Abstract.
To achieve and sustain malaria elimination, identification and treatment of the asymptomatic infectious reservoir is critical. Malaria rapid diagnostic tests (RDTs) are frequently used to identify asymptomatic, Plasmodium-infected individuals through test-and-treat strategies, but their sensitivity is low when used in low transmission settings. Characteristics of individuals with subpatent (RDT-negative but polymerase chain reaction [PCR]–positive) Plasmodium parasitemia were evaluated in southern Zambia where malaria transmission has declined and efforts to achieve malaria elimination are underway. Simple random sampling based on satellite imagery was used to select households for participation in community-based, cross-sectional surveys between 2008 and 2013. Questionnaires were administered to collect information on age, gender, recent history of malaria symptoms, and recent antimalarial drug use. Blood samples were collected by finger prick for Plasmodium falciparum histidine-rich protein 2 RDT, blood smears for microscopy, and dried blood spots for molecular analysis to detect malaria parasites and their sexual stage. Of 3,863 participants with complete data, 102 (2.6%) were positive by microscopy, RDT, or PCR. Of these, 48 (47%) had subpatent parasitemia. Most individuals with subpatent parasitemia were asymptomatic (85%). Compared with individuals without parasitemia, individuals with subpatent parasitemia were significantly more likely to be aged 5–25 years. Approximately one quarter (27%) of those with subpatent parasitemia had detectable gametocytemia. These findings suggest that strategies based on active or reactive case detection can identify asymptomatic individuals positive by RDT, but more sensitive diagnostic tests or focal drug administration may be necessary to target individuals with subpatent parasitemia to achieve malaria elimination.
INTRODUCTION
As a result of increased funding and international commitment supporting control interventions, malaria incidence worldwide fell by 37% between 2000 and 2015.1 Over the same period, malaria mortality fell by 60% in all age groups and by 65% in children younger than 5 years.1 These epidemiological trends were primarily driven by the scale-up of insecticide-treated nets and indoor residual spraying, as well as early diagnosis by rapid diagnostic tests (RDTs) and prompt treatment with artemisinin combination therapy (ACT).2 After 2015, the Global Technical Strategy for Malaria set ambitious global targets for 2030: to reduce malaria incidence and mortality rates by at least 90%, eliminate malaria from at least 35 countries, and prevent the re-establishment of local transmission in malaria-free areas.2 Many countries have made remarkable progress in reducing malaria morbidity and mortality and are shifting their focus from malaria control to elimination.
To achieve and sustain malaria elimination, timely identification, and treatment of both symptomatic and asymptomatic infected individuals is critical. Passive case detection at health facilities misses asymptomatic individuals who do not seek medical care.3,4 Infected individuals without clinical signs and symptoms can harbor gametocytes and serve as reservoirs for transmission.5–7 The proportion of individuals harboring malaria parasites who are asymptomatic varies depending on transmission intensity and levels of clinical immunity.6 In high transmission settings, asymptomatic malaria infections are typically observed in older individuals with premunition following repeated parasite exposure. As transmission declines, the proportion of asymptomatic, infected individuals may increase until clinical immunity wanes and more infected individuals become symptomatic.8 Although the prevalence of asymptomatic parasitemia may decrease as transmission declines, most infected individuals are asymptomatic even in low transmission settings.4,6,7 However, there is large variation in the prevalence of asymptomatic parasitemia even in settings with similarly low transmission. The use of different definitions of asymptomatic malaria and diagnostic tests may, in part, explain this heterogeneity.4
Routinely used diagnostic tools, such as microscopy and RDTs, are not sufficiently sensitive to detect low parasite levels typically found in asymptomatic individuals (< 100 parasite/μL).7 The use of more sensitive tests such as polymerase chain reaction (PCR) can more accurately estimate parasite prevalence and characterize asymptomatic individuals with low-level parasitemia, such as those who are RDT negative but PCR positive (defined here as subpatent malaria).
Numerous studies have compared microscopy and PCR results to characterize the extent of submicroscopic malaria.6,9,10 However, few studies have directly compared RDT and PCR results, and the magnitude of asymptomatic, subpatent, and infectious parasitemia in pre-elimination settings remains poorly understood. The primary objective of this study was to investigate the prevalence of subpatent and asymptomatic Plasmodium falciparum infection and gametocytemia in the catchment area of Macha Hospital, Southern Province, Zambia, where malaria transmission has declined more than 90% since 2000.11,12 Zambia has adopted the goal of achieving malaria elimination by 2021, and Southern Province is one of the regions that has been the focus of recent operational strategies for malaria elimination.13 Understanding characteristics of individuals with subpatent and asymptomatic parasitemia will help guide these elimination strategies.
METHODS
Study site.
The study was conducted in the catchment area of Macha Hospital, a rural settlement in Southern Province, Zambia. The study area lies about 1,100 meters above sea level and 70 km from the nearest town of Choma. The climate is tropical savannah, with distinct wet and dry seasons. Malaria transmission is highest during the single rainy season from November to April, and the primary vector is Anopheles arabiensis.11,14 Subsistence farming and animal rearing are the primary sources of livelihood. Most residents live with extended family in scattered households comprising one or more structures.
