Abstract.
The pork tapeworm, Taenia solium, is among the leading causes of preventable epilepsy in the world and is common in rural areas of developing countries where sanitation is limited and pigs have access to human feces. Prior studies in rural villages of Peru have observed clusters of T. solium cysticercosis among pigs that live near human tapeworm carriers. Such spatial analyses, however, have been limited by incomplete participation and substandard diagnostic tests. In this study, we evaluated the association between necropsy-confirmed cysticercosis in pigs and their distance to T. solium tapeworm carriers in six villages in northern Peru. A total of six (1.4%) tapeworm carriers were detected using copro-antigen enzyme-linked immunosorbent assay and seven of 10 (70%) pigs belonging to the tapeworm carriers were found with viable cyst infection on necropsy. This was significantly greater than the prevalence of viable cyst infection among pigs living < 500 m (11%) and > 500 m (0.5%) from a tapeworm carrier (P < 0.001 for distance trend). Similar statistically significant prevalence gradients were observed after adjustment for possible confounders and for other pig-level outcomes including infection with > 10 viable cysts, degenerated cyst infection, and serological outcomes. This investigation confirms that porcine cysticercosis clusters strongly around tapeworm carriers in endemic rural regions of northern Peru and supports interventions that target these hotspots.
INTRODUCTION
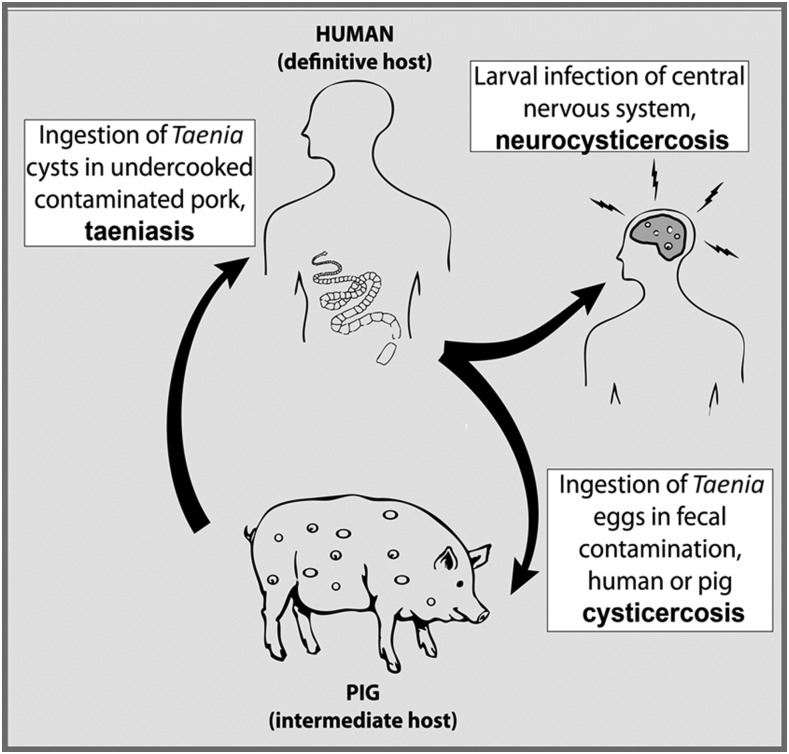
Neurocysticercosis is an important cause of acquired epilepsy in poor rural areas of the world and an urgent public health problem.1 Neurocysticercosis is caused by infection of the brain by Taenia solium, a zoonotic cestode transmitted between humans and pigs. T. solium is common in areas of the world where sanitation is limited and domestic pigs are allowed to roam freely and forage on human feces (Figure 1).
Figure 1.
Life cycle of Taenia solium (adapted with permission from O’Neal et al.5).
In rural villages of Peru where T. solium is endemic, infected pigs (porcine cysticercosis) have been found to cluster spatially around human tapeworm carriers (taeniasis).2–4 Our group has previously observed significant clustering of both seropositive pigs (based on enzyme-linked immunoelectrotransfer blot [EITB] for T. solium antibody response)2 and pigs with viable cyst infection (based on necroscopic examination)4 around human tapeworm carriers in rural areas of northern Peru. The incidence in these hotspots was strongest among pigs living within 50 m of identified carriers and did not depend on whether the tapeworm carrier was the pig’s owner or a neighbor within 50 m. Collectively, these findings have provided evidence to support geographically targeted screening and treatment for human tapeworm carriers. This approach, known as “ring strategy,” targets humans for screening and treatment if they reside within 100 m of pigs found to be infected with T. solium cysts. When piloted in northern Peru, ring strategy successfully identified and treated tapeworm carriers and reduced seroincidence in pigs while requiring fewer doses of antihelminthic therapy compared with mass-applied interventions.5
Despite these compelling initial findings, important gaps remain in our understanding of the spatial dynamics of T. solium transmission. Previous spatial analyses conducted by our group have been limited by both inadequate porcine diagnostics and low levels of participation in human and porcine screening, factors that have reduced the precision of spatial associations, and the resulting strength of conclusions drawn from these analyses.
In the present study, we follow-up our previous investigations by examining the spatial relationship between T. solium tapeworm carriers and porcine cysticercosis hotspots in six villages of northern Peru. Specifically, we improve on prior work by taking advantage of a robust sample of humans and pigs (93% of eligible humans screened, 70% of pigs necropsied) and the use of necroscopic examination as a gold-standard diagnostic for porcine cysticercosis. Together, these features will allow us to more precisely assess the presence of spatial clustering in endemic rural villages of northern Peru, seek more specific attribution for the source of infection within clusters, and address possible explanations for non-clustered infection.
MATERIALS AND METHODS
Study site and timeline.
Data collection for this analysis took place between 2005 and 2006 as part of the Cysticercosis Elimination Demonstration Project (CEDP) in the Tumbes region of Peru.6 This region is located on the northern coast of Peru, where there is little annual rainfall, and the primary economic activities are agriculture and smallholder farming. Raising pigs by allowing them to roam freely through the community is a common practice in these areas. The six villages included in this analysis were assigned to participate in a “pig replacement” intervention in which all pigs were offered to be purchased and euthanized by the CEDP and all humans were offered two rounds of mass antihelminthic treatment. An initial census was conducted in May 2005, pigs were purchased for serological and necroscopic analysis in July/August 2005, and mass antihelminthic treatment with posttreatment stool screenings were offered to the human population in September 2005 and January 2006.
