Abstract
Human growth hormone (GH) is a classical pituitary endocrine hormone that is essential for normal postnatal growth and has pleiotropic effects across multiple physiological systems. GH is also expressed in extrapituitary tissues and has localized autocrine/paracrine effects at these sites. In adults, hypersecretion of GH causes acromegaly, and strategies that block the release of GH or that inhibit GH receptor (GHR) activation are the primary forms of medical therapy for this disease. Overproduction of GH has also been linked to cancer and the microvascular complications that are associated with diabetes. However, studies to investigate the therapeutic potential of GHR antagonism in these diseases have been limited, most likely due to difficulty in accessing therapeutic tools to study the pharmacology of the receptor in vivo. This review will discuss current and emerging strategies for antagonizing GH function and the potential disease indications.
Subject terms: Endocrine system and metabolic diseases, Molecular medicine, Drug development, Drug screening
Growth hormone overproduction: the current state of suppressive treatments
Emerging therapies are offering an expanded toolkit for combatting the effects of human growth hormone overproduction. Human growth hormone (GH) is a major driver of postnatal growth; however, systemic or localized overproduction is implicated in the aberrant growth disease acromegaly, cancer, and diabetes. In this review, researchers led by Jo Perry, from the University of Auckland, New Zealand, discuss strategies that either inhibit GH production, block its systemic receptor, or interrupt its downstream signaling pathways. The only licensed GH receptor blocker is pegvisomant, but therapies are in development that include long-acting protein and antibody-based blockers, and nucleotide complexes that degrade GHR production have also shown promise. Studies investigating GHR antagonism are limited, partly due to difficulty in accessing therapeutic tools which block GHR function, but overcoming these obstacles may yield advances in alleviating chronic disease.
Introduction
Human growth hormone (GH) is a peptide hormone that is secreted from the anterior pituitary. It has a central function of regulating postnatal growth and metabolism and exhibits pleiotropic effects on various human tissues. Chronic hypersecretion of GH into the circulation, usually from a GH-secreting pituitary adenoma, is classically associated with acromegaly, a debilitating disease characterized by excessive skeletal growth, soft tissue enlargement, insulin resistance, and cardiovascular and gastrointestinal morbidities.1 Increased GH levels have also been implicated in cancer and diabetes.2–5 Pegvisomant, a GH analog, is the only clinically used antagonist of the GH receptor (GHR).6,7 However, other antagonists are in clinical trials or preclinical development. This review will focus on current strategies for antagonizing GH function and the related disease indications and will discuss considerations associated with an increasingly complex GH signal transduction network. Due to space limitations, reviews have been used in the place of original articles in some instances.
GH secretion and physiological function
GH is released from the somatotroph cells of the anterior pituitary in a pulsatile fashion. Release is primarily regulated by the hypothalamic hormones, growth hormone-releasing hormone (GHRH; positive regulation), and somatostatin (negative regulation) (Fig. 1).8 GHRH is a peptide hormone that interacts with a G protein-coupled receptor (GHRHR) in somatotroph cells to activate the cAMP signaling pathway, which leads to increased GH mRNA transcription and release. GHRH upregulates the pituitary-specific POU homeodomain transcription factor, Pit-1, which in turn, transcriptionally upregulates the GH1, GHRHR and Pit-1 genes (auto-upregulation). Activation of GHRHR signaling in somatotroph cells also induces the release of GH from secretory vesicles as a result of the influx of extracellular Ca2+.8 A complex series of short and long feedback loops negatively regulates GH secretion. Increased levels of GH and IGF1 in the circulation stimulate the release of somatostatin, which interacts with somatostatin receptors and negatively regulates GH secretion from the anterior pituitary.
Fig. 1.
Endocrine regulation of GH and therapeutic blockade. GH is secreted from the anterior pituitary under the control of hypothalamic hormones, growth hormone releasing hormone (GHRH) and somatostatin (SSTN), and ghrelin, which is predominantly secreted in the stomach. Endocrine secretion of GH impacts numerous physiological systems with wide-ranging effects in various tissues. GH is also expressed in extrapituitary tissues in which it has localized autocrine/paracrine effects. Strategies to antagonize GH signaling are shown and are described in detail below
GH secretion is also influenced by ghrelin, a GH secretagogue that is produced primarily by the endocrine cells of the stomach, but also by the intestinal tract and hypothalamus.9 In addition, secretion is regulated by thyroid hormones, leptin, androgens, and estrogen. Other key stimuli for secretion include nutrition, exercise, body composition, and the onset of deep sleep.10–13 Distinct sex-specific secretion patterns are apparent.14,15
Once released into the circulation, GH binds and activates the cell-surface GHR, as well as the related prolactin receptor in target tissues such as liver, muscle, bone, and adipose tissue (Fig. 1). It is the key regulator of insulin-like growth factor 1 (IGF1), which is secreted from target tissues, particularly the liver. Increased serum GH and IGF1 produce feedback loops that lead to inhibition of GHRH, release of somatostatin, and consequently inhibition of GH secretion from the pituitary. Whereas the endocrine system is the main secretory pathway, GH is also expressed in many extrapituitary tissues in which it has autocrine and paracrine effects.4,16,17
The primary function of GH is to promote postnatal longitudinal growth. It induces bone growth and is involved in the regulation of lipid, carbohydrate, nitrogen, and mineral metabolism and electrolyte balance. It increases lipolysis in adipocytes and decreases body fat; it increases amino acid uptake and nitrogen retention in muscle and maintains muscle mass and strength.8,18 GH has effects on the immune system, cardiovascular system, neurogenesis and the central nervous system, and aging.3,19–21 As a consequence, abnormal GH secretion has the potential to impact multiple tissues and organs. In particular, GH hypersecretion leads to gigantism in childhood and acromegaly in adults, whereas congenital disruption of GH signaling causes short stature and in rare cases Laron syndrome. In adults, deficiency is known as GH deficiency syndrome.
Growth hormone receptor signal transduction
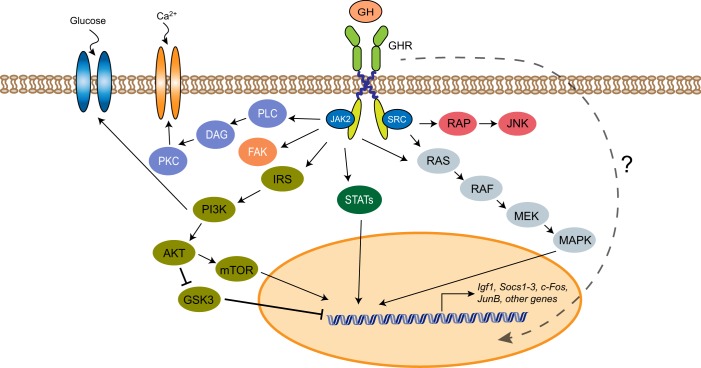
The GHR is a type I cytokine receptor that lacks intrinsic kinase activity and requires recruitment of the nonreceptor tyrosine kinase, Janus kinase 2 (JAK2), for activation.2,3,22,23 Substantial evidence also supports the concept that SRC family kinases, in particular LYN, are recruited to the receptor. These kinases participate in GHR signal transduction.2,3 A predimerized GHR homodimer interacts with the GH ligand through two binding sites, which have different affinities for the receptor. Binding leads to a rotational change in the receptor transmembrane domain, which leads to transphosphorylation and activation of two JAK2 molecules that are associated with the cytoplasmic domain of the receptor.24,25 Phosphorylated JAK2 then phosphorylates tyrosines in the cytoplasmic domain of GHR, and this facilitates recruitment of signaling molecules to the receptor. The primary signaling pathway activated by GH is the JAK-STAT (signal transducer and activator of transcription) pathway (Fig. 2). The STAT molecules that are activated by GH signaling are STAT1, 3, 5a, and 5b. Other key signaling pathways that are utilized are the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin (PI3K/AKT/mTOR) pathways, as well as SH2B1β, a scaffold protein that interacts with JAK2 and mediates GH-induced changes in the cytoskeleton.22 The GHR has also been observed to rapidly translocate to the nucleus following activation, but its role there remains unclear.
Fig. 2.
GHR signal transduction. A predimerized GHR interacts with the GH ligand and activates the associated kinases, JAK2 and SRC. Key signal transduction pathways activated by the GHR include the JAK-STAT, MEK/MAPK, PI3K/AKT/mTOR, and PLC/DAG/PKC pathways. The GHR can also translocate to the nucleus (dotted line), but the function remains unclear. GHR growth hormone receptor, GH growth hormone, JAK2 janus kinase 2, SRC SRC proto-oncogene, STAT signal transducer and activator of transcription, MEK mitogen-activated protein kinase kinase, MAPK mitogen-activated protein kinase, PI3K/AKT/mTOR phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin, GSK3 glycogen synthase kinase 3, IRS insulin receptor substrate, PLC/DAG/PKC phospholipase C/diacylglycerol/protein kinase C, FAK focal adhesion kinase, RAP rap guanine nucleotide exchanger
Once activated, GHR signal transduction is downregulated by suppressor of cytokine signaling proteins 1–3 (SOCS1–3); these are negative regulators that facilitate the ubiquitination and degradation of the receptor. Downregulation of the receptor also occurs through dephosphorylation by several protein tyrosine phosphatases and protein inhibitor of activated STATs (PIAS).2
Activation of the GHR and GHR-stimulated signal transduction pathways has been comprehensively reviewed elsewhere, and we refer the reader to recent reviews for a more detailed description of GHR-mediated signaling.2,3,18,22,26
In addition to activating the GHR, human GH can bind and activate a second cytokine receptor, the prolactin receptor.27 The GHR also cross-talks and/or forms complexes with several other growth factor and hormone receptors (Fig. 3). GH increases the phosphorylation of the epidermal growth factor receptor (EGFR) and promotes downstream ERK signaling.28–30 It has been proposed that a GHR-JAK2-IGF1 receptor complex is formed, which is activated by GH stimulation and inhibited by a soluble IGF1 receptor extracellular domain fragment.31,32 The androgen receptor also cross-talks with GH signaling at the level of STAT5 and SOCS2 in prostate cancer cells.33 More recently it has been demonstrated that EphA4, a member of the Eph family of receptor tyrosine kinases, may interact with the GHR.34 EphA4 knockout mice have dramatically reduced body size and impaired GHR signaling: they have normal levels of GH mRNA expression in the pituitary but have reduced plasma IGF1 and reduced IGF1 mRNA expression in the liver. The authors demonstrated that EphA4 forms a complex with GHR and JAK2 and enhances IGF1 production in response to GH.34 Furthermore, recent studies from Stuart Frank’s laboratory have shown that the GHR and prolactin receptor form complex heteromultimeric structures that may affect GH signal transduction in some cell lines.35,36
Fig. 3.