Sampling and data collection.
The study design and procedures were described previously.12 Briefly, satellite images of the catchment area were used to create a sampling frame from which households were randomly selected for participation in community-based, serial cross-sectional surveys. Trained local field workers used global positioning system coordinates to locate selected households for initial notification visits and data collection. All household residents present at the time of the visit were eligible for enrollment, and written informed consent was obtained from all adults or caregivers of children who agreed to participate. Questionnaires were administered to collect information on age, gender, recent history of malaria, signs and symptoms of malaria, and recent antimalarial therapy. Self-reported history of malaria included the occurrence of fever, headache, chills, vomiting, diarrhea, and cough within the prior 48 hours and the prior 2 weeks. For recent antimalarial drug use, surveys conducted from 2008 to 2011 used a 2-week reference period, whereas surveys thereafter used a 1-month reference period to better identify individuals with potential prolonged P. falciparum histidine-rich protein 2 (PfHRP2) antigenemia following treatment. Body temperature on the date of assessment was recorded using a digital ear thermometer (ThermoScan®, Braun, Kronberg, Germany), and a temperature equal to or greater than 38°C was considered fever.
Laboratory procedures.
Blood samples were collected by finger prick and screened for malaria using a PfHRP2-based RDT (ICT Malaria P.f.; ICT Diagnostics, Cape Town, South Africa). In 2013, the Zambian Ministry of Health switched to another PfHRP2-based RDT, the SD Bioline Malaria Ag P.f. (Standard Diagnostics, Inc., Gyeonggi-do, Republic of Korea) as the standard RDT. Both RDTs met World Health Organization procurement criteria and reliably detected parasite densities of 200 parasites/μL or higher.15 In accordance with the national guidelines, RDT-positive individuals were treated with artemether/lumefantrine, whereas pregnant RDT-positive women were referred to local health centers for treatment with quinine in the first trimester and ACT in the second and third trimesters. Finger prick blood samples were also used to prepare blood smears for microscopic examination and dried blood spots (DBS) on filter paper (Whatman 903™ Protein Saver Card; GE Healthcare Bio-Sciences, Pittsburgh, PA). Blood smears were Giemsa-stained and independently examined by two microscopists. When microscopy results were discordant, a third reader examined the slide to make the final determination. Blood spots were dried overnight and individually stored in a Ziploc bag with a desiccant at −20°C. Approximately 50 μL of dried blood was excised and used for each molecular assay for asexual and sexual stages of P. falciparum.
Parasite DNA was extracted from the DBS using a Chelex-saponin method as previously described.16 Nested PCR was used to detect the presence of Plasmodium mitochondrial DNA using genus-specific primers targeting the cytochrome b (cytb) gene.12,17 The detection limit of the genus-specific PCR was 1 parasite/μL. Polymerase chain reaction–, RDT- or microscopy-positive samples were re-analyzed at the Johns Hopkins Bloomberg School of Public Health for species confirmation and determination of parasite densities. Quantitative PCR (q-PCR) was performed using SYBR® Green super mix (Bio-Rad, Hercules, CA) and reactions were run in a CFX384 real time thermo cycler (Bio-Rad). Species-specific primers targeting the cytb gene, and thermocycling conditions were described.12 The limit of detection for the P. falciparum cytb q-PCR was 0.04 copies/μL.12 To detect P. falciparum gametocytes, RNA was extracted from the DBS, cDNA was made by reverse transcriptase, and a nested PCR was performed to amplify cDNA to detect pfs25 transcripts.18 Samples from 2008 to 2009 were analyzed to detect gametocytes regardless of PCR status; however, because of the low parasite prevalence, only PCR-positive samples were analyzed by reverse transcriptase-PCR (RT-PCR) for samples collected from 2010 to 2013. The limit of detection for the pfs25 RT-PCR was 10 gametocyte/μL. Assays were performed at Macha Research Trust in Zambia except species confirmation by q-PCR.
Statistical analysis.
Participants who reported antimalarial drug use within the prior month were excluded as they were more likely to be asymptomatic yet test positive by RDT because of residual antigenemia.19,20 To establish criteria for asymptomatic malaria, signs and symptoms that were more prevalent among RDT-positive individuals were identified using the χ2 test for proportions.
To assess factors associated with subpatent parasitemia compared with uninfected individuals, including demographic characteristics (age and gender), individual characteristics (signs and symptoms of malaria and reported bed net use), household characteristics (presence of children younger than 5 years and RDT-positive household residents) and seasonality, the χ2 test for proportions was applied and Fisher’s exact test was used when sample sizes were fewer than five observations. Variables associated with the outcome with a P value ≤ 0.2 were included in a logistic regression model and tested by stepwise regression. Variables with P < 0.05 were included in the final model.
