Abstract
F-box only protein 22 (FBXO22), a substrate receptor of the SKP1-Cullin 1-F-box protein (SCF) E3 ubiquitin ligase that targets key regulators of cellular activities for ubiquitylation and degradation, plays important roles in the progression of human cancer. However, little is known about the role of FBXO22 in renal cell carcinoma (RCC). This study aims to explore the biological function of FBXO22 in RCC progression and its specific regulation mechanism. We performed immunohistochemistry analysis and found that the expression level of FBXO22 was significantly lower in RCC tissues than in normal renal tissues. Reduced FBXO22 expression in RCC tissues is related to tumor size and TNM stage and to worse overall and disease-free survival. Through an in vitro assay, we demonstrated that FBXO22 has no effect on renal cancer cells proliferation, whereas FBXO22 remarkably restricted RCC cell migration and invasion, thereby reversing EMT transition and elevating the activity of tissue inhibitor of matrix metalloproteinase-1, which subsequently inhibited metalloproteinase-9 (MMP-9) expression and activity in vitro. We also found that FBXO22 suppresses tube formation by disrupting the secretion of vascular endothelial growth factor. Meanwhile, in vivo studies verified that FBXO22 suppresses RCC metastasis. These findings suggested that FBXO22 is a novel prognostic indicator and plays an important role in RCC metastasis.
Keywords: FBXO22, proliferation, metastasis, angiogenesis, renal cell carcinoma
Introduction
Renal cell carcinoma (RCC) is one of the most common urological malignant tumors 1. Among all RCC histological subtypes, clear cell renal cell carcinoma (ccRCC) is the most common form, which metastasis occurs in the advanced stages and predicts very poor clinical outcome 2. The canonical molecular alteration in RCC is the von Hippel-Lindau tumor suppressor (VHL) gene 3. The inactivation of VHL increases activity of the hypoxia-induced factor (HIF), which is essential to angiogenesis, anaerobic metabolism, inflammation, and metastasis 4. In the past years, owing to resistance to chemotherapy and radiation therapy, cytokine therapy is ineffective in late-stage disease management, and molecular targeting drugs are limited to a short interval; these conditions result in poor overall survival 5-7. Thus, discovering novel biological markers that improve early diagnosis and patient therapy is essential.
The ubiquitin-proteasome system, is a common posttranslational regulation mechanism involved in many biological processes, including cell cycle progression, transcription, signal transduction, and tumorigenesis 8-11. It is carried out by a three-step cascade of ubiquitin-transfer reactions, namely, activation, conjugation, and ligation, which are performed by ubiquitin-activating enzymes (E1s), ubiquitin-conjugating enzymes (E2s), and ubiquitin ligases (E3s), respectively 12. The largest subfamily of E3s in mammalian is the Skp1-Cul1-F box protein ubiquitin ligases (SCFs), which is composed of four main subunits: SKP1, CUL1, RBX1 and one of many F-box proteins (FBPs) 13. The F-box proteins directly bind to substrates, using various domains, including Leucine rich repeats, WD40 motifs, and other domains, which are the basis for categorizing FBPs into subfamilies, such as FBXW, FBXL, and FBXO 14. Recent studies showed that FBPs exhibit tumor suppressive activities 15-18. FBXO22, which was first identified as a p53-targeting gene, is not yet well characterized, although it was reported to target KDM4A and p53 for degradation and thus regulates histone H3 methylation at lysines 9 and 36 and senescence 19, 20. Moreover, previous studies demonstrated that FBXO22 promotes hepatocellular carcinoma progression through targeting tumor suppressor krüppel-like factor 4 (KLF4) for degradation, while plays anti-metastatic roles in breast by suppressing EMT and promoting proteasomal degradation of SNAIL 21, 22. Nevertheless, the expression levels and functions of FBXO22 in RCC remain unknown.
In the current study, we aim to examine the expression levels of FBXO22 in RCC and explored the relationships of FBXO22 with clinicopathological characteristics by using immunostaining of RCC tissue microarrays. We found that FBXO22 is negatively related to the aggressive phenotype of RCC. Then, we demonstrated that FBXO22 suppresses the migration and invasion of RCC in vitro and in vivo by regulating EMT, MMP-2, and TIMP-2. We also demonstrated that the increasing rate of vascular endothelial growth factor (VEGF) secretion, which is induced by knocking down FBXO22, promotes RCC angiogenesis. These data provide new insights into the mechanisms of RCC tumorigenesis and support the potential value of FBXO22 as a novel prognostic marker for RCC treatment.
Materials and Methods
Patients and specimens
Retrospective RCC cohorts with tissue microarrays (TMAs) containing 277 RCC tissues and 35 normal renal tissues were constructed by a contract service at the National Engineering Centre for Biochip (Shanghai, China). The tissues, which were embedded in paraffin blocks, were collected from the Pathology Department of Affiliated Hospital of Xuzhou Medical University. All the patients got a definitive diagnosis of RCC and then underwent treatment of radical surgery at the above hospital. Detailed clinical information of each specimen was recorded accurately and completely, and all the RCC patients were termly followed up for 4-81 months for the evaluation of postoperative survival. All the tissue specimens were obtained for the present research with the informed consent of each patient, and the use of human specimens was approved by the Review Board of the Affiliated Hospital of Xuzhou Medical College.
TMA immunohistochemistry
TMA immunohistochemistry was implemented according to the streptavidin-peroxidase (Sp) method. A standard Sp Kit (Zhongshan biotech, Beijing, China) was used. Before immunostaining, TMA slides were dewaxed at 60 °C for 2 h, then deparaffinized with xylene and hydrated with graded ethanol and distilled water. Endogenous peroxidases were inhibited with 3% H2O2 for 30 min. Antigen retrieval was performed by heating the TMA slides immersed in a retrieval solution (10 mM sodium citrate buffer, pH 6.0) at 100 °C for 6 min in a pressure boiler. After 30 min blocking with 5% normal goat serum, the sections were incubated with polyclonal rabbit anti-FBXO22 antibody (1:100 dilution, Proteintech) overnight at 4 °C. The slides were then with a biotin-labeled secondary antibody (1:500; ZB-2050; Beijing Zhongshan Golden Bridge Biotechnology, Beijing, China) for1 h at room temperature and then with avidin-peroxidase reagent and 3, 3′-diaminobenzidine (DAB; Zhongshan biotech, Beijing, China) substrate. After hematoxylin counterstain and dehydration, the sections were sealed with cover slips.