GHR crosstalk. In addition to the GHR, GH can bind and activate the PRLR, and the GHR can form heteromultimers with PRLR. Furthermore, GHR cross-talks and/or forms complexes with several other growth factor and hormone receptors, such as EphA4, EGFR, and IGF1R, which enhances the stimulation of downstream signaling pathways. GHR growth hormone receptor, GH growth hormone, JAK2 janus kinase 2, STAT signal transducer and activator of transcription, MEK mitogen-activated protein kinase kinase, MAPK mitogen-activated protein kinase, PI3K/AKT/mTOR phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin, PRLR prolactin receptor, EGFR epidermal growth factor receptor, IGF1R insulin-like growth factor 1 receptor, AR androgen receptor
GH excess: disease indications
Acromegaly
One of the most well-described diseases associated with excess GH is acromegaly, a chronic disease that is generally caused by benign pituitary adenomas, with rare exceptions that include secretion from tumors at other sites.37 Patients may exhibit clinical features, such as abnormal growth of the hands, feet and facial features, and enlarged organs. Other pathologic features linked to effects of excessive GH may be observed, such as vertebral deformities and abnormal calcium levels, increased risk of cardiovascular disease, respiratory comorbidities, and glucose intolerance.1,38,39 Hypersecretion of GH leads to elevated concentrations of circulating IGF1 in acromegalic patients, and these two hormones may have different metabolic features and target tissue responses.40
Treatment options for acromegaly include surgery, medical therapy and radiotherapy. The aim of treatment is to reduce both GH and IGF-1 to within normal limits.37,40,41 The primary choice for therapy is surgical removal of the tumor; however, there are some situations in which this may not achieve an optimal outcome, for example in the case of larger tumors with significantly high levels of GH. Therefore, more than one intervention may be necessary to achieve symptom remission and GH/IGF1 normalization. Medical therapies are an important treatment option, particularly for patients who may not be suitable for surgery or those with persistently high levels of GH/IGF1 following surgery. There are three main types of medical therapy, including somatostatin receptor ligands (SRL), GHR antagonists and dopamine agonizts42,43 (Table 1). The GHR antagonist pegvisomant is an analog of GH that competes with GH for receptor binding and consequently blocks GHR signal transduction. SRLs and pegvisomant are described in more detail in the following sections. Other strategies include dopamine agonists that bind to the dopamine receptors in the pituitary gland and block the secretion of prolactin and GH. These agents may benefit patients in whom hypersecretion of both prolactin and GH occurs. Radiotherapy is another therapeutic option but is reserved for aggressive tumors that are not controlled by surgery or medical treatment due to the high risk of hypopituitarism and other complications.37,41
Table 1.
GH signaling inhibitors
| Strategy/drug name | Target | Company |
|---|---|---|
| SSTN receptor ligands | ||
| Octreotide LAR42,43 | GH secretion | Novartis; clinical use |
| Lanreotide autogel42,43 | GH secretion | Ipsen; clinical use |
| Pasireotide LAR42,43 | GH secretion | Novartis; clinical use |
| Octreolin42,43 | GH secretion | Chiasma; clinical development |
| Intravail Octreotide ProTek42,43 | GH secretion | Aegis; clinical use |
| Somatoprim (DG3173)42,43 | GH secretion | Aspireo; clinical development |
| Dopamine agonist | ||
| Cabergoline42,43 | PRL/GH secretion | Par Pharmaceutical; clinical use |
| GHRH antagonists111 | GH secretion | Preclinical development |
| GH analogs | ||
| Pegvisomant/B20366,114 | GHR | Pfizer; clinical use |
| GH-G120R118 | GHR/PRLR | |
| Fusion proteins | ||
| Antagonist–GHBP fusion128 | GHR | Asterion Ltd.; clinical development |
| Antisense oligonucleotide | ||
| ATL1103 (Atesidorsen)132 | GHR | Antisense Therapeutics Ltd.; clinical development |
| Anti-GHR antibody | ||
| Anti-GHRcyt-mAb133,137 | GHR | |
| GF185136 | GHR | |
| RN172135 | GHR | Pfizer; preclinical |
| CG-86134 | Ghr (porcine) | |
| Small molecule compound | ||
| BVT-A138 | Unknown | |
| GH signaling pathway inhibitors | ||
| JAK-STAT, MAPK, PI3K/AKT/mTOR140–144 | ||
GHR growth hormone receptor, SSTN somatostatin, PRLR prolactin receptor, PRL prolactin, JAK-2 Janus kinase 2, STAT signal transducer and activator of transcription, MAPK mitogen-activated protein kinase, PI3K/AKT/mTOR phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin
Cancer
There is a growing body of evidence that implicates GH in multiple cancer types, particularly breast, colon and endometrial cancer. Individuals with GH resistance, as seen in Laron syndrome, a rare genetic condition resulting from an inactivating mutation in the GHR, are protected from cancer and diabetes.44–46 Conversely, patients with acromegaly have a higher risk of developing certain cancers, although the extent of that risk remains a topic of debate with conflicting observations.47,48 This is partly due to limitations in the ability to quantify the risk of cancer in patients with a rare disease and differences in the methodological approaches. Recent nationwide cohort studies in Italy and Denmark both found an increased cancer risk in patients with acromegaly.48,49 This was supported by a meta-analysis of 23 studies, which observed a slightly elevated overall risk of cancer in these patients.49
Such observations are supported by numerous in vitro and in vivo studies that have demonstrated multiple effects on tumor development.4,47,50,51 GH accelerates tumor progression through autocrine/paracrine effects on cancer cell behavior and neighboring cells within the tumor microenvironment. These effects include promoting cell survival/proliferation,52,53 migration/invasion,53,54 oncogenic transformation,55,56 and epithelial-to-mesenchymal transition,54,57,58 as well as promoting tumor angiogenesis59 and lymphangiogenesis60 and enhancing a cancer stem cell-like phenotype.57,61 These effects have been observed in multiple tumor types, including breast, endometrial, and hepatocellular carcinomas, and lung cancer, melanoma, prostate cancer, and colon cancer.17,47,57,61–63 The effects are mediated through altered transcription of numerous genes that are associated with various aspects of cancer progression. A recent study demonstrated that disrupted GH signaling is associated with elevated p53 in colon tissue in humans and mice and that GH may act as a tumor promoter by suppressing p53, PTEN, and APC levels.64 In addition, GH regulates the function of other receptors that promote cancer progression.65,66
Single-nucleotide polymorphisms in GH-related genes are associated with the risk of developing osteosarcoma, breast, and colon cancer.67–69 Recently, a P495T variant of the GHR that is associated with increased incidence of lung cancer in various ethnic groups was shown to prolong GH signaling, which leads to increased expression of genes associated with tumor proliferation and epithelial-to-mesenchymal transition.70 The amino acid change at position 495 impairs SOCS2 binding and leads to a reduced downregulation of the receptor.
Such studies support the rationale for testing GHR antagonism in cancer, but only a small number of preclinical studies have used pegvisomant in an oncology setting. Single agent growth-inhibitory effects have been reported in breast, colon, and meningioma tumor xenografts, which suggests that GHR antagonism as a monotherapy may have efficacy in some tumor types.71–74 However, it is clear from these studies that not all tumor types tested were responsive, and currently the field is lacking diagnostic biomarkers capable of predicting response.
As with many targeted therapies, improved anticancer responses may be observed with combination therapeutic approaches. In this regard, GH promotes chemo- and radioresistance, and GHR antagonism/suppression may be beneficial when combined with radiotherapy and certain chemotherapeutic drugs.47,75–77 Recently, we reported that GH promotes radioresistance in cancer cell lines78 and that pegvisomant suppresses endometrial tumor regrowth following radiotherapy in a xenograft model, which highlights the potential utility of this combinational approach and identifies GHR antagonism as a potential molecularly targeted radiosensitizing strategy.76 This finding is of interest because for many common cancers, adding molecularly targeted agents to radiotherapy can increase preclinical cure rates,79–81 and currently the only targeted agent clinically approved for this application is the EGFR antagonist, cetuximab.
