Abstract.
Prompt and effective treatment is key to malaria control and prevention, as it reduces disease morbidity and mortality and minimizes the number of transmission reservoirs. Transmission reduction may be particularly important among school-age children (SAC, 5–15 years old), who have the highest prevalence of Plasmodium falciparum infection in southern Malawi. We hypothesized that one factor contributing to this difference in prevalence is that SAC are less likely to seek appropriate treatment for fever than children younger than 5 years. In this study, we assessed treatment-seeking behaviors of people of all ages between 2012 and 2014 in Malawi. During each of the five cross-sectional surveys, all members of ∼900 households reported on fever and treatment-seeking in the previous 2 weeks. Multilevel logistic regression was used to analyze predictors of whether febrile people sought treatment and whether they did so at formal (government/private clinics) or informal sources (primarily shops). Twenty-two percent of participants (3,579/16,621) reported fever, and 2,715 of those (75.9%) sought treatment. Seeking treatment exclusively from local shops remains a common practice, although use of recommended diagnostic testing and antimalarial drugs was infrequently reported there. Although SAC were not significantly less likely than children aged < 5 years to seek treatment, SAC and adults (age ≥ 16 years) were significantly less likely to use formal sources. Our results indicate that encouraging treatment at government/private clinics and increasing retail access to appropriate antimalarial testing and treatment, especially among SAC, could help remedy inadequate treatment of symptomatic disease and potentially reduce Plasmodium transmission in Malawi.
INTRODUCTION
Although intensive scale-up in global malaria control efforts made significant progress since the year 2000, the most recent World Malaria Report indicated that progress has recently stagnated, and the majority of malaria deaths (91%) continue to be concentrated in the World Health Organization (WHO) Africa Region.1 Assessing gaps in these control efforts is necessary for continued progress.
Universal diagnosis and prompt effective treatment is one of the pillars of the WHO’s Global Technical Strategy for malaria control.2 Not only does treatment directly reduce disease morbidity and mortality, but the shorter infection duration also indirectly benefits the population by clearing and/or preventing the development of infectious gametocytes in the human population, thereby decreasing human-to-mosquito transmission. The effectiveness of treatment as a control measure depends on: 1) the proportion of infections that are symptomatic, 2) how many symptomatic people choose to seek treatment and where they seek it, 3) whether health care providers can detect infections and provide appropriate treatment, and 4) whether treatment effectively clears the patients’ Plasmodium infections. The present analysis focuses on understanding the second element—which factors affect when and where a symptomatic person chooses to seek care.
Malawi, a small, landlocked nation in sub-Saharan Africa is one place with persistent endemicity, with 4.5 million cases and 7,000 deaths estimated in 2016.1 The Malawian government provides medical consultation and antimalarial medication free of charge in government health-care facilities,3–5 and these facilities are supplied with malaria rapid diagnostic tests (RDTs) and antimalarial drugs. Nonetheless, previous research has reported that limited access to such treatment, based on distance, wait-times, and medicine shortages, may still motivate people to seek care through informal sources such as local shops and traditional healers.6 Treatment is less likely to be appropriate and effective from informal sources because there is no organized effort to train providers about the appropriate diagnosis and management of malaria, there is little monitoring of drug quality in these settings, and compliance with national treatment guidelines tends to be low.7,8 In addition, people pay for their own choice of medication, so there is incentive not to purchase diagnostics and to choose less expensive treatments, which may not be adequate or appropriate.
Many previous reports on treatment-seeking behaviors have focused on young children (age < 5 years), but our research in southern Malawi has highlighted school-age children (SAC, 5–15 years) as key transmission reservoirs for their high Plasmodium falciparum infection prevalence and relatively low rates of bed-net use.9–11 Some previous studies of fever treatment-seeking in sub-Saharan Africa have reported that older children and adults were less likely to attend formal health-care facilities than children younger than 5 years,6,12–15 but this finding was not universal.16 These considerations prompted us to evaluate reported treatment-seeking behaviors in an all-ages sample in Malawi to identify differences in treatment-seeking by age that might represent a potential gap for targeted expansion of control efforts.