To identify characteristics of individuals with subpatent parasitemia, and thus missed by current RDTs, individuals with subpatent parasitemia were compared with those who had parasitemia confirmed by both RDT and PCR by age (dichotomized as younger or older than 15 years), gender, symptoms of malaria, and presence of gametocytemia. The χ2 test for proportions was applied and Fisher’s exact test was used when sample sizes were fewer than five observations. Statistical analyses were performed using Stata version 14 (StataCorp LP, College Station, TX).
Ethical considerations.
The study was approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board and the Zambian Tropical Diseases Research Center Ethics Research Committee.
RESULTS
Characteristics of study participants.
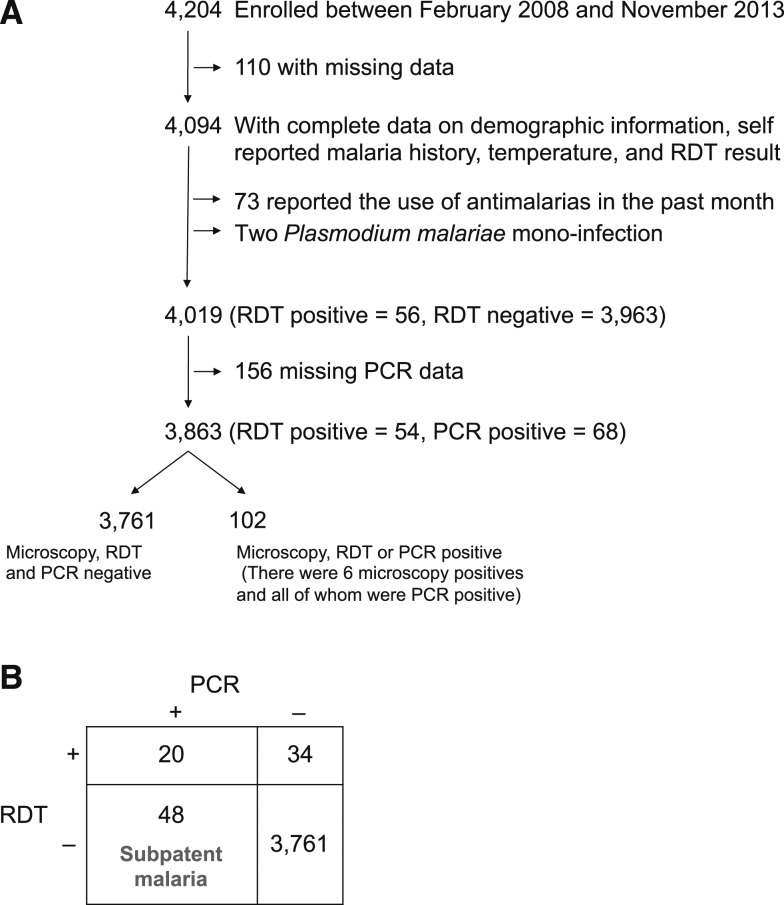
Between February 2008 and November 2013, 4,204 participants were enrolled in the community-based, serial cross-sectional surveys. Excluded from analysis were those with 1) incomplete data on demographic characteristics, self-reported malaria history, temperature on the day of the study visit, and RDT results (N = 110); 2) reported antimalarial drug use within the prior month (N = 73); 3) Plasmodium malariae monoinfection (N = 2); and 4) missing PCR results (N = 156). The remaining 3,863 (92%) participants were included in the final analysis (Figure 1).
Figure 1.
Flow chart showing the selection of participants included in the analysis (A) and contingency table of rapid diagnostic test (RDT) and polymerase chain reaction (PCR) results (B).
Of the 73 participants who reported taking antimalarial drugs within the prior month, 76% reported at least one symptom (fever, chills, headache, diarrhea, vomiting, or cough) in the past 2 weeks, and this proportion was significantly higher than those who did not report taking an antimalarial drug (53%, P < 0.005). All 73 participants who reported antimalarial use within the prior month had RDT and microscopy results, 69 had genus-specific nested PCR results, and 35 had gametocyte RT-PCR results available. Test positivity was 1.4% by microscopy, 2.7% by RDT, 2.9% by genus-specific nested PCR, and 2.9% by gametocyte RT-PCR.
Approximately half of the participants were male (47%) and about half were aged 15 years or younger (51%) (Supplemental Table 1), consistent with the age distribution of the general population in Zambia.21 Test positivity was 1.8% by PCR, 1.4% by RDT and 0.2% by microscopy. Seven percent of households had at least one RDT-positive resident. On the day of the study visit, 1.5% of participants had documented fever (tympanic temperature ≥ 38°C). The most prevalent self-reported symptoms were cough and headache within either the prior 48 hours or 2 weeks (Supplemental Table 1). Sixty-two percent of households reported owning at least one bed net.
Parasite densities.