Diabetes mellitus
Diabetes mellitus is a chronic metabolic disorder that is characterized by elevated blood glucose caused by deficiency or resistance to insulin. Glucose homeostasis is primarily regulated by insulin, which lowers blood glucose by increasing the uptake into cells and increasing its utilization and storage as fat and glycogen in peripheral tissues. Insulin also decreases gluconeogenesis (synthesis of glucose from noncarbohydrate carbon substrates) and glycogenolysis (breakdown of glycogen). GH has well-described diabetogenic actions. In particular, GH opposes the actions of insulin: it increases glucose production through gluconeogenesis and glycogenolysis in the liver and kidney, suppresses glucose uptake in adipose tissues, and is lipolytic. In healthy adults and adolescents during puberty, increased GH levels impair glucose tolerance and induce insulin resistance.82,83 Conversely, adults with Laron syndrome have reduced circulating IGF1 and increased insulin sensitivity.45,46,84 This is supported by studies in mice85 and dogs.86 Blocking the effects of GH in patients with acromegaly improves diabetes and glucose metabolism. However, a one-month treatment with a GHR antagonist had no effect on insulin sensitivity in insulin-resistant nondiabetic men.87
In addition to systemic effects on glucose metabolism and insulin sensitivity, GH may contribute to diabetes-associated complications, such as diabetic retinopathy and diabetic renal disease, through localized expression and autocrine/paracrine effects in tissues,88–93 although published research in these areas is limited. Diabetic retinopathy is one of the most frequent complications of diabetes and is a leading cause of blindness. The proliferative form is a more advanced stage of the disease and is characterized by retinal neovascularization. Extrapituitary expression of GH has been detected in the human retina and vitreous fluid,94 and GH has been demonstrated to directly stimulate the proliferation of human retinal microvascular endothelial cells in vitro.95 In vivo, ischemia-induced retinal neovascularization was inhibited in transgenic mice expressing a GH antagonist gene.96 Increased serum levels of GH and IGF1 have been observed in type 2 diabetes mellitus (T2D) patients with proliferative diabetic retinopathy compared to T2D patients with nonproliferative retinopathy or with no evidence of this complication.97
An increased prevalence of proliferative retinopathy is also observed in patients with acromegaly.98 Whether GHR-targeted strategies will be an effective treatment for retinopathy is still a matter of debate: a small clinical study using the GHR antagonist, pegvisomant, did not observe regression of proliferative diabetic retinopathy. However, it has been suggested that this may have been due to insufficient suppression of IGF1.99,100
In diabetic nephropathy, excess GH stimulates glomerular growth, affects the structure and function of the kidney, and is associated with glomerular podocyte dysfunction.89,101–103
Longevity
The GH/IGF1 axis has an intriguing association with longevity in animals. Numerous studies, particularly in rodents, indicate that suppression of the GH/IGF1 axis has multiple benefits in terms of aging. Disruption of GH and IGF1 signaling extends lifespan, enhances insulin sensitivity, decreases DNA and protein oxidation in the liver, reduces cancer incidence and may reduce age-related inflammation.20,104–108 GHR dysfunction clearly protects individuals with Laron Syndrome against aging-related diseases such as cancer and diabetes, but it is unclear whether GH/IGF1 deficiency increases human lifespan, although a handful of studies have indicated that attenuated GH/IGF1 function is associated with human longevity.109,110
Strategies that target GHR signaling
The most successful strategy to date for directly inhibiting GHR function has undoubtedly been peptide receptor antagonists, exemplified by the clinically used GHR antagonist pegvisomant (see below). However, therapeutic options to target the GHR are still limited, and pegvisomant can be difficult for researchers to access. Given the growing body of evidence that has suggested a role for this receptor in cancer and other diseases, there is certainly room for the development of alternative targeted therapeutics and several approaches are in preclinical and clinical development (Figs. 1 and 4, Table 1).
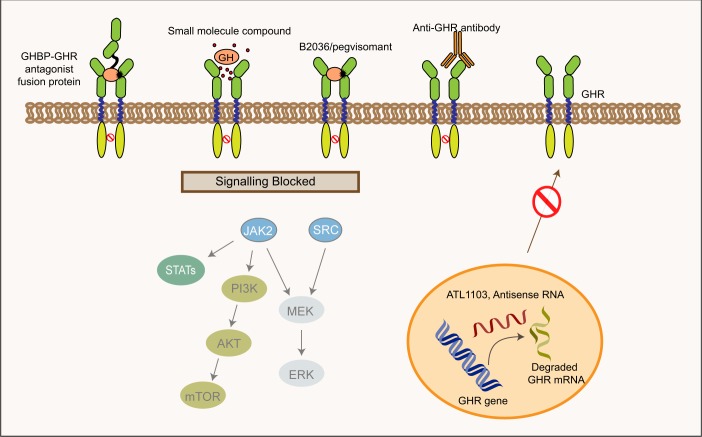
Fig. 4.
Strategies targeting the GHR One protein-derived GHR antagonist is clinically approved (pegvisomant), and several other GHR-targeted approaches are in development. These include an antagonist-GHBP fusion protein and anti-GHR antibodies, which inhibit the activation of GHR and block downstream signaling. Another approach is atesidorsen (ATL1103), an antisense oligonucleotide (ASO), that binds and induces the degradation of GHR mRNA. Small molecule compounds may also have applications; however, there are currently limited reports in this area
Inhibitory strategies broadly fall into three categories: those that inhibit inhibition of GH secretion from the pituitary (pre-receptor), those that directly inhibit the GHR, and drugs which inhibit downstream components of GHR signaling pathways (post-receptor).
Inhibitors of GH secretion (pre-receptor)
Inhibitors of GH secretion include SRLs, dopamine agonizts and GHRH antagonists.42,43,111 SRLs bind to the somatostatin receptors that are present in the tumor and suppress the secretion of GH from the pituitary. These inhibitors include the first-generation SRLs, octreotide and lanreotide, and the second-generation SRLs, pasireotide, octreolin, and somatoprim (Table 1). Currently, SRLs are used to treat acromegaly and neuroendocrine tumors. Other applications include diabetes, obesity and cancer.112 SRLs also have direct anticancer activity through their actions on tumor cells that express somatostatin receptors, but it is unclear whether they suppress tumoral (autocrine/paracrine) secretion of GH.
Peptide GHRH analog antagonists have been developed in several labs. In particular, studies by Schally et al.111 have led to a series of well-characterized inhibitors.113 GHRH and GHRH receptors are expressed in many cancer cells and tumor tissues. Antagonists for GHRH inhibit the proliferation of a wide range of cancer cell lines in vitro and inhibit xenograft tumor growth, which demonstrates their potential clinical utility.111 Similar to SRLs, GHRH antagonists inhibit endocrine secretion of GH from the anterior pituitary to varying extents. However, the main action of these antagonists is through direct inhibition of GHRH receptors in tumor tissues. Potential applications besides oncology include acromegaly, diabetic retinopathy, and nephropathy.111
GHR antagonists
Pegvisomant
Pegvisomant is a chemically modified (PEGylated) analog of GH that was discovered in John Kopchick’s lab and developed by Pfizer. It is the only clinically approved GHR antagonist and has been approved by the FDA for the treatment of acromegaly [reviewed in ref. 6,114,115]. The first reported GHR antagonist was a bovine GH protein that was mutated with three amino acid substitutions located in helix 3, which resulted in a dwarf phenotype in transgenic mice.6,116,117 A similar result was observed with a single amino acid substitution of glycine to arginine at position 120 on helix 3 of human GH (G120R).118 Pegvisomant contains a G120K mutation (substitution to a lysine also allows PEGylation at this site). Eight more mutations (H18D, H21N, R167N, K168A, D171S, K172R, E174S, and I179T) were introduced at binding site 1 on the molecule to prevent PEGylation and to increase the affinity for the receptor at this site.6 This antagonist, which is the protein component of pegvisomant, is known as B2036. The half-life of native GH (as well as B2036) in the circulation is short, which limits its in vivo efficacy. To reduce clearance and increase the serum half-life, polyethylene glycol-5000 (PEG5000) was conjugated to the molecule.119 PEGylation extends the serum half-life, delays renal clearance, and reduces the immunogenicity of pegvisomant (B2036-PEG). B2036 competes with GH in vitro on the basis of an increased affinity for the GHR. However, PEGylation reduces the affinity for the receptor somewhat. Because pegvisomant targets the GHR instead of GH, it results in reduced IGF1 and enhanced GH levels through the negative-feedback loop. Therefore, measurement of GH is not useful to monitor treatment of acromegaly with pegvisomant: instead IGF1 is determined as a surrogate biomarker.120,121 In addition, pegvisomant treatment decreases the insulin and glucose concentrations.122
Pegvisomant is highly efficacious with only mild-side effects. One concern was potential adenoma growth caused by the increased levels of circulating GH. From the clinical trials conducted so far, changes in the tumor size or recurrence were infrequent, but further assessment needs to be carried out. An evaluation of pegvisomant as long-term monotherapy in acromegalic patients from the global safety surveillance study ACROSTUDY reported increases or increases/decreases in the tumor sizes in 12 of 542 subjects (2.2%).7 Another study reviewed the efficacy of pegvisomant as a monotherapy for acromegaly over a 10-year period, and showed 6 of 64 (9.4%) cases with tumor growth.123 Pegvisomant has also been evaluated in a combination therapy with SRL, particularly with respect to normalizing the IGF1 concentration in acromegalic patients who have failed SRL monotherapy. The outcome of a 42-week study of active acromegalic patients demonstrated that the combined therapy was effective in normalizing the levels of IGF1 and that there was no indication of tumor growth.124 However, an analysis of 62 SRL-resistant acromegalic patients indicated better IGF1 normalization with pegvisomant monotherapy compared to combined pegvisomant/SRL treatment.125 Although pegvisomant is well tolerated and highly effective, some limitations need to be considered. Pegvisomant is more costly than SRL, and daily injection is required. Treatment side-effects include elevated aminotransferase levels and injection site reactions (lipohypertrophy).