A repeated-sample, cross-sectional study collected data on the treatment-seeking practices of individuals in the 2 weeks before the surveys at the end of rainy and dry seasons during 2012 through 2014 in households sampled from three districts in southern Malawi. The present analysis aimed to: 1) assess the general predictors for seeking any treatment for fever over time, and 2) analyze the predictors of seeking treatment for fever from the formal sector (government or private facilities or community health workers [CHWs]) compared with informal sources (shops, traditional healers, or other). We hypothesized that residents of more rural areas, particularly those areas farther from the health facilities, would be less likely to seek formal treatment sources when febrile. We also hypothesized that SAC, who have high prevalence of infection in this population,9 are less likely to seek effective antimalarial treatment for fever through formal health-care facilities than children younger than 5 years.
MATERIALS AND METHODS
The study was carried out as part of the Malawi International Center of Excellence for Malaria Research (U19AI089683) and was approved by the independent institutional review boards of the University of Malawi College of Medicine, the University of Maryland School of Medicine, and Michigan State University.
Study design.
Data were collected in three ecologically diverse districts of southern Malawi during biannual cross-sectional surveys at the end of each rainy (April–May) and dry season (September–October) from 2012 to 2014. The three districts represent different transmission patterns. Blantyre is an urban highlands area with expected low transmission, Thyolo is semi-rural highlands with expected moderate transmission, and Chikhwawa is rural lowlands with expected high transmission. The sampling methods were reported previously.9 Briefly, 10 enumeration areas (EAs) were randomly selected using probability proportional to size in each of the three districts. One compact segment of approximately 30 households was randomly selected and sampled from each EA. In total, ∼900 households were selected and visited for each survey.
During each visit, the field team interviewed household members in the local language (Chichewa) to obtain information about household- and individual-level variables. Data were collected on all household members, whether present or absent at the time of survey, if they had slept in the house more than 2 weeks of the previous month. An adult caregiver responded for all children in the household. Standardized questionnaires based on the Malaria Indicator Survey17 were used to collect household- and individual-level data. Household data included socioeconomic status (SES) variables, house construction, indoor residual spraying, and insecticide treated net (ITN) ownership and quality. Individual-level data included demographics, use of ITNs, receipt of intermittent preventive therapy in pregnancy, and reported recent symptoms of disease and treatments. Specifically, participants or their parents/guardians, in the case of children, were asked whether they had experienced a fever in the previous 2 weeks, whether they had sought treatment or medical advice related to any illness or health concern in the previous 2 weeks, where such treatment was obtained, whether a finger or heel stick had been performed to enable diagnostic testing, and what medications were taken. Some people who said they had not sought treatment outside of the home did report taking medications in the previous 2 weeks; for the purposes of this analysis, these subjects were not classified as having sought treatment. Subjects that reported seeking treatment for fever were also asked the number of days from the onset of fever until they sought care.
Treatment sources were classified as “formal” or “informal.” Formal sources included government hospitals, government health centers, government health posts, government mobile clinics, CHWs, private hospitals/clinics, private pharmacies, private doctors, and private mobile clinics. Informal sources included shops, traditional practitioners, or “other.” Data were not collected on the order of visits, but participants were classified as seeking formal health care if they sought treatment in at least one formal source, regardless of whether they sought additional treatment through informal sources.
Data management and analysis.
All study data were collected and managed in the Research Electronic Data Capture (REDCap) system (Vanderbilt University) hosted at the University of Malawi College of Medicine.18 Data were collected electronically in the field on android-based tablets using OpenDataKit (http://opendatakit.org) and uploaded to REDCap at the end of each day. Geographic locations of study households were captured using the global positioning system (GPS) embedded in the tablets starting from the dry season of 2013. Earlier surveys may not have included precisely the same households, but were sampled from the same compact segment. Secondary data on the locations of health facilities was gathered from the Malawi Spatial Data Platform (MASDAP, www.masdap.mw), administrated by the National Spatial Data Center in Lilongwe, Malawi. Geographic data were managed and all maps created using ArcGIS version 10.4.1 (Esri, Redlands, CA).
Distance to health-care facilities was calculated as a potential predictor of interest. The “mean center” method in ArcMap was used to estimate the centroid of each compact segment. Locations of health-care facilities were provided by MASDAP. The proximity analysis tool in ArcMap was used to calculate the Euclidean distance in kilometers (km) from the centroid of households in each EA to the nearest health facility of any kind.
Socioeconomic status variables were collected at the household level. Ten variables pertaining to asset ownership and wealth were combined into a single indicator variable based on the method suggested by Filmer and Pritchett.19 The 10 variables (radio, bike, car, house, phone, and television ownership, access to electricity in the home, whether or not the head of household had earned income, food shortages in the previous month, and highest education achieved by the head of household or spouse) were weighted based on principal component analysis using proc factor. Households were then grouped into quartiles of weighted index scores for each survey.