Household census.
Each household in the study area was visited for an initial census to record the age and gender of all household members, type of sanitary facilities available (latrine or open field defecation), and geographic coordinates of each household. Household coordinates were recorded using global positioning system (GPS) handheld receivers (GeoExplorer II, Trimble, Sunnyvale, CA) with sub-meter accuracy after differential correction. No formal borders delimit communities but inhabitants reported consistently which village they live in.
Pig serology and necropsy.
In July 2005, 1 month before necroscopic analysis, all pigs in the study village were counted and tested for serological evidence of cysticercosis. For this, a 6- to 8-mL serum sample was obtained from vena cava puncture and was analyzed with the serum EITB assay.7,8 This assay measures antibody reactivity of pig serum to seven lentil–lectin-purified glycoprotein antigens isolated from native cysts and is highly sensitive (89%) but poorly specific (48%) for detecting active T. solium cyst infection.9,10
Following serological analysis, study staff returned to the study villages and offered to purchase all pigs from pig owners at fair market prices. All pigs residing in the study villages were considered eligible and targeted for purchase. Purchased pigs were transported to a field laboratory where necroscopic examinations were performed. To euthanize pigs for necroscopic analysis, a fatal dose of tranquilizers and anesthetics was administered, corresponding to the pigs’ weight. The ages of piglets were estimated by the pig owners and confirmed using conventional teeth-eruption indicators.11
Trained veterinary technicians under supervision of senior staff conducted necroscopic procedures. The entire carcass, including brain and organs, was dissected and 0.5 cm slices were evaluated to determine the presence of T. solium cysticerci. Identified cysts were classified into one of three categories: 1) viable cysts if a defined cystic structure with liquid content was still present, 2) nonviable or “degenerated” cysts if the liquid content had been replaced by semi-solid contents, or 3) calcified lesions without a cystic structure. The total number of viable and degenerated cysts was recorded for each pig, but calcified lesions were not counted systematically.
Mass treatment and screening for taeniasis.
After all available pigs in the villages were purchased and necropsied, two rounds of mass treatment for human taeniasis were administered (September 2005 and January 2006). In each round, consenting residents excluding children under 2 and pregnant women received a single oral dose of niclosamide according to their age (1 g for children < 6 years old and 2 g otherwise) to treat T. solium taeniasis. Niclosamide was chosen to treat intestinal tapeworms because it is highly effective (single-dose efficacy of 77%,12 with cure rates of 95% or more with follow-up treatment12–14) and has a lower incidence of adverse events compared with available alternatives.15
A single posttreatment stool sample was requested from all participants aged 2 years or older, regardless if they received treatment or not. Inhabitants were provided with disposable 500 mL plastic containers for stool collection as well as toilet paper and soap, and instructed in basic hygiene procedures to avoid self-contamination. All stool specimens were evaluated for T. solium coproantigens using the enzyme-linked immunosorbent assay (CoAg-ELISA),13 which is highly sensitive and specific for detecting active infection of tapeworms from the Taenia spp. genus.16 The optical density ratio (ODR) for each CoAg-ELISA sample was calculated by comparing the optical density of each sample to a known positive control. All samples were further examined microscopically using the test tube spontaneous sedimentation technique.17 When tapeworm segments were found, definitive speciation for T. solium was performed by counting uterine branches and by polymerase chain reaction (PCR).18 For this analysis, confirmed cases of taeniasis were those for whom ODR ≥ 40% on CoAg-ELISA, or T. solium proglottids were definitively identified in stool. A case definition of ODR ≥ 40% was chosen with the goal of reducing false positives due to nonspecific binding and cross-reaction with other Taenia spp., which may occur at lower ODR values.19,20 After each round of mass treatment and screening, positively identified cases were followed up with multiple rounds of niclosamide therapy and stool screening until parasite clearance was confirmed.
Statistical analysis.
Three necropsy-defined primary outcomes were analyzed separately: 1) the presence of one or more viable T. solium cysts, 2) the presence of 10 or more viable cysts, and 3) the presence of one or more nonviable degenerated cysts. We chose to analyze viable and degenerated cysts separately because degenerated cysts may not represent recent infections (degenerated cysts take up to 6 months to clear from muscles21), and are not reliably identified on necroscopic examination without histopathological confirmation, which was not available here. In addition, we evaluated pigs for their serologic response to ≥ 1, ≥ 2, ≥ 3, and ≥ 4 EITB bands. Distance in meters from the pig-owner’s home to the location of the nearest confirmed tapeworm carrier was calculated using equator equivalences for latitude and longitude as previously described.2 We first compared the prevalence of each pig outcome at increasing distances from identified tapeworm carriers. Using Chi-square trend tests for P-values, we assessed the prevalence of pig outcomes at > 500 m from tapeworm carrier households, 2–500 m, and within the same household of tapeworm carriers. Although a variety of distance thresholds were assessed, these cutoffs were chosen because they represent three distinct exposures for foraging pigs—the immediate home environment, other households within the small villages (< 500 m), and neighboring small villages (> 500 m). We considered using a 50-m distance threshold for this analysis to achieve consistency with our prior analyses, but we ultimately rejected this because of the limited number of pigs residing within this distance from a tapeworm carrier.
We also examined pig- and household-level predictors for viable cyst infection using Poisson family generalized linear models. The Poisson regression with log-link function was used for increased stability of regression coefficients and improved convergence.22 Bivariate prevalence ratios were calculated for the distance gradient and relevant covariates including the presence of degenerated cysts, pig age and sex, housing density (number of neighboring houses within 100 m), size of the household pig herd, and human waste management (latrine or indoor bathroom versus open field defecation), while adjusting for clustering of pigs purchased from the same household. Our final adjusted Poisson regression model included distance to tapeworm carriers and covariates that were significant in the bivariate analysis. Ninety-five percent confidence intervals (CIs) and Wald P-values (< 0.05) were used to determine statistical significance for model predictors. Statistical analyses were performed using Stata 13.1 (Stata Corporation, College Station, TX), and maps were prepared with ArcMap 10.3 (Environmental Sciences Research Institute, Redlands, CA).
Ethics.
The study protocol was approved by the institutional review boards of the Cayetano Heredia Peruvian University, the Centers for Disease Control and Prevention, and the Johns Hopkins Bloomberg School of Public Health. In addition, the study was reviewed and approved by the Animal Ethics Committee of the School of Veterinary Medicine, Universidad de San Marcos, Lima, Peru. All study subjects provided written informed consent for their participation. Approval from legal guardians and child assent was obtained for legal minors. A single consent form was used for all study procedures, and prospective participants were told that they could refuse to participate in specific procedures.
