Abstract.
Cystic echinococcosis (CE) is a parasitic zoonosis caused by the larval stage of the tapeworm Echinococcus granulosus. Detection of the adult stage in the canine definitive host is essential for estimating infection rates, surveillance and monitoring of CE control programs. This study sought to develop and validate a coproantigen sandwich enzyme–linked immunosorbent assay (copro-ELISA), based on antibodies against E. granulosus–soluble membrane antigens (EGMA), that is capable of distinguishing infected and noninfected dogs. Anti-E. granulosus polyclonal immunoglobulin G antibodies were obtained from rabbit antiserum against EGMA. Optimization of the test was performed with 51 positive and 56 negative stool samples of canine echinococcosis. Specificity, sensitivity, cross-reactivity, intra- and inter-assay precision, and over time detection were evaluated. According to the receiver operating characteristic analysis, the diagnostic sensitivity and specificity were 96.1% (CI: 85.9–99.6) and 98.2% (CI: 89.5–100), respectively. Negative and positive predictive values were 96.5% (CI: 91.7–100) and 98% (CI: 94.1–100), respectively. No cross-reactivity with Taenia hydatigena, Dipylidium caninum, or Toxocara canis was observed. Intra- and inter-assay repeatability showed values of less than 15% of the variation coefficient. The over time detection was from 20 to 27 days postinfection with E. granulosus. The copro-ELISA based on EGMA detection offers a simplified in-house development of diagnostic testing. This assay showed high specificity and sensitivity and had no cross-reactivity with other parasites. Further studies and development of this test in a kit format may be useful for the detection of active infection in dogs living in CE endemic regions.
INTRODUCTION
Cystic echinococcosis (CE) or hydatidosis is a neglected tropical zoonosis that affects low-income countries worldwide. Human CE prevalence is around 10% in endemic countries and leads to a mortality rate of up to 5%.1,2 Cystic echinococcosis is an important public health problem in South America. In Peru, for example, 3,000 new human cases are reported annually, and the endemic areas of the country have an annual incidence of more than 100 cases/10,000 inhabitants.3
The CE disease is caused by the larval stage of the cestode Echinococcus granulosus. The adult worm infects dogs and other canids, which serve as definitive hosts. These animals spread eggs through their feces to the environment during the approximately 3-year patent period.4 Humans and livestock, the intermediate hosts, become orally infected with oncospheres that develop from larval protoscoleces into cysts, mainly within the lungs or liver.5 Clinical symptoms depend on the location of the cyst in affected organs; in addition, spontaneous rupture of the cysts may cause secondary infections or anaphylaxis.6
Echinococcus granulosus sensu stricto (genotype 1) is responsible for a majority of human CE and has a wide distribution often associated with transmission by sheep, as intermediate hosts,7 to dogs, as definitive hosts.8 In endemic areas, the prevalence of this helminth infection in dogs has been reported to range from 32% to 88%.9 The detection of the parasite in the definitive host is crucial for estimating the frequency of canine echinococcosis as well as important for surveillance and monitoring of CE control programs in humans.10
Conventional diagnostic techniques in dogs include microscopic observation of eggs in feces, counting of adult worms after purgation with arecoline hydrobromide, molecular tools such as polymerase chain reaction (PCR), and immunoassays such as indirect or capture enzyme–linked immunosorbent assay (ELISA) and western blot. Microscopic observation is not convenient and not recommended for the detection and differentiation of E. granulosus eggs because of their similarity with other members of the Taeniidae family. By comparison, molecular tools provide improved specificity and variable sensitivity during the prepatent period.11
Coproantigen sandwich ELISA (copro-ELISA) is another specific and sensitive laboratory test for diagnosing canine echinococcosis in stool samples.11 As parasites release metabolic products into the definitive host intestine, coproantigens can be used for immunological detection.12 This tool has been used in different CE geographical regions, and its utility and ease of use has been confirmed in epidemiological surveys.13 However, there are currently no commercially available kits for E. granulosus coproantigen diagnosis.
Several copro-ELISA tests have been developed in different countries with variable sensitivity and specificity, 78–100% and 85–95%, respectively.11 Traditional copro-ELISA includes a preliminary step of pre-adsorption of polyclonal antibodies against E. granulosus somatic antigens that are then treated with normal feces from noninfected dogs. This reduces the background of cross-reactions with noninfected samples.14 Nonetheless, cross-reaction with Taenia hydatigena remains problematic for E. granulosus differentiation, and efforts to improve assay specificity using monoclonal antibodies have not decreased the cross-reactivity.11
A copro-ELISA technique with higher sensitivity and specificity is needed to implement CE surveillance and control programs, which requires a precise, reproducible, and relatively affordable test to diagnose the active parasite infection in dogs. This study sought to evaluate and validate a copro-ELISA that avoids pre-adsorption step, detects the soluble antigens of the adult stage of E. granulosus, and distinguishes between the stool samples of infected and noninfected dogs.
