Abstract
Optical coherence tomography angiography (OCTA) is a non-invasive retinal imaging innovation that has been gaining popularity for the evaluation of the retinal vasculature. Of clinical importance is its current use either as an alternative or in conjunction with conventional dye-based angiography in neovascular age-related macular degeneration. OCTA is not without limitations and these include image artefact, a relatively small field of view and failure of the segmentation algorithms, which can confound the interpretation of findings. While there are numerous publications on OCTA in neovascular AMD, few have examined the diagnostic accuracy of this new technology compared with the accepted gold standard of fundus fluorescein angiography (FFA). In this review, we summarise the literature on the clinical application of OCTA in nAMD. In particular, we have reviewed the published articles that have reported the sensitivity and specificity of OCTA in the diagnosis of nAMD, and those that have described and or correlated the morphological findings and compared them to dye-based angiography.
Subject terms: Tomography, Macular degeneration
摘要
光学相干断层扫描血管造影 (OCTA) 是一种无创、新型的视网膜成像技术, 在视网膜血管性疾病的评估方面应用得越来越广泛。其临床重要性在于, 目前可替代作为新生血管性年龄相关性黄斑变性 (nAMD) 常规检查的血管造影或与常规血管造影相补充。 OCTA 也有其局限性, 如图像伪影、观察范围有限以及分割算法的失误, 由此可能会影响对结果解释的准确性。目前有大量关于OCTA在nAMD诊断中应用的文献报道, 但很少有人将这种新技术与公认的金标准眼底荧光血管造影 (FFA) 在诊断的准确性方面进行对比。在这篇综述中, 我们总结了 OCTA 在 nAMD 诊疗中的临床应用, 特别是总结了OCTA 在 nAMD 诊断中的敏感性和特异性以及形态学改变与常规 FFA 发现的相关性。
Introduction
Optical coherence tomography angiography (OCTA) has gained enormous popularity since its introduction into the commercial sector in recent years [1, 2]. Its main advantages in comparison to traditional techniques for visualisation of the posterior pole vasculature include the ease of image capture, rapid processing of the digital information and the high-resolution display of the retinal and choroidal vasculature profiles without the use of intravenous contrast agents and dyes [1, 2]. These features of OCTA imaging have overcome some of the challenges and risks, albeit small, of dye-based angiography, such as the need for cannulation and administration of intravenous substances. Acquisition of the OCTA image is quick and the processing immediate, and thus information on the retinal and choroidal circulation can be obtained almost immediately and with greater resolution than possible with traditional dye-based angiography [1, 2].
Despite these obvious advantages of OCTA, the segmentation of the individual layer boundaries, which is critical for displaying high-resolution images of the vascular profiles, can fail. Therefore, there are concerns relating to the use of OCTA alone to diagnose neovascular age-related macular degeneration (nAMD). nAMD markedly alters the contours and interfaces between the layers of the outer retina, retinal pigment epithelium and inner choroid through deposition of drusenoid material, the presence of neovascular complexes, exudation of blood and lipid and the development of fibrosis.
The Early Detection of Neovascular Age-Related Macular Degeneration (EDNA) study is a multicentre prospective cohort diagnostic accuracy study assessing the sensitivity and specificity of comparator tests on detection of nAMD in the fellow eye following commencement of Anti-VEGF treatment in the affected eye [3]. The study aims to identify an optimal monitoring regime for early detection of nAMD in the second eye of patients diagnosed with nAMD in one eye. To enable widespread applicability of results, all comparator tests are routinely used in NHS outpatient settings. As many units do not yet have access to OCTA technology, this has not been included as a comparator test in the EDNA study. However, given the increasing evidence suggesting that this emerging technology may in future aid diagnosis and monitoring of nAMD, trainees from the EDNA clinical sites who had been inducted as co principal investigators (co-PIs) were tasked with summarising the current knowledge of OCTA in nAMD.
Methods
A literature review of EMBASE, MEDLINE and PUBMED databases to cover the period from 01 January 2014 to 31 July 2017 was undertaken. Search terms used were “optical coherence tomography angiography” OR “OCT angiography” OR “OCT-A”, AND “neovascular macular degeneration” OR “neovascular age-related macular degeneration” OR “neovascular AMD” OR “nAMD” OR “wet age-related macular degeneration” OR “wet AMD” OR “wet ARMD”. The literature review was performed by multiple members of the EDNA Co-PI Writing Group searching the above databases directly or via the NICE Healthcare Databases Advanced Search tool. Only articles published in English and peer reviewed were included. Studies of OCTA in non-neovascular AMD were excluded. Due to the emerging nature of OCTA technology, many published studies include only small numbers of participants, therefore no studies were excluded on the basis of sample size.