RESULTS
Census and tapeworm detection.
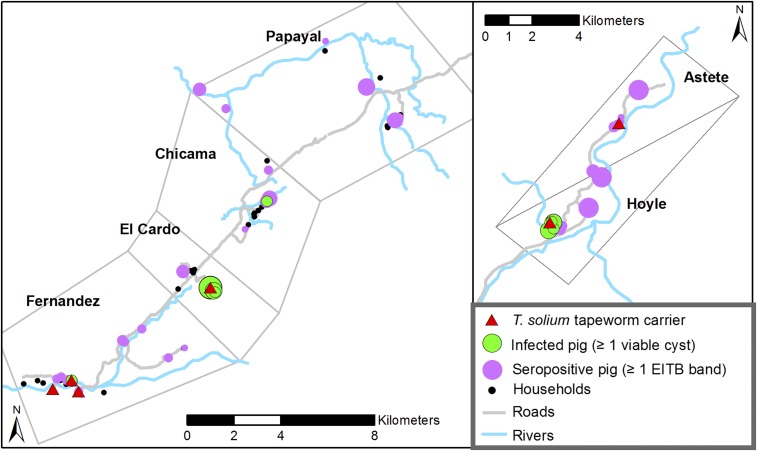
The census showed that there were a total of 474 people living in 118 households across the six study villages (Table 1). All study villages were relatively small and spread out, each consisting of a small cluster of 10–20 homes in the center of town with homesteads dispersed 1–2 km apart on the periphery of the town (Figure 2). Villages ranged between nine and 41 households each and averaging 1.8 neighboring households per 100 m radius. Few households had drinking water on the premises, none had sewage connection or electricity, and 49% did not have a latrine or indoor bathroom on site. Sixty-one (52%) of the 118 study households raised pigs, and the median herd size among these households was four pigs (range: 1–35).
Table 1.
Characteristics of human and porcine population by village
| Village | Total | ||||||
|---|---|---|---|---|---|---|---|
| Astete | Chicama | El Cardo | Papayal | Fernandez | Hoyle | ||
| Households | 9 | 11 | 11 | 15 | 41 | 31 | 118 |
| Open field defecation | 4 (44%) | 8 (73%) | 10 (91%) | 0 (0%) | 13 (32%) | 21 (70%) | 56 (49%) |
| Pig owners | 6 (67%) | 4 (36%) | 4 (36%) | 10 (67%)` | 18 (44%) | 19 (61%) | 61 (52%) |
| Herd size (med, range) | 16 (2–35) | 10.5 (3–15) | 16.5 (12–20) | 3.5 (1–15) | 2 (1–15) | 5 (1–31) | 4 (1–35) |
| Housing density (mean number of houses within 100 m) | 0.25 | 0.18 | 0.55 | 0.53 | 3.37 | 1.86 | 1.83 |
| Human population | 42 | 44 | 46 | 53 | 171 | 118 | 474 |
| Age, mean (SD) | 25 (16) | 36 (25) | 35 (24) | 28 (22) | 27 (20) | 26 (18) | 28 (21) |
| Female (%) | 18 (43%) | 25 (57%) | 20 (43%) | 19 (36%) | 86 (50%) | 55 (47%) | 223 (47%) |
| Provided stool sample | 39 (93%) | 41 (93%) | 39 (85%) | 49 (92%) | 155 (91%) | 99 (84%) | 422 (89%) |
| Taenia solium taeniasis (%) | 1 (2.6%) | 0 | 1 (2.6%) | 0 | 3 (1.9%) | 1 (1.0%) | 6 (1.4%) |
| Pig serology population | 90 | 39 | 65 | 49 | 52 | 169 | 464 |
| Age in months, med (IQR) | 8 (3–15) | 5 (4–18) | 12 (6–16) | 3 (3–12) | 5 (3–17) | 9 (6–18) | 7 (3–18) |
| Female (%) | 51 (57%) | 23 (59%) | 44 (68%) | 27 (55%) | 34 (65%) | 89 (53%) | 268 (58%) |
| EITB serology | |||||||
| ≥ 1 band | 56 (62%) | 19 (49%) | 42 (65%) | 37 (75%) | 34 (65%) | 101 (60%) | 267 (58%) |
| ≥ 2 bands | 15 (17%) | 3 (8%) | 24 (37%) | 13 (27%) | 14 (27%) | 41 (24%) | 110 (23%) |
| ≥ 3 bands | 12 (13%) | 1 (3%) | 22 (34%) | 7 (14%) | 13 (25%) | 26 (15%) | 81 (17%) |
| ≥ 4 bands | 1 (1%) | 0 | 3 (5%) | 0 | 3 (6%) | 1 (1%) | 8 (2%) |
| Pig necropsy population | 73 (81%) | 26 (67%) | 30 (46%) | 35 (71%) | 26 (50%) | 136 (80%) | 326 (70%) |
| ≥ 1 degenerated cyst* | 2 (3%) | 4 (15%) | 8 (27%) | 4 (11%) | 3 (12%) | 10 (7%) | 31 (9.5%) |
| ≥ 1 viable cyst* | 0 | 1 (4%) | 8 (27%) | 0 | 2 (8%) | 7 (5%) | 18 (5.5%) |
| ≥ 10 viable cysts* | 0 | 0 | 2 (7%) | 0 | 0 | 4 (3%) | 6 (1.8%) |
EITB = enzyme-linked immunoelectrotransfer blot.
Cyst infection categories are not mutually exclusive; pigs may be represented in multiple categories.
Figure 2.
Geographic distribution of humans and pigs in six study villages. The size of the purple (no outline) and green (with outline) circles represents the number of positive pigs at each household (range: 1–20 for seropositive pigs and 1–5 for necropsy-confirmed infected pigs). This figure appears in color at www.ajtmh.org.