As described above, a small number of preclinical studies have demonstrated efficacy for pegvisomant in certain tumor models.71–73,76 One obstacle to preclinical use in animal models is the species specificity of the drug. Both human GH and the protein component of pegvisomant (B2036) can bind the human and mouse GHR, but pegylation significantly reduces the affinity for the mouse GHR. Consequently, much higher concentrations of pegvisomant are necessary to reduce serum IGF1 in mice.47 In addition, rodent GH does not activate the human or primate GHR, so the use of pegvisomant in animal models of disease does not address the effect of the blockade of systemic GH. Therefore, although early animal studies have been promising, these limitations combine to reduce the potential of these in vivo studies to support clinical translation.
Antagonist–GHBP fusion proteins
An alternative approach that is used to generate long-acting forms of protein therapeutics involves generating larger chimeric proteins that avoid kidney filtration. Richard Ross and colleagues from Asterion Ltd. have developed fusion proteins composed of the GH ligand or a GHR antagonist fused to GH-binding protein (GHBP), the extracellular domain of the GHR, which is proteolytically cleaved from the receptor and exists in the circulation (Fig. 4). The fusion of GH to its natural binding protein decreases its immunogenicity and prolongs its half-life in the circulation.126 Initially, a GH–GHBP fusion protein agonist was generated for the treatment of GH deficiency.127 This chimeric protein was found to exist in solution as both a monomer and a dimer. In the dimer form, the GH portion of one molecule bound to the receptor portion of another molecule in a head-to-tail reciprocal dimer. More recently Wilkinson et al. demonstrated that fusion of GHBP to a GHR antagonist protein similar to B2036 significantly reduced IGF1 by 14% after a single subcutaneous injection in rabbits and may be useful for treating acromegaly.128 Introduction of a W104A mutation in the fused GHBP prevented intra- and inter-molecular binding. Three chimeric GHR antagonists were generated with extended in vivo clearance times; the terminal half-life of the fusion proteins was greater than 20 h in rats.128
Antisense oligonucleotides
Advances in antisense therapy have led to development of novel GHR antagonists (Fig. 4). Antisense oligonucleotides (ASOs) are short single-stranded DNA or RNA molecules (or chemical analogs) that bind and induce the degradation of target RNAs.129 Early studies reported an inhibitory effect of GHR-targeted antisense oligonucleotides on GHR expression and IGF1 production in mice.130,131 Antisense Therapeutics Ltd. is developing an antisense oligonucleotide drug, ATL1103 (now atesidorsen). Atesidorsen is a 20-mer ASO that has been modified to enhance its stability and circulating half-life. In Phase II outcomes, twice-weekly treatment with 200 mg atesidorsen was well tolerated and decreased the serum IGF1 concentration by 27.8% at week 14 and 18.7% at week 21 in acromegalic patients.132 In addition, interim analysis from a small higher dose study using twice-weekly 300 mg atesidorsen for 13 weeks demonstrated results consistent with the Phase II trial outcomes (Antisense Therapeutic Ltd.).
Anti-GHR antibodies
GHR antibodies that inhibit GHR-mediated signal transduction have been reported.133–136 Stuart Frank’s lab has developed an inhibitory conformation-sensitive monoclonal antibody (Anti-GHRcyt-mAb) that targets the extracellular domain of the rabbit GHR. Anti-GHRcyt-mAb effectively inhibits activation and downstream signal transduction of the rabbit and human GHR.133,137 Similarly, Sun et al.136 have reported a monoclonal antibody (GF185) that targets the human GHR, which acts as a full competitor for GH binding and also inhibits GHR signaling. Lan et al.134 have generated an anti-idiotypic antibody (CG-86) which mimics an epitope on porcine GH and investigated the inhibitory effects on GH signaling and cell proliferation, which demonstrated the potential utility of this approach for generating GH antagonists. More recently Pfizer has reported development of a humanized GHR monoclonal antagonist antibody (RN172), which blocked GH signaling in vitro and reduced IGF1 production in monkeys following a single-intravenous injection.135
Small molecules
The only small molecule GHR antagonist that has been reported is an orally available compound BVT-A (N-[5-(aminosulfonyl)-2-methylphenyl]-5-bromo-2-furamide). In two studies, BVT-A suppressed GH induction of IGF1 expression in hepatocytes in vitro and reduced GH stimulation of IGF1 secretion and body weight in hypophysectomized rats.138,139 However, it is unclear where in the GH signaling pathway this compound acts, and no subsequent activity has been reported in this area since the original publication.
Inhibitors of GH signal transduction pathways (post-receptor)
Given that GH activates JAK-STAT, PI3K/AKT/mTOR and MAPK signaling, agents which target components of these signaling pathways would be expected to modulate pathway-specific GH effects. However, targeting GH signal transduction pathways will unlikely be specific to GH signaling because other receptors and cell signaling pathways can also utilize the same signaling pathways as GH. The potential for small molecules to inhibit these pathways and non-selectively inhibit GHR signaling needs to be considered in small molecule discovery studies. A detailed description of therapeutic drugs that target molecules in the GHR signal transduction pathways is beyond the scope of the current review, and we refer the reader to recent reviews.140–144
Targeting autocrine/paracrine, and systemic functions: therapeutic considerations
GHR antagonism suppresses the endocrine, autocrine, and paracrine functions of GH, and this is an important consideration that has particular relevance for cancer on the basis that both systemic and tumor-derived GH contribute to cancer progression.4,47,50 Furthermore, it is quite clear from earlier studies by Lobie and colleagues that GH has differential effects depending on whether the source of GH is pulsatile secretion from the pituitary or secretion from cancer cells or other cells in the tumor microenvironment. For example, in breast cancer, autocrine expression of GH from the tumor cells promotes a more aggressive cellular phenotype, compared to exogenously added GH, which mimics endocrine secretion.52,54,145,146 This phenomenon may not be limited to cancer. For example, autocrine/paracrine actions may also be important in conditions such as diabetic retinopathy because GH expression occurs in ocular tissues.88 Notably, antagonism of GH signaling has the added benefit of suppressing the IGF1-mediated effects, which contribute to the etiology of several disease indications in a manner similar to that described above.
As with any therapeutic agents, the potential for side effects associated with suppression of GH should be considered. Apart from the known side effects associated with pegvisomant, which are described above, potential adverse effects may be extrapolated from adult GH deficiency (AGHD). The clinical features of AGHD includes abnormal body composition, decreased cardiac capacity, increased risk of fracture, insulin resistance, and decreased quality of life.147–150 The abnormal body composition is characterized by increased adipose tissue mass, decreased lean body mass, decreased muscle mass and strength.148,149 Cardiac changes, such as reduced aortic area and left ventricular mass index, are also reported in adult-onset GH deficiency.151 A study of adults with isolated GH deficiency (i.e., AGHD that does not involve abnormalities in other pituitary hormones) reported decreased total IgG levels but no increased risk of infectious disease in the subjects.152 In addition, abnormal vascular and neural retinal morphology was observed in adults with isolated GH deficiency.153 It was demonstrated that anaerobic capacity was impaired in GH-deficient adults, and this decreases exercise tolerance and quality of life.154 Skin and sleep changes were also reported in GH-deficient adults.148,155
Conclusion
Suppression of the GH/IGF1 axis is a key medical therapy that is indicated for acromegaly and may also be useful in other diseases such as cancer. Currently, pegvisomant is the only clinically used GHR inhibitor. However, a small number of GHR antagonists are in clinical trials or preclinical development, and we anticipate that these will eventually expand the options available for clinical and research applications.
Clearly, the most successful strategy for targeting GHR signaling to date has involved protein-based therapeutics. However, other strategies are showing promise, particularly antisense oligo and antibody-based approaches. One avenue that has been underexplored is the development of small molecule therapeutic agents to target the receptor. The GHR is a challenging target in this regard in that it lacks a kinase domain and the ligand binding surface involves a large, relatively featureless protein-protein interface. Identification of inhibitors of protein-protein interactions has been a major challenge in drug discovery, yet screening strategies, including high-throughput screening and fragment-based drug design, are able to identify compounds suitable for drug development.156,157 Recent successes with such strategies have resulted in high impact therapies including agents that block the p53-MDM protein interaction and the formation of antiapoptotic complexes by the Bcl2 protein with Navitoclax and Venetoclax.157 Conceivable mechanisms for on-target GHR inhibition involve disruption of the formation of the signaling complex. Indeed, this type of inhibitory mechanism was described for blocking the interaction between another cytokine receptor, the interleukin-2 receptor, and its ligand.158 Recent advances in our understanding of GHR activation24 should contribute to the development of small molecule-based targeting strategies.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Melmed S. Acromegaly pathogenesis and treatment. J. Clin. Invest. 2009;119:3189–3202. doi: 10.1172/JCI39375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dehkhoda F, Lee CMM, Medina J, Brooks AJ. The growth hormone receptor: mechanism of receptor activation, cell signaling, and physiological aspects. Front. Endocrinol. 2018;9:35. doi: 10.3389/fendo.2018.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks AJ, Waters MJ. The growth hormone receptor: mechanism of activation and clinical implications. Nat. Rev. Endocrinol. 2010;6:515–525. doi: 10.1038/nrendo.2010.123. [DOI] [PubMed] [Google Scholar]
- 4.Perry JK, Emerald BS, Mertani HC, Lobie PE. The oncogenic potential of growth hormone. Growth Horm. IGF Res. 2006;16:277–289. doi: 10.1016/j.ghir.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Thankamony GA, Dunger DB, Acerini CL. Pegvisomant: current and potential novel therapeutic applications. Expert. Opin. Biol. Ther. 2009;9:1553–1563. doi: 10.1517/14712590903449222. [DOI] [PubMed] [Google Scholar]
- 6.Kopchick JJ, Parkinson C, Stevens EC, Trainer PJ. Growth hormone receptor antagonists: discovery, development, and use in patients with acromegaly. Endocr. Rev. 2002;23:623–646. doi: 10.1210/er.2001-0022. [DOI] [PubMed] [Google Scholar]
- 7.Freda P, et al. Long-term treatment with pegvisomant as monotherapy in patients with acromegaly: experience from acrostudy. Endocr. Pract. 2015;21:264–274. doi: 10.4158/EP14330.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonert, V. S. & Melmed, S. in The Pituitary: Fourth Edition 85–127. 10.1016/B978-0-12-804169-7.00004-0 (2017).