Evaluation of immunostaining
The positive immunostaining of FBXO22 was predominantly located in the nucleus and partially in the cytoplasm. Two pathologists separately examined the TMAs under blinded experimental conditions. The staining scores of FBXO22 were evaluated according to the intensity and percentage of cells with positive staining. The staining intensity of FBXO22 was scored 0, 1, 2, or 3 (0, negative; 1, weak; 2, moderate; 3, strong); the percentage of the FBXO22‑positive stained cells was graded as 1 (0%-25%), 2 (26%-50%), 3 (51%-75%), and 4 (76%-100%). The immunoreactive score (IRS) of each section was calculated by multiplying the scores of staining intensity and the percentage of positive cells. On the basis of the IRS, staining patterns were divided into two classes: low (IRS: 0-6) and high (IRS: 8-12) expression.
Cell lines and cell culture
Human RCC cell lines (ACHN, 786‑O) and human umbilical vein endothelial cells (HUVECs) were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). The ACHN cells were cultured in an MEM medium supplemented with 10% fetal bovine serum. The 786‑O cell lines were cultured in an RPMI 1640 medium supplemented with 10% fetal bovine serum. The HUVECs were cultured in an ECM medium supplemented with 10% fetal bovine serum. Then, 100 U/mL streptomycin/penicillin was added to the MEM, RPMI 1640, and ECM medium. All the cells were cultured in an incubator at 37 °C with 5% CO2.
FBXO22 siRNA transfection and viral transduction
Small interfering RNA (siRNA) specific for FBXO2 (siFBXO22) and non-specific control (siCtrl) were purchased from Gene-Pharma (Shanghai, China) and transfected with siLentFect lipid reagent (Bio-Rad Laboratories, Inc.) according to the manufacturer's protocol when the RCC cells grew to 30%-50% confluency. About 6 h after transfection, the medium containing transfection reagents was replaced with a fresh medium. The FBXO22 knockdown 786-O cell lines and control 786-O cell lines were established by transfection with lentivirus packing FBXO22 shRNA expression and control vector, respectively (Shanghai GenePharma). The target cells were transfected with lentivirus for 48 h and then selected with 5 µg/ml puromycin (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 2 weeks. The siRNA sequences are as follows: siCtrl: sense, 5'-UUCUCCGAACGUGUCACGUTT-3'; antisense, 5' -ACGUGACACGUUCGGAGATT-3'. siFBXO22: sense, 5'-GGUGGGAGCCAGUAAUUAUTT-3'; antisense, 5'-AUAAUUACUGGCUCCCACCTT-3'.
Western blotting analysis
The proteins were separated on a 10% SDS-PAGE and transferred to a nitrocellulose membrane, which was blocked with 5% skim milk for 2 h at room temperature and then incubated overnight at 4 °C with primary antibody: anti-FBXO22 (1:2000 for WB, Proteintech); anti-N-Cadherin(1:2000 for WB, Proteintech); anti-MMP-9(1:200 for WB, Cell Signaling Technology); anti-TIMP-1(1:200 for WB, Santa Cruz); anti-VEGF (1:1000 for WB, Proteintech) and anti-GAPDH (1:5000 for WB, Santa Cruz). The membrane was washed and then incubated with corresponding HRP secondary antibodies (goat anti-rabbit and goat anti-mouse, IgG) for 2 h at room temperature. Finally, the protein signals were detected semi quantitatively with TanonTM High-sig ECL Western blotting substrate (Tanon, Shanghai, China).
Cell proliferation assay
Cell proliferation was assessed with a cell counting kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Shanghai, China). The transfected 786-O and ACHN cells (4 × 103) were seeded into 96-well plates. Exactly 10 μl of CCK-8 solution was then added to each well containing 100 μl of complete medium at indicated time points. The cells were incubated for 1 h at 37 °C. Absorbance was measured at 450 nm with an Epoch 2 microplate spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA). All the assays were repeated three times.
Cell migration and invasion assay
Migration and invasion assays were performed on the transwell chambers in the absence (migration) and presence (invasion) of growth factor-reduced matrigel (BD Biosciences, Mississauga, Canada). Then, 8 ×104 and 4 × 104 cells in FBS-free medium were plated in the top chamber for the invasion and migration assays, respectively. The cells were incubated at 37 °C with 5% CO2 for 24 and 12 h successively, and then the cells that traversed the membrane were fixed in 90% methanol and stained with crystal violet. Meanwhile, the cells in the upper chamber were carefully removed with a cotton swab. The cells that traversed the upper chamber were dried and then counted.
Tube formation assay
The transfected 786-O and ACHN cells were cultured with fresh complete medium for 24 h, and the supernatants were collected and used as conditioned media. A 48-well plate was coated with 100 µL of matrigel and maintained at 37 °C for solidification. A total of 40,000 HUVECs were suspended in 100 mL of conditioned medium and cultured at 37 °C for 4 h. Subsequently, the number of capillary-like tubes was visualized in 5 random fields with a microscope (Olympus, DP80) at ×200 magnification.