The human population was aged 28 years on average (range: 0–86) and 47% female. Among a total census population of 474 inhabitants, 453 (96%) were eligible for participation in taeniasis treatment and screening based on age and pregnancy status. Of those, 416 (92%) received at least one dose of niclosamide, and 422 (93%) provided at least one stool sample. Six confirmed T. solium tapeworm carriers were detected by CoAg-ELISA results (ELISA ODR ≥ 40%). One carrier expelled proglottid segments and a scolex, which was confirmed as T. solium by PCR and morphology, whereas all other carriers were positive based on the results of the CoAg-ELISA alone. The prevalence of taeniasis among all study villages was 1.4% (6/422) and ranged from 0% to 2.6% across the six villages. No tapeworms were detected in the villages of Chicama and Papayal, whereas one tapeworm each was detected in Astete, El Cardo, and Hoyle, and three tapeworms were found in Fernandez, the largest of the six villages.
Porcine cysticercosis.
A total of 464 pigs were counted and tested with EITB serology in the pre-necropsy serum sample. Fifty-eight percent of pigs were female, and their median age was 7 months. Including all pigs and humans, the human-to-pig ratio among all study villages was 1 to 0.97. Porcine cysticercosis seropositivity was 58% for ≥ 1 positive EITB band, whereas 23% had ≥ 2 positive EITB bands, 17% had ≥ 3 positive EITB bands, 2% had ≥ 4 bands, and only one pig (0.2%) had five EITB bands.
Of the 464 pigs identified in the study communities, staff were able to purchase 326 (70%) for necroscopic examination. Necroscopic examination identified 18 (5.5%) of 326 pigs that had at least one viable T. solium cyst. Of those, nine (50%) were infected with a single viable cyst, three (17%) had between two and 10 viable cysts, and six (33%) had more than 10 viable cysts (maximum: 2,698 cysts). Compared with uninfected pigs, pigs with viable cyst infection did not have a significantly different age (mean age 15 versus 11 months, t-test P-value = 0.11) or sex (72% versus 53% female, chi-square P-value = 0.13), but originated from households that had significantly smaller pig herds (mean herd size of 12.1 versus 17.5, t-test P-value = 0.03) and were located in more dense areas of the villages (mean of 2.1 versus 1.0 neighbors within 100 m, t-test P-value = 0.001). A total of 31 (9.5%) pigs had degenerated cysts. Ten of 31 pigs with degenerated cysts also had viable cysts, and seven had more than 10 degenerated cysts (maximum: 175 cysts).
Infection distance gradients.
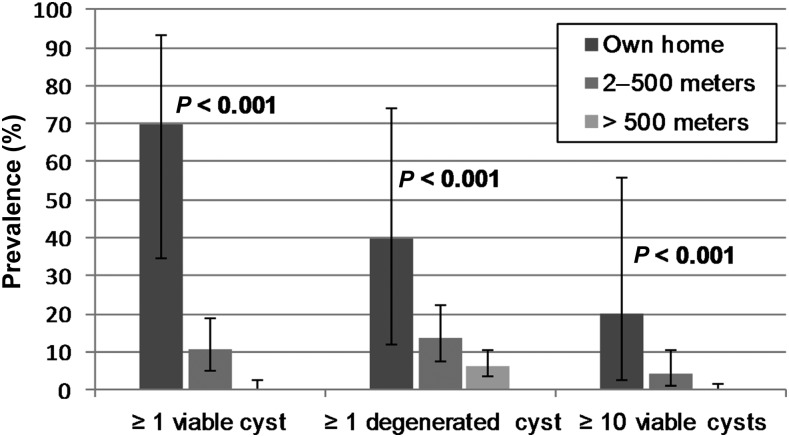
Pig infection prevalence presented a clear distance gradient with higher rates near tapeworm carriers (Figure 3). The prevalence of viable infection was 70% (7/10) among pigs owned by a tapeworm carrier, and decreased to 10.6% (10/94) among pigs 2–500 m from a tapeworm carrier. Only 0.5% (1/222) of pigs living > 500 m from a carrier were infected (P < 0.001 for trend). Among pigs with viable cyst infection, 39% (7/18) were found at the home of a tapeworm carrier and 94% (17/18) were within 500 m of a carrier. The prevalence of infection with 10 or more viable cysts was 20% (2/10) among pigs owned by a tapeworm carrier and 4.3% (4/94) among pigs 2–500 m from a tapeworm carrier. No pigs with ≥ 10 viable cysts were located > 500 m from a tapeworm carrier (P < 0.001 for trend).
Figure 3.
Prevalence and 95% confidence intervals (exact binomial) for pig necropsy outcomes at increasing distances from identified tapeworm carriers. P-values are chi-square tests of trend for distance.
Evaluating the 50 m distance threshold used in prior analyses, 53.3% (8/15) of pigs living < 50 m from a tapeworm carrier had viable cyst infection, and a significant prevalence gradient was observed between pigs residing < 50 m, 50–500 m, and > 500 m from a tapeworm carrier (P < 0.001 for trend). The elevated prevalence of viable infection among these pigs, however, was entirely driven by the high prevalence among pigs belonging to tapeworm carriers. Of the five pigs residing within 50 m but not belonging to a tapeworm carrier, only one pig (20%) had a single viable cyst. This is less than but not significantly different from the 70% (7/10) prevalence among pigs belonging to tapeworm carriers (P = 0.067, two-sample test of proportion).
Among pigs with viable cysts, distance to the nearest tapeworm carrier was significantly associated with the number of viable cysts found during necroscopic examination. This correlation was significant when cyst burden was assessed as a continuous variable (P < 0.001, ρ = −0.29, Spearman’s nonparametric correlation) and categorically (P < 0.001 for uninfected versus 1–9 viable cysts versus ≥ 10 viable cysts, Cuzick’s trend test).
The prevalence of infection with degenerated cysts followed a similar but less dramatic pattern of clustering: 40% (4/10) among pigs belonging to tapeworm carriers, 13.8% (13/94) at 2–500 m, and 6.3% (14/222) > 500 m from a tapeworm carrier (P < 0.001 for trend). The median distance to the nearest tapeworm carrier was not significantly different between uninfected pigs (1,821 m) and pigs with degenerated cysts only (1,769 m) (Mann–Whitney P-value = 0.79).