Not all participants knew their exact age in months or years, but virtually everyone could be categorized as either: young children (age < 5 years), SAC (age, 5–15 years), or adults (age ≥ 16 years). The ownership of bed nets was assessed at the household level; in households that owned at least one bed net, we identified which individuals were reported to have slept under a net on the previous night. Net ownership/usage was included as a potential predictor as it may reflect an overall perception of malaria risk that could also influence treatment-seeking behavior.
All data analysis was performed using Statistical Analysis Systems (SAS) version 9.4 (SAS Institute, Cary, NC). Analyzed outcomes were either binomial (sought treatment or not or sought treatment at a formal source versus informal source) or time to event (time from onset of fever to seeking treatment). All predictors were categorical except the distances to health facilities, which were estimated continuously in kilometers from the center of each EA’s compact segment of 30 households. For univariate analyses of binomial outcomes, Wilcoxon–Mann–Whitney and Chi-squared tests of association were used for the continuous predictors and categorical predictors, respectively. Fisher’s exact test was used when cells had expected values less than five.
Logistic regression was used to analyze the predictors of seeking treatment when febrile. Multilevel models with random intercepts were developed to account for clustering of treatment-seeking behaviors at the household and EA levels. For those who reported fever, the Kaplan–Meier method was used to plot the time in days from the onset of fever to seeking treatment, stratified by various potential predictors. The log-rank test was applied as a test for homogeneity of the stratified survival curves, and Cox proportional hazards regression was used to calculate unadjusted and adjusted hazard ratios for the key predictors. Among those who reported being febrile and seeking treatment in the previous 2 weeks, multilevel logistic regression was also used to assess the predictors of seeking any formal as opposed to exclusively informal treatment sources, again including random intercepts for the household and EA levels.
RESULTS
Symptom and treatment-related questionnaire data were available for between 3,175 and 3,528 participants per survey, including three dry season surveys and two rainy season surveys (Table 1). Overall, 20.2% of people in the dry season and 23.5% in the rainy season reported a fever in the previous 2 weeks (P < 0.0001). People reporting fever were significantly more likely to be from the rural districts than from Blantyre (P < 0.0001), to live in households from lower wealth quartiles (P < 0.0001), and to be from households where the head or their spouse had lower levels of schooling (P < 0.0001). School-age children were less likely to have had a fever (15.5%) than adults (23.4%) or children younger than 5 years (28.7%, P < 0.0001).
Table 1.
Characteristics of the study population
| Characteristic | Febrile* | ||
|---|---|---|---|
| n | n (%) | P-value | |
| Season | |||
| Dry | 10,090 | 2,042 (20.2%) | < 0.0001 |
| Rainy | 6,531 | 1,537 (23.5%) | |
| Survey | |||
| Dry season 2012 | 3,528 | 848 (24.0%) | < 0.0001 |
| Rainy season 2013 | 3,356 | 727 (21.7%) | |
| Dry season 2013 | 3,216 | 630 (19.6%) | |
| Rainy season 2014 | 3,175 | 810 (25.5%) | |
| Dry season 2014 | 3,346 | 564 (16.9%) | |
| Household-level factors | |||
| District | |||
| Blantyre | 5,389 | 1,006 (18.7%) | < 0.0001 |
| Thyolo | 5,393 | 1,105 (20.5%) | |
| Chikhwawa | 5,839 | 1,468 (25.1%) | |
| Household wealth index | |||
| Lowest quartile | 3,943 | 959 (24.3%) | < 0.0001 |
| Second quartile | 4,031 | 865 (21.5%) | |
| Third quartile | 4,192 | 882 (21.0%) | |
| Highest quartile | 4,339 | 834 (19.2%) | |
| House construction quality | |||
| Mostly unfinished | 7,494 | 1,782 (23.8%) | < 0.0001 |
| Mostly finished | 9,121 | 1,796 (19.7%) | |
| Eaves | |||
| Closed | 12,362 | 2,542 (20.6%) | < 0.0001 |
| Open | 4,251 | 1,036 (24.4%) | |
| Highest education level achieved by the head of household or spouse | |||
| No schooling | 2,484 | 613 (24.7%) | 0.0006 |
| Some standard level school | 9,050 | 1,908 (21.1%) | |
| Some education past standard 8 | 5,060 | 1,053 (20.8%) | |
| Unknown | 27 | 5 (18.5%) | |
| Individual-level factors | |||
| Gender | |||
| Female | 9,794 | 2,214 (22.6%) | < 0.0001 |
| Male | 6,827 | 1,365 (20.0%) | |
| Net use/access on previous night | |||
| No nets owned in household | 2,806 | 594 (21.2%) | < 0.0001 |
| Nets owned but not used by person | 4,111 | 764 (18.6%) | |
| Person slept under a net | 9,701 | 2,221 (22.9%) | |
| Age | |||
| Children younger than 5 years | 3,184 | 914 (28.7%) | < 0.0001 |
| School-age children (age 5–15 years) | 6,114 | 945 (15.5%) | |
| Adults (age > 15 years) | 7,299 | 1,711 (23.4%) | |
| Unknown | 24 | 9 (37.5%) | |
Bolded P-values were those < 0.05.