Gelatin zymography
The proteins in the conditioned medium were harvested. The protein samples were mixed with two SDS-PAGE nonreducing buffers (1:1 ratio) and then placed on 10% SDS-PAGE containing 0.1% gelatin (Sigma, Shanghai, China). After electrophoresis, the gel was incubated in Triton X-100 exchange buffer (20 mM Tris-HCl, pH 8.0), 150 mM NaCl, 5 mM CaCl, and 2.5% Triton X-100) for 30 min, then washed with an incubation buffer (same buffer without Triton X-100) for 10 min 3 times. The gel was incubated in incubation buffer overnight at 37 °C. After incubation, the gel was stained in 0.5% Coomassie Blue R-250 (Sigma, Shanghai, China) for 1 h, then distained by 30% methanol and 10% glacial acetic acid for 1 h. The gel was photographed and quantitatively measured by scanning densitometry.
ELISA for VEGF
The transfected 786-O and ACHN cells were cultured with fresh complete medium for 24 h, and the supernatants were collected and used as conditioned media. VEGF concentration was measured with ELISA kits (R&D Systems, MN, USA) according to the manufacturer's instructions.
Tail vein assay of metastasis in vivo
For the experimental metastasis, the BALB/c nude mice were randomly divided into two groups consisting of 6 mice each. The mice were injected intravenously with 3x106 luciferase lentivirus-infected stable FBXO22-knockdown and control 786-O cells in 0.2 mL of PBS through tail vein. After 6 weeks, we injected the two groups of mice with pentobarbital sodium to induce anesthesia. Subsequently, bioluminescence imaging protocol was used according to the manufacturer's protocol (Night OWL II LB983; Berthold Technologies, Bad Wildbad, Germany). Finally, the survival times of the nude mice were monitored.
Statistical analysis
All statistical analyses were determined with the SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). The χ2 or Fisher's exact test was applied for the analysis of the relationship between FBXO22 expression and the clinicopathological parameters of RCC patients. Survival analysis was investigated through the Kaplan-Meier method and the log-rank test. Statistical significance of Student's t-test was used for two-group comparisons. A P value of < 0.05 was considered significant.
Results
FBXO22 expression is down-regulated in RCC tissues
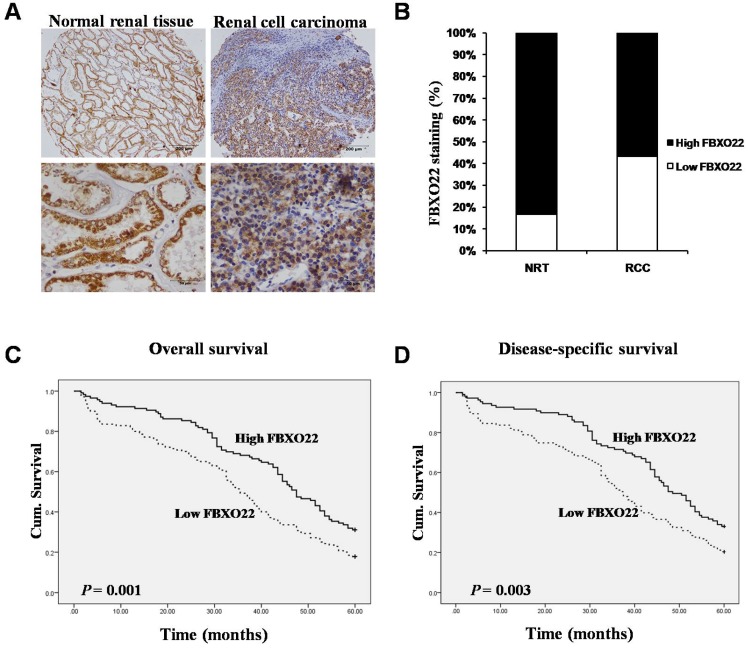
To evaluate whether the FBXO22 protein expression is related to RCC tissues, we carried out immunostaining analysis on 35 normal renal tissues and 277 RCC tissues. By removing the samples that we through antigen retrieval, we found that FBXO22 is predominantly located in the cytoplasm (Figure 1A). Tissue specimens were classified as low (IRS: 0-6) and high (IRS: 8-12) FBXO22 expression through the quantitative analysis of the protein staining results. The high expression level of FBXO22 was observed in 30 (85.7%) normal renal tissues and 157 (56.7%) RCC tissues. Our results showed that FBXO22 expression level was significantly lower in the tumors than in the normal renal tissues (Figure 1B; P < 0.001).
Figure 1.
Expression of FBXO22 was down-regulated in RCC tissues and positive associated with overall and disease-free survival of RCC patients. (A) IHC assay of FBXO22 protein expression in normal Renal tissues and RCC tissues. Top panel, magnification, x100; Bottom panel, magnification, x200. (B) Quantification of the relative protein level of FBXO22 in normal Renal tissues and RCC tissues, the overall expression level of FBXO22 in the RCC tissues was significantly lower (P<0.01, χ2test). (C) High FBXO22 expression related to greater favorable overall cumulative survival of RCC patients (P = 0.001, log-rank test). (D) High FBXO22 expression related to greater favorable disease-free cumulative survival of RCC patients (P=0.003, log-rank test).
Low expression of FBXO22 is positively correlated with clinicopathological characteristics
We evaluated the correlation between FBXO22 expression and the clinicopathological parameters of RCC with Fisher's exact test. Statistical results suggested that loss of FBXO22 is strongly correlated with some clinicopathological parameters of RCC (Table 1), including tumor size (P = 0.047) and TNM stage (P = 0.020). No compelling correlations were found between FBXO22 expression and patient's age or gender.
Table 1.