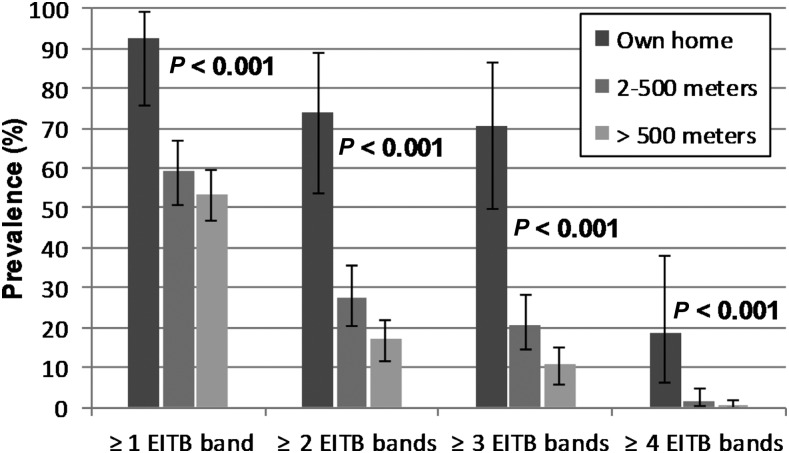
Similar to necroscopic examination, the prevalence of pig seropositivity followed a decreasing trend as distance from tapeworm carriers increased (Figure 4). Among pigs belonging to tapeworm carriers, 70% (19/27) had ≥ 3 positive EITB bands, whereas the prevalence of ≥ 3 positive EITB bands was 20% (31/149) and 11% (31/288) at 2–500 m and > 500 m from a tapeworm carrier, respectively (P < 0.001 for trend). Using case definitions of ≥ 2 and ≥ 1 positive EITB bands, the distance gradients followed similar statistically significant trends (P < 0.001 for each), but had decreased magnitudes. At ≥ 4 EITB bands, a significant distance trend (P < 0.001) was observed despite only eight seropositive pigs at this level. Overall, the number of EITB bands in seropositive pigs was inversely correlated with the distance to the nearest carrier (P < 0.01, ρ = −0.15, Spearman’s non-parametric correlation).
Figure 4.
Prevalence and 95% confidence intervals (exact binomial) for pig serological outcomes at increasing distances from identified tapeworm carriers. P-values are chi-square tests of trend for distance.
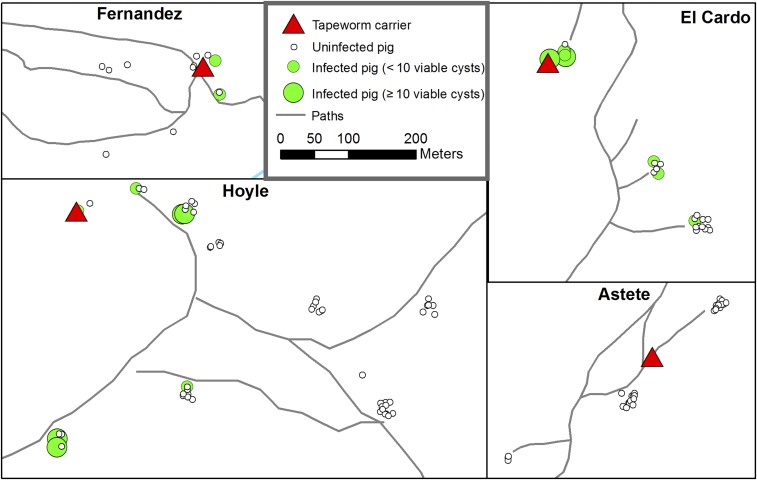
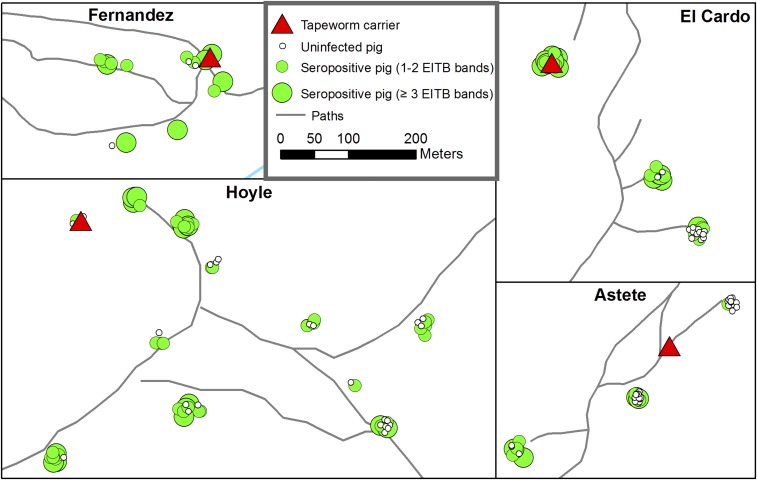
Figures 5 and 6 show the locations of four of the six identified tapeworm carriers and the spatial distribution of pigs with viable cyst infection and seropositivity surrounding them (the remaining two tapeworm carriers in the village of Fernandez were isolated with no nearby study pigs). Strong clustering of both viable cyst infection and seropositivity around tapeworm carriers can be observed, but clustering of seropositive pigs is less intense because of widespread antibody response among pigs with one and two EITB bands.
Figure 5.
Locations of four of six tapeworm carriers and pigs with viable cyst infection. The remaining two tapeworms carriers in Fernandez did not have nearby pigs. Points are jittered to show all pigs from each household—each small cluster of dots represents pigs from a single household. This figure appears in color at www.ajtmh.org.
Figure 6.
Locations of four of six tapeworm carriers and seropositive (enzyme-linked immunoelectrotransfer blot serology). The remaining two tapeworm carriers in Fernandez did not have nearby pigs. Points are jittered to show all pigs from each household—each small cluster of dots represents pigs from a single household. This figure appears in color at www.ajtmh.org.
Poisson regression analysis.
Table 2 presents pig- and household-level factors associated with viable cyst infection in a bivariate Poisson regression model adjusted for household clustering. The distance between a pig and its nearest tapeworm carrier was the factor associated with the greatest difference in the prevalence of viable infection, but pig age, the presence of degenerated cysts, and residence in a denser area of town were also associated with viable cyst infection. These covariates were retained in the final multivariable Poisson regression model (Table 3). In this adjusted model, the prevalence of viable cyst infection among pigs belonging to tapeworm carriers was 64 times (95% CI: 7.7, 532.6) greater than the prevalence among pigs living > 500 m from a carrier and 6.8 (95% CI: 4.0, 11.4) times greater than pigs living < 500 m from a carrier. Among pigs living < 500 m from a carrier, the prevalence of viable cyst infection was 9.4 (95% CI: 1.1, 83.1) times greater than those > 500 m from a carrier.
Table 2.