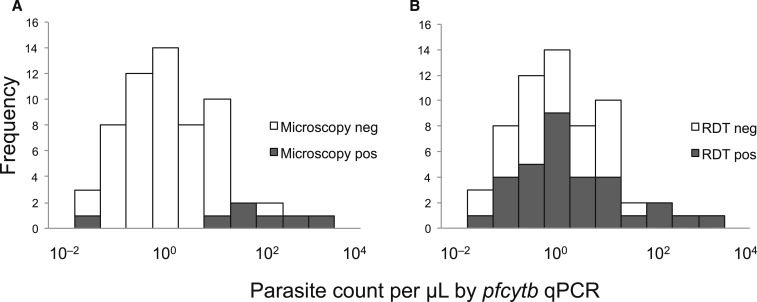
Of the 102 microscopy-, RDT- or genus-specific PCR–positive samples, 87 were tested by pfcytb q-PCR to determine the parasite density and 61 (70% of 87) were q-PCR positive. Based on the q-PCR results, the median parasite density was 2 parasites/μL, suggesting most parasitemic individuals had low-level parasitemia (Figure 2). Most (89%) infected individuals also had submicroscopic parasitemia. Some individuals with low-level parasitemia were RDT positive, but more than half (58%) of individuals with parasitemia less than the median parasite density were RDT negative (Figure 2).
Figure 2.
Parasite density distribution and detection of parasitemia by (A) microscopy or (B) rapid diagnostic test (RDT). Parasite densities quantified by pfcytb q-PCR are presented in log10 scale. All q-PCR–positive samples were included regardless of symptomatic or asymptomatic status (N = 61).
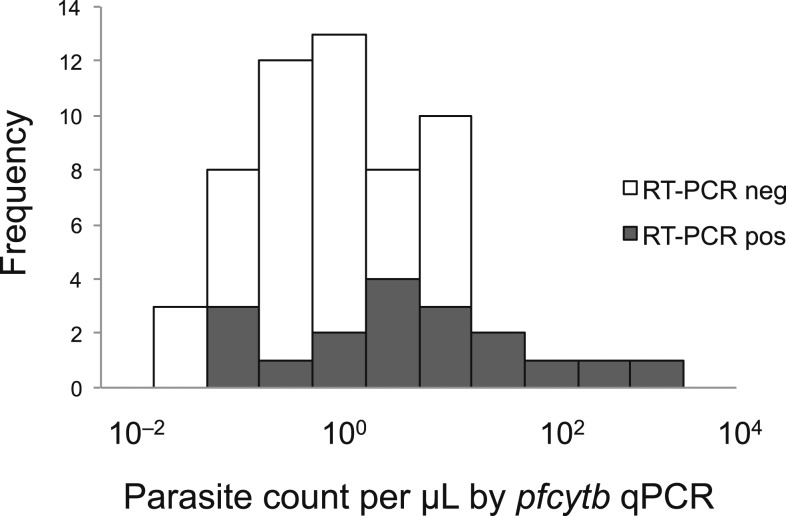
Of the 61 samples with q-PCR results, 59 had gametocyte-specific pfs25 RT-PCR data available, 18 of which had detectable gametocyte-specific mRNA (31% of 59). Of the seven microscopy-positive samples, two had detectable gametocyte mRNA. Although the sample size was small, most individuals with detectable gametocyte mRNA had submicroscopic and subpatent parasitemia (Figure 3).
Figure 3.
Parasite density distribution and detection of gametocyte-specific mRNA by reverse transcriptase polymerase chain reaction (RT-PCR). Of those pfcytb Quantitative PCR (q-PCR)–positive samples, 59 were tested for the detection of gametocyte-specific mRNA.
Characteristics of symptomatic and asymptomatic parasitemia.
To identify signs and symptoms associated with malaria confirmed by RDT, 4,019 participants with data on RDT status and complete demographic information, self-reported malaria history, and temperature on the day of the visit were included (Figure 1). Among these, 56 (1.4%) were RDT positive. Rapid diagnostic test–positive individuals were more likely to be aged 5–25 years and less likely to be younger than 5 years or older than 25 years than individuals who were RDT negative (P = 0.001) (Supplemental Table 2). Rapid diagnostic test–positive individuals were also more likely to have fever on the day of the study visit (P < 0.0005) and self-reported fever (P = 0.03) and chills (P = 0.02) within the previous 48 hours (Supplemental Table 2). Based on these results, symptomatic malaria was defined as individuals with confirmed P. falciparum infection by microscopy, RDT, or PCR with documented fever or self-reported fever with chills within the prior 48 hours. Approximately two-thirds (64%) of RDT-positive participants were asymptomatic using this definition.
Are individuals with subpatent parasitemia different from those without parasitemia?