FBXO22 staining and clinicopathological characteristics of 277 renal cancer patients.
|
Variables |
FBXO22 staining | |||
|---|---|---|---|---|
| Low (%) | High (%) | Total | P * | |
|
All cases
Age |
148 (53.4) | 129 (46. 6) | 277 | |
| ≤56 years | 65 (50.8) | 63 (49.2) | 128 | 0.413 |
| >56 years | 83 (55.7) | 66 (44.3) | 149 | |
| Gender | ||||
| Male | 98 (54.1) | 83 (45.9) | 181 | 0.744 |
| Female | 50 (52.1) | 46 (47.9) | 96 | |
| Tumor size | ||||
| ≤7 cm | 108 (50.2) | 107 (49.8) | 215 | 0.047 |
| >7 cm | 40 (64.5) | 22 (35.5) | 62 | |
| pT status | ||||
| pT1 | 89 (48.9) | 93 (51.1) | 182 | 0.036 |
| pT2-T4 | 59 (62.1) | 36 (37.9) | 95 | |
| pN status | ||||
| pN0 | 131 (52.0) | 121 (48.0) | 252 | 0.023 |
| pN1-N3 | 15 (78.9) | 4 (21.1) | 19 | |
| TNM stage | ||||
| I | 77 (47.5) | 85 (52.5) | 162 | 0.020 |
| II-IV | 53 (63.1) | 31 (36.9) | 84 | |
* Two sided Fisher's exact tests.
Some cases were not available for the information.
FBXO22 expression is positive correlated with the prognosis of RCC patients
To further understand the impact of FBXO22 on clinical outcome of RCC patients, we used the Fisher's exact test to examine the relationship of FBXO22 expression with the overall and disease-free patient survival of the RCC patients. Our data suggested that high FBXO22 expression in RCC patients is correlated with favorable 5-year overall and disease-specific survival in contrast to low FBXO22 expression (Figure 1C-D).
Silencing of FBXO22 exerts no effect on RCC proliferation in vitro
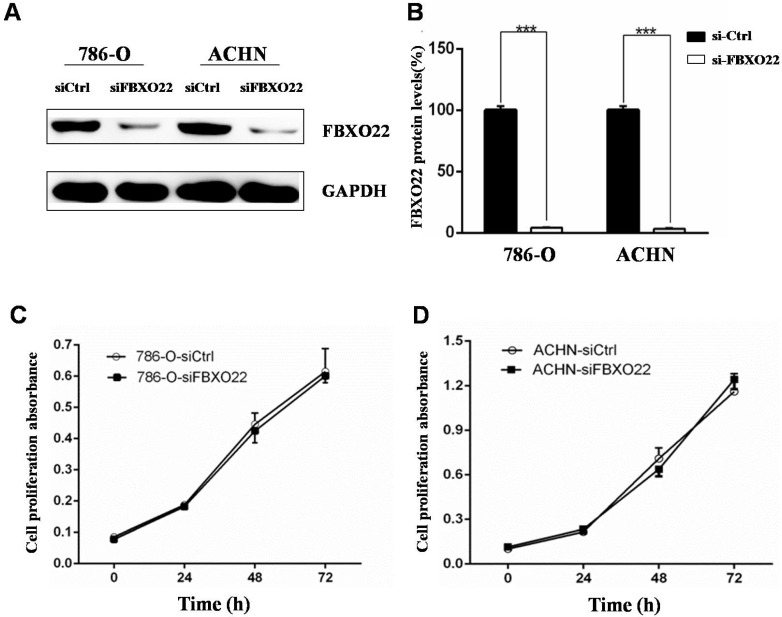
The above results showed that low FBXO22 expression is associated with poor progression of RCC. In this regard, we investigated the biological function of FBXO22 in RCC. Given that the expression of FBXO22 is associated with tumor size, we first examined the effect of FBXO22 on RCC cell proliferation in vitro. The 786‑O and ACHN cells were transfected with siCtrl and siRNAs targeting FBXO22, respectively (Figure 2A-B). Then, we performed CCK-8 cell proliferation assays to validate the effect of FBXO22 on the proliferation of RCC cells. The results revealed that FBXO22 has no effect on cell progression (Figure 2C-D).
Figure 2.
Silencing of FBXO22 exerts no effect on clear RCC cell proliferation in vitro. (A, B) Knockdown of FBXO22 was confirmed at the protein level in 786‑O and ACHN cells by western blotting. (C, D) Knockdown of FBXO22 exerts no effect on RCC cell proliferation, as shown by CCK-8 assays. Data are presented as the mean ± SD for three independent experiments, ***, P < 0.001.
Silencing of FBXO22 promotes RCC cells migration and invasion in vitro
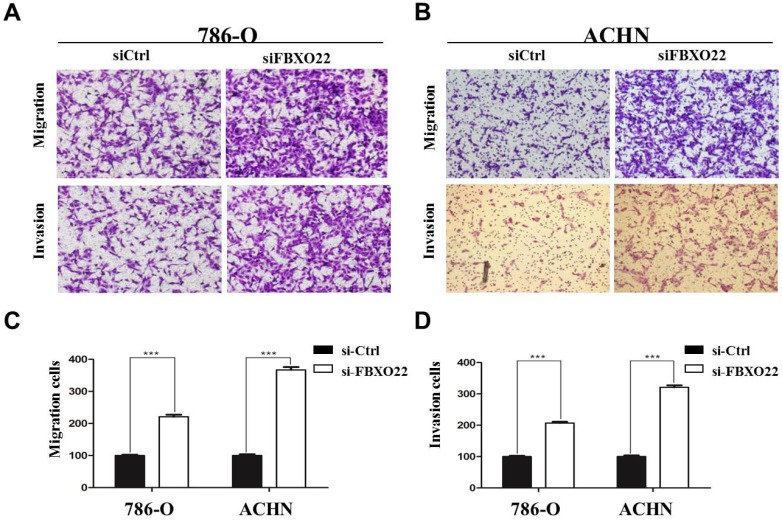
Given that the correlation between low FBXO22 expression and cancer metastasis is significant and migration and invasion ability is crucial to tumor metastasis 28, we performed Transwell assays to investigate the effect of FBXO22 on RCC cells migration and invasion. Our data revealed that knocking down FBXO22 promotes cell migration and invasion in 786‑O cells compared with the corresponding controls (Figure 3A). In the ACHN cell, the results were consistent with those in the 786-O cell (Figure 3B). Cell migration and invasion number per field were counted in five random fields in the ACHN and 786‑O cells (Figure 3C-D).