- 9.Okada S, Kopchick JJ. Biological effects of growth hormone and its antagonist. Trends Mol. Med. 2001;7:126–132. doi: 10.1016/S1471-4914(01)01933-5. [DOI] [PubMed] [Google Scholar]
- 10.Frenette E, Lui A, Cao M. Neurohormones and sleep. Vitam. Horm. 2012;89:1–17. doi: 10.1016/B978-0-12-394623-2.00001-9. [DOI] [PubMed] [Google Scholar]
- 11.Berryman, D. E. & List, E. O. Growth hormone’s effect on adipose tissue: quality versus quantity. Int. J. Mol. Sci. 18, 10.3390/ijms18081621 (2017). [DOI] [PMC free article] [PubMed]
- 12.Ho KY, et al. Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man. J. Clin. Invest. 1988;81:968–975. doi: 10.1172/JCI113450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunawardane, K., Krarup Hansen, T., Sandahl Christiansen, J. & Lunde Jorgensen, J. O. Normal Physiology of Growth Hormone in Adults. Endotext [Internet] (MDText.com, Inc., South Dartmouth (MA), 2000). https://www.ncbi.nlm.nih.gov/books/NBK279056/.
- 14.Vijayakumar A, Novosyadlyy R, Wu YJ, Yakar S, LeRoith D. Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Horm. IGF Res. 2010;20:1–7. doi: 10.1016/j.ghir.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaffe CA, et al. Regulatory mechanisms of growth hormone secretion are sexually dimorphic. J. Clin. Invest. 1998;102:153–164. doi: 10.1172/JCI2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harvey S. Extrapituitary growth hormone. Endocrine. 2010;38:335–359. doi: 10.1007/s12020-010-9403-8. [DOI] [PubMed] [Google Scholar]
- 17.Perry JK, Liu DX, Wu ZS, Zhu T, Lobie PE. Growth hormone and cancer: an update on progress. Curr. Opin. Endocrinol. Diabetes Obes. 2013;20:307–313. doi: 10.1097/MED.0b013e328363183a. [DOI] [PubMed] [Google Scholar]
- 18.Troike, K. M. et al. Impact of growth hormone on regulation of adipose tissue. Compr. Physiol.7, 819–840 (2017). [DOI] [PubMed]
- 19.Waters MJ, Blackmore DG. Growth hormone (GH), brain development and neural stem cells. Pediatr. Endocrinol. Rev. 2011;9:549–553. [PubMed] [Google Scholar]
- 20.Gesing A, et al. A long-lived mouse lacking both growth hormone and growth hormone receptor: a new animal model for aging studies. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017;72:1054–1061. doi: 10.1093/gerona/glw193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartke A, List EO, Kopchick JJ. The somatotropic axis and aging: Benefits of endocrine defects. Growth Horm. IGF Res. 2016;27:41–45. doi: 10.1016/j.ghir.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carter-Su C, Schwartz J, Argetsinger LS. Growth hormone signaling pathways. Growth Horm. IGF Res. 2016;28:11–15. doi: 10.1016/j.ghir.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu T, Goh ELK, Graichen R, Ling L, Lobie PE. Signal transduction via the growth hormone receptor. Cell. Signal. 2001;13:599–616. doi: 10.1016/S0898-6568(01)00186-3. [DOI] [PubMed] [Google Scholar]
- 24.Waters MJ, Brooks AJ. JAK2 activation by growth hormone and other cytokines. Biochem. J. 2015;466:1–11. doi: 10.1042/BJ20141293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brooks AJ, et al. Mechanism of activation of protein kinase JAK2 by the growth hormone receptor. Science. 2014;344:1249783. doi: 10.1126/science.1249783. [DOI] [PubMed] [Google Scholar]
- 26.Ceseña, T. I. et al. Multiple mechanisms of growth hormone-regulated gene transcription. Mol. Genet. Metab. 90, 126–133. [DOI] [PMC free article] [PubMed]
- 27.Goffin, V., Shiverick, K. T., Kelly, P. A. & Martial, J. A. Sequence-function relationships within the expanding family of prolactin, growth hormone, placental lactogen, and related proteins in mammals. Endocr. Rev. 17, 385–410. [DOI] [PubMed]
- 28.Li X, Huang Y, Jiang J, Frank SJ. Synergy in ERK activation by cytokine receptors and tyrosine kinase growth factor receptors. Cell. Signal. 2011;23:417–424. doi: 10.1016/j.cellsig.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y, Kim SO, Jiang J, Frank SJ. Growth hormone-induced phosphorylation of epidermal growth factor (EGF) receptor in 3T3-F442A cells: modulation of EGF-induced trafficking and signaling. J. Biol. Chem. 2003;278:18902–18913. doi: 10.1074/jbc.M300939200. [DOI] [PubMed] [Google Scholar]
- 30.Yamauchi T, et al. Tyrosine phosphorylation of the EGF receptor by the kinase Jak2 is induced by growth hormone. Nature. 1997;390:91. doi: 10.1038/36369. [DOI] [PubMed] [Google Scholar]
- 31.Gan Y, et al. Human GH receptor-IGF-1 receptor interaction: implications for GH signaling. Mol. Endocrinol. 2014;28:1841–1854. doi: 10.1210/me.2014-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang Y, Kim SO, Yang N, Jiang J, Frank SJ. Physical and functional interaction of growth hormone and insulin-like growth factor-I signaling elements. Mol. Endocrinol. 2004;18:1471–1485. doi: 10.1210/me.2003-0418. [DOI] [PubMed] [Google Scholar]
- 33.Iglesias-Gato D, et al. SOCS2 mediates the cross talk between androgen and growth hormone signaling in prostate cancer. Carcinogenesis. 2014;35:24–33. doi: 10.1093/carcin/bgt304. [DOI] [PubMed] [Google Scholar]
- 34.Jing X, et al. Crosstalk of humoral and cell–cell contact-mediated signals in postnatal body growth. Cell Rep. 2012;2:652–665. doi: 10.1016/j.celrep.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, et al. GHR/PRLR heteromultimer is composed of GHR homodimers and PRLR homodimers. Mol. Endocrinol. 2016;30:504–517. doi: 10.1210/me.2015-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu J, et al. The role of prolactin receptor in GH signaling in breast cancer cells. Mol. Endocrinol. 2013;27:266–279. doi: 10.1210/me.2012-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dineen R, Stewart PM, Sherlock M. Acromegaly—diagnosis and clinical management. QJM. 2016;110:411–420. doi: 10.1093/qjmed/hcw004. [DOI] [PubMed] [Google Scholar]
- 38.Pivonello R, et al. Complications of acromegaly: cardiovascular, respiratory and metabolic comorbidities. Pituitary. 2017;20:46–62. doi: 10.1007/s11102-017-0797-7. [DOI] [PubMed] [Google Scholar]
- 39.Lombardi G, et al. The cardiovascular system in growth hormone excess and growth hormone deficiency. J. Endocrinol. Invest. 2012;35:1021–1029. doi: 10.3275/8717. [DOI] [PubMed] [Google Scholar]
- 40.Melmed S, et al. Guidelines for acromegaly management: an update. J. Clin. Endocrinol. Metab. 2009;94:1509–1517. doi: 10.1210/jc.2008-2421. [DOI] [PubMed] [Google Scholar]
- 41.Giustina A, et al. Expert consensus document: a consensus on the medical treatment of acromegaly. Nat. Rev. 2014;10:243–248. doi: 10.1038/nrendo.2014.21. [DOI] [PubMed] [Google Scholar]
- 42.Melmed S. New therapeutic agents for acromegaly. Nat. Rev. 2016;12:90–98. doi: 10.1038/nrendo.2015.196. [DOI] [PubMed] [Google Scholar]
- 43.Maffezzoni F, Frara S, Doga M, Mazziotti G, Giustina A. New medical therapies of acromegaly. Growth Horm. IGF Res. 2016;30–31:58–63. doi: 10.1016/j.ghir.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Laron Z, Kauli R, Lapkina L, Werner H. IGF-I deficiency, longevity and cancer protection of patients with Laron syndrome. Mutat. Res.Rev. Mutat. Res. 2017;772:123–133. doi: 10.1016/j.mrrev.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Guevara-Aguirre J, et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci. Transl. Med. 2011;3:70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steuerman R, Shevah O, Laron Z. Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur. J. Endocrinol. 2011;164:485–489. doi: 10.1530/EJE-10-0859. [DOI] [PubMed] [Google Scholar]
- 47.Perry JK, Wu ZS, Mertani HC, Zhu T, Lobie PE. Tumour-derived human growth hormone as a therapeutic target in oncology. Trends Endocrinol. Metab. 2017;28:587–596. doi: 10.1016/j.tem.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Terzolo M, et al. Acromegaly is associated with increased cancer risk: a survey in Italy. Endocr. Relat. Cancer. 2017;24:495–504. doi: 10.1530/ERC-16-0553. [DOI] [PubMed] [Google Scholar]
- 49.Dal J, et al. Cancer Incidence in patients with acromegaly: a cohort study and meta-analysis of the literature. J. Clin. Endocrinol. Metab. 2018;103:2182–2188. doi: 10.1210/jc.2017-02457. [DOI] [PubMed] [Google Scholar]