Three groups of published material were identified: (a) studies comparing the diagnostic accuracy of OCTA versus FFA and/or ICGA in nAMD with sensitivity and specificity values or positive and negative predictive values (Table 1); (b) studies describing OCTA features of nAMD (Table 2) and (c) review articles and perspectives.
Table 1.
Diagnostic accuracy of OCTA
| Author and date | Study design | Equipment/algorithm | Study sample | Key findings | Comment |
|---|---|---|---|---|---|
| De Carlo et al. (2015) | Observational, retrospective study to describe the characteristics of CNV on OCTA and to determine the sensitivity and specificity of OCTA in detecting CNV as compared with FFA. CNV of any type. | Angiovue (Optovue, Inc, Fremont, CA). SSADA (split-spectrum amplitude-decorrelation angiography) software algorithm with 70,000 A scans per second. | Study involved 72 eyes, 61 patients. Of these, 30 eyes of 24 patients underwent same-day FFA and OCTA as part of the diagnostic study. | CNV on FFA n = 8, CNV on OCTA n = 4, sensitivity = 50%, no CNV on FFA n = 22, no CNV on OCTA n = 20, specificity = 91%. | Very small sample size of n = 8 to determine sensitivity of OCTA vs. FFA. All CNV subtypes were included. |
| Gong et al. (2016) | Retrospective, observational study describing the morphological characteristics and efficacy of OCTA in detecting nAMD. | Angiovue (Optovue, Inc, Fremont, CA). SSADA (split-spectrum amplitude-decorrelation angiography) software algorithm with 70,000 A scans per second. | Eighty-six eyes of 53 patients. Twelve eyes were excluded due to poor-quality images, 8 eyes were excluded due to the absence of OCTA or FFA results. | nAMD on FFA n = 52, nAMD on OCTA n = 45, sensitivity = 86.5%, no nAMD on FFA n = 34, no nAMD on OCTA n = 23, specificity = 67.6%, OCTA false positives n = 11 and false negatives n = 7 PPV 80.4% and NPV 76.7%. | Images of poor-quality were excluded. Therefore the applicability of OCTA across a wider population cannot be assessed. |
| Inoue et al. (2016) | Multicentre, retrospective cohort study to determine the sensitivity and specificity of OCTA and OCT to detect type 1 CNV as compared with FFA. type 1 nCNVM, OCTA alone, FFA alone, OCTA and OCT vs. FFA and OCT as reference standard. | Angiovue (Optovue, Inc, Fremont, CA). SSADA (split-spectrum amplitude-decorrelation angiography) software algorithm with 70,000 A scans per second. | Study involved 105 eyes of 92 patients with type 1 CNV as confirmed on FFA and OCT. Ten eyes with other types of CNV excluded. | CNV on en-face OCTA and structural OCT n = 90, sensitivity 85.7%, CNV on FFA alone n = 70, sensitivity 66.7%, CNV on en-face OCTA alone n = 70, sensitivity 66.7%. | Not treatment-naive subjects. Unable to assess specificity. Only relates to type 1 CNV. En-face OCTA with structural OCT more sensitive than en-face OCTA alone. |
| Carnevali et al. (2016) | Study to determine the detection rate of treatment-naive quiescent choroidal neovascularization due to AMD by OCTA, compared with FFA/ICGA. | Two instruments used AngioPlex CIRRUS HD-OCT. Carl Zeiss Meditec Inc. Dublin CA. OMAG (optical microangiography) algorithm with 68,000 A scans per second. Angiovue (Optovue, Inc, Fremont California, CA). SSADA with 70,000 A scans per second. | Twenty-two eyes of 20 patients with treatment-naive CNV due to AMD. Twenty-two eyes of 22 patients with non-neovascular AMD acted as the control group. | Quiescent CNV on OCTA n = 18, sensitivity = 81.8%. No CNV on OCTA n = 22, specificity = 100%. | Small sample. Treatment-naive quiescent CNV patients instead of eyes with active nAMD. Control group with non-neovascular AMD. |
| Liang et al. (2016) | Observational, retrospective case series to characterise the features of CNV on OCTA and to evaluate whether OCTA can be used to identify clinical activity of CNV as compared with FFA. | Angiovue (Optovue, Inc, Fremont, CA). SSADA (split-spectrum amplitude-decorrelation angiography) software algorithm with 70,000 A scans per second. | Forty-five eyes from 35 patients with nAMD. Six eyes had same-day FFA and OCTA, used as sample for measurement of sensitivity and specificity. | Twenty-five of 45 eyes were found to be clinically active. Of these 25 eyes, 22 (88%) had a CNV on OCTA. Of six eyes with same-day FFA and OCTA, active nAMD on FFA n = 3, active nAMD on OCTA n = 2, sensitivity 66.7%. Non-active nAMD on FFA n = 3, non-active nAMD on OCTA n = 3, specificity 100%. | Sensitivity calculations based on a very small sample. OCTA features of CNV did not correlate with activity. |