* Denotes subjects who were reported as having fever in the previous 2 weeks, the previous 48 hours, or both.
Treatment-seeking when febrile.
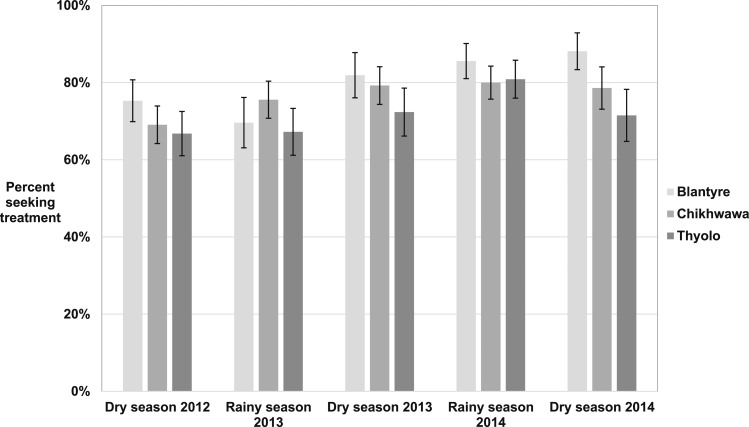
Overall, a high proportion of febrile people (70.2–81.9% each survey) reported seeking treatment throughout the study. The proportion seeking treatment generally increased over time, and was higher in Blantyre than Thyolo or Chikhwawa, although there was considerable heterogeneity within each district (Figure 1). No clear difference between rainy and dry seasons was apparent.
Figure 1.
Percent of febrile people that reported seeking treatment in the previous 2 weeks by district over five cross-sectional surveys in southern Malawi, 2012–2014.
The spatial pattern of treatment-seeking proportions varied among EAs and in relation to the health facility locations (Supplemental Figure 1). In Blantyre, the distance from each EA to the nearest health facility was shortest, ranging from 0.24 to 2.36 km, with an average of 1.26 km (standard deviation [SD]: 0.64) and a median of 1.11 km. In Thyolo, the distance ranged from 1.51 to 5.03 km, with an average of 2.83 km (SD: 1.09) and a median of 2.46 km. In Chikhwawa, the distance ranged from 0.58 to 7.65 km, with an average of 4.69 km (SD: 2.24) and a median of 4.88 km.
Predictors of treatment-seeking following reported fever in the previous 2 weeks were analyzed using multilevel logistic regression models with random intercepts to account for potential household- and EA-level clustering (Table 2). The random intercept was statistically significant in the household level of the final model (P < 0.0001), suggesting that there was additional unexplained variation among households. District was crudely associated with odds of seeking treatment, but the association was not significant after adjusting for distance from health facilities. District was nonetheless retained in the final model because it had a significant interaction with distance to health facility. Each 1 km increase in distance to the nearest dispensary/maternity ward was associated with statistically significantly lower odds of seeking treatment with a febrile illness in Thyolo (OR = 0.77, 95% CI = 0.68–0.88) and Chikhwawa (OR = 0.90, 95% CI = 0.84–0.95), but not Blantyre (OR = 1.10, 95% CI = 0.84–1.43).
Table 2.