METHODS
Study design.
Three dogs were infected with protoscoleces of E. granulosus. The adult parasites were recovered and the soluble membrane antigens were isolated. A pair of rabbits were immunized to produce antisera against E. granulosus–soluble membrane antigens (EGMA). A copro-ELISA was optimized for the detection of parasitic antigens in 51 and 56 stool samples of dogs infected and noninfected with E. granulosus, respectively. Another three dogs only infected with T. hydatigena, Dipylidium caninum or Toxocara canis were also included. Repeatability, specificity, sensitivity, positive and negative predictive values, cross-reactivity with the non-E. granulosus parasites, and over time detection of the coproantigens from the three experimentally infected dogs with protoscoleces were evaluated.
Ethics statements.
Experimentally infected dogs and immunized rabbits were raised according to the Institutional Animal Care and Use Committee. The protocol of this study No. 2016-001 was approved by the Ethics and Animal Welfare Committee of the Facultad de Medicina Veterinaria, Universidad Nacional Mayor de San Marcos, Lima, Peru. Dogs were handled according to biosafety guidelines of the institution and received two antiparasitic treatments (5 mg/kg praziquantel, 45 mg/kg fenbendazole, and 10 mg/kg pyrantel pamoate) at 10 weeks of the experimental infection.
Isolation of EGMA.
Three healthy male mixed breed dogs of 5 months of age were orally infected with 100,000 viable protoscoleces obtained from a pool of hydatid cysts from naturally infected sheep from the Peruvian Central Highlands. The viability of the protoscoleces was determined by staining with 0.1% eosin.15 The E. granulosus parasites were confirmed as genotype 1 by PCR.16 After 68 days of experimental infection, the adult worms were recovered from the dogs using arecoline hybromide purgation (4 mg/kg). The parasites were washed and centrifuged several times with cold phosphate-buffered saline (PBS) and then placed in Tris-buffered saline (10 mM Tris, 140 mM NaCl, pH 8.02) mixed with 2% Triton X-114 (Sigma-Aldrich, St. Louis, MO) for 2 hours at 4°C while slightly shaking.17 Inhibitory proteases such as pepstatin, leupeptin (1/1,000; Sigma-Aldrich), and pefabloc (1/100; Sigma-Aldrich) were used to avoid protein degradation. The parasites in the buffer were centrifuged at 10,000 × g for 15 minutes at 4°C, and the supernatant was collected and incubated at 37°C for 5 minutes to induce phase separation. The hydrophilic EGMA proteins (upper phase) were recovered and treated again as described previously. The protein concentration was measured with the Bradford assay as previously described.18
Obtaining of purified polyclonal rabbit antibodies against EGMA.
Two-month-old New Zealand rabbits weighing approximately 1.8 kg were immunized with a single batch of 50 µg/mL of EGMA that had been homogenized with Freund’s adjuvant (v/v). The homogenized antigen was administered subcutaneously four times (the first time with complete Freund’s adjuvant, the other times with incomplete Freund’s adjuvant) every 2 weeks. Blood samples were taken each time and 2 weeks after the final immunization to determine the titer of the anti-EGMA hyperimmune serum by indirect ELISA.19 The rabbits were euthanized by intravenous administration of pentobarbital (60 mg/kg). Total serum was obtained from centrifugation of the exsanguinated blood at 5,000 × g for 10 minutes, and it was stored at −70°C until use.
A single batch of rabbit immunoglobulin G (IgG) antibodies was purified from the serum by chromatographic affinity using a column prepacked with Protein G Sepharose according to the manufacturer’s instructions (HiTrap Protein G; General Electric, Boston, MA). The Bradford assay was used to quantify the recovered IgG antibodies. Conjugation of anti-EGMA IgG antibodies with peroxidase was performed with a commercial kit using the manufacturer’s instructions (SureLINK HRP Conjugation Kit; KPL, Milford, MA).
In-house copro ELISA.
The stool samples of 51 dogs infected with adult E. granulosus (diagnosed using the gold standard of necropsy) were used as positive samples of canine echinococcosis. The feces (5 g) had been previously mixed and stored with 5% neutral buffered formalin (v/v 1:4). These positive samples were obtained from the fecal bank of the Laboratorio de Epidemiología y Economía Veterinaria, Facultad de Medicina Veterinaria, Universidad Nacional Mayor de San Marcos, Lima, Peru. Samples of the fecal bank were from experimentally dogs infected with E. granulosus. The sampling day, postinfection with E. granulosus, and the parasite burden at the time of the necropsy for each sample were registered.