| Faridi et al. (2017) | Prospective case series to determine the sensitivity and specificity of OCTA in diagnosing nAMD compared with FFA/OCT. | Angiovue (Optovue, Inc, Fremont, CA). SSADA (split-spectrum amplitude-decorrelation angiography) software algorithm with 70,000 A scans per second. | Seventy-two eyes of patients with treatment-naive nAMD, non-neovascular AMD or normal controls | nAMD on FFA n = 32. nAMD on en-face OCTA alone n = 26, sensitivity 81.3%. nAMD on en-face and cross-sectional OCTA n = 32, sensitivity 100%. No nAMD on FFA n = 40. No nAMD on en-face OCTA alone n = 37 (grader A) / n = 39 (grader B), specificity 92.5% (grader A)/97.5% (grader B). No AMD on en-face and cross-sectional OCTA n = 39 (grader A), n = 40 (grader B), specificity 97.5% (grader A)/100% (grader B). | Small sample. Images of poor-quality were excluded. |
Table 2.
OCTA features in nAMD
| Author and year | Study | Equipment used | Study sample | Key findings | Comment |
|---|---|---|---|---|---|
| Jia et al. (2014) | Observational study to detect and quantify CNV using OCTA—treatment-naive CNV in nAMD. | Prototype swept-source OCTA built by the Massachusetts Institute of Technology. Axial speed of 100 kHz with a 1050 nm laser. The SSADA algorithm was employed. | Ten eyes (five with nAMD, five control) | OCTA can detect CNV based on detection of blood flow in the outer retina, which can be displayed on cross-sectional and en-face OCTA. | Small cross-sectional study. FFA was used to identify cases with nAMD. |
| Kuehlewein et al. (2015) | Prospective study of type 1 nAMD, correlating OCTA morphological characteristics with imaging and clinical criteria. Analysis of the structural features of type 1 CNV at baseline and sequentially after anti-VEGF therapy. | AngioVue. Optovue Inc, Fremont, CA | Thrity-three eyes of 25 patients. Eighteen eyes had follow-up on OCT A. | In 75% of eyes, a highly organised vascular complex could be identified. In 72% of these eyes, a large main central vessel, trunk/feeder vessel, could be seen. Minimal change in lesion area and vessel density following treatment. | Small cross-sectional study only included type 1 CNV. |
| Dansingani et al. (2015) | Retrospective study of morphology of nAMD on OCTA after >50 anti-VEGF treatment. | AngioVue. Optovue Inc, Fremont, CA | Nine eyes of eight patients. | All 9 had type 1 CNV and received a mean of 65.8 injections during a mean follow-up of 9.1 years. OCTA revealed tangled vascular networks of large calibre vessels with high flow. | Small cross-sectional study. Historical multimodal imaging was reviewed, but no FFA to coincide with OCTA visit. |
| Roisman et al. (2016) | Prospective observational study to establish whether OCTA identifies subclinical type 1 CNV in asymptomatic intermediate AMD. | Modified CIRRUS prototype (Carl Zeiss Meditec, Inc. Dublin, CA). | Eleven patients with intermediate AMD in one eye and CNV in their fellow eye. | OCTA identified type 1 CNV corresponding to ICGA plaques in three of the fellow asymptomatic eyes with intermediate AMD. | Small cross-sectional study with ICGA as comparator. |
| Lindner et al. (2016) | Prospective study investigating the potential of OCTA to identify and quantify the neovascular network in treatment-naive exudative AMD compared with FFA. | Spectralis HRA+OCTA, which uses the full-spectrum amplitude decorrelation angiography. (Heidelberg Engineering, Heidelberg, Germany). | Thrity-one eyes from 27 patients, three nAMD types (type 1, 2 and 3). | Type 1 in five, type 2 in 11, type 3 in nine and mixed in six eyes, Interreader agreement for measurements of neovascular network was 0.884 for OCTA and 0.636 for FFA, Overlap coefficient was similar for both technologies, Area agreement was weaker for type 3 and increasing lesion size. | Small cross-sectional study. Twelve eyes were excluded, as neovascular network exceeded OCTA image frame size. |