- 50.Harvey S, Martínez-Moreno CG, Luna M, Arámburo C. Autocrine/paracrine roles of extrapituitary growth hormone and prolactin in health and disease: an overview. Gen. Comp. Endocrinol. 2015;220:103–111. doi: 10.1016/j.ygcen.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Chhabra Y, Waters MJ, Brooks AJ. Role of the growth hormone—IGF-1 axis in cancer. Carcinogenesis. 2011;6:71–84. doi: 10.1586/eem.10.73. [DOI] [PubMed] [Google Scholar]
- 52.Kaulsay KK, et al. Autocrine stimulation of human mammary carcinoma cell proliferation by human growth hormone. Exp. Cell Res. 1999;250:35–50. doi: 10.1006/excr.1999.4492. [DOI] [PubMed] [Google Scholar]
- 53.Pandey V, et al. Autocrine human growth hormone stimulates oncogenicity of endometrial carcinoma cells. Endocrinology. 2008;149:3909–3919. doi: 10.1210/en.2008-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mukhina S, et al. From the cover: phenotypic conversion of human mammary carcinoma cells by autocrine human growth hormone. Proc. Natl Acad. Sci. 2004;101:15166–15171. doi: 10.1073/pnas.0405881101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu T, et al. Oncogenic transformation of human mammary epithelial cells by autocrine human growth hormone. Cancer Res. 2005;65:317–324. [PubMed] [Google Scholar]
- 56.Ogawa Y, Watanabe M, Tominaga T. Prognostic factors of craniopharyngioma with special reference to autocrine/paracrine signaling: underestimated implication of growth hormone receptor. Acta Neurochir. 2015;157:1731–1740. doi: 10.1007/s00701-015-2519-0. [DOI] [PubMed] [Google Scholar]
- 57.Wang JJ, et al. Autocrine hGH stimulates oncogenicity, epithelial–mesenchymal transition and cancer stem cell-like behavior in human colorectal carcinoma. Oncotarget. 2017;8:103900–103918. doi: 10.18632/oncotarget.21812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Basu R, Wu S, Kopchick JJ. Targeting growth hormone receptor in human melanoma cells attenuates tumor progression and epithelial mesenchymal transition via suppression of multiple oncogenic pathways. Oncotarget. 2017;8:21579–21598. doi: 10.18632/oncotarget.15375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brunet-Dunand SE, et al. Autocrine human growth hormone promotes tumor angiogenesis in mammary carcinoma. Endocrinology. 2009;150:1341–1352. doi: 10.1210/en.2008-0608. [DOI] [PubMed] [Google Scholar]
- 60.Banziger-Tobler NE, Halin C, Kajiya K, Detmar M. Growth hormone promotes lymphangiogenesis. Am. J. Pathol. 2008;173:586–597. doi: 10.2353/ajpath.2008.080060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen, Y. J. et al. Autocrine human growth hormone promotes invasive and cancer stem cell-like behavior of hepatocellular carcinoma cells by STAT3 dependent inhibition of CLAUDIN-1 expression. Int. J. Mol. Sci. 18, E1274 (2017). [DOI] [PMC free article] [PubMed]
- 62.Chhabra Y, Waters MJ, Brooks AJ. Role of the growth hormone–IGF-1 axis in cancer. Expert Rev. Endocrinol. Metab. 2011;6:71–84. doi: 10.1586/eem.10.73. [DOI] [PubMed] [Google Scholar]
- 63.Brittain AL, Basu R, Qian Y, Kopchick JJ. Growth hormone and the epithelial-to-mesenchymal transition. J. Clin. Endocrinol. Metab. 2017;102:3662–3673. doi: 10.1210/jc.2017-01000. [DOI] [PubMed] [Google Scholar]
- 64.Chesnokova V, et al. Growth hormone is permissive for neoplastic colon growth. Proc. Natl Acad. Sci. 2016;113:E3250–E3259. doi: 10.1073/pnas.1600561113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gonzalez L, et al. Attenuation of epidermal growth factor (EGF) signaling by growth hormone (GH) J. Endocrinol. 2017;233:175–186. doi: 10.1530/JOE-16-0606. [DOI] [PubMed] [Google Scholar]
- 66.Recouvreux MV, et al. Androgen receptor regulation of local growth hormone in prostate cancer cells. Endocrinology. 2017;158:2255–2268. doi: 10.1210/en.2016-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wagner K, Hemminki K, Försti A. The GH1/IGF-1 axis polymorphisms and their impact on breast cancer development. Breast Cancer Res. Treat. 2007;104:233–248. doi: 10.1007/s10549-006-9411-9. [DOI] [PubMed] [Google Scholar]
- 68.Le Marchand L, et al. Association of a common polymorphism in the human GH1 gene with colorectal neoplasia. J. Natl. Cancer Inst. 2002;94:454–460. doi: 10.1093/jnci/94.6.454. [DOI] [PubMed] [Google Scholar]
- 69.Shi J, Tong JH, Cai S. GH1 T1663A polymorphism and cancer risk: a meta-analysis of case–control studies. Tumor Biol. 2014;35:4529–4538. doi: 10.1007/s13277-013-1596-z. [DOI] [PubMed] [Google Scholar]
- 70.Chhabra Y, et al. A growth hormone receptor SNP promotes lung cancer by impairment of SOCS2-mediated degradation. Oncogene. 2018;37:489–501. doi: 10.1038/onc.2017.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Divisova J, et al. The growth hormone receptor antagonist pegvisomant blocks both mammary gland development and MCF-7 breast cancer xenograft growth. Breast Cancer Res. Treat. 2006;98:315–327. doi: 10.1007/s10549-006-9168-1. [DOI] [PubMed] [Google Scholar]
- 72.Dagnaes-Hansen F, Duan H, Rasmussen LM, Friend KE, Flyvbjerg A. Growth hormone receptor antagonist administration inhibits growth of human colorectal carcinoma in nude mice. Anticancer Res. 2004;24:3735–3742. [PubMed] [Google Scholar]
- 73.McCutcheon IE, et al. Antitumor activity of the growth hormone receptor antagonist pegvisomant against human meningiomas in nude mice. J. Neurosurg. 2001;94:487–492. doi: 10.3171/jns.2001.94.3.0487. [DOI] [PubMed] [Google Scholar]
- 74.Friend, K. E. Cancer and the potential place for growth hormone receptor antagonist therapy. Growth Horm. IGF Res. Suppl A, 121–123 (2001). [DOI] [PubMed]
- 75.Basu R, Baumgaertel N, Wu S, Kopchick JJ. Growth hormone receptor knockdown sensitizes human melanoma cells to chemotherapy by attenuating expression of ABC drug efflux pumps. Horm. Cancer. 2017;8:143–156. doi: 10.1007/s12672-017-0292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Evans A, Jamieson SMF, Liu DX, Wilson WR, Perry JK. Growth hormone receptor antagonism suppresses tumour regrowth after radiotherapy in an endometrial cancer xenograft model. Cancer Lett. 2016;379:117–123. doi: 10.1016/j.canlet.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 77.Minoia M, et al. Growth hormone receptor blockade inhibits growth hormone-induced chemoresistance by restoring cytotoxic-induced apoptosis in breast cancer cells independently of estrogen receptor expression. J. Clin. Endocrinol. Metab. 2012;97:E907–E916. doi: 10.1210/jc.2011-3340. [DOI] [PubMed] [Google Scholar]
- 78.Bougen NM, et al. Autocrine human GH promotes radioresistance in mammary and endometrial carcinoma cells. Endocr. Relat. Cancer. 2012;19:625–644. doi: 10.1530/ERC-12-0042. [DOI] [PubMed] [Google Scholar]
- 79.Harrington KJ, et al. Guidelines for preclinical and early phase clinical assessment of novel radiosensitisers. Br. J. Cancer. 2011;105:628–639. doi: 10.1038/bjc.2011.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lawrence YR, et al. NCI-RTOG translational program strategic guidelines for the early-stage development of radiosensitizers. J. Natl. Cancer Inst. 2013;105:11–24. doi: 10.1093/jnci/djs472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharma RA, et al. Clinical development of new drug-radiotherapy combinations. Nat. Rev. Clin. Oncol. 2016;13:627–642. doi: 10.1038/nrclinonc.2016.79. [DOI] [PubMed] [Google Scholar]
- 82.Hannon TS, Janosky J, Arslanian SA. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr. Res. 2006;60:759–763. doi: 10.1203/01.pdr.0000246097.73031.27. [DOI] [PubMed] [Google Scholar]
- 83.Nellemann B, et al. Growth hormone-induced insulin resistance in human subjects involves reduced pyruvate dehydrogenase activity. Acta Physiol. 2014;210:392–402. doi: 10.1111/apha.12183. [DOI] [PubMed] [Google Scholar]