Forty-eight individuals with subpatent parasitemia were compared with 3,761 individuals without evidence of parasitemia (Table 1). A higher proportion of individuals with subpatent parasitemia were aged 5–25 years compared with individuals without parasitemia (71% versus 47%, P = 0.001). Most individuals with subpatent parasitemia were asymptomatic, and the prevalence of fever or malaria-specific symptoms (fever on the day of the visit or self-reported fever with chills in the last 48 hours) was not significantly different from those without parasitemia (afebrile: 97% versus 99%, P = 0.10; absence of malaria-specific symptoms: 85% versus 90%, P = 0.35). The prevalence of subpatent parasitemia did not vary by season. In the final logistic regression model, the odds of subpatent parasitemia was significantly higher for participants who were aged 5–15 years (odds ratio [OR] = 7.3 [95% confidence interval [CI]: 1.7, 31], P = 0.007) and 15–25 years (OR = 9.2 [95% CI: 2.1, 41], P = 0.004) compared with children younger than 5 years, adjusting for gender, household bed net ownership, and households with at least one RDT-positive resident.
Table 1.
Characteristics of individuals with subpatent parasitemia (RDT negative but PCR positive) and those without parasitemia
| RDT negative but PCR positive (N = 48) | RDT and PCR negative (N = 3,761) | ||||
|---|---|---|---|---|---|
| N | % | N | % | P value | |
| Male | 28 | 58 | 1,753 | 47 | 0.11 |
| Age | 0.001 | ||||
| < 5 years | 2 | 4.2 | 783 | 21 | – |
| 5–15 years | 21 | 44 | 1,160 | 31 | – |
| 15–25 years | 13 | 27 | 584 | 16 | – |
| > 25 years | 12 | 25 | 1,234 | 32 | – |
| At least one child younger than 5 years in the household | 34 | 71 | 2,836 | 75 | 0.47 |
| Presence of bed nets in the house where sleeping | 24 | 50 | 2,323 | 62 | 0.10 |
| At least one RDT-positive household member | 5 | 10 | 219 | 5.8 | 0.20 |
| Absence of fever on the day of the visit | 46 | 96 | 3,710 | 99 | 0.10 |
| Absence of malaria-specific symptoms | 41 | 85 | 3,368 | 90 | 0.35 |
| Seasonality | |||||
| Rainy season (November–April) | 25 | 52 | 1,951 | 52 | 0.98 |
| Hot and dry season (August–October) | 10 | 21 | 657 | 17 | 0.54 |
| Cool and dry season (May–July) | 13 | 27 | 1,153 | 31 | 0.59 |
PCR = polymerase chain reaction; RDT = rapid diagnostic test.
Are individuals with subpatent parasitemia different from those who are RDT positive?
Twenty individuals with parasitemia by RDT and 48 with subpatent parasitemia were compared (Table 2). Of the 48 individuals with subpatent parasitemia, two had parasites observed by microscopy, but the level of parasitemia was less than 200 parasites/μL, the reported limit of detection for the RDT. Because of the small sample size, age was dichotomized as younger or older than 15 years. Individuals with parasitemia by RDT were more likely to report malaria-specific symptoms than those with subpatent parasitemia (45% versus 15%, P = 0.01). A higher proportion of individuals with subpatent parasitemia were older than 15 years (52% versus 25%, P = 0.06). Importantly, a higher proportion of individuals with parasitemia by RDT had detectable gametocytemia (50% versus 27%, P = 0.09), but approximately one-quarter of individuals with subpatent parasitemia had detectable gametocyte-specific mRNA (Table 2). Of the 34 RDT-positive but PCR-negative samples (Figure 1), 27 (79%) were tested for gametocyte-specific mRNA and all were negative (0% [one-sided, 97.5% CI: 0, 0.13]).
Table 2.
Characteristics of individuals with subpatent parasitemia (RDT negative but PCR positive) and those with patent parasitemia
| RDT and PCR positive (N = 20) | RDT negative but PCR positive (N = 48) | ||||
|---|---|---|---|---|---|
| N | % | N | % | P value | |
| Younger than 15 years | 15 | 75 | 23 | 48 | 0.06 |
| Male | 8 | 40 | 28 | 58 | 0.19 |
| Presence of fever on the day of the visit | 6 | 30 | 2 | 4.2 | 0.01 |
| Presence of malaria-specific symptoms | 9 | 45 | 7 | 15 | 0.01 |
| Gametocytes by RT-PCR | 10 | 50 | 13 | 27 | 0.09 |
PCR = polymerase chain reaction; RDT = rapid diagnostic test; RT-PCR = reverse transcriptase PCR.
DISCUSSION
Because of their low cost and ease of use, requiring minimal training and equipment, RDTs are widely used as diagnostics for malaria, improving patient care. More recently, their use has extended beyond the clinical diagnosis of symptomatic malaria. Rapid diagnostic test are increasingly used for active surveillance in active or reactive test-and-treat programs. Despite this expansion, more information is needed to optimize such use and better understand their limitations in different epidemiological settings. In a low transmission setting in southern Zambia, individuals with subpatent parasitemia were more likely to be older than uninfected individuals and those with patent parasitemia confirmed by both RDT and PCR. The absence of clinical symptoms makes these individuals difficult to identify. Although the prevalence of gametocytemia was lower than in those with a positive RDT, approximately one-quarter of individuals with subpatent parasitemia had detectable gametocyte-specific mRNA, suggesting these individuals potentially could transmit P. falciparum parasites.