| Costanzo et al. (2016) | Prospective study of patients diagnosed with type 1 CNV, to compare lesion size as measured by OCTA vs. ICGA. | AngioVue. Optovue Inc, Fremont, CA | Nineteen eyes of 17 patients. | Lesions appear significantly smaller on OCTA (p < 0.05). | Small cross-sectional study with ICG as comparator. Poor-quality OCTA scans and lesions larger than OCTA size were excluded, but there was no data on how many eyes were excluded. |
| Kuehlewein et al. (2015) | Case study of the structural features of type 2 CNV using OCTA at baseline and following anti-VEGF therapy. | AngioVue. Optovue Inc, Fremont, CA | One eye | Reduction in size and density of neovascular complex, but central vessel trunk remained unchanged. | Descriptive single case study with longitudinal follow-up. Microvascular components are delineated with precision. |
| Mastropasqua et al. (2017) | Descriptive longitudinal study of OCTA features in treatment-naive nAMD at baseline and following anti-VEGF therapy over a 4-month period. | AngioVue. Optovue Inc, Fremont, CA | Fifteen eyes of 15 patients. | Although foveal and parafoveal superficial vascular plexus flow area decreased after treatment, CNV lesion area remained unchanged. | Small sample longitudinal study without a comparator. Only enrolled type 1 CNV. |
| Phasukkijwatana et al. (2016) | Longitudinal study analysing the OCTA morphological features before and after anti-VEGF therapy in type 3 nAMD diagnosed by FFA. | AngioVue. Optovue Inc, Fremont, CA | Seventeen eyes of 14 patients were analysed (13 eyes were excluded). | One month after the first injection, nCNVM was undetectable in five (29%) eyes and lesion reduced in all other eyes, In 11 eyes with visible trunk vessels, treatment resulted in regression of fine nCNVM tufts, but not trunk vessels. | Small sample longitudinal study enrolled type 3 CNV only. FFA not available at follow-up. |
| Sulzbacher et al. (2017) | Retrospective study to classify nAMD based on OCTA. | AngioVue. Optovue Inc, Fremont, CA | Eighty-eight eyes of 67 patients were evaluated (61 eyes were excluded), Treatment naive, n = 14, Previous anti-VEGF therapy, n = 74. | Treatment-naive eyes more likely to have dense net configuration, while eyes with long-standing disease duration have loose net configuration. | Cross-sectional study with a high exclusion rate due to motion artefact. Initial diagnosis of nAMD made with FFA. |
| Tan et al. (2017) | Descriptive study of active and inactive type 3 CNV studied with OCTA, FFA, ICGA, OCT, AF. | AngioVue. Optovue Inc, Fremont, CA | Twenty-seven eyes of 23 patients, nine eyes had consecutive follow-up. | Fifty-seven percent inactive untreated eyes had high flow vessel tuft visible on OCTA. Persistent high flow tuft on OCTA in 57% of treated, now inactive nCNVM, reduced flow signal and intensity of NV complex after treatment, increase in size and intensity of high flow tuft during progression or recurrence. | Small sample enrolled type 3 CNV only. Treated and treatment-naive eyes included. Two flow patterns observed: (a) signal confined to the neurosensory retina, (b) signal extends through RPE. |
| Amoroso et al. (2017) | Prospective study assessing the reproducibility and reliability of vessel area measurements by OCTA in nAMD. | AngioVue. Optovue Inc, Fremont, CA | Forty-eight eyes of 46 patients. | The difference between first and second measurements of mean CNV area was 0.03 mm2. Intra grader agreement was 0.98 (CI 0.98 to 0.99) and intergrader agreement was 0.98 (0.97 to 0.99). | Cross-sectional study with type 1, 2 and 3 CNV were included. OCTA measurements are more reproducible than FA. |
Findings
Sensitivity and specificity of OCTA in the diagnosis of nAMD
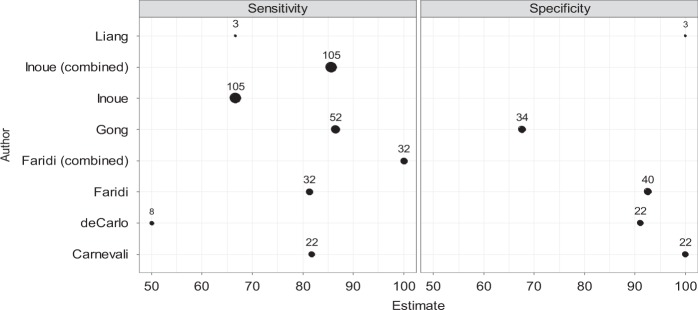
Six publications providing information on the diagnostic accuracy (sensitivity, specificity or calculations of positive and negative predictive values) of OCTA in nAMD are summarised in Table 1 [4–9]. Most studies compared sensitivity and specificity with the gold standard of FFA and one study used both FFA and ICGA. Figure 1 is a representative forest plot of the sensitivity and specificity of OCTA, and it can be seen that there is only moderate consistency of sensitivity and specificity. The majority of studies had small sample sizes and none reported confidence intervals, thus limiting the accuracy of their results.