Predictors of seeking any treatment in the previous 2 weeks among people reporting fever
| Characteristic | Febrile n | Sought any treatment n (%) | Crude OR (95% CI) | Multilevel* adjusted OR (95% CI) |
|---|---|---|---|---|
| Season | ||||
| Dry | 2,042 | 1,533 (75.1%) | 1.0 (ref) | – |
| Rainy | 1,537 | 1,182 (76.9%) | 1.10 (0.95–1.29) | – |
| Survey | ||||
| Dry season 2012 | 848 | 595 (70.2%) | 1.0 (Ref) | 1.0 (ref) |
| Rainy season 2013 | 727 | 519 (71.4%) | 1.06 (0.85–1.32) | 1.05 (0.82–1.34) |
| Dry season 2013 | 630 | 490 (77.8%) | 1.49 (1.17–1.89) | 1.58 (1.21–2.07) |
| Rainy season 2014 | 810 | 663 (81.9%) | 1.92 (1.52–2.42) | 1.95 (1.51–2.53) |
| Dry season 2014 | 564 | 448 (79.4%) | 1.64 (1.28–2.11) | 1.69 (1.28–2.24) |
| Household-level factors | ||||
| District | ||||
| Blantyre | 1,006 | 804 (79.9%) | 1.0 (ref) | 1.0 (ref)† |
| Thyolo | 1,105 | 793 (71.8%) | 0.64 (0.52–0.78) | 0.75 (0.50–1.13) |
| Chikhwawa | 1,468 | 1,118 (76.2%) | 0.80 (0.66–0.98) | 1.11 (0.72–1.71) |
| Distance to the nearest health facility (km) | Median: 2.456 | 0.92 (0.89–0.95) | – | |
| Range: 0.243–7.653 | ||||
| Blantyre | – | – | – | 1.10 (0.84–1.43) |
| Thyolo | – | – | – | 0.77 (0.68–0.88) |
| Chikhwawa | – | – | – | 0.90 (0.84–0.95) |
| Household wealth index | ||||
| Lowest quartile | 959 | 684 (71.3%) | 0.58 (0.47–0.73) | 0.67 (0.50–0.90) |
| Second quartile | 865 | 654 (75.6%) | 0.73 (0.57–0.91) | 0.81 (0.60–1.08) |
| Third quartile | 882 | 676 (76.6%) | 0.77 (0.61–0.97) | 0.82 (0.62–1.08) |
| Highest quartile | 834 | 676 (81.1%) | 1.0 (ref) | 1.0 (ref) |
| House construction quality | ||||
| Mostly unfinished | 1,782 | 1,319 (74.0%) | 1.0 (ref) | – |
| Mostly finished | 1,796 | 1,395 (77.7%) | 1.22 (1.05–1.42) | – |
| Eaves | ||||
| Closed | 2,542 | 1,910 (75.1%) | 1.0 (ref) | – |
| Open | 1,036 | 805 (77.7%) | 1.15 (0.97–1.37) | – |
| Highest education achieved by head of household or spouse | ||||
| No schooling | 613 | 438 (71.5%) | 0.58 (0.46–0.73) | – |
| Some standard level school | 1,908 | 1,417 (74.3%) | 0.67 (0.56–0.81) | – |
| Some education past standard 8 | 1,053 | 855 (81.2%) | 1.0 (Ref) | – |
| Individual-level factors | ||||
| Gender | ||||
| Female | 2,214 | 1,676 (75.7%) | 1.0 (ref) | – |
| Male | 1,365 | 1,039 (76.1%) | 1.02 (0.87–1.20) | – |
| Net use/access on previous night | ||||
| No nets in household | 594 | 420 (70.7%) | 0.68 (0.55–0.83) | 0.67 (0.53–0.85) |
| Nets in household, but not used by person | 764 | 561 (73.4%) | 0.78 (0.64–0.94) | 0.78 (0.63–0.97) |
| Person slept under a net | 2,221 | 1,734 (78.1%) | 1.0 (ref) | 1.0 (ref) |
| Age | ||||
| Children younger than 5 years | 914 | 732 (80.1%) | 1.0 (ref) | 1.0 (ref) |
| School-aged children (age 5–15 years) | 945 | 723 (76.5%) | 0.81 (0.65–1.01) | 0.87 (0.68–1.10) |
| Adults (age > 15 years) | 1,711 | 1,253 (73.2%) | 0.68 (0.56–0.83) | 0.72 (0.58–0.88) |
Bolded values are those that are significant at P < 0.05.
* Random intercepts included at the enumeration area (EA) and household levels. There was significant unexplained variation at the household level in the final model (P < 0.0001) but not the EA level. Estimates are adjusted for all other variables listed in this column.