Figure 3.
FBXO22 inhibited the migration and invasion of RCC cells. (A) Knockdown FBXO22 significantly promoted the migration and invasion of 786-O cell. (B) Knockdown FBXO22 significantly promoted the migration and invasion of ACHN cell. (C, D) The cell migration and invasion number per field was counted in three random fields in ACHN and 786‑O cells. Data are presented as the mean ± SD for three independent experiments, ***, P < 0.001.
Depletion of FBXO22 enhanced angiogenesis in vitro
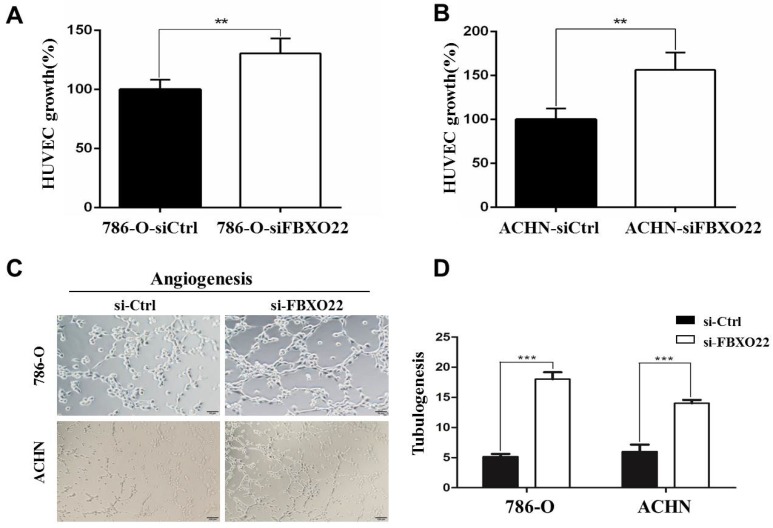
VHL alteration is accountable for ~90% of all RCC 23, and VHL inactivation increases HIF activity. The subsequent activation of hypoxic gene downstream has been thought to play an important role in RCC development 24. The well-described HIF target gene VEGF, which is predominantly expressed in endothelial cells, is involved in angiogenesis regulation. Consequently, we investingated whether FBXO22 affects angiogenesis in vitro. The angiogenic potentials of the supernatant of the 786-O and ACHN cells transfected with siCtrl or siRNAs targeting FBXO22 were determined by endothelial cell proliferation and tube formation assays. In the cell proliferation assay, we found that the conditioned medium from the 786-O and ACHN cells transfected with siCtrl inhibits the proliferation of endothelial cells in contrast to those transfected with siRNAs (Figure 4A-B). In the tube formation assay, the average number of complete tubular structures formed by the HUVECs was significantly decreased in the conditioned medium from knocked down FBXO22 in contrast to that in the controls (Figure 4C-D).
Figure 4.
Depletion of FBXO22 enhanced Angiogenesis in vitro. (A, B) Knockdown of FBXO22 promote HUVECs proliferation, as shown by CCK-8 assays. (C, D) Representative pictures were taken in situ for tube formation in the supernatant of 786-O and ACHN cells transfected with control siRNA (siCtrl) or small interfering RNAs (siRNAs) targeting FBXO22. Data are presented as the mean ± SD for three independent experiments, **P<0.01,***, P < 0.001.
FBXO22 inhibits RCC cells progression by regulating EMT, MMP-9, and VEGF
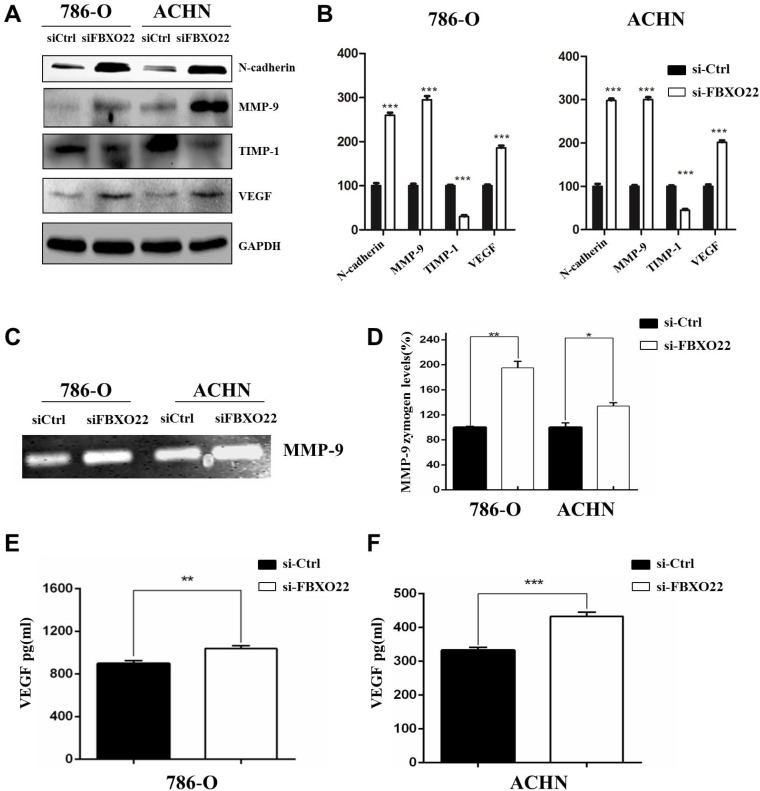
EMT is essential to tumor metastasis, so we evaluated the function of FBXO22 on the expression of EMT markers, such as N-cadherin. Our results indicated that silencing FBXO22 significantly increases N-cadherin expression (Figure 5A-B). MMP-9 is an important matrix metalloproteinase that mediates Epithelial-Mesenchymal Transition and cell metastasis, and TIMP1 is the specific tissue inhibitor of MMP-9. Our results indicated that MMP-9 expression increased and TIMP1 levels were reduced in 786-O and ACHN cells after the silencing of FBXO22 (Figure 5A-B). Additionally, our data revealed that knockdown FBXO22 significantly increases VEGF expression (Figure 5A-B). We carried out gelatin zymography analysis and ELISA assay to confirm the regulatory role of FBXO22 in MMP-9 (Figure 5C-D) and VEGF (Figure 5E-F) expression.
