1021/cr068072h. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida Ji, Kataoka K, Horcajada R, Nagaki A. Modern strategies in electroorganic synthesis. Chem. Rev. 2008;108:2265–2299. doi: 10.1021/cr0680843. [DOI] [PubMed] [Google Scholar]
- 32.Francke R, Little RD. Redox catalysis in organic electrosynthesis: basic principles and recent developments. Chem. Soc. Rev. 2014;43:2492–2521. doi: 10.1039/c3cs60464k. [DOI] [PubMed] [Google Scholar]
- 33.Horn EJ, Rosen BR, Baran PS. Synthetic organic electrochemistry: an enabling and innately sustainable method. ACS Cent. Sci. 2016;2:302–308. doi: 10.1021/acscentsci.6b00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan M, Kawamata Y, Baran PS. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 2017;117:13230–13319. doi: 10.1021/acs.chemrev.7b00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang S, Liu Y, Lei A. Electrochemical oxidative cross-coupling with hydrogen evolution: a green and sustainable way for bond formation. Chem. 2018;4:27–45. doi: 10.1016/j.chempr.2017.10.001. [DOI] [Google Scholar]
- 36.Wiebe A, et al. Electrifying organic synthesis. Angew. Chem. Int. Ed. 2018;57:5594–5619. doi: 10.1002/anie.201711060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang Y, Xu K, Zeng C. Use of electrochemistry in the synthesis of heterocyclic structures. Chem. Rev. 2017;118:4485–4540. doi: 10.1021/acs.chemrev.7b00271. [DOI] [PubMed] [Google Scholar]
- 38.Badalyan A, Stahl SS. Cooperative electrocatalytic alcohol oxidation with electron-proton-transfer mediators. Nature. 2016;535:406–410. doi: 10.1038/nature18008. [DOI] [PubMed] [Google Scholar]
- 39.Horn EJ, et al. Scalable and sustainable electrochemical allylic C–H oxidation. Nature. 2016;533:77–81. doi: 10.1038/nature17431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu N, Sauer GS, Saha A, Loo A, Lin S. Metal-catalyzed electrochemical diazidation of alkenes. Science. 2017;357:575–579. doi: 10.1126/science.aan6206. [DOI] [PubMed] [Google Scholar]
- 41.Ma C, Fang P, Mei TS. Recent advances in C–H functionalization using electrochemical transition metal catalysis. ACS Catal. 2018;8:7179–7189. doi: 10.1021/acscatal.8b01697. [DOI] [Google Scholar]
- 42.Sauermann N, Meyer TH, Qiu Y, Ackermann L. Electrocatalytic C–H activation. ACS Catal. 2018;8:7086–7103. doi: 10.1021/acscatal.8b01682. [DOI] [Google Scholar]
- 43.Morofuji T, Shimizu A, Yoshida Ji. Electrochemical C–H amination: synthesis of aromatic primary amines via N-arylpyridinium ions. J. Am. Chem. Soc. 2013;135:5000–5003. doi: 10.1021/ja402083e. [DOI] [PubMed] [Google Scholar]
- 44.Morofuji T, Shimizu A, Yoshida Ji. Direct C–N coupling of imidazoles with aromatic and benzylic compounds via electrooxidative C–H functionalization. J. Am. Chem. Soc. 2014;136:4496–4499. doi: 10.1021/ja501093m. [DOI] [PubMed] [Google Scholar]
- 45.Gao X, Wang P, Zeng L, Tang S, Lei A. Cobalt(II)-catalyzed electrooxidative C–H amination of arenes with alkylamines. J. Am. Chem. Soc. 2018;140:4195–4199. doi: 10.1021/jacs.7b13049. [DOI] [PubMed] [Google Scholar]
- 46.Sauermann N, Mei R, Ackermann L. Electrochemical C−H amination by cobalt catalysis in a renewable solvent. Angew. Chem. Int. Ed. 2018;57:5090–5094. doi: 10.1002/anie.201802206. [DOI] [PubMed] [Google Scholar]
- 47.Wang P, Tang S, Huang P, Lei A. Electrocatalytic oxidant-free dehydrogenative C−H/S−H cross-coupling. Angew. Chem. Int. Ed. 2017;56:3009–3013. doi: 10.1002/anie.201700012. [DOI] [PubMed] [Google Scholar]
- 48.Tang S, Wang S, Liu Y, Cong H, Lei A. Electrochemical oxidative C−H amination of phenols: access to triarylamine derivatives. Angew. Chem. Int. Ed. 2018;57:4737–4741. doi: 10.1002/anie.201800240. [DOI] [PubMed] [Google Scholar]
- 49.Jaworski, J. S., Kalinowski, M. K. in: The Chemistry of Anilines (eds Patai, S. & Rappoport, Z.). (John Wiley & Sons, Ltd, 2007).