The prevalence and proportion of low-density parasitemia reflects recent malaria transmission dynamics and the impact of interventions.22 In southern Zambia, the prevalence of microscopic parasitemia was low (0.2%), with 91% of individuals with parasitemia by PCR having submicroscopic parasitemia. The proportion of submicroscopic infections in southern Zambia is consistent with that predicted by models of the inverse relationship between the prevalence of parasitemia by PCR and the proportion of submicroscopic infections.9 More specifically, the study area experienced a decline in malaria transmission over the past decade, and the high proportion of submicroscopic infections is consistent with models that suggest such high proportions can follow recent, large decreases in malaria transmission.9,22
In southern Zambia, characteristics of individuals with subpatent parasitemia were similar to those without parasitemia except for their age distribution, with subpatent parasitemia more common among school-aged children and young adults. In malaria-endemic areas, most clinical cases occur among children younger than 5 years. Age is a proxy for malaria exposure and older individuals are more likely to have developed premunition, or clinical immunity. In the highlands of western Kenya, PCR-confirmed, parasitemic individuals older than 20 years had reduced odds of RDT-confirmed infection, whereas children younger than 5 years were more likely to be RDT and PCR positive.23,24 A recent pooled analysis of published and unpublished studies on the relationships between RDT and PCR positivity across settings with different malaria endemicity showed that the odds of subpatent infection in adults was 5-fold higher than those younger than 5 years, even after adjusting for transmission intensity.25 School-age children traditionally have not been a focus of malaria control programs, but more recent data suggest that this age group has high parasite prevalence and can harbor gametocytes.26 Despite their high risk of parasitemia, low bed net use among school-age children has been widely reported.27–29 Low bed net use among school-age children was previously observed in southern Zambia, perhaps contributing to the high parasite prevalence in this age group.30,31
A small proportion (4%) of individuals with subpatent parasitemia had fever on the day of the study visit and 15% had malaria-specific symptoms. However, the prevalence of fever and symptoms was not significantly different from individuals without parasitemia, consistent with previous findings that low-density parasitemia can be associated with fever but not more frequently than those without parasitemia.32
Compared with individuals with patent, RDT-positive parasitemia, individuals with subpatent parasitemia were more likely to be older and asymptomatic. Infected individuals with subpatent parasitemia will not be detected through active or reactive test-and-treat programs that rely on RDTs as the diagnostic test. Plasmodium falciparum histidine-rich protein 2 deletions have been reported in sub-Saharan Africa,33–38 and parasites with Pfhrp2 deletions can result in RDT-negative parasitemia. We have not identified Pfhrp2 deletions in southern Zambia and RDT-negative parasitemia is likely due to low-level parasitemia, less than the limit of detection of RDTs.12 Importantly, approximately one-quarter of individuals with subpatent parasitemia had detectable gametocyte-specific mRNA, highlighting their potential contribution to malaria transmission.
Further complicating the interpretation of RDT results when used for active or reactive case detection in a low transmission setting was the fact that more than half of RDT-positive participants were PCR negative, consistent with false-positive RDT results due to persistent antigenemia after clearance of parasites.19 Importantly, among individuals with a positive RDT but negative PCR for P. falciparum who had RT-PCR data available, none had detectable gametocyte-specific mRNA, suggesting that these individuals were not at risk of transmitting parasites and thus do not need to be identified and treated through active or reactive test-and-treat programs. As the storage conditions of filter papers and the duration of storage can affect the efficiency of RNA extraction,39 some of the samples negative by RT-PCR could be misclassified. Nevertheless, detectable gametocytemia among RDT-positive but PCR-negative individuals is unlikely.
One limitation of this study was the small number of individuals with parasitemia, a problem common to studies in low transmission settings. However, understanding the characteristics of the parasite reservoir in settings with low parasite prevalence is critical to the design of effective and efficient elimination strategies. A second limitation was imperfect assessment of the infectiousness of individuals with subpatent parasitemia and detectable gametocyte-specific mRNA, and their role in sustaining transmission in this low transmission setting. Individuals with higher gametocyte burden are more infectious to mosquitoes,40 but further study of the infectiousness of individuals with subpatent parasitemia in this setting will be important to guide elimination strategies.
The high prevalence of asymptomatic and subpatent parasitemia in this area of declining malaria transmission in southern Zambia presents significant challenges to malaria elimination. Strategies based on active or reactive case detection are important to identify asymptomatic, infected individuals but more sensitive screening tools or focal drug administration strategies may be needed to further reduce malaria transmission and achieve elimination.
Supplementary Material
Acknowledgments:
This work was supported by the Johns Hopkins Malaria Research Institute, Bloomberg Philanthropiesthe, and the Division of Microbiology and Infectious Diseases, National Institutes of Allergies and Infectious Diseases, National Institutes of Health as part of the International Centers of Excellence for Malaria Research (U19 AI089680). We thank the field team and laboratory staff at the Macha Research Trust and, most importantly, residents of the Macha community who participated in this study.