Figure 5.
Target genes regulated by FBXO22. (A, B) Western blot analysis was used to detect the relative protein levels of N-cadherin, MMP‑9, TIMP1 and VEGFA in FBXO22 low expression group and control group for both 786-O and ACHN cell lines. GAPDH was used as a loading control. (C, D) The activity of MMP-9 were evaluated by Gelatin zymography. (E, F) ELISA for the secretion of VEGF in 786-O and ACHN cells Transduced with control siRNA (siCtrl) or small interfering RNAs (siRNAs) targeting FBXO22. Data are presented as the mean ± SD for three independent experiments, *P < 0.05, **P<0.01,***, P < 0.001.
Knockdown of FBXO22 promotes RCC cell lung metastasis in vivo
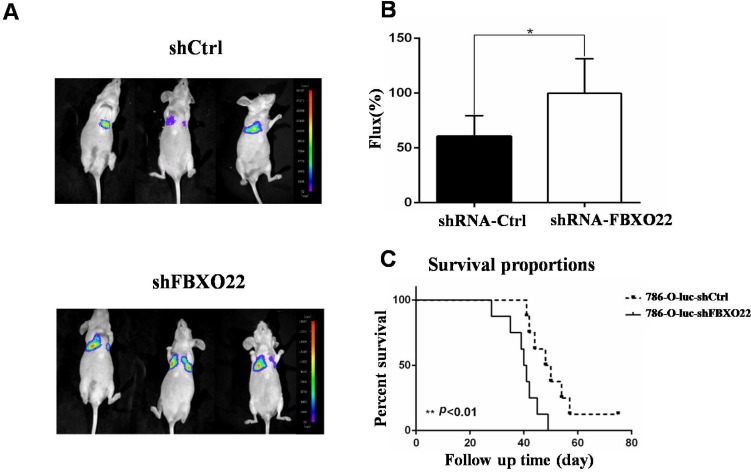
To further validate whether FBXO22 has anticancer effects in vivo, we knocked down FBXO22 in 786-O RCCs, which were stably marked with luciferase. Then, we utilized LLC-luc-shCtrl and LLC-luc-shFBXO22 to construct transferring nude mouse models to study the role of FBXO22 in tumor metastasis (Figure 6A). After 6 weeks, bioluminescence imaging was used in the measurement of metastatic lesions. According to bioluminescence imaging results, the nude mice bearing shFBXO22 are more prone to metastasize than the mice bearing shCtrl cells (Figure 6B). The FBXO22‑silenced group demonstrated lower survival rates than the control group (Figure 6C).
Figure 6.
FBXO22 deprivation promoted RCC cell lung metastasis in vivo. (A) Stably carrying LLC-luc-shGFP and LLC-luc-shFBXO22 cells were injected into nude mice through tail vein. Bioluminescence images were shown. (B) The relative Firefly luciferase was shown in column diagram. Compared with the shCtrl group, the Firefly luciferase activity was increased following FBXO22 knockdown. (C) Statistical analysis of survival, the nude mice survival time was significantly shortened compared with the shCtrl group (n = 10 in each group). Data are presented as the mean ± SD, *P<0.05, **P<0.01.
Discussion
F-box proteins are the substrate receptors of SCF E3 ubiquitin ligase, which plays important roles in recognizing and recruiting substrate proteins for polyubiquitylation 13. A total of 69 FBPs are categorized within three families according to their protein interaction domains: WD40 domains (10 FBXW proteins), leucine-rich repeats (21 FBXL proteins) and FBXO (F-box only), which is more or less a “catch-all” group with several members containing various domains 25. Many studies showed that FBPs are closely associated with human cancers and regulate the expression levels and activities of oncogenes and tumor suppressor genes, such as SKP2, which is the most studied F-box oncoprotein. Meanwhile, FBXW7 (also known as CDC4 and FBW7) is a well-established tumor suppressor gene 26. However, the majority of FBPs are not completely characterized. FBXO22 is a member of the F-box protein family, which has been proved promote hepatocellular carcinoma progression and play anti-metastatic roles in breast cancer 21, 22. However, the effects of FBXO22 on RCC have never been reported.
In this study, we presented several unexpected findings concerning the important roles of FBXO22 in RCC. We first investigated FBXO22 expression in RCC tissues. The protein level of FBXO22 was low in the RCC tissues relative to that in normal renal tissues. This finding indicated that FBXO22 has a vital role in the progression of RCC. We further evaluated the correlations between FBXO22 expression and clinicopathological parameters. We discovered that FBXO22 is negative associated with tumor size and TNM stage. Moreover, RCC patients with high FBXO22 expression levels had a favorable overall and disease-free survival. These results indicated that FBXO22 is a prognostic factor for RCC patients and a tumor suppressor gene.
The programs involved in cancer progression include proliferation, angiogenesis, and metastasis 27. Previous studies reported that FBXO22 participates in the regulation breast cancer cell proliferation and metastasis. Our present data suggested that the abilities of RCC cells for migration and invasion are dramatically enhanced after knocking down FBXO22. This enhancement is consistent with the function of FBXO22 in vivo. Additionally, the knockdown of FBXO22 distinctly enhances tube formation in HMVECs.