Fig. 1.
Forest plots showing sensitivity and specificity of studies, including sample size
De Carlo et al. [4] found the sensitivity to be 50% in a small study of eight eyes, whilst Gong et al. [5] with a larger sample size of 52 eyes found sensitivity to be 86.5%. In both studies, the reasons identified for false negatives were the presence of tall pigment epithelial detachments and haemorrhage. Liang et al. [6] reported the detection of neovascular complexes on OCTA in patients found to have active leakage on FFA with a sensitivity of 66.7% and a specificity of 100%. Between these, the reported specificity for OCTA ranged from 67.6% [5] to 100% [7]. One study enroled only type 1 CNV and compared the detection of the neovascular complexes by OCTA alone and in combination with structural OCT to the gold standard of FFA [7]. When FFA and OCTA were graded independently, sensitivity was 66.7% for both. Using en-face OCTA there were six false negative, almost two thirds of which were associated with a large sub retinal haemorrhage. On combining the OCTA information with the cross-sectional structural OCT, the sensitivity for detection of type 1 CNV increased to 85.7% and specificity was also improved. The authors attribute the false positives to projection artefact, and it would appear that the use of the cross-sectional images helped avoid falsely attributing signals to potentially avascular layers. The higher proportion of eyes in which type 1 CNV was detected suggests that the case mix of nAMD can influence variation in the sensitivity and specificity of detection by OCTA. Conversely, limiting the case mix to a single homogeneous subtype could give rise to falsely high levels of sensitivity. A single study by Gong et al. has provided both positive and negative predictive values for the detection of CNV, and these were estimated to be 80.4% and 76.7%, respectively [5].
Features of the nAMD lesion on OCTA
We found 12 publications with descriptions of morphology, and occasionally the size of the neovascular complexes of AMD, with some limiting their study participants to specific subtypes of nAMD (Table 2) [10–21]. Concurrent FFA and or ICGA were infrequently performed, but when available, were used to compare and contrast the features observed on these traditional imaging modalities with those of OCTA [6, 13]. Roisman et al. [14] observed that plaques seen on ICGA correspond to sub-pigment epithelial choroidal neovascular complexes but often without any evidence of active leakage. A small number of studies have measured the area of the CNV complex on OCTA and FFA [15, 16]. Costanzo et al. [15] showed that the size of the lesion was around 25% smaller on OCTA compared with FFA. Lindner et al. [17] reported similar overlap coefficients for OCTA and FFA for CNV lesions, but intergrader agreement on size was higher for OCTA when compared with FFA (0.884 vs. 0.636), indicating better consistency of size determination for the former. However, agreement was poor for OCTA in eyes with retinal angiomatous proliferation (RAP) lesions. Amoroso et al. [18] also reported high intergrader agreement for determination of lesion size using OCTA, but comparison with FFA was not undertaken in this study.
A subgroup of studies [16, 19–21] have observed patients over short follow-up periods, not exceeding 6 months, and have described changes in the OCTA findings following anti-vascular endothelial growth factor (anti-VEGF) therapy. Post-treatment changes that have been noted include regression of peripheral capillary tufts with persistence of central trunk vessels and change from a dense to a loose net configuration of the CNV complexes. However, not all studies are concordant in their views on the change in CNV size, with some reporting reduction in CNV area [20, 21], while others have found no change in this parameter although observing a fall in blood flow [13, 18].
Many of the studies listed in Table 2 were limited by the exclusion of eyes with lesions that exceeded the OCTA image frame size. Furthermore, eyes with poor-quality OCTA scans were also excluded, and thus the ability of OCTA to detect nAMD in eyes with media opacities and poor fixation remains unevaluated.