In addition, 56 dogs from nonendemic areas of CE were included as known negative field samples of canine echinococcosis to evaluate the diagnostic specificity. The negative animals were treated at least twice with anthelminthic drugs (as described previously) before the samples were obtained. A copro-parasitological sedimentation test20 was performed to determine whether any parasite eggs remained after treatment (parasite-free status). The negative stool samples were collected 2 weeks after the last treatment and homogenized with 5% neutral buffered formalin (v/v 1:4). Each sample was then centrifuged at 3,500 × g for 30 minutes, and the supernatant was collected and stored at room temperature until use.
A sandwich copro-ELISA based on the capture and detection of somatic E. granulosus antigens in stool samples14 was used with considerable modifications. A 96-well microplate (Immulon 4HBX; Thermo Fisher Scientific, Waltham, MA) was coated with 0.8 µg/mL of anti-EGMA polyclonal IgG antibodies in 0.05 M carbonate/bicarbonate buffer at pH 9.6 and incubated at 4°C overnight. Five washes with 200 µL of 0.15 M PBS mixed with 0.1% polysorbate 20 (Tween 20; Sigma-Aldrich) were performed after each incubation. Blocking of the micro-wells was performed with 200 µL of PBS mixed with 0.3% Tween 20 for 1 hour at room temperature. The stool sample supernatant (50 µL) was dispersed with 50 µL of heat inactivated fetal bovine serum (Corning, Corning, NY) and then incubated for 1 hour at room temperature while being slightly shaken. Detection was performed with 0.9 µg/mL of the anti-EGMA polyclonal IgG antibodies previously conjugated with peroxidase and incubated for 1 hour at room temperature while being slightly shaken. The commercial chromogenic substrate 3,3′,5,5′-tetramethylbenzidine (TMB) (100 µL) was mixed with 0.02% hydrogen peroxide (v/v) to reveal the reaction in the dark according to the manufacturer’s instruction (TMB 2-Component Microwell Peroxidase Substrate Kit; SeraCare, Milford, MA). The reaction was stopped with 50 µL of 2 M sulfuric acid, and the results were immediately read by a spectrophotometer at 450 nm (Molecular Devices, San Jose, CA). Optical densities (OD) were registered by the SoftMax Pro 5.0 software (Molecular Devices).
Repeatability.
The intra-assay precision was evaluated in one microplate using 21 replicates representing four concentrations of EGMA: low (1 µg/mL), medium (2.5 µg/mL), high (5 µg/mL), and not detectable (negative for the presence of EGMA). The inter-assay precision was also performed with 21 replicates of the concentration types described previously over four different microplates on the same day. The mean OD and the coefficient of variation (CV) for each sample type was calculated for between one and four microplates.21
Diagnostic sensitivity and specificity.
Fifty-one positive and 56 negative samples for canine echinococcosis were blind tested for sensitivity and specificity. The negative predicted value (NPV) and the positive predictive value (PPV) were estimated; 95% confidence intervals were calculated for all data. Positive (stool samples with EGMA) and negative (stool samples without EGMA) controls were included for the validation of each run per microplate. Normalization of the OD values was performed according to the sample-to-positive (S/P) ratio for each sample:22 (OD sample − OD negative control)/(OD positive control − OD negative control). The optimal cutoff value was estimated using the receiver operating characteristic (ROC) analysis from all the S/P ratios of the positive and negative samples of canine echinococcosis. The diagnostic sensitivity was calculated as true positives (over the cutoff value)/(true positives + false negatives), whereas specificity was calculated as true negatives (under the cutoff value)/(true negatives + false positives). Positive predictive value was calculated as true positives/(true positives + false positives) and NPV was calculated as true negatives/(false negatives + true negatives).23
Detection limit and cross-reactivity.
Serial dilutions of known concentrations of EGMA were artificially spiked with the supernatant of a negative stool sample. Three replicates per point were run, and the detection limit was considered as the minimum concentration more than the cutoff value.
The analytical specificity (cross-reactivity) was also evaluated in triplicate using three stool samples containing proglottids or eggs from one dog experimentally infected with eight cysts of T. hydatigena (sampled at 68 days post-infection), and two more dogs naturally infected either with T. canis or D. caninum.
Over time determination.
Stool samples from the three dogs infected with the protoscoleces of E. granulosus were collected weekly after infection until the day of purgation with arecoline (after 10 weeks). The copro-ELISA was performed as described previously to determine the first day after the infection in which the test was positive.
Statistical analysis.
Data analysis and the ROC curve were used to estimate the diagnostic sensitivity and specificity, and the NPV and PPV. The Spearman’s correlations of the S/P ratio with the parasite burden (quantity of adult worms per dog) and the S/P ratio with the sampling day (postinfection with protoscoleces of E. granulosus) of the 51 positive samples were also calculated. All statistical analyses were carried out using the SPSS 25 software, and the significance level was set at 0.05.
RESULTS
The intra-assay repeatability of the 21 replicates resulted in average CV values of less than 15% for all stool sample concentration types: high (8%), medium (6%), low (7%), and negative (6%). Inter-assay repeatability of the 84 replicates distributed on the four microplates (336 replicates) on the same day resulted in CV values of less than 15% for all concentration types: high (7%), medium (4%), low (6%), and negative (13%).