Our study is the first to show that the abilities of RCC cells for migration, invasion, and angiogenesis are dramatically enhanced after the knocking down of FBXO22 through in vitro and in vivo experiments. However, the molecular mechanism involved in the regulation of migration, invasiveness, and angiogenesis by FBXO22 remains unclear. Substantial evidence demonstrated that EMT plays a critical role in cancer invasion and metastasis, primary events in an EMT are down-regulation of E-cadherin expression and up-regulation of N-cadherin expression 28. In the present study, the knockdown of FBXO22 markedly increased N-cadherin levels. Thus, the inhibitory effects on RCC cell migration and invasion are associated with reduced N-cadherin expression. This discovery is consistent with a previous report showing that the FBXO22 suppresses the migration and invasion of breast cancer cells after by inhibiting EMT expression.
Matrix metalloproteinase (MMP) is a family of neutral proteinases that degrade the components of the basement membrane and extracellular matrix, thereby allowing cancer cells to invade and migrate 29. MMP-9 is one of the MMP family members that degrade collagen, a major component of the basement membrane, and are strongly associated with tumor invasion and metastasis 27. MMP-9 is a negative prognostic factor in RCC 30, 31. However, the relationship between FBXO22 and MMP-9 in RCC is currently unknown. TIMP1 is the specific tissue inhibitor of MMP-9. Our present study demonstrated that after knockdown of FBXO22, MMP-9 expression increased along with the reduction of TIMP1 expression, suggesting that the FBXO22 gene, through TIMP1, induces MMP-9 to affect the abilities of RCC cells for migration and invasion. Tumor growth requires the induction and maintenance of a blood supply, which is affected by the activities of angiogenic factors, such as VEGF 32. Given the prominent role of VEGF in tumor angiogenesis, we detected the effect of knockdown FBXO22 expression on VEGF activity. Our data showed that VEGF secretion increases by silencing FBXO22 in RCC cells.
In summary, this study is the first to report that FBXO22 inhibits RCC migration, invasion, and angiogenesis through the following mechanisms: (1) Reversal the endogenous EMT, (2) suppression MMP-9 expression through the inactivation of TIMP1, and (3) inhibition of VEGF expression. These findings indicated that FBXO22 is an effective target for the targeted therapy of RCC patients and plays an important role in treatment against RCC metastasis. Future study will explore the underlying mechanism involved in the FBXO22-mediated regulation of EMT, TIMP1, and VEGF expression.
Acknowledgments
This study was funded by grants from the National Natural Science Foundation of China (No. 81672845, 81872304), the Project of Invigorating Health Care through Science, Technology and Education from Jiangsu Province (ZDRCC2016009), the Shanghai Municipal Commission of Health and Family Planning (No. 20164Y0236), the Medical-Engineering Joint Funds of Shanghai Jiao Tong University (No.YG2016QN50) and the Science and Technology Commission of Shanghai Municipality (No. 17ZR1416700), the Cultivating Fund of shanghai Renji Hospital (PYIII-17-016).
Authorship
Junqing Han, Jin Bai, You Wang designed the experiments; Feng Guo, Jinjin Liu, Xiao Han performed the experiments; Xuping Zhang and Tian Lin drafted the manuscript.
Abbreviations
- RCC
renal cell carcinoma
- ccRCC
clear cell renal cell carcinoma
- TMA
tissue microarray
- CCK-8
cell counting kit-8
- VHL
the von Hippel-Lindau tumor suppressor
- EMT
epithelial-mesenchymal transition
- MMP-9
metalloproteinase-9
- TIMP-1
matrix metalloproteinase-1
- VEGF
vascular endothelial growth factor.
References
- 1.Quan J, Pan X, He T, Lin C, Lai Y, Chen P. et al. Tumor suppressor miR-211-5p is associated with cellular migration, proliferation and apoptosis in renal cell carcinoma. Exp Ther Med. 2018;15:4019–28. doi: 10.3892/etm.2018.5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas JS, Kabbinavar F. Metastatic clear cell renal cell carcinoma: A review of current therapies and novel immunotherapies. Critical Reviews in Oncology Hematology. 2015;96:527–33. doi: 10.1016/j.critrevonc.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Young AC, Craven RA, Cohen D, Taylor C, Booth C, Harnden P. et al. Analysis of VHL Gene Alterations and their Relationship to Clinical Parameters in Sporadic Conventional Renal Cell Carcinoma. Clinical Cancer Research. 2009;15:7582–92. doi: 10.1158/1078-0432.CCR-09-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lendahl U, Lee KL, Yang H, Poellinger L. Generating specificity and diversity in the transcriptional response to hypoxia. Nat Rev Genet. 2009;10:821–32. doi: 10.1038/nrg2665. [DOI] [PubMed] [Google Scholar]
- 5.Genetics of Kidney Cancer (Renal Cell Cancer) (PDQ(R)) Health Professional Version. PDQ Cancer Information Summaries. Bethesda (MD); 2002. [Google Scholar]
- 6.Weinstock M, McDermott D. Targeting PD-1/PD-L1 in the treatment of metastatic renal cell carcinoma. Ther Adv Urol. 2015;7:365–77. doi: 10.1177/1756287215597647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randall JM, Millard F, Kurzrock R. Molecular aberrations, targeted therapy, and renal cell carcinoma: current state-of-the-art. Cancer Metastasis Rev. 2014;33:1109–24. doi: 10.1007/s10555-014-9533-1. [DOI] [PubMed] [Google Scholar]
- 8.Lipford JR, Deshaies RJ. Diverse roles for ubiquitin-dependent proteolysis in transcriptional activation. Nat Cell Biol. 2003;5:845–50. doi: 10.1038/ncb1003-845. [DOI] [PubMed] [Google Scholar]