Bivariate associations between viable cyst infection in pigs and selected household and pig-level risk factors (n = 326 pigs)
| N (% of total) | Number positive (≥ 1 viable cyst) | Prevalence ratio of viable cyst infection (95% CI) | P-value | |
|---|---|---|---|---|
| Distance to nearest tapeworm carrier | ||||
| Carrier’s home | 10 (3%) | 7 (70.0%) | 155.4 (21.2, 1,140) | < 0.001 |
| 2–500 m | 94 (29%) | 10 (10.6%) | 23.6 (2.94, 189) | 0.003 |
| > 500 m | 222 (68%) | 1 (0.5%) | Ref. | – |
| Alternative distance categorization | ||||
| < 50 m | 15 (5%) | 8 (53.3%) | 118 (15.1, 930) | < 0.001 |
| 50–500 m | 89 (27%) | 9 (10.1%) | 22.4 (2.8, 183) | 0.004 |
| > 500 m | 222 (68%) | 1 (0.5%) | Ref. | – |
| Degenerated cyst infection | ||||
| Yes | 31 (10%) | 10 (32.3%) | 11.9 (5.47, 25.8) | < 0.001 |
| No | 295 (90%) | 8 (2.7%) | Ref. | – |
| Pig sex | ||||
| Male | 147 (55%) | 5 (3.4%) | 0.47 (0.16, 1.33) | 0.15 |
| Female | 179 (45%) | 13 (7.3%) | Ref. | – |
| Geographic density (houses within 100 m) | ||||
| ≥ 2 | 65 (20%) | 11 (16.9%) | 6.31 (1.30, 30.7) | 0.022 |
| 0–1 | 261 (80%) | 7 (2.7%) | Ref. | – |
| Number of pigs in household | ||||
| 1–5 | 60 (18%) | 4 (6.7%) | 1.27 (0.34, 4.69) | 0.72 |
| > 5 | 266 (82%) | 14 (5.3%) | Ref. | – |
| Sanitation | ||||
| Open field defecation | 195 (60%) | 12 (6.2%) | 1.31 (0.32, 5.33) | 0.71 |
| Latrine | 128 (40%) | 6 (4.7%) | Ref. | – |
| Pig age, per additional month | – | – | 1.03 (1.01, 1.06) | 0.009 |
Table 3.
Adjusted associations between distance to tapeworm carriers and viable cyst infection
| Viable cyst infection PR (95% confidence interval)*† | ||
|---|---|---|
| Distance to nearest tapeworm carrier | ||
| Carrier’s home | 63.9 (7.67, 532.6) | 6.73 (4.02, 11.4) |
| 2–500 m | 9.42 (1.06, 83.1) | Ref. |
| > 500 m | Ref. | 0.11 (0.01, 0.94) |
* Adjusted for pig age, housing density, and degenerated cyst infection.
† All associations significant (P < 0.05).
DISCUSSION
Our results show significant clustering of confirmed and viable porcine cysticercosis near T. solium tapeworm carriers. Seventy percent of pigs owned by tapeworm carriers were infected with viable T. solium cysts compared with just 0.5% of pigs living more than 500 m from a tapeworm carrier. These findings confirm previous reports that pigs with viable cyst infection cluster around human tapeworm carriers in this region and support the view that human-to-pig transmission of T. solium is highly focal and dependent on local environmental contamination by tapeworm carriers.
In previous work conducted in a similar region of northern Peru, we found that a 50 m radius surrounding the household of a tapeworm carrier represented an area of significantly elevated pig seroprevalence.2 We later confirmed this finding of a 50 m radius of elevated prevalence using necroscopic examination of pigs as a gold-standard diagnostic.4 In the current investigation, the observed clusters of pig infection were concentrated in the households of tapeworm carriers without extending to neighboring households.
The difference between our current findings and our previous work with respect to the distance at which clustering occurs is likely because of local variations in geography and pig roaming. The villages included in this study were considerably more rural and dispersed than in prior analysis (average of 1.8 households within 100-m radius compared with 6.6 in our prior analysis2). Greater distances between households likely led to less interaction of pigs with neighboring households and the concentration of clusters of infected pigs within the household of tapeworm carriers. This mechanism of within-household transmission of T. solium from human carriers to their pig herd is consistent with a GPS tracking study previously conducted by our group in this region.23 In that study, we found that both pig roaming patterns and reported human defecation locations were concentrated in the immediate vicinity of households, highlighting the potential for focal transmission through exposure to T. solium eggs in cases where the pig owner has taeniasis.
Although our study’s findings were clear in demonstrating that both seropositive pigs and pigs with viable cyst infection cluster around tapeworm carriers, the spatial associations were not absolute. Many pigs with viable infection were not found in the immediate proximity of tapeworm carriers. In fact, 11 of 18 pigs with viable cyst infection did not belong to a tapeworm carrier, and one infected pig was found in the village of Chicama, where no tapeworm carriers were detected. Similarly, seropositivity among pigs was widespread in study villages (58% of pigs with ≥ 1 EITB band) and seropositive pigs were routinely found distant from tapeworm carriers. There are a few possible explanations for these findings. First, pigs residing far from a tapeworm carrier could have been exposed to T. solium eggs through roaming and foraging outside of their home areas. Whereas our GPS tracking study found that this distant roaming is not typical,23 even a brief visit to the contaminated environment of a tapeworm carrier could result in seropositivity and/or frank cyst infection during this distant roaming. In addition, despite our high level of human participation in taeniasis screening (89% of all human inhabitants were tested for taeniasis), it is possible that we did not detect all tapeworm carriers in the study villages. The presence of undetected tapeworm carriers could have contributed to infection or seropositivity in pigs that would have appeared to be distant from identified tapeworm carriers, thereby biasing the results toward observing large distance values for some pigs despite their proximity to an unknown carrier. Finally, regarding the widespread seropositivity found in this study, the high molecular weight band (GP50) of the EITB assay used to assess antibody response is known to be cross-reactive with other coendemic parasites. This could have caused some low-level seropositivity among uninfected pigs and could explain the occurrence of seropositive pigs distant from identified tapeworm carriers.10
An important unanswered question about cysticercosis is the mechanism by which pigs are exposed to and infected with T. solium eggs. Our observations of strong clustering of infected pigs around tapeworm carriers combined with the lack of human sanitation found in these villages (49% of households report open field defecation) are consistent with coprophagic transmission—that is, pigs directly ingesting human feces that contains T. solium eggs. Under this scenario, tapeworm carriers practicing open field defecation would produce hotspots of environmental contamination in their immediate vicinity, and cause high rates of infection among pigs in their own herd, as we observed.