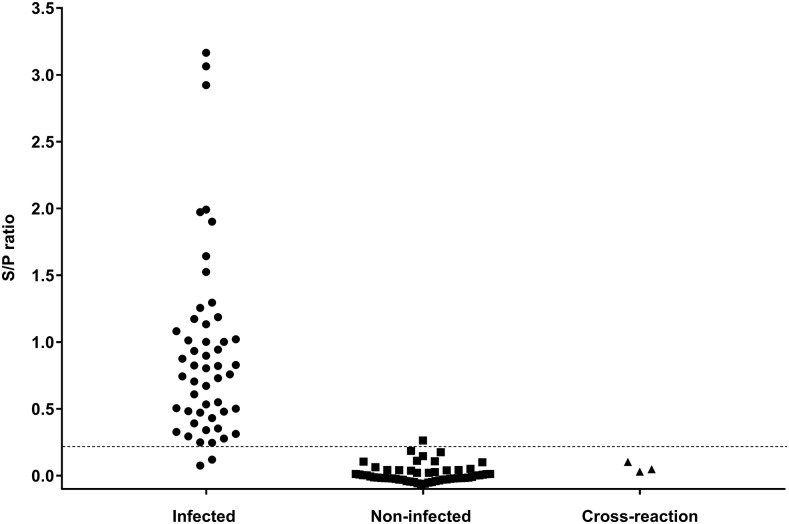
The cutoff value of the copro-ELISA was calculated based on 51 positive and 56 negative samples for canine echinococcosis (Table 1). The optimal cutoff value was 0.216, according to the ROC analysis with an area under the curve (AUC) of 0.995 (CI: 95%: 0.988–1). Therefore, the sensitivity and specificity of the test were 96.1% (CI: 95%: 85.9–99.6) and 98.2% (CI: 95%: 89.5–100), respectively. The NPV and PPV were 96.5% (CI: 95%: 91.7–100) and 98% (CI: 95%: 94.1–100), respectively. The distribution of all positive and negative samples with respect to the cutoff value is summarized in Figure 1.
Table 1.
Distribution of the echinococcal samples according to the copro-ELISA and the gold standard test
| Infected with Echinococcus granulosus | Total | |||
|---|---|---|---|---|
| Yes* | No† | |||
| Copro-ELISA | Positive | 49 | 1 | 50 |
| Negative | 2‡ | 55 | 57 | |
| Total | 51 | 56 | 107 | |
Copro-ELISA = coproantigen sandwich enzyme-linked immunosorbent assay.
* Diagnosed by necropsy as the gold standard test.
† Diagnosed by copro-parasitological sedimentation test.
‡ One dog had only one E. granulosus and another one was sampled at 20 days postinfection.
Figure 1.
Distribution of infected (n = 51), noninfected (n = 56), and cross-reaction (n = 3) dogs diagnosed by Copro-enzyme–linked immunosorbent assay for Echinococcus granulosus. The dotted line determines the cutoff value (0.216). Circles show dogs infected with E. granulosus, squares show noninfected dogs, and triangles show dogs infected with Taenia hydatigena, Dipylidium caninum, or Toxocara canis.
The detection limit was 0.5–1 µg of EGMA per 1 mL of stool supernatant, and there was no cross-reaction with the T. hydatigena (eight adult worms), T. canis, or D. caninum samples (Figure 1). All 56 negative samples for canine echinococcosis were negative for Taenia spp. eggs as confirmed by the copro-parasitological sedimentation test. One sample of this group was positive for Giardia sp. and one was positive for Ancylostoma sp. by copro-parasitological sedimentation test, but neither of these samples showed cross-reaction in the copro-ELISA for E. granulosus.