- 50.Yang H, Bard AJ. The application of fast scan cyclic voltammetry. Mechanistic study of the initial stage of electropolymerization of aniline in aqueous solutions. J. Electroanal. Chem. 1992;339:423–449. doi: 10.1016/0022-0728(92)80466-H. [DOI] [Google Scholar]
- 51.Treat NJ, et al. Metal-free atom transfer radical polymerization. J. Am. Chem. Soc. 2014;136:16096–16101. doi: 10.1021/ja510389m. [DOI] [PubMed] [Google Scholar]
- 52.Pan X, et al. Mechanism of photoinduced metal-free atom transfer radical polymerization: experimental and computational studies. J. Am. Chem. Soc. 2016;138:2411–2425. doi: 10.1021/jacs.5b13455. [DOI] [PubMed] [Google Scholar]
- 53.Chen M, et al. Logic-controlled radical polymerization with heat and light: multiple-stimuli switching of polymer chain growth via a recyclable, thermally responsive gel photoredox catalyst. J. Am. Chem. Soc. 2017;139:2257–2266. doi: 10.1021/jacs.6b10345. [DOI] [PubMed] [Google Scholar]
- 54.Gong H, Zhao Y, Shen X, Lin J, Chen M. Organocatalyzed photocontrolled radical polymerization of semifluorinated (meth)acrylates driven by visible light. Angew. Chem. Int. Ed. 2018;57:333–337. doi: 10.1002/anie.201711053. [DOI] [PubMed] [Google Scholar]
- 55.Zhang K, et al. Novel aromatic polyimides with pendent triphenylamine units: synthesis, photophysical, electrochromic properties. J. Electroanal. Chem. 2012;682:101–109. doi: 10.1016/j.jelechem.2012.06.018. [DOI] [Google Scholar]
- 56.Yen HJ, Liou GS. Enhanced near-infrared electrochromism in triphenylamine-based aramids bearing phenothiazine redox centers. J. Mater. Chem. 2010;20:9886–9894. doi: 10.1039/c0jm01889a. [DOI] [Google Scholar]
- 57.Wu CS, Chen Y. Copolyfluorenes containing pendant bipolar groups: synthesis, optoelectronic properties and applications. J. Mater. Chem. 2010;20:7700–7709. doi: 10.1039/c0jm00707b. [DOI] [Google Scholar]
- 58.Wang J, Liu K, Ma L, Zhan X. Triarylamine: versatile platform for organic, dye-sensitized, and perovskite solar cells. Chem. Rev. 2016;116:14675–14725. doi: 10.1021/acs.chemrev.6b00432. [DOI] [PubMed] [Google Scholar]
- 59.Hindson JC, et al. All-aromatic liquid crystal triphenylamine-based poly(azomethine)s as hole transport materials for opto-electronic applications. J. Mater. Chem. 2010;20:937–944. doi: 10.1039/B919159C. [DOI] [Google Scholar]
- 60.Hartwig JF, Kawatsura M, Hauck SI, Shaughnessy KH, Alcazar-Roman LM. Room-temperature palladium-catalyzed amination of aryl bromides and chlorides and extended scope of aromatic C−N bond formation with a commercial ligand. J. Org. Chem. 1999;64:5575–5580. doi: 10.1021/jo990408i. [DOI] [PubMed] [Google Scholar]
- 61.Surry DS, Buchwald SL. Selective palladium-catalyzed arylation of ammonia: synthesis of anilines as well as symmetrical and unsymmetrical di- and triarylamines. J. Am. Chem. Soc. 2007;129:10354–10355. doi: 10.1021/ja074681a. [DOI] [PubMed] [Google Scholar]
- 62.Fors BP, Buchwald SL. A multiligand based Pd catalyst for C−N cross-coupling reactions. J. Am. Chem. Soc. 2010;132:15914–15917. doi: 10.1021/ja108074t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goodbrand HB, Hu NX. Ligand-accelerated catalysis of the Ullmann condensation: application to hole conducting triarylamines. J. Org. Chem. 1999;64:670–674. doi: 10.1021/jo981804o. [DOI] [Google Scholar]
- 64.Li C, et al. Rainbow perylene monoimides: easy control of optical properties. Chem. – A Eur. J. 2009;15:878–884. doi: 10.1002/chem.200802126. [DOI] [PubMed] [Google Scholar]
- 65.Hsiao SH, Lin JW. Facile preparation of electrochromic poly(amine–imide) films from diimide compounds with terminal triphenylamino groups via electrochemical oxidative coupling reactions. Polym. Chem. 2014;5:6770–6778. doi: 10.1039/C4PY00610K. [DOI] [Google Scholar]
- 66.Chae HK, et al. Tertiary building units: synthesis, structure, and porosity of a metal−organic dendrimer framework (MODF-1) J. Am. Chem. Soc. 2001;123:11482–11483. doi: 10.1021/ja011692+. [DOI] [PubMed] [Google Scholar]
- 67.Eberson L, Persson O, Hartshorn MP. Detection and reactions of radical cations generated by photolysis of aromatic compounds with tetranitromethane in 1,1,1,3,3,3-hexafluoro-2-propanol at room temperature. Angew. Chem. Int. Ed. 1995;34:2268–2269. doi: 10.1002/anie.199522681. [DOI] [Google Scholar]
- 68.Eberson, L., Hartshorn, M. P. & Persson, O. Generation of solutions of highly persistent radical cations by 4-tolylthallium(III) bis(trifluoroacetate) in 1,1,1,3,3,3-hexafluoropropan-2-ol. J. Chem. Soc. Chem. Commun. 10.1039/C39950001131 (1995).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number CCDC 1554125 (4k). The data can be obtained free of charge from The Cambridge Crystallographic Data Centre [http://www.ccdc.cam.ac.uk/data_request/cif]. The data supporting the findings of this study are available within the article and its Supplementary Information files. Any further relevant data are available from the authors on request.