- 9.O'Connell BC, Harper JW. Ubiquitin proteasome system (UPS): what can chromatin do for you? Curr Opin Cell Biol. 2007;19:206–14. doi: 10.1016/j.ceb.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Liu P, Inuzuka H, Wei W. Roles of F-box proteins in cancer. Nat Rev Cancer. 2014;14:233–47. doi: 10.1038/nrc3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vucic D, Dixit VM, Wertz IE. Ubiquitylation in apoptosis: a post-translational modification at the edge of life and death. Nat Rev Mol Cell Biol. 2011;12:439–52. doi: 10.1038/nrm3143. [DOI] [PubMed] [Google Scholar]
- 12.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–79. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 13.Heo J, Eki R, Abbas T. Deregulation of F-box proteins and its consequence on cancer development, progression and metastasis. Seminars in cancer biology. 2016;36:33–51. doi: 10.1016/j.semcancer.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin J, Cardozo T, Lovering RC, Elledge SJ, Pagano M, Harper JW. Systematic analysis and nomenclature of mammalian F-box proteins. Genes Dev. 2004;18:2573–80. doi: 10.1101/gad.1255304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Signoretti S, Di Marcotullio L, Richardson A, Ramaswamy S, Isaac B, Rue M. et al. Oncogenic role of the ubiquitin ligase subunit Skp2 in human breast cancer. J Clin Invest. 2002;110:633–41. doi: 10.1172/JCI15795. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Ougolkov A, Zhang B, Yamashita K, Bilim V, Mai M, Fuchs SY. et al. Associations among beta-TrCP, an E3 ubiquitin ligase receptor, beta-catenin, and NF-kappaB in colorectal cancer. Journal of the National Cancer Institute. 2004;96:1161–70. doi: 10.1093/jnci/djh219. [DOI] [PubMed] [Google Scholar]
- 17.Kwon YW, Kim IJ, Wu D, Lu J, Stock WA Jr, Liu Y. et al. Pten regulates Aurora-A and cooperates with Fbxw7 in modulating radiation-induced tumor development. Mol Cancer Res. 2012;10:834–44. doi: 10.1158/1541-7786.MCR-12-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lian Z, Lee EK, Bass AJ, Wong KK, Klein-Szanto AJ, Rustgi AK. et al. FBXO4 loss facilitates carcinogen induced papilloma development in mice. Cancer Biol Ther. 2015;16:750–5. doi: 10.1080/15384047.2015.1026512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan MK, Lim HJ, Harper JW. SCF(FBXO22) regulates histone H3 lysine 9 and 36 methylation levels by targeting histone demethylase KDM4A for ubiquitin-mediated proteasomal degradation. Molecular and cellular biology. 2011;31:3687–99. doi: 10.1128/MCB.05746-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johmura Y, Sun J, Kitagawa K, Nakanishi K, Kuno T, Naiki-Ito A. et al. SCF(Fbxo22)-KDM4A targets methylated p53 for degradation and regulates senescence. Nat Commun. 2016;7:10574. doi: 10.1038/ncomms10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun R, Xie HY, Qian JX, Huang YN, Yang F, Zhang FL. et al. FBXO22 Possesses Both Protumorigenic and Antimetastatic Roles in Breast Cancer Progression. Cancer Res. 2018;78:5274–86. doi: 10.1158/0008-5472.CAN-17-3647. [DOI] [PubMed] [Google Scholar]
- 22.Tian X, Dai S, Sun J, Jin G, Jiang S, Meng F. et al. F-box protein FBXO22 mediates polyubiquitination and degradation of KLF4 to promote hepatocellular carcinoma progression. Oncotarget. 2015;6:22767–75. doi: 10.18632/oncotarget.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young AC, Craven RA, Cohen D, Taylor C, Booth C, Harnden P. et al. Analysis of VHL Gene Alterations and their Relationship to Clinical Parameters in Sporadic Conventional Renal Cell Carcinoma. Clin Cancer Res. 2009;15:7582–92. doi: 10.1158/1078-0432.CCR-09-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du W, Zhang L, Brett-Morris A, Aguila B, Kerner J, Hoppel CL. et al. HIF drives lipid deposition and cancer in ccRCC via repression of fatty acid metabolism. Nat Commun. 2017;8:1769. doi: 10.1038/s41467-017-01965-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Mallampalli RK. Small molecule therapeutics targeting F-box proteins in cancer. Semin Cancer Biol. 2016;36:105–19. doi: 10.1016/j.semcancer.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakayama KI, Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nature Reviews Cancer. 2006;6:369–81. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]
- 27.Sun GG, Wei CD, Jing SW, Hu WN. Interactions between filamin A and MMP-9 regulate proliferation and invasion in renal cell carcinoma. Asian Pacific journal of cancer prevention: APJCP. 2014;15:3789–95. doi: 10.7314/apjcp.2014.15.8.3789. [DOI] [PubMed] [Google Scholar]
- 28.Wu K, Xu K, Liu K, Huang J, Chen J, Zhang J. et al. Long noncoding RNA BC200 regulates cell growth and invasion in colon cancer. Int J Biochem Cell Biol. 2018;99:219–25. doi: 10.1016/j.biocel.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Yin LL, Chung CM, Chen J, Fok KL, Ng CP, Jia RR. et al. A suppressor of multiple extracellular matrix-degrading proteases and cancer metastasis. J Cell Mol Med. 2009;13:4034–41. doi: 10.1111/j.1582-4934.2008.00576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Sun B, Zhao X, Liu Z, Wang X, Yao X. et al. Clinical significances and prognostic value of cancer stem-like cells markers and vasculogenic mimicry in renal cell carcinoma. J Surg Oncol. 2013;108:414–9. doi: 10.1002/jso.23402. [DOI] [PubMed] [Google Scholar]
- 31.Awakura Y, Ito N, Nakamura E, Takahashi T, Kotani H, Mikami Y. et al. Matrix metalloproteinase-9 polymorphisms and renal cell carcinoma in a Japanese population. Cancer Lett. 2006;241:59–63. doi: 10.1016/j.canlet.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Mukhopadhyay D, Datta K. Multiple regulatory pathways of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) expression in tumors. Semin Cancer Biol. 2004;14:123–30. doi: 10.1016/j.semcancer.2003.09.019. [DOI] [PubMed] [Google Scholar]