† The final model included a significant interaction between district and distance to the nearest health facility. The estimated OR for each district was presented at 2.5 km from the nearest health facility.
People in the lowest wealth quartile were significantly less likely to seek treatment than people in the highest wealth quartile (OR = 0.67, 95% CI = 0.50–0.90). After adjusting for distance to health facility and household wealth index, other variables reflecting lower SES (education level of the head of household or their spouse) were not significantly associated with odds of treatment-seeking.
The proportion of febrile people seeking treatment decreased with age, from 80.1% of children younger than 5 years, to 76.5% of SAC, to 73.2% of adults. The difference between SAC and children younger than 5 years was not statistically significant (OR = 0.87, 95% CI = 0.68–1.10), but the decreased odds for adults were significantly lower than those of young children (OR = 0.72, 95% CI = 0.58–0.88).
Results of the Cox regression for time to seeking treatment after fever onset were similar to those presented in Table 2 and have been included in Supplemental Table 2.
Sources of treatment and diagnosis.
Although the proportion of febrile people seeking treatment was high, use of only informal sources was common. Among the 2,715 people whose treatment source was known, 1,114 (41.0%) visited informal sources, primarily shops (n = 1,102). Very few reported visiting a traditional practitioner (n = 6) or “other” source (n = 6). Only 32 people (1.2%) reported using multiple treatment sources—most commonly a formal source and a shop.
Multilevel logistic regression was used to assess predictors of seeking treatment through a formal source among the febrile people who sought treatment (Table 3). People with a lower household wealth index were less likely to seek treatment at formal sources, although adjustment for distance to health facilities accounted for part of the observed crude association and the difference was not statistically significant in the final multilevel model (lowest versus highest quartile, OR = 0.88, 95% CI = 0.65–1.20). Other predictors of seeking treatment at formal sources—season of survey, gender, and age category—corresponded with expected perceptions of malaria risk. Regarding the key hypothesis of this analysis, young children who sought treatment for fever were more likely to use a formal source (69.3%) than both SAC and adults (57.1% and 55.9%, respectively). These differences were statistically significant in the final model, with the OR for SAC estimated at 0.59 (95% CI = 0.46–0.74) and the OR for adults at 0.52 (95% CI = 0.42–0.65) relative to children younger than 5 years.
Table 3.
Predictors of seeking treatment in a formal vs. informal source in the previous 2 weeks among people reporting fever that sought any treatment
| Characteristic | Sought any treatment n | Used a formal* treatment source n (%) | Crude OR (95% CI) | Multilevel† adjusted OR (95% CI) |
|---|---|---|---|---|
| Season | ||||
| Dry | 1,533 | 876 (57.1%) | 1.0 (ref) | 1.0 (ref) |
| Rainy | 1,182 | 747 (63.2%) | 1.29 (1.10–1.51) | 1.28 (1.07–1.54) |
| Survey | ||||
| Dry season 2012 | 595 | 329 (55.3%) | 1.0 (ref) | – |
| Rainy season 2013 | 519 | 323 (62.2%) | 1.33 (1.05–1.69) | – |
| Dry season 2013 | 490 | 286 (58.4%) | 1.13 (0.89–1.44) | – |
| Rainy season 2014 | 663 | 424 (64.0%) | 1.43 (1.14–1.80) | – |
| Dry season 2014 | 448 | 261 (58.3%) | 1.13 (0.88–1.45) | – |
| Household-level factors | ||||
| District | ||||
| Blantyre | 804 | 538 (66.9%) | 1.0 (ref) | 1.0 (ref)*** |
| Thyolo | 793 | 455 (57.4%) | 0.67 (0.54–0.82) | 0.77 (0.32–1.81) |
| Chikhwawa | 1,118 | 630 (56.4%) | 0.64 (0.53–0.77) | 0.95 (0.38–2.37) |
| Distance to the nearest health facility of any kind (km) | Median: 2.36 | 0.92 (0.89–0.95) | – | |