Note: Supplemental tables appear at www.ajtmh.org.
REFERENCES
- 1.WHO , 2015. World Malaria Report Available at: http://www.who.int/malaria/publications/world-malaria-report-2015/report/en/. Accessed November 13, 2018.
- 2.WHO , 2016. World Malaria Report Available at: http://www.who.int/malaria/publications/world-malaria-report-2016/report/en/. Accessed November 13, 2018.
- 3.Searle KM, Hamapumbu H, Lubinda J, Shields TM, Pinchoff J, Kobayashi T, Stevenson JC, Bridges DJ, Larsen DA, Thuma PE, Moss WJ; Southern Africa International Centers of Excellence for Malaria Research , 2016. Evaluation of the operational challenges in implementing reactive screen-and-treat and implications of reactive case detection strategies for malaria elimination in a region of low transmission in southern Zambia. Malar J 15: 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L, 2013. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther 11: 623–639. [DOI] [PubMed] [Google Scholar]
- 5.Bousema JT, Gouagna LC, Drakeley CJ, Meutstege AM, Okech BA, Akim IN, Beier JC, Githure JI, Sauerwein RW, 2004. Plasmodium falciparum gametocyte carriage in asymptomatic children in western Kenya. Malar J 3: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin JT, Saunders DL, Meshnick SR, 2014. The role of submicroscopic parasitemia in malaria transmission: what is the evidence? Trends Parasitol 30: 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bousema T, Okell L, Felger I, Drakeley C, 2014. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol 12: 833–840. [DOI] [PubMed] [Google Scholar]
- 8.de Mast Q, Brouwers J, Syafruddin D, Bousema T, Baidjoe AY, de Groot PG, van der Ven AJ, Fijnheer R, 2015. Is asymptomatic malaria really asymptomatic? Hematological, vascular and inflammatory effects of asymptomatic malaria parasitemia. J Infect 71: 587–596. [DOI] [PubMed] [Google Scholar]
- 9.Okell LC, Bousema T, Griffin JT, Ouedraogo AL, Ghani AC, Drakeley CJ, 2012. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Commun 3: 1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okell LC, Ghani AC, Lyons E, Drakeley CJ, 2009. Submicroscopic infection in Plasmodium falciparum-endemic populations: a systematic review and meta-analysis. J Infect Dis 200: 1509–1517. [DOI] [PubMed] [Google Scholar]
- 11.Mharakurwa S, Thuma PE, Norris DE, Mulenga M, Chalwe V, Chipeta J, Munyati S, Mutambu S, Mason PR; Southern Africa International Centers of Excellence for Malaria Research , 2012. Malaria epidemiology and control in southern Africa. Acta Trop 121: 202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laban NM, Kobayashi T, Hamapumbu H, Sullivan D, Mharakurwa S, Thuma PE, Shiff CJ, Moss WJ; Southern Africa International Centers of Excellence for Malaria Research , 2015. Comparison of a PfHRP2-based rapid diagnostic test and PCR for malaria in a low prevalence setting in rural southern Zambia: implications for elimination. Malar J 14: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.PMI , 2018. FY 2018 Zambia Malaria Operational Plan Available at: https://www.pmi.gov/where-we-work/zambia. Accessed November 13, 2018.
- 14.Kent RJ, Mharakurwa S, Norris DE, 2007. Spatial and temporal genetic structure of Anopheles arabiensis in southern Zambia over consecutive wet and drought years. Am J Trop Med Hyg 77: 316–323. [PMC free article] [PubMed] [Google Scholar]
- 15.WHO , 2017. Malaria Rapid Diagnostic Test Performance—Results of WHO Product Testing of Malaria RDTs: Round 7 (2015–2016) Available at: https://www.who.int/malaria/publications/atoz/978924151268/en/. Accessed November 13, 2018.
- 16.Kain KC, Lanar DE, 1991. Determination of genetic variation within Plasmodium falciparum by using enzymatically amplified DNA from filter paper disks impregnated with whole blood. J Clin Microbiol 29: 1171–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steenkeste N, et al. 2009. Towards high-throughput molecular detection of Plasmodium: new approaches and molecular markers. Malar J 8: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mlambo G, Vasquez Y, LeBlanc R, Sullivan D, Kumar N, 2008. A filter paper method for the detection of Plasmodium falciparum gametocytes by reverse transcription polymerase chain reaction. Am J Trop Med Hyg 78: 114–116. [PubMed] [Google Scholar]
- 19.Mayxay M, Pukrittayakamee S, Chotivanich K, Looareesuwan S, White NJ, 2001. Persistence of Plasmodium falciparum HRP-2 in successfully treated acute falciparum malaria. Trans R Soc Trop Med Hyg 95: 179–182. [DOI] [PubMed] [Google Scholar]
- 20.Marquart L, Butterworth A, McCarthy JS, Gatton ML, 2012. Modelling the dynamics of Plasmodium falciparum histidine-rich protein 2 in human malaria to better understand malaria rapid diagnostic test performance. Malar J 11: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CIA , 2017. The World Factbook—Zambia Available at: https://www.cia.gov/library/publications/the-world-factbook/geos/za.html. Accessed August 8, 2018.
