Cycloplegic retinoscopy is the gold standard refraction approach in children. Various protocols are in use to achieve cycloplegia during outpatient appointments. Many use not only the muscarinergic antagonist, cyclopentolate (CP), which paralyses the ciliary muscle, but also the alpha-adrenergic agonist, phenylephrine (PE), which as a mydriatic paralyses the iris constrictor, but has no effect on the ciliary muscle. Whilst mydriasis facilitates visualisation of the retinoscopy reflex, there is a risk of underestimating hypermetropia. Published evidence suggests that repeated instillation of CP only is effective even for brown and very dark irides [1–3].
To develop a Patient Group Directive (PGD) we carried out a two-cycle audit (CA18/PA/02). The first round evaluated our current protocol: blue iris, CP 1% once (0.5% if age < 3 months); brown iris, CP/PE 2.5% once; very dark iris, CP/PE twice, 10–15 min apart; repeated if pupils still constrict on pentorch illumination. As standard, we set full dilation in 90% within 30 min, i.e. the level expected for blue irides with a single CP drop [4].
In the second round, we only included children with brown or very dark iris, administering CP twice or three times, respectively, 10–15 min apart.
We assessed pupil constriction to pentorch illumination, standard when deciding whether additional drops are required. We also noted time from instillation of the first drop to pentorch test.
We evaluated 107 consecutive children (Table 1). Round 1 found that pupils were dilated in 97% of children with blue, 73% with brown, and 23% with very dark iris (Fig. 1), at a mean 24 min (SD 18) after first drop. Repeat instillation increased success to 100%, 91%, and 85%, respectively. In the second round, pupil dilation was achieved in all children with brown iris, and in 89% with very dark iris, at a mean 32 min (SD 13) after first instillation.
Table 1.
Ethnic background and iris colour of children assessed in this audit
| Round 1 | Round 2 | |||
|---|---|---|---|---|
| n | % | n | % | |
| Ethnicity | ||||
| Afro-Caribbean | 5 | 9 | 10 | 20 |
| Asian | 7 | 13 | 19 | 37 |
| Caucasian | 44 | 79 | 20 | 39 |
| Chinese | 1 | 2 | ||
| Other | 1 | 2 | ||
| Iris colour | ||||
| Blue | 32 | 57 | ||
| Brown | 11 | 20 | 14 | 27 |
| Very dark | 13 | 23 | 37 | 73 |
Fig. 1.
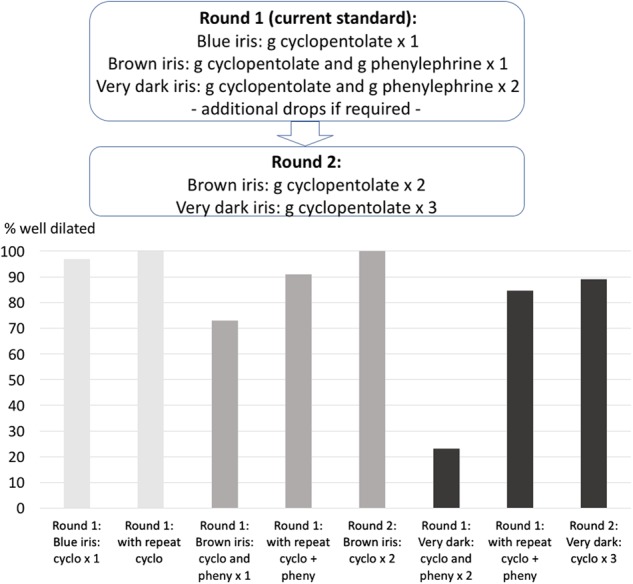
Proportion of well-dilated pupils in rounds 1 and 2 of the audit. Eyes with blue irides are well dilated after one application of cyclopentolate (CP). In brown irides, application of CP twice increases the proportion of well-dilated pupils, and in very dark irides, application of CP three times is effective. Phenylephrine does not appear to enhance this effect
Our use of pupil constriction as outcome measure, which assesses mydriasis, not cycloplegia, is a limitation. However, as we found that better dilation is achieved without the use of PE, this audit has changed our practice and contributed positively to the development of the PGD.
Acknowledgements
This work was supported by the National Institute for Health Research (NIHR) Moorfields Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Farhood Q. Cycloplegic refraction in children with cyclopentolate versus atropine. J Clin Exp Ophthalmol. 2012;3:239. doi: 10.4172/2155-9570.1000239. [DOI] [Google Scholar]
- 2.Ebri A, Kuper H, Wedner S. Cost-effectiveness of cycloplegic agents: results of a randomized controlled trial in nigerian children. Invest Ophthalmol Vis Sci. 2007;48:1025–31. doi: 10.1167/iovs.06-0604. [DOI] [PubMed] [Google Scholar]
- 3.Celebi S, Aykan U. The comparison of cyclopentolate and atropine in patients with refractive accommodative esotropia by means of retinoscopy, autorefractometry and biometric lens thickness. Acta Ophthalmol Scand. 1999;77:426–9. doi: 10.1034/j.1600-0420.1999.770414.x. [DOI] [PubMed] [Google Scholar]
- 4.Zurevinsky J, Sawchuk K, Lim HJ, Lee CH, Rubab S. A clinical randomized trial comparing the cycloplegic effect of cyclopentolate drops applied to closed eyelids versus open eyelids. Am Orthopt J. 2016;66:114–21. doi: 10.3368/aoj.66.1.114. [DOI] [PubMed] [Google Scholar]