- 84.Guevara-Aguirre J, et al. GH receptor deficiency in Ecuadorian adults is associated with obesity and enhanced insulin sensitivity. J. Clin. Endocrinol. Metab. 2015;100:2589–2596. doi: 10.1210/jc.2015-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boparai RK, Arum O, Khardori R, Bartke A. Glucose homeostasis and insulin sensitivity in growth hormone-transgenic mice: a cross-sectional analysis. Biol. Chem. 2010;391:1149–1155. doi: 10.1515/bc.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Strage EM, et al. Relationship among insulin resistance, growth hormone, and insulin-like growth factor I concentrations in diestrous Swedish Elkhounds. J. Vet. Intern. Med. 2014;28:419–428. doi: 10.1111/jvim.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee AP, Mulligan K, Schambelan M, Murphy EJ, Weiss EJ. Growth hormone receptor antagonism with pegvisomant in insulin resistant non-diabetic men: a phase II pilot study. F1000Research. 2017;6:614. doi: 10.12688/f1000research.11359.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harvey S, Martinez-Moreno CG. Growth hormone and ocular dysfunction: endocrine, paracrine or autocrine etiologies? Growth Horm. IGF Res. 2016;29:28–32. doi: 10.1016/j.ghir.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 89.Mukhi D, Nishad R, Menon RK, Pasupulati AK. Novel actions of growth hormone in podocytes: Implications for diabetic nephropathy. Front. Med. 2017;4:102. doi: 10.3389/fmed.2017.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kamenicky P, Mazziotti G, Lombes M, Giustina A, Chanson P. Growth hormone, insulin-like growth factor-1, and the kidney: pathophysiological and clinical implications. Endocr. Rev. 2014;35:234–281. doi: 10.1210/er.2013-1071. [DOI] [PubMed] [Google Scholar]
- 91.Holly JM, Amiel SA, Sandhu RR, Rees LH, Wass JA. The role of growth hormone in diabetes mellitus. J. Endocrinol. 1988;118:353–364. doi: 10.1677/joe.0.1180353. [DOI] [PubMed] [Google Scholar]
- 92.Pasupulati AK, Menon RK. Growth hormone and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2018;28:10–15. doi: 10.1097/MNH.0000000000000468. [DOI] [PubMed] [Google Scholar]
- 93.Bermea KC, Rodríguez-García A, Tsin A, Barrera-Saldaña HA. Somatolactogens and diabetic retinopathy. Growth Horm. IGF Res. 2018;41:42–47. doi: 10.1016/j.ghir.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 94.Harvey S, Parker E, Macdonald I, Sanders EJ. Growth hormone is present in the human retina and vitreous fluid. Neurosci. Lett. 2009;455:199–202. doi: 10.1016/j.neulet.2009.03.073. [DOI] [PubMed] [Google Scholar]
- 95.Rymaszewski Z, Cohen RM, Chomczynski P. Human growth hormone stimulates proliferation of human retinal microvascular endothelial cells in vitro. Proc. Natl Acad. Sci. USA. 1991;88:617–621. doi: 10.1073/pnas.88.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smith LE. Essential role of growth hormone in ischemia-induced retinal neovascularization. Science. 1997;276:1706–1709. doi: 10.1126/science.276.5319.1706. [DOI] [PubMed] [Google Scholar]
- 97.Zhang J, et al. Correlation of retinopathy with serum levels of growth hormones and insulin-like growth factor-1 in patients with diabetic retinopathy. Int. J. Clin. Exp. Med. 2017;10:1325–1329. [Google Scholar]
- 98.Wu TE, Chen HS. Increased prevalence of proliferative retinopathy in patients with acromegaly. J. Chin. Med. Assoc. 2018;81:230–235. doi: 10.1016/j.jcma.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 99.Group, G. H. A. for P. D. R. S.. The effect of a growth hormone receptor antagonist drug on proliferative diabetic retinopathy. Ophthalmology. 2001;108:2266–2272. doi: 10.1016/S0161-6420(01)00853-3. [DOI] [PubMed] [Google Scholar]
- 100.Chantelau E. Effect of a growth hormone receptor antagonist on proliferative diabetic retinopathy. Ophthalmology. 2002;109:2187–2188. doi: 10.1016/S0161-6420(02)01270-8. [DOI] [PubMed] [Google Scholar]
- 101.Blutke, A., Schneider, M. R., Wolf, E. & Wanke, R. Growth hormone (GH)-transgenic insulin-like growth factor 1 (IGF1)-deficient mice allow dissociation of excess GH and IGF1 effects on glomerular and tubular growth. Physiol. Rep. 4, 10.14814/phy2.12709 (2016). [DOI] [PMC free article] [PubMed]
- 102.Grunenwald S, Tack I, Chauveau D, Bennet A, Caron P. Impact of growth hormone hypersecretion on the adult human kidney. Ann. Endocrinol. 2011;72:485–495. doi: 10.1016/j.ando.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 103.Kumar PA, et al. Growth hormone (GH)-dependent expression of a natural antisense transcript induces zinc finger E-box-binding homeobox 2 (ZEB2) in the glomerular podocyte: a novel action of GH with implications for the pathogenesis of diabetic nephropathy. J. Biol. Chem. 2010;285:31148–31156. doi: 10.1074/jbc.M110.132332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Anisimov VN, Bartke A. The key role of growth hormone–insulin–IGF-1 signaling in aging and cancer. Crit. Rev. Oncol. Hematol. 2013;87:201–223. doi: 10.1016/j.critrevonc.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bartke, A. & Darcy, J. GH and ageing: Pitfalls and new insights. Best Pract. Res. Clin. Endocrinol. Metab.31, 113–125 (2017). [DOI] [PMC free article] [PubMed]
- 106.Junnila RK, List EO, Berryman DE, Murrey JW, Kopchick JJ. The GH/IGF-1 axis in ageing and longevity. Nat. Rev. Endocrinol. 2013;9:366–376. doi: 10.1038/nrendo.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Spadaro O, et al. Growth hormone receptor deficiency protects against age-related NLRP3 inflammasome activation and immune senescence. Cell Rep. 2016;14:1571–1580. doi: 10.1016/j.celrep.2016.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bartke A. Healthspan and longevity can be extended by suppression of growth hormone signaling. Mamm. Genome. 2016;27:289–299. doi: 10.1007/s00335-016-9621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Milman S, et al. Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. Aging Cell. 2014;13:769–771. doi: 10.1111/acel.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van der Spoel E, et al. Growth hormone secretion is diminished and tightly controlled in humans enriched for familial longevity. Aging Cell. 2016;15:1126–1131. doi: 10.1111/acel.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schally AV, Varga JL, Engel JB. Antagonists of growth-hormone-releasing hormone: an emerging new therapy for cancer. Nat. Clin. Pract. Endocrinol. Metab. 2008;4:33–43. doi: 10.1038/ncpendmet0677. [DOI] [PubMed] [Google Scholar]
- 112.Rai U, Thrimawithana TR, Valery C, Young SA. Therapeutic uses of somatostatin and its analogues: current view and potential applications. Pharmacol. Ther. 2015;152:98–110. doi: 10.1016/j.pharmthera.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 113.Zarandi M, et al. Synthesis and structure-activity studies on novel analogs of human growth hormone releasing hormone (GHRH) with enhanced inhibitory activities on tumor growth. Peptides. 2017;89:60–70. doi: 10.1016/j.peptides.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 114.Van Der Lely AJ, Kopchick JJ. Growth hormone receptor antagonists. Neuroendocrinology. 2006;83:264–268. doi: 10.1159/000095537. [DOI] [PubMed] [Google Scholar]
- 115.Pradhananga S, Wilkinson I, Ross RJMM. Pegvisomant: structure and function. J. Mol. Endocrinol. 2002;29:11–14. doi: 10.1677/jme.0.0290011. [DOI] [PubMed] [Google Scholar]
- 116.Chen WY, Wight DC, Wagner TE, Kopchick JJ. Expression of a mutated bovine growth hormone gene suppresses growth of transgenic mice. Proc. Natl Acad. Sci. 1990;87:5061–5065. doi: 10.1073/pnas.87.13.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kopchick JJ. History and future of growth hormone research. Horm. Res. 2003;60(Suppl 3):S103–S112. doi: 10.1159/000074510. [DOI] [PubMed] [Google Scholar]
- 118.Chen WY, Chen NY, Yun J, Wagner TE, Kopchick JJ. In vitro and in vivo studies of antagonistic effects of human growth hormone analogs. J. Biol. Chem. 1994;269:15892–15897. [PubMed] [Google Scholar]
- 119.Clark R, et al. Long-acting growth hormones produced by conjugation with polyethylene glycol. J. Biol. Chem. 1996;271:21969–21977. doi: 10.1074/jbc.271.36.21969. [DOI] [PubMed] [Google Scholar]
- 120.Sheppard MC. Primary medical therapy for acromegaly. Clin. Endocrinol. 2003;58:387–399. doi: 10.1046/j.1365-2265.2003.01734.x. [DOI] [PubMed] [Google Scholar]
- 121.Sherlock M, Woods C, Sheppard MC. Medical therapy in acromegaly. Nat. Rev. Endocrinol. 2011;7:291–300. doi: 10.1038/nrendo.2011.42. [DOI] [PubMed] [Google Scholar]
- 122.van der Lely AJ, et al. Long-term treatment of acromegaly with pegvisomant, a growth hormone receptor antagonist. Lancet. 2001;358:1754–1759. doi: 10.1016/S0140-6736(01)06844-1. [DOI] [PubMed] [Google Scholar]