Equipment and software algorithms tested in the included studies. Various OCTA systems can use different algorithms to compute and display retinal vessels that have flow [22]. OCTA techniques can be intensity signal based (speckle and phase variance), amplitude-decorrelation (split spectrum angiography; SSADA and full spectrum; FSADA) and complex signal based (optical microangiography; OMAG). Amongst the studies that estimated sensitivity and specificity (Table 1) and in the studies that documented the features of neovascular complexes in AMD, we observed that they were mainly limited to the AngioVue (Optovue Inc, Fremont, CA) and the AngioPlex (Carl Zeiss Meditec. Inc, Fremont, CA), and with only one study using the Heidelberg system that employs the FSADA algorithm.
Discussion
In contrast to FFA and ICGA, OCTA is an entirely non-invasive method for imaging vascular profiles in the retina and the choroid. It is rapidly gaining popularity both for the detection of nAMD and the subsequent monitoring of the response to treatment [2]. Detecting the flow through blood vessels is the fundamental concept upon which OCTA is based. This principle has been utilised in the detection of normal and abnormal vasculature and is now being applied successfully to the visualisation of CNV in nAMD. OCTA acquisition systems have in-built segmentation algorithms for automated detection of flow and will display vascular profiles by retinal layer, however, none are currently validated, thus expert scrutiny followed by adjustment of the segmentation lines are required. The presence of a CNV on OCTA correlates well with findings on structural OCT and FFA [5]. The sensitivity and specificity values across studies vary (Fig. 1) and appear to be influenced by the both methodology and equipment used, and also by the subtype classification of nAMD. In this context, it is worth noting that type 1 or type 2 CNV appear to be more easily visualised on OCTA compared with RAP or polypoidal complexes.
The increased definition with which the vascular network of a CNV can be visualised on OCTA has led to a better understanding of the structural evolution of these lesions with anti-angiogenic treatment. Despite inactivity on FFA, a vascular network can remain persistent on OCTA [6]. Lesions have been described as exhibiting a central trunk vessel with an extending vascular network. With treatment, these complexes may show a reduction in the overall size, however, the main trunk vessels remain unchanged [13, 16, 19, 21]. Also the changing retinal architecture due to atrophy and or fibrosis can confound the interpretation of lesion size, activity and vascularity based on OCTA. Interestingly, CNV lesions when measured are smaller on OCTA when compared with dye-based angiography [15], but the interobserver coefficient of variation is smaller with the former. This may be due to the better delineation of the margins of the neovascular complex on OCTA resulting in the outlining of a smaller area of involvement as compared with FFA, where leakage can obscure the margins and suggest a greater area of involvement.
The advantages of OCTA are the easy, non-invasive and repeatable quantitative measurement of the dimensions of the vascular network in nAMD. As mentioned previously, interobserver reproducibility in the size measurements of both type 1 and type 2 CNV lesions are better than that made using FFA. Findings on OCTA are concordant with those seen on FFA and ICGA [8, 15, 17]. There is a general consensus on the higher resolution of OCTA in outlining the profiles of the retinal microvasculature, and this attribute has allowed investigators to describe features of the neovascular complexes in AMD that were previously not visible with contrast-based angiography and afforded a better understanding of post-treatment pathophysiology [6].
However, the modality is not without its limitations. Almost all of the studies to date were limited by the sample size, which tended to be small, a lack of negative controls and, in some, the restriction of the study to a single type of nAMD. Even more importantly, no study provided details on the reasons for exclusion such as the presence of media opacities, poor fixation and restricted mobility, which are likely to be commonly encountered in the older population. Therefore, the evidence at present does not fully support the exclusive use of OCTA in the diagnosis of CNV in the nAMD population.
Vascular leakage cannot be seen using OCTA, as the technology requires flow within the vessels. Furthermore, when vascular pathology results in very fast, very slow or turbid flow, such vessels may not be consistently visualised with the current generation of OCTA machines, where the interscan time is fixed. Variable interscan time technology may overcome these obstacles.
There are other limitations that currently exist and reduce the clinical potential of OCTA, many of which have been highlighted by Spaide et al. [23]. The vascular signal can be blocked by overlying pathology [4, 5, 8], and this may be interpreted as reduced flow within the retinal vascular plexuses or choriocapillaris. Projection artefacts from the superficial or deep retinal plexus vessels can interfere with the signal from the choroidal vasculature. Motion artefacts cause poor-quality scans. False-positive scans have been described when the CNV is visible on OCTA, however the lesions are inactive on FFA, and this finding has been attributed mainly to projection artefact and inaccurate segmentation [15, 23].