- 22.Gatton ML, Cheng Q, 2010. Interrupting malaria transmission: quantifying the impact of interventions in regions of low to moderate transmission. PLoS One 5: e15149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stresman GH, Stevenson JC, Ngwu N, Marube E, Owaga C, Drakeley C, Bousema T, Cox J, 2014. High levels of asymptomatic and subpatent Plasmodium falciparum parasite carriage at health facilities in an area of heterogeneous malaria transmission intensity in the Kenyan highlands. Am J Trop Med Hyg 91: 1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevenson JC, Stresman GH, Baidjoe A, Okoth A, Oriango R, Owaga C, Marube E, Bousema T, Cox J, Drakeley C, 2015. Use of different transmission metrics to describe malaria epidemiology in the highlands of western Kenya. Malar J 14: 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu L, van den Hoogen LL, Slater H, Walker PG, Ghani AC, Drakeley CJ, Okell LC, 2015. Comparison of diagnostics for the detection of asymptomatic Plasmodium falciparum infections to inform control and elimination strategies. Nature 2015 Dec 3 (7580): S86–S93. DOI: 10.1038/nature16039. [DOI] [PubMed]
- 26.Ouedraogo AL, et al. 2016. Dynamics of the human infectious reservoir for malaria determined by mosquito feeding assays and ultrasensitive malaria diagnosis in Burkina Faso. J Infect Dis 213: 90–99. [DOI] [PubMed] [Google Scholar]
- 27.Nankabirwa J, Brooker SJ, Clarke SE, Fernando D, Gitonga CW, Schellenberg D, Greenwood B, 2014. Malaria in school‐age children in Africa: an increasingly important challenge. Trop Med Int Health 19: 1294–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walldorf JA, et al. 2015. School-age children are a reservoir of malaria infection in Malawi. PLoS One 10: e0134061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noor AM, Kirui VC, Brooker SJ, Snow RW, 2009. The use of insecticide treated nets by age: implications for universal coverage in Africa. BMC Public Health 9: 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinchoff J, Hamapumbu H, Kobayashi T, Simubali L, Stevenson JC, Norris DE, Colantuoni E, Thuma PE, Moss WJ; Southern Africa International Centers of Excellence for Malaria Research , 2015. Factors associated with sustained use of long-lasting insecticide-treated nets following a reduction in malaria transmission in southern Zambia. Am J Trop Med Hyg 93: 954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanyangarara M, Hamapumbu H, Mamini E, Lupiya J, Stevenson JC, Mharakurwa S, Chaponda M, Thuma PE, Gwanzura L, Munyati S, Mulenga M, Norris DE, Moss WJ; Southern Africa International Centers of Excellence for Malaria Research , 2018. Malaria knowledge and bed net use in three transmission settings in southern Africa. Malar J 17: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Proietti C, Pettinato DD, Kanoi BN, Ntege E, Crisanti A, Riley EM, Egwang TG, Drakeley C, Bousema T, 2011. Continuing intense malaria transmission in northern Uganda. Am J Trop Med Hyg 84: 830–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koita OA, et al. 2012. False-negative rapid diagnostic tests for malaria and deletion of the histidine-rich repeat region of the hrp2 gene. Am J Trop Med Hyg 86: 194–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wurtz N, et al. 2013. Pfhrp2 and pfhrp3 polymorphisms in Plasmodium falciparum isolates from Dakar, Senegal: impact on rapid malaria diagnostic tests. Malar J 12: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parr JB, et al. 2017. Pfhrp2-deleted Plasmodium falciparum parasites in the Democratic Republic of the Congo: a national cross-sectional survey. J Infect Dis 216: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menegon M, L’Episcopia M, Nurahmed AM, Talha AA, Nour BYM, Severini C, 2017. Identification of Plasmodium falciparum isolates lacking histidine-rich protein 2 and 3 in Eritrea. Infect Genet Evol 55: 131–134. [DOI] [PubMed] [Google Scholar]
- 37.Berhane A, et al. 2018. Major threat to malaria control programs by Plasmodium falciparum lacking histidine-rich protein 2, Eritrea. Emerg Infect Dis 24: 462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kozycki CT, Umulisa N, Rulisa S, Mwikarago EI, Musabyimana JP, Habimana JP, Karema C, Krogstad DJ, 2017. False-negative malaria rapid diagnostic tests in Rwanda: impact of Plasmodium falciparum isolates lacking hrp2 and declining malaria transmission. Malar J 16: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wampfler R, Mwingira F, Javati S, Robinson L, Betuela I, Siba P, Beck HP, Mueller I, Felger I, 2013. Strategies for detection of Plasmodium species gametocytes. PLoS One 8: e76316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bousema T, Drakeley C, 2011. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev 24: 377–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.