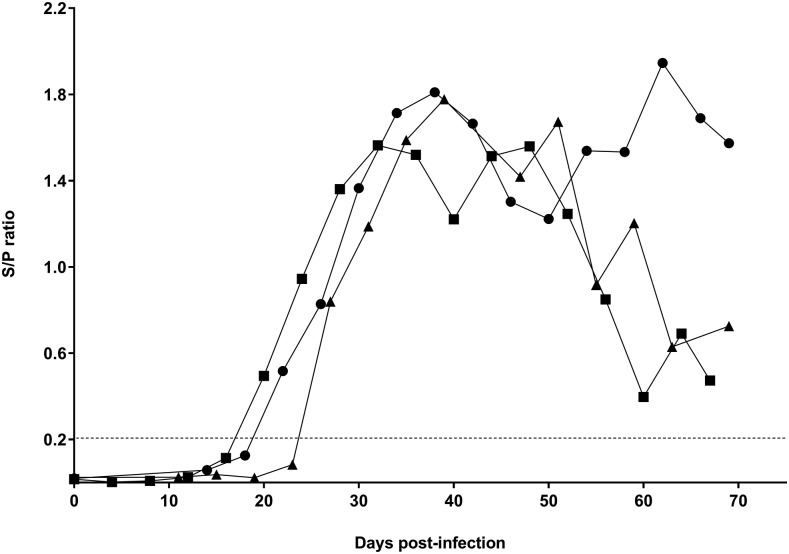
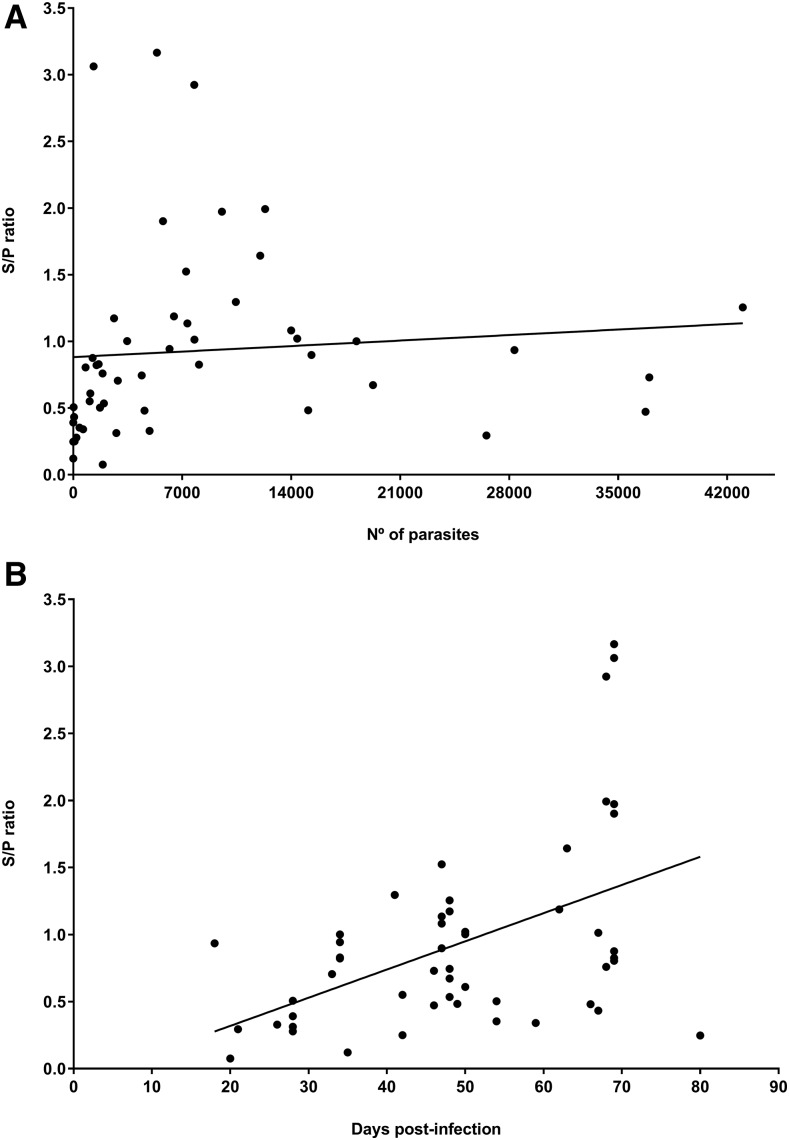
The over time determination of the three infected dogs with protoscoleces of E. granulosus was positive by copro-ELISA from 20 to 27 days postinfection (Figure 2). The Spearman’s correlation of the S/P ratio and the parasite burden was statistically moderate (ρ = 0.476) (Figure 3A). The minimum detectable parasite load by copro-ELISA was two worms. The correlation of the S/P ratio and the postinfection day was also significant but low (ρ = 0.419) (Figure 3B). The minimum detectable sampling day by copro-ELISA was 21 days postinfection with E. granulosus.
Figure 2.
Over time detection by Copro-enzyme–linked immunosorbent assay from the three dogs infected with protoscoleces of Echinococcus granulosus. The dotted line represents the cutoff value (0.216). Circles show dog No. 1 (12,321 adult parasites), squares show dog No. 2 (56 adult parasites), and triangles show dog No. 3 (56 adult parasites).
Figure 3.
Spearman’s correlation. (A) the sample-to-positive (S/P) ratio with the parasite burden (ρ = 0.476, P < 0.001), and (B) the S/P ratio with the postinfection day (ρ = 0.419, P = 0.002) from positive samples of canine echinococcosis (n = 51).
DISCUSSION
This study validated an in-house copro-ELISA for the diagnosis of canine echinococcosis. The sensitivity and specificity were 96.1% and 98.2%, respectively, whereas the NPV and PPV were 96.5% and 98%, respectively.