- 123.Ramos-Levi AM, et al. Long-term treatment with pegvisomant for acromegaly: a 10-year experience. Clin. Endocrinol. 2016;84:540–550. doi: 10.1111/cen.12993. [DOI] [PubMed] [Google Scholar]
- 124.Feenstra J, et al. Combined therapy with somatostatin analogues and weekly pegvisomant in active acromegaly. Lancet. 2005;365:1644–1646. doi: 10.1016/S0140-6736(05)63011-5. [DOI] [PubMed] [Google Scholar]
- 125.Bianchi A, et al. Long-term treatment of somatostatin analog-refractory growth hormone-secreting pituitary tumors with pegvisomant alone or combined with long-acting somatostatin analogs: a retrospective analysis of clinical practice and outcomes. J. Exp. Clin. Cancer Res. 2013;32:40. doi: 10.1186/1756-9966-32-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cawley P, Wilkinson I, Ross RJ. Developing long‐acting growth hormone formulations. Clin. Endocrinol. 2013;79:305–309. doi: 10.1111/cen.12240. [DOI] [PubMed] [Google Scholar]
- 127.Wilkinson IR, et al. A ligand-receptor fusion of growth hormone forms a dimer and is a potent long-acting agonist. Nat. Med. 2007;13:1108–1113. doi: 10.1038/nm1610. [DOI] [PubMed] [Google Scholar]
- 128.Wilkinson IR, et al. A long-acting GH receptor antagonist through fusion to GH binding protein. Sci. Rep. 2016;6:35072. doi: 10.1038/srep35072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Crooke ST. Molecular mechanisms of antisense oligonucleotides. Nucleic Acid. Ther. 2017;27:70–77. doi: 10.1089/nat.2016.0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tachas G, et al. A GH receptor antisense oligonucleotide inhibits hepatic GH receptor expression, IGF-I production and body weight gain in normal mice. J. Endocrinol. 2006;189:147–154. doi: 10.1677/joe.1.06553. [DOI] [PubMed] [Google Scholar]
- 131.Wilkinson-Berka JL, et al. An antisense oligonucleotide targeting the growth hormone receptor inhibits neovascularization in a mouse model of retinopathy. Mol. Vis. 2007;13:1529–1538. [PubMed] [Google Scholar]
- 132.Trainer PJ, et al. A randomised, open-label, parallel group phase 2 study of antisense oligonucleotide therapy in acromegaly. Eur. J. Endocrinol. 2018;179:97–108. doi: 10.1530/EJE-18-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jiang J, et al. A conformationally sensitive growth hormone receptor antibody: impact on GH signaling and GHR proteolysis. Mol. Endocrinol. 2004;18:2981–2996. doi: 10.1210/me.2004-0102. [DOI] [PubMed] [Google Scholar]
- 134.Lan H, Zheng X, Khan MA, Li S. Anti-idiotypic antibody: a new strategy for the development of a growth hormone receptor antagonist. Int. J. Biochem. Cell. Biol. 2015;68:101–108. doi: 10.1016/j.biocel.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 135.Siebel, S. et al. Humanization and characterization of a human growth hormone receptor (GHR) antagonist antibody RN172. Endocr. Rev. 35 (2014). https://www.endocrine.org/meetings/endo-annual-meetings.
- 136.Sun F, Liu Y, Sun H, Tian B. Development and characterization of a novel GHR antibody antagonist, GF185. Int. J. Biol. Macromol. 2015;79:864–870. doi: 10.1016/j.ijbiomac.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 137.Yang N, et al. Activation of growth hormone receptors by growth hormone and growth hormone antagonist dimers: insights into receptor triggering. Mol. Endocrinol. 2008;22:978–988. doi: 10.1210/me.2007-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rosengren L, Parrow V, Chmielewska J, Mode A, Fhölenhag K. In vivo evaluation of a novel, orally bioavailable, small molecule growth hormone receptor antagonist. Growth Horm. IGF Res. 2007;17:47–53. doi: 10.1016/j.ghir.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 139.Rosengren L, et al. Antisense and sense RNA probe hybridization to immobilized crude cellular lysates: a tool to screen growth hormone antagonists. J. Biomol. Screen. 2005;10:260–269. doi: 10.1177/1087057104273802. [DOI] [PubMed] [Google Scholar]
- 140.Fruman DA, et al. The PI3K pathway in human disease. Cell. 2017;170:605–635. doi: 10.1016/j.cell.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schwartz DM, et al. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug. Discov. 2017;16:843–862. doi: 10.1038/nrd.2017.201. [DOI] [PubMed] [Google Scholar]
- 142.Schwartz DM, Bonelli M, Gadina M, O’Shea JJ. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat. Rev. Rheumatol. 2016;12:25–36. doi: 10.1038/nrrheum.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dankner M, Rose AAN, Rajkumar S, Siegel PM, Watson IR. Classifying BRAF alterations in cancer: new rational therapeutic strategies for actionable mutations. Oncogene. 2018;37:3183–3199. doi: 10.1038/s41388-018-0171-x. [DOI] [PubMed] [Google Scholar]
- 144.Caunt CJ, Sale MJ, Smith PD, Cook SJ. MEK1 and MEK2 inhibitors and cancer therapy: the long and winding road. Nat. Rev. Cancer. 2015;15:577–592. doi: 10.1038/nrc4000. [DOI] [PubMed] [Google Scholar]
- 145.Perry JK, Mohankumar KM, Emerald BS, Mertani HC, Lobie PE. The contribution of growth hormone to mammary neoplasia. J. Mammary Gland Biol. Neoplasia. 2008;13:131–145. doi: 10.1007/s10911-008-9070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mertani HC, et al. Autocrine human growth hormone (hGH) regulation of human mammary carcinoma cell gene expression. Identification of CHOP as a mediator of hGH-stimulated human mammary carcinoma cell survival. J. Biol. Chem. 2001;276:21464–21475. doi: 10.1074/jbc.M100437200. [DOI] [PubMed] [Google Scholar]
- 147.Fukuda I, Hizuka N, Muraoka T, Ichihara A. Adult growth hormone deficiency: current concepts. Neurol. Med. Chir. 2014;54(Suppl 3):599–605. doi: 10.2176/nmc.ra.2014-0088. [DOI] [PubMed] [Google Scholar]
- 148.Kargi AY, Merriam GR. Diagnosis and treatment of growth hormone deficiency in adults. Nat. Rev. 2013;9:335–345. doi: 10.1038/nrendo.2013.77. [DOI] [PubMed] [Google Scholar]
- 149.Reed ML, Merriam GR, Kargi AY. Adult growth hormone deficiency - benefits, side effects, and risks of growth hormone replacement. Front. Endocrinol. 2013;4:64. doi: 10.3389/fendo.2013.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Monson, J. P., Brooke, A. M. & Akker, S. in Endotext (eds. De Groot, L. J. et al.) (MDText.com, Inc., South Dartmouth (MA), 2000). https://www.ncbi.nlm.nih.gov/books/NBK278982/.
- 151.Thomas JD, et al. Characterisation of myocardial structure and function in adult-onset growth hormone deficiency using cardiac magnetic resonance. Endocrine. 2016;54:778–787. doi: 10.1007/s12020-016-1067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Campos VC, et al. Infectious diseases and immunological responses in adult subjects with lifetime untreated, congenital GH deficiency. Endocrine. 2016;54:182–190. doi: 10.1007/s12020-016-1061-z. [DOI] [PubMed] [Google Scholar]
- 153.Pereira-Gurgel VM, et al. Abnormal vascular and neural retinal morphology in congenital lifetime isolated growth hormone deficiency. Growth Horm. IGF Res. 2016;30–31:11–15. doi: 10.1016/j.ghir.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 154.Chikani V, Cuneo RC, Hickman I, Ho KK. Impairment of anaerobic capacity in adults with growth hormone deficiency. J. Clin. Endocrinol. Metab. 2015;100:1811–1818. doi: 10.1210/jc.2015-1006. [DOI] [PubMed] [Google Scholar]
- 155.Tanriverdi F, Karaca Z, Unluhizarci K, Kelestimur F. Unusual effects of GH deficiency in adults: a review about the effects of GH on skin, sleep, and coagulation. Endocrine. 2014;47:679–689. doi: 10.1007/s12020-014-0276-0. [DOI] [PubMed] [Google Scholar]
- 156.Ivanov AA, Khuri FR, Fu H. Targeting protein–protein interactions as an anticancer strategy. Trends Pharmacol. Sci. 2013;34:393–400. doi: 10.1016/j.tips.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Arkin MR, Tang Y, Wells JA. Small-molecule inhibitors of protein–protein interactions: progressing toward the reality. Chem. Biol. 2014;21:1102–1114. doi: 10.1016/j.chembiol.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Thanos CD, Randal M, Wells JA. Potent small-molecule binding to a dynamic hot spot on IL-2. J. Am. Chem. Soc. 2003;125:15280–15281. doi: 10.1021/ja0382617. [DOI] [PubMed] [Google Scholar]