An unexpected finding of this analysis that is inconsistent with this proposed mechanism, however, was the low burden of cysts found in infected pigs. Half (9/18) of pigs with viable cyst infection in this study had only a single cyst found in necroscopic examination, and 67% (12/18) had fewer than 10 viable cysts. Direct ingestion of feces containing T. solium eggs would likely result in higher cyst burdens than those seen in most of the infected pigs found in this study (proglottid segments found in human feces each contain 20,000 or more eggs24). It is possible, therefore, that the low-burden cyst infections found here were caused by alternate mechanisms of infection that have not been sufficiently studied. Alternate exposure routes that have been proposed include the contamination of pig feed with T. solium eggs25 and the persistence of low concentrations of T. solium eggs in the environment. Evidence for environmental persistence of T. solium eggs is the most developed, as eggs have been found in the soil of some endemic communities,26 and eggs of related cestode species have been found to remain viable in moist environments for between 6 months and 4 years.27–30 There is also evidence that T. solium eggs can be dispersed by arthropod vectors.31,32 While these studies provide compelling hypotheses to explain the proliferation of low-burden cyst infections and seropositivity found in the present analysis, further research is needed to confirm and elaborate these possible mechanisms.
Our findings of significant clustering of porcine cysticercosis are also important to consider in the context of existing control interventions. “Ring strategy” is a control intervention that has been successful in Peru through targeting antiparasitic treatment to humans who live within 100 m of positively identified pigs.5 In the present analysis, three of six identified tapeworm carriers owned a necropsy-positive pig and would have been targeted if a ring strategy were applied in these villages. Although this coverage (50% of teaniasis cases identified and treated) is less than what would be expected in a mass treatment intervention, the clustering of human taenasis and pig infection found here means that the intervention would be targeted more efficiently to a smaller and higher risk population. Ultimately, decisions regarding whether to adopt a focal (i.e., ring strategy) or mass (i.e., mass drug administration) approach to control should be based on local conditions, with the end goal being a reduction in transmission to a locally acceptable level, even if some infection is missed. Factors such as the degree of clustering present, resources and infrastructure available, and community preference should all be considered in determining the best approach.
There are a few important strengths and limitations of this study that must be highlighted. First, because of the low prevalence of taeniasis and the small size of villages, two of the study villages had no tapeworm carriers detected, and only one village had more than one tapeworm carrier detected. This low prevalence limited our ability to make inferences about infection risk among pigs belonging to and living near tapeworm carriers. Second, because of the small size of study villages, the generalizability of these results to other populations in the region and around the world is not known. Spatial patterns of infection may vary greatly based on local geographic features and pig husbandry practices, and the patterns observed in these small villages of northern Peru may be different from other endemic areas. Finally, two important strengths of our study were our use of a highly specific, gold-standard diagnostic for porcine cysticercosis (necroscopic examination), and our high level of participation in pig necropsy (70% of all village pigs), both factors which set this study apart from prior analyses conducted by our group. Despite these strengths, we were still only able to purchase 70% of pigs for necroscopic examination, meaning that some infected pigs were likely not included in the analysis. Furthermore, among those pigs that were included, we were unable to attribute the source of infection for all infected pigs, as some pigs were found far from detected tapeworm carriers. Future research using microsatellite DNA markers to link cysts found in pigs to their specific parent tapeworm may help to better understand the spatial and environmental mechanism of T. solium transmission.33
In conclusion, we demonstrated significant clustering of viable porcine cysticercosis infection in the immediate surroundings of confirmed T. solium tapeworm carriers. This study contributes to a growing body of evidence that T. solium tapeworm carriers represent important sources of infection to pigs raised in their immediate vicinity. The highly focal transmission pattern that was described here is relevant for geographically targeted control interventions that have shown early success in Peru. Continued investigation of the spatial dynamics of T. solium transmission will allow for further improvements in the effectiveness and efficiency of targeted approaches to control T. solium transmission in Peru and around the world.
Acknowledgments:
We thank the population of the study sites and local health workers for their support and cooperation.
REFERENCES
- 1.Commission on Tropical Diseases of the International League Against Epilepsy , 1994. Relationship between epilepsy and tropical diseases. Epilepsia 35: 89–93. [PubMed] [Google Scholar]
- 2.Lescano AG, García HH, Gilman RH, Guezala MC, Tsang VCW, Gavidia CM, Rodriguez S, Moulton LH, Green JA, Gonzalez AE; Cysticercosis Working Group in Peru , 2007. Swine cysticercosis hotspots surrounding Taenia solium tapeworm carriers. Am J Trop Med Hyg 76: 376–383. [PubMed] [Google Scholar]
- 3.O’Neal SE, et al. 2012. Geographic correlation between tapeworm carriers and heavily infected cysticercotic pigs. PLoS Negl Trop Dis 6: e1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pray IW, Ayvar V, Gamboa R, Muro C, Moyano LM, Benavides V, Flecker RH, Garcia HH, O’Neal SE, 2017. Spatial relationship between Taenia solium tapeworm carriers and necropsy cyst burden in pigs. PLoS Negl Trop Dis 11: e0005536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Neal SE, Moyano LM, Ayvar V, Rodriguez S, Gavidia C, Wilkins PP, Gilman RH, Garcia HH, Gonzalez AE; Cysticercosis Working Group in Peru , 2014. Ring-screening to control endemic transmission of Taenia solium. PLoS Negl Trop Dis 8: e3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia HH, et al. 2016. Elimination of Taenia solium transmission in northern Peru. N Engl J Med 374: 2335–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsang VC, Brand JA, Boyer AE, 1989. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J Infect Dis 159: 50–59. [DOI] [PubMed] [Google Scholar]