Few copro-ELISA tests based on the detection of somatic, excretory, or secretory antigens of E. granulosus have been developed with variable sensitivity and specificity.24–27 All of these tests have a higher sensitivity than arecoline purgation and PCR for the detection of prepatent infection in dogs.28 The sensitivity of our test was higher than previously reported copro-ELISAs that used polyclonal antibodies.13,24–26 Two false-negative samples were observed in the present study; however, one of these had only one parasite, whereas the other was sampled at 20 days postinfection with E. granulosus. The parasite burden and the postinfection time may have affected detection due to fewer parasitic antigens being produced by a lower number of worms29 or due to the antigen presentation of immature worms in early infection. Our results suggest that in dogs, our test can detect parasites from 20 days postinfection. By contrast, other copro-ELISA tests have reported early detection before 10 days postinfection with the use of antibodies to anti-E. granulosus somatic crude proteins obtained by sonication.13 It may detect more antigens but are less specific as compared with our anti-EGMA obtained by nonionic detergent which probably isolates outer surface proteins instead of somatic proteins.
The specificity of our test was higher (98.2%) than other published copro-ELISAs, including those that have used monoclonal antibodies.27,30 Our copro-ELISA used anti-EGMA polyclonal antibodies, which probably explains the presence of the one false-positive sample that did not have any parasite eggs in the copro-parasitological sedimentation test. Some fecal sample proteins from the noninfected dogs may have cross-reacted with the polyclonal antibodies because the source of the E. granulosus antigens may have been contaminated with some dog fecal components during the worm isolation step.14 The coproantigens of E. granulosus have been partially characterized; a peptide core and carbohydrate moieties content (α-mannose and/or α-glucose, βgalactose and N-acetyl-β-glucosamine) are likely to be related to molecules derived from the surface tegument of the adult worms.31 Coproantigens of other Echinococcus species such as Echinococcus vogeli, Echinococcus canadensis, or Echinococcus ortleppi have not been studied to be similar each other or to react with anti-E. granulosus antibodies. The total characterization of the EGMA and coproantigens in infected and noninfected dogs may improve the sensitivity and specificity of the polyclonal antibodies against specific EGMA for copro-ELISA tests.
The detection of coproantigens in host feces has several advantages over classical parasitological diagnosis14 and other techniques. For instance, necropsy and examination of a dog’s small intestine for the presence of adult E. granulosus tapeworms is considered as the gold standard technique. However, conducting necropsies is a biohazardous risk, laborious and an ethically questionable procedure.32 By contrast, serological screening is considered unsuitable for reliable diagnosis because there is a poor correlation between serum antibodies and worms29 and because it does not differentiate between present and past infections.13 Finally, although copro-PCR has a high specificity, it presents less sensitivity than coproantigen detection tests during prepatent periods.28 PCR is also a relatively complex and expensive procedure requiring good laboratory facilities; therefore, it has limited application in routine CE surveillance or control programs.5
An important feature of our copro-ELISA is that the anti-EGMA polyclonal antibodies do not require a complex pre-adsorption step with noninfected fecal samples. This reduces the time of development of the specific antibodies and assay compared with the original and previous copro-ELISA that was based on antibodies from total somatic antigens. Polyclonal antibodies also have increased stability and can be generated faster in rabbits, at a lower cost and with less sophisticated technical skills than monoclonal antibodies.33 By contrast to the other copro-ELISA tests developed to date, our test did not show any cross-reactivity with Giardia sp., Ancylostoma sp. or any other common helminths of dogs (mainly T. hydatigena). Despite not displaying cross-reaction with the eight large T. hydatigena worms that were present in one sample, it will be necessary to evaluate the cross-reactivity of the test with other parasites and with a larger number of stool specimens.
One disadvantage of the production of anti-E. granulosus antibodies is the high cost, labor, and biohazardous risk involved in obtaining mature worms in the natural definitive host. In addition, the use of dogs for antigen production may be ethically controversial. The identification of the specific antigens from the protein components of E. granulosus is necessary for further molecular cloning studies that will allow for the recombinant production of EGMA and, therefore, the development of more specific antibodies.
Cystic echinococcosis control programs have been administered in several South American countries with the strategy of treating dog populations with praziquantel to decrease CE until transmission is interrupted.5 It is therefore necessary to form a regional consensus on a reproducible test for diagnosing active canine infection. The utility of a copro-ELISA test for this purpose in endemic areas has been reported in Argentina.11 The AUC of our copro-ELISA was very high (0.995), almost a perfect diagnostic test measurement (AUC = 1) for the differentiation between diseased and nondiseased samples.34 The ROC analysis uses both positive and negative samples and also showed optimal cutoff values depending on different scenarios of sensitivity and specificity that may be considered according to the prevalence of geographical regions worldwide. Considering the high prevalence of dog infection up to 50% in endemic areas of Peru,35 our Copro-ELISA would have good PPV and NPV contributing to the detection of true positive and negative cases. Interlaboratory assays should be used to evaluate the reproducibility (stability) of our copro-ELISA, particularly in other countries with a prevalence of canine echinococcosis. Notably, the test may provide rapid diagnosis of active infections in Peru or other endemic regions. Screening by coproantigen detection may be useful at the beginning and during control programs because the effect of the antiparasitic treatment in dogs may be monitored in real time in different areas and compared with other CE interventions.