The currently available commercial instruments are limited in their scan area. The region of the fundus covered generally lies between 3 mm × 3 mm and 12 mm × 12 mm. The smaller the field size, the faster the acquisition and the better the resolution. However, the smaller the scan area, the greater the likelihood that a lesion may lie outside of it and areas are missed.
In the majority of the studies that fulfilled the criteria for selection in the present review, we noted that the AngioVue had been used as the OCTA acquisition system. The high frequency of use of the angioVue likely reflected the commercial availability of this instrument, which was the first to be introduced into the market. As the angioVue uses optical microangiography, which is only one of many algorithms deployed in the available instruments, the sensitivity and specificity of other available systems also require validation.
Despite these caveats, our review suggests increasing confidence in the use of OCTA. As more robust evidence accrues, it is likely to become more widely accepted as a form of rapid, sensitive and non-invasive imaging in the detection and management of nAMD.
Disclaimer
The views and opinions expressed are those of the authors and do not necessarily reflect those of the EDNA programme, NIHR, the UK National Health Service or the Department of Health.
Acknowledgements
We wish to acknowledge the support of the EDNA study office based at the University of Aberdeen, with special thanks to Dr Kathryn Bannister. With thanks to David Wright, MRC Innovation Fellow, Health Data Research UK, Centre for Public Health, Queen’s University Belfast for generation of the Forest plots.
Compliance with ethical standards
Conflict of interest
Professor Chakravarthy is the Chief Investigator for EDNA and has received honoraria from Zeiss and Heidleberg. EDNA is an National Institute for Health Research (NIHR) funded project; HTA Project: 12/142/07—Early detection of neovascular age-related macular degeneration. None of the other Authors have any financial disclosures.
Footnotes
All authors are members of the EDNA Co-PI Writing Committee.
References
- 1.Cole ED, Ferrara EA, Novis EA, Louzada RN, Waheed NK. Clinical trial endpoints for optical coherence tomography angiography in neovascular age related macular degeneration. Retina. 2016;36:S83–S96. doi: 10.1097/IAE.0000000000001338. [DOI] [PubMed] [Google Scholar]
- 2.Chalam KV, Sambhav K. Optical coherence tomography angiography in retinal diseases. J Ophthalmic Vis Res. 2016;11:84–92. doi: 10.4103/2008-322X.180709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.EDNA; https://www.journalslibrary.nihr.ac.uk/programmes/hta/1214207#/
- 4.De Carlo TE, Bonini Filho MA, Chin AT, Adhi M, Ferrara D, Baumal CR, et al. Spectral domain optical coherence tomography angiography of choroidal neovascularization. Ophthalmology. 2015;122:1228–38. doi: 10.1016/j.ophtha.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 5.Gong Jingwen, Yu Suqin, Gong Yuanyuan, Wang Fenghua, Sun Xiaodong. The Diagnostic Accuracy of Optical Coherence Tomography Angiography for Neovascular Age-Related Macular Degeneration: A Comparison with Fundus Fluorescein Angiography. Journal of Ophthalmology. 2016;2016:1–8. doi: 10.1155/2016/7521478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang MC, DeCarlo TE, Baumal CR, Reichel E, Waheed NK, Duker JS, et al. Correlation of spectral domain coherence tomography angiography and clinical activity in neovascular age related macular degeneration. Retina. 2016;36:2265–73. doi: 10.1097/IAE.0000000000001102. [DOI] [PubMed] [Google Scholar]
- 7.Carnevali A, Cicinelli MV, Capuana V, Corvi F, Mazzaferro A, Querques L, et al. Optical coherence tomography angiography: a useful tool for diagnosis of treatment naïve quiescent choroidal neovascularization. Am J Ophthalmol. 2016;169:189–98. doi: 10.1016/j.ajo.2016.06.042. [DOI] [PubMed] [Google Scholar]
- 8.Inoue M, Jung JJ, Balaratnasingam C, Dansingani KK, Dharami-Gavazi E, Suzuki M et al. for the COFT-1 Study group. A comparison between optical coherence tomography angiography for the imaging of type 2 neovascularization. Invest Ophthalmol Vis Sci. 2016;57:OCT314–OCT323. [DOI] [PubMed]