- 8.Tsang VCW, Pilcher JA, Zhou W, Boyer AE, Kamango-Sollo EIP, Rhoads ML, Murrell KD, Schantz PM, Gilman RH, 1991. Efficacy of the immunoblot assay for cysticercosis in pigs and modulated expression of distinct IgM/ IgG activities to Taenia solium antigens in experimental infections. Vet Immunol Immunopathol 29: 69–78. [DOI] [PubMed] [Google Scholar]
- 9.Jayashi CM, Gonzalez AE, Castillo Neyra R, Rodríguez S, García HH, Lightowlers MW, 2014. Validity of the enzyme-linked immunoelectrotransfer blot (EITB) for naturally acquired porcine cysticercosis. Vet Parasitol 199: 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muro C, et al. 2017. Porcine cysticercosis: possible cross-reactivity of taenia hydatigena to GP50 antigen in the enzyme-linked immunoelectrotransfer blot assay. Am J Trop Med Hyg 97: 1830–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engel HN, Claire LE, 1986. Anatomy. Leman AD, Straw BE, Mengelin WL, eds. Diseases of Swine. Ames, IA: Iowa State University Press, 3–25. [Google Scholar]
- 12.Bustos JA, et al. 2012. Detection of Taenia solium taeniasis coproantigen is an early indicator of treatment failure for taeniasis. Clin Vaccine Immunol 19: 570–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allan JC, Avila G, Garcia Noval J, Flisser A, Craig PS, 1990. Immunodiagnosis of taeniasis by coproantigen detection. Parasitology 101: 473–477. [DOI] [PubMed] [Google Scholar]
- 14.Pearson RD, Hewlett EL, 1985. Niclosamide therapy for tapeworm infections. Ann Intern Med 102: 550–551. [DOI] [PubMed] [Google Scholar]
- 15.Flisser A, Madrazo I, Plancarte A, Schantz P, Allan J, Craig P, Sarti E, 1993. Neurological symptoms in occult neurocysticercosis after single taeniacidal dose of praziquantel. Lancet 342: 748. [DOI] [PubMed] [Google Scholar]
- 16.Allan JC, Velasquez-Tohom M, Torres-Alvarez R, Yurrita P, Garcia-Noval J, 1996. Field trial of the coproantigen-based diagnosis of Taenia solium taeniasis by enzyme-linked immunosorbent assay. Am J Trop Med Hyg 54: 352–356. [DOI] [PubMed] [Google Scholar]
- 17.Tello R, Terashima A, Marcos LA, Machicado J, Canales M, Gotuzzo E, 2012. Highly effective and inexpensive parasitological technique for diagnosis of intestinal parasites in developing countries: spontaneous sedimentation technique in tube. Int J Infect Dis 16: 2011–2013. [DOI] [PubMed] [Google Scholar]
- 18.Mayta H, Talley A, Gilman RH, Jimenez J, Verastegui M, Ruiz M, Garcia HH, Gonzalez AE, 2000. Differentiating Taenia solium and Taenia saginata infections by simple hematoxylin-eosin staining and PCR-restriction enzyme analysis. J Clin Microbiol 38: 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yolken RH, Stopa PJ, 1979. Analysis of nonspecific reactions in enzyme-linked immunosorbent assay testing for human rotavirus. J Clin Microbiol 10: 703–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allan JC, Wilkins PP, Tsang VCW, Craig PS, 2003. Immunodiagnostic tools for taeniasis. Acta Trop 87: 87–93. [DOI] [PubMed] [Google Scholar]
- 21.Sikasunge CS, Johansen MV, Willingham AL, Leifsson PS, Phiri IK, 2008. Taenia solium porcine cysticercosis: viability of cysticerci and persistency of antibodies and cysticercal antigens after treatment with oxfendazole. Vet Parasitol 158: 57–66. [DOI] [PubMed] [Google Scholar]
- 22.Nelder JA, Wedderburn RWM, 1972. Generalized lineal models. J R Stat Soc Ser A 135: 370–384. [Google Scholar]
- 23.Pray IW, Swanson DJ, Ayvar V, Muro C, Moyano LM, Gonzalez AE, Garcia HH, O’Neal SE; Cysticercosis Working Group in Peru , 2016. GPS tracking of free-ranging pigs to evaluate ring strategies for the control of cysticercosis/taeniasis in Peru. PLoS Negl Trop Dis 10: e0004591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pawlowsky Z, 2002. Taenia solium: basic biology and transmission. Singh G, Prabhakar S, eds. Taenia solium Cysticercosis: From Basic to Clinical Science. New York, NY: CABI Publishing, 1–14. [Google Scholar]
- 25.Braae UC, Harrison W, Lekule F, Magnussen P, Johansen MV, 2015. Feedstuff and poor latrines may put pigs at risk of cysticercosis—a case-control study. Vet Parasitol 214: 187–191. [DOI] [PubMed] [Google Scholar]
- 26.Huerta M, et al. 2008. Parasite contamination of soil in households of a Mexican rural community endemic for neurocysticercosis. Trans R Soc Trop Med Hyg 102: 374–379. [DOI] [PubMed] [Google Scholar]
- 27.Thevenet PS, Jensen O, Drut R, Cerrone GE, Grenóvero MS, Alvarez HM, Targovnik HM, Basualdo JA, 2005. Viability and infectiousness of eggs of Echinococcus granulosus aged under natural conditions of inferior arid climate. Vet Parasitol 133: 71–77. [DOI] [PubMed] [Google Scholar]
- 28.Wachira TM, Macpherson CN, Gathuma JM, 1991. Release and survival of Echinococcus eggs in different environments in Turkana, and their possible impact on the incidence of hydatidosis in man and livestock. J Helminthol 65: 55–61. [DOI] [PubMed] [Google Scholar]
- 29.Ilsøe B, Kyvsgaard NC, Nansen P, Henriksen SA, 1990. A study on the survival of Taenia saginata eggs on soil in Denmark. Acta Vet Scand 31: 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coman BJ, Rickard MD, 1977. A comparison of in vitro and in vivo estimates of the viability of Taenia pisiformis eggs aged under controlled conditions, and their ability to immunise against a challenge infection. Int J Parasitol 7: 15–20. [DOI] [PubMed] [Google Scholar]
- 31.Lawson JR, Gemmell MA, 1990. Transmission of taeniid tapeworm eggs via blowflies to intermediate hosts. Parasitology 100: 143–146. [DOI] [PubMed] [Google Scholar]
- 32.Gomez-Puerta LA, Lopez-Urbina MT, Garcia HH, Gonzalez AE, 2014. Longevity and viability of Taenia solium eggs in the digestive system of the beetle Ammophorus rubripes. Rev Bras Parasitol Veterinária 23: 94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pajuelo MJ, et al. 2015. Identification and characterization of microsatellite markers derived from the whole genome analysis of Taenia solium. PLoS Negl Trop Dis 9: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]