| Range: 0.24–7.65 | ||||
| Blantyre | – | – | – | 0.89 (0.51–1.56) |
| Thyolo | – | – | – | 1.12 (0.80–1.55) |
| Chikhwawa | – | – | – | 0.92 (0.79–1.07) |
| Household wealth index | ||||
| Lowest quartile | 684 | 384 (56.1%) | 0.65 (0.52–0.81) | 0.88 (0.65–1.20) |
| Second quartile | 654 | 368 (56.3%) | 0.65 (0.52–0.81) | 0.84 (0.62–1.12) |
| Third quartile | 676 | 402 (59.5%) | 0.74 (0.59–0.93) | 0.91 (0.68–1.21) |
| Highest quartile | 676 | 449 (66.4%) | 1.0 (ref) | 1.0 (ref) |
| House construction quality | ||||
| Mostly unfinished | 1,319 | 739 (56.0%) | 1.0 (ref) | – |
| Mostly finished | 1,395 | 884 (63.4%) | 1.36 (1.16–1.58) | – |
| Eaves | ||||
| Closed | 1,910 | 1,186 (62.1%) | 1.0 (ref) | – |
| Open | 805 | 437 (54.3%) | 0.73 (0.61–0.86) | – |
| Highest education achieved by head of household or spouse | ||||
| No schooling | 438 | 251 (57.3%) | 0.76 (0.60–0.96) | – |
| Some standard level school | 1,417 | 821 (57.9%) | 0.78 (0.65–0.93) | – |
| Some education past standard 8 | 855 | 546 (63.9%) | 1.0 (Ref) | – |
| Individual-level factors | ||||
| Gender | ||||
| Female | 1,676 | 1,020 (60.9%) | 1.0 (ref) | 1.0 (ref) |
| Male | 1,039 | 603 (58.0%) | 0.89 (0.76–1.04) | 0.81 (0.67–0.97) |
| Net use/access on previous night | ||||
| No nets in household | 420 | 244 (58.1%) | 0.92 (0.74–1.14) | – |
| Nets in household, but not used by person | 561 | 336 (59.9%) | 0.99 (0.82–1.20) | – |
| Person slept under a net | 1,734 | 1,043 (60.1%) | 1.0 (ref) | – |
| Age | ||||
| Child (age < 5 years) | 732 | 507 (69.3%) | 1.0 (ref) | 1.0 (ref) |
| School-aged child (age 5–15 years) | 723 | 413 (57.1%) | 0.59 (0.48–0.73) | 0.59 (0.46–0.74) |
| Adult (age ≥ 16 years) | 1,253 | 700 (55.9%) | 0.56 (0.46–0.68) | 0.52 (0.42–0.65) |
* Formal treatment sources were any government or private health-care sources, including hospitals/centers/posts, mobile clinics, private doctors, and community health workers. Informal treatment sources were shops, traditional practitioners, or “other,” which was typically described as the use of treatments provided by neighbors or family members. People were classified as seeking formal treatment if they reported using at least one formal treatment source.
† Random intercepts included at the enumeration area (EA) and household levels. There was significant unexplained variation at the household level in the final model (P < 0.0001) but not the EA level. Final model included all variables that were significant in the multivariable model with fixed effects.
‡ The final model included an interaction between district and distance to the nearest health facility. The estimated OR for each district was presented for a distance of 2.5 km from the nearest health facility.
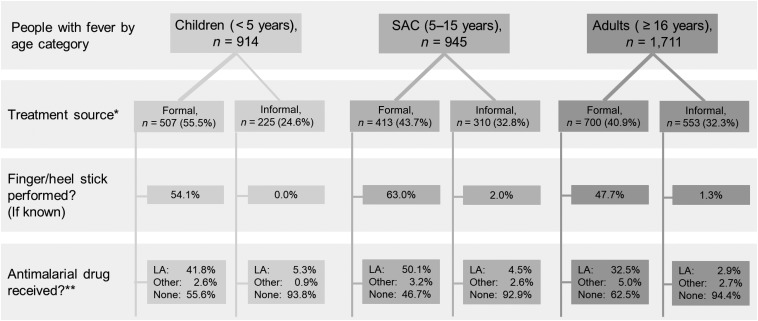
In Malawi, the Ministry of Health recommends that patients reporting with fever be tested for Plasmodium parasites with malaria RDTs or microscopy and given artemisinin combination therapies (ACTs), typically lumefantrine artemether (LA), if positive.4 Only 1.2% using the informal sector and 53.5% using the formal sector reported having a finger or heel stick for diagnostic testing. Although antimalarial drugs are potentially available, in practice, antimalarial treatment remains relatively uncommon in the informal sector. During the dry and rainy season surveys, respectively, 35.2% and 53.6% of febrile people seeking formal care reported taking an antimalarial. However, from those seeking exclusively informal sources of care, the corresponding numbers were 4.1% and 9.4%. School-age children more frequently received a heel stick and an antimalarial drug than children of other age groups when they sought treatment from formal sources, but diagnostic testing and antimalarial drug use were low in all age groups that used informal sources (Figure 2).