In conclusion, the new copro-ELISA based on the detection of EGMA in dogs infected with E. granulosus reduced the time of antibodies development, increased sensitivity, and specificity compared with other copro-ELISAs, detected positive cases during the prepatent period, and did not show any cross-reactivity with common canine helminths tested in this study (T. hydatigena, D. caninum, and T. canis).
Acknowledgments:
We would like to thank Nora Pierangeli, PhD, Yesenia Castillo, BSc, Marco Quispe, MLT, Cristian Roca, MSc, and Karen Ann Alroy, DVM.
REFERENCES
- 1.Chrieki M, 2002. Echinococosis-an emerging parasite in the immigrant population. Am Fam Physician 66: 817–820. [PubMed] [Google Scholar]
- 2.Agudelo Higuita NI, Brunetti E, Mccloskey C, 2015. Cystic echinococcosis. J Clin Microbiol 54: 518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pavletic CF, et al. 2017. Cystic echinococcosis in South America: a call for action. Rev Panam Salud Publica 41: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spickler AR, Roth JA, Galyon J, Lofstedt MV, 2010. Enfermedades Emergentes y Exóticas de los Animales. Ames, IA: Center for Food Security and Public Health. [Google Scholar]
- 5.Craig PS, Hegglin D, Lightowlers MW, Torgerson PR, Wang Q, 2017. Echinococcosis: control and prevention. Adv Parasitol 96: 55–158. [DOI] [PubMed] [Google Scholar]
- 6.Brunetti E, Garcia HH, Junghanss T, 2011. Cystic echinococcosis: chronic, complex, and still neglected. PLoS Negl Trop Dis 5: e1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvarez Rojas CA, Romig T, Lightowlers MW, 2014. Echinococcus granulosus sensu lato genotypes infecting humans—review of current knowledge. Int J Parasitol 44: 9–18. [DOI] [PubMed] [Google Scholar]
- 8.Carmena D, Cardona GA, 2013. Canine echinococcosis: global epidemiology and genotypic diversity. Acta Trop 128: 441–460. [DOI] [PubMed] [Google Scholar]
- 9.Grosso G, Gruttadauria S, Biondi A, Marventano S, Mistretta A, 2012. Worldwide epidemiology of liver hydatidosis including the Mediterranean area. World J Gastroenterol 18: 1425–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casas N, Costas Otero S, Céspedes G, Sosa S, Santillán G, 2013. Detección de coproantígenos para el diagnóstico de echinococosis canina en la zona fronteriza de La Quiaca-Villazón. Rev Argent Microbiol 45: 154–159. [DOI] [PubMed] [Google Scholar]
- 11.Craig P, Mastin A, van Kesteren F, Boufana B, 2015. Echinococcus granulosus: epidemiology and state-of-the-art of diagnostics in animals. Vet Parasitol 213: 132–148. [DOI] [PubMed] [Google Scholar]
- 12.Allan JC, Craig PS, 2006. Coproantigens in taeniasis and echinococcosis. Parasitol Int 55: 75–80. [DOI] [PubMed] [Google Scholar]
- 13.Pierangeli NB, Soriano SV, Roccia I, Bergagna HF, Lazzarini LE, Celescinco A, Kossman AV, Saiz MS, Basualdo JA, 2010. Usefulness and validation of a coproantigen test for dog echinococcosis screening in the consolidation phase of hydatid control in Neuquén, Argentina. Parasitol Int 59: 394–399. [DOI] [PubMed] [Google Scholar]
- 14.Allan JC, et al. 1992. Coproantigen detection for immunodiagnosis of echinococcosis and taeniasis in dogs and humans. Parasitology 104: 347–356. [DOI] [PubMed] [Google Scholar]
- 15.Himonas C, Antoniadou-Sotiriadou K, Papadopoulos E, 1994. Hydatidosis of food animals in Greece: prevalence of cysts containing viable protoscoleces. J Helminthol 68: 311–313. [DOI] [PubMed] [Google Scholar]
- 16.Stefanić S, Shaikenov BS, Deplazes P, Dinkel A, Torgerson PR, Mathis A, 2004. Polymerase chain reaction for detection of patent infections of Echinococcus granulosus (“sheep strain”) in naturally infected dogs. Parasitol Res 92: 347–351. [DOI] [PubMed] [Google Scholar]
- 17.Santos AL, et al. 2006. Phytomonas serpens: cysteine peptidase inhibitors interfere with growth, ultrastructure and host adhesion. Int J Parasitol 36: 47–56. [DOI] [PubMed] [Google Scholar]