- 9.Faridi A, Jia Y, Gao SS, Huang D, Bhavsar KV, Wilson DJ, et al. Sensitivity and specificity of OCT angiography to detect choroidal neovascularization. Ophthalmol Retin. 2017;1:294–303. doi: 10.1016/j.oret.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia Y, Bailey ST, Wilson DJ, Tam O, Klein ML, Flaxel CJ, et al. Quantitative optical coherence tomography angiography of choroidal neovascularization in age related macular degeneration. Ophthalmology. 2014;121:1435–44. doi: 10.1016/j.ophtha.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuehlewein L, Bansal M, Lenis TL, Iafe NA, Sadda SR, Bonini Filho MA, et al. Optical coherence tomography angiography of type 1 neovascularization in age related macular degeneration. Am J Ophthalmol. 2015;160:739–48. doi: 10.1016/j.ajo.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 12.Dansingani KK, Freund KB. Optical coherence tomography angiography reveals mature, tangled vascular networks in eyes with neovascular age-related macular degeneration showing resistance to geographic atrophy. Ophthalmic Surg Laser Imaging Retin. 2015;46:907–12. doi: 10.3928/23258160-20151008-02. [DOI] [PubMed] [Google Scholar]
- 13.Tan A, Dansingani KK, Yannuzzi LA, Sarraf D, Freund KB. Type 3 neovascularization imaged with cross-sectional and enface optical coherence tomography angiography. Retina. 2017;37:234–46. doi: 10.1097/IAE.0000000000001343. [DOI] [PubMed] [Google Scholar]
- 14.Roisman L, Zhang Q, Wang RK, Gregori G, Zhang A, Chen CL, et al. Optical coherence tomography angiography of asymptomatic neovascularization in intermediate age related macular degeneration. Ophthalmology. 2016;123:1309–19. doi: 10.1016/j.ophtha.2016.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costanzo E, Meire A, Quereques G, Capuano V, Jung C. Soied. Type 1 choroidal neovascularization lesion size: Indocyanine green angiography versus optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:OCT307–OCT313. doi: 10.1167/iovs.15-18830. [DOI] [PubMed] [Google Scholar]
- 16.Phaskkijwatana N, Tan ACS, Chen X, Freund KB. Sarraf. Optical coherence tomography angiography of type 3 neovascularization in age related macular degeneration after antiangiogenic therapy. Br J Ophthalmol. 2017;101:597–602. doi: 10.1136/bjophthalmol-2016-308815. [DOI] [PubMed] [Google Scholar]
- 17.Linder M, Fang PP, Steinberg JS, Domdei N, Pfau M, Krohne TU, et al. OCT Angiography-based detection and quantification of the neovascular network in exudative AMD. Invest Ophthalmol Vis Sci. 2016;57:6342–8. doi: 10.1167/iovs.16-19741. [DOI] [PubMed] [Google Scholar]
- 18.Amoroso Francesca, Miere Alexandra, Semoun Oudy, Jung Camille, Capuano Vittorio, Souied Eric H. Optical coherence tomography angiography reproducibility of lesion size measurements in neovascular age-related macular degeneration (AMD) British Journal of Ophthalmology. 2017;102(6):821–826. doi: 10.1136/bjophthalmol-2017-310569. [DOI] [PubMed] [Google Scholar]
- 19.Mastropasqua L, Toto L, Borrelli E, Carpineto P, Antonio LD, Mastropasqua R. Optical coherence tomography angiography assessment of vascular effects occurring after aflibercept intravitreal injections in treatment naïve patients with wet age related macular degeneration. Retina. 2017;37:247–56. doi: 10.1097/IAE.0000000000001145. [DOI] [PubMed] [Google Scholar]
- 20.Sulzbacher Florian, Pollreisz Andreas, Kaider Alexandra, Kickinger Stefan, Sacu Stefan, Schmidt-Erfurth Ursula. Identification and clinical role of choroidal neovascularization characteristics based on optical coherence tomography angiography. Acta Ophthalmologica. 2017;95(4):414–420. doi: 10.1111/aos.13364. [DOI] [PubMed] [Google Scholar]
- 21.Kuehlewein L, Sadda SR, Sarraf D. OCT angiography and sequential quantitative analysis of type 2 neovascularization after ranibizumab therapy. Eye. 2015;29:932–5. doi: 10.1038/eye.2015.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen CL, Wang RK. Optical coherence tomography based angiography. Biomed Opt Express. 2017;8:1056–82. doi: 10.1364/BOE.8.001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spaide RF, Fujimoto JG, Waheed NK. Image artefacts in optical coherence angiography. Retina. 2015;35:2163–80. doi: 10.1097/IAE.0000000000000765. [DOI] [PMC free article] [PubMed] [Google Scholar]