- 18.Bradford MM, 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 19.Alzamora-Gonzales L, Echevarria RJ, Colona-Vallejos EH, Aguilar-Luis MA, de Amat-Herbozo CCD, 2016. Desarrollo de ELISA sándwich indirecto para la determinación de antígenos de excreción-secreción de Fasciola hepatica. Rev Peru Biol 23: 47–52. [Google Scholar]
- 20.Tello R, Terashima A, Marcos LA, Machicado J, Canales M, Gotuzzo E, 2012. Highly effective and inexpensive parasitological technique for diagnosis of intestinal parasites in developing countries: spontaneous sedimentation technique in tube. Int J Infect Dis 16: e414–e416. [DOI] [PubMed] [Google Scholar]
- 21.Hanneman SK, Cox CD, Green KE, Kang DH, 2011. Estimating intra- and inter-assay variability in salivary cortisol. Biol Res Nurs 13: 243–250. [DOI] [PubMed] [Google Scholar]
- 22.Giménez-Lirola LG, et al. 2016. Detection of African swine fever virus antibodies in serum and oral fluid specimens using a recombinant protein 30 (p30) dual matrix indirect ELISA. PLoS One 11: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greiner M, Pfeiffer D, Smith RD, 2000. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med 45: 23–41. [DOI] [PubMed] [Google Scholar]
- 24.Deplazes P, Gottstein B, Eckert J, Jenkins DJ, Ewald D, Jimenez-Palacios S, 1992. Detection of Echinococcus coproantigens by enzyme-linked immunosorbent assay in dogs, dingoes and foxes. Parasitol Res 78: 303–308. [DOI] [PubMed] [Google Scholar]
- 25.Elayoubi FA, Fraser A, Jenkins DJ, Craig PS, 2003. Partial characterisation of carbohydrate-rich Echinococcus granulosus coproantigens. Int J Parasitol 33: 1553–1559. [DOI] [PubMed] [Google Scholar]
- 26.Benito A, Carmena D, 2005. Double-antibody sandwich ELISA using biotinylated antibodies for the detection of Echinococcus granulosus coproantigens in dogs. Acta Trop 95: 9–15. [DOI] [PubMed] [Google Scholar]
- 27.Huang Y, et al. 2014. Echinococcus infections in Chinese dogs: a comparison of coproantigen kits. J Helminthol 88: 189–195. [DOI] [PubMed] [Google Scholar]
- 28.Morel N, Lassabe G, Elola S, Bondad M, Herrera S, Marí C, Last JA, Jensen O, Gonzalez-Sapienza G, 2013. A monoclonal antibody-based copro-ELISA kit for canine echinococcosis to support the PAHO effort for hydatid disease control in South America. PLoS Negl Trop Dis 7: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lahmar S, Lahmar S, Boufana B, Bradshaw H, Craig PS, 2017. Screening for Echinococcus granulosus in dogs: comparison between arecoline purgation, coproELISA and coproPCR with necropsy in pre-patent infections. Vet Parasitol 144: 287–292. [DOI] [PubMed] [Google Scholar]
- 30.Siles-Lucas M, Casulli A, Conraths FJ, Müller N, 2017. Laboratory diagnosis of Echinococcus spp. in human patients and infected animals. Adv Parasitol 96: 159–257. [DOI] [PubMed] [Google Scholar]
- 31.Malgor R, Nonaka N, Basmadjian I, Sakai H, Carámbula B, Oku Y, Carmona C, Kamiya M, 1997. Coproantigen detection in dogs experimentally and naturally infected with Echinococcus granulosus by a monoclonal antibody-based enzyme-linked immunosorbent assay. Int J Parasitol 27: 1605–1612. [DOI] [PubMed] [Google Scholar]
- 32.Benito A, Carmena D, Joseph L, Martínez J, Guisantes JA, 2006. Dog echinococcosis in northern Spain: comparison of coproantigen and serum antibody assays with coprological exam. Vet Parasitol 142: 102–111. [DOI] [PubMed] [Google Scholar]
- 33.Lipman N, Jackson LR, Trudel LJ, Weis-Gracia F, 2005. Monoclonal versus polyclonal antibodies: distinguishing characteristics, applications, and information resources. ILAR J 46: 258–268. [DOI] [PubMed] [Google Scholar]
- 34.Hajian-Tilaki K, 2013. Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Caspian J Intern Med 4: 627–635. [PMC free article] [PubMed] [Google Scholar]
- 35.Montalvo R, Clemente J, Castañeda L, Caro E, Cente Y, Nuñez M, 2018. Coproprevalence of canine infestation by Echinococcus granulosus in an endemic hidatidosis district in Peru. Rev Inv Vet Perú 29: 263–269. [Google Scholar]