Figure 2.
Treatment source, diagnostic testing, and antimalarial drug use reported among febrile people by age. LA = lumefantrine artemether; SAC = school-age children. *Remainder of febrile people did not report seeking treatment. **Other antimalaria drug options included chloroquine, quinine, or sulfadoxine/pyrimethamine.
DISCUSSION
Like many other sub-Saharan nations, malaria persists in Malawi, and progress in control efforts has stagnated.1 Previous investigations into treatment-seeking in Malawi, and other malaria-endemic nations, tended to focus solely on children younger than 5 years.6,20–24 We studied treatment-seeking among people of all ages from several different transmission settings in Malawi, and these data support the findings from several other analyses: school-age children are likely important P. falciparum transmission reservoirs in this area. We previously found that SAC have the highest prevalence of P. falciparum infections,9 the highest prevalence of gametocytes,25 and are the least likely to sleep under ITNs.10 The present analysis found that febrile SAC are also less likely than children younger than 5 years to seek treatment from formal sources. In informal sources, the recommended use of diagnostic testing and antimalarial treatment was rare. This represents a missed opportunity for malaria control, as incompletely treated infections in SAC may persist for months, are associated with adverse health effects, and may contribute to ongoing parasite transmission.26–32
To design interventions to effectively increase rates of appropriate care, qualitative and quantitative studies are needed to identify why SAC and adults in Malawi are less likely to seek formal health care for fever than younger children. Several studies have suggested that the perception of lower malaria severity in older children is the primary explanation for this difference.12,33 Mothers in these studies further noted that older children were more capable of taking tablets and explaining their symptoms, minimizing the need for formal evaluation by health-care providers.12,33 School-age children may also prioritize school and work commitments, as informal sources of care may necessitate less time loss than formal sources.13 These decisions may also be impacted by beliefs that health-care facilities with limited resources would prioritize treatment of children younger than 5 years, although a recent study of formal health facilities in the area reported high compliance with universal diagnosis and treatment guidelines for all age groups.34 It is also possible that SAC or their caregivers may have reduced power to negotiate for household time and resources to travel to a formal clinic for care than for children younger than 5 years. Many studies have researched caretaker decision-making for treatment of children younger than 5 years, but SAC have increasing independence from their caretakers and may gradually acquire responsibility for treatment decisions. Studies must therefore assess these and other hypotheses in both the children and their caretakers, and the degree of decision-making agency of each, to fully characterize these patterns.
Overall, treatment-seeking for fever was common, with higher proportions compared with previous reports from Malawi6,21–24 and a statistically significant increase from 2012 (70.2%) to 2014 (79.4%) in this study. This phenomenon may reflect some degree of biased self-reporting to conform to study staff expectations across the multiple survey interactions; however, it supports the idea that efforts to control malaria have been successful in encouraging prompt treatment-seeking behaviors in the community. Nonetheless, the use of informal health facilities such as retail shops continues to be common despite policies for free provision of antimalarial treatment through government facilities. Our analysis showed that ∼40% of people who sought care for fever, did so only from informal sources such as local retail shops where diagnostic testing and antimalarial drug use were rarely reported. Effective solutions depend on the reasons for choosing informal facilities and the reasons RDTs and ACTs are not commonly purchased there. If these choices are motivated by lack of availability or cost limitations, in the absence of more accessible government facilities, expanded distribution of free or subsidized malaria RDTs and ACTs through CHWs and local shops for people of all ages might help increase the use of appropriate treatment measures from the informal sector. Several trials across Africa have found that subsidies on RDTs and ACTs substantially increase their purchase and appropriate use for antimalaria treatment in retail shops.35–40 If age-related differences are more attributable to perceptions of malaria risks, informational campaigns would be needed to encourage the use of recommended care. This study did not disentangle the reasons for different treatment-seeking choices.
There were some additional limitations of this analysis. First, we did not have access to RDT or microscopy results from the dates of treatment-seeking to evaluate whether the reported use or nonuse of ACTs was appropriate. A recent analysis reported that adherence to recommendations was high in formal health-care facilities of Malawi where diagnostic testing was available34; however, the extremely low use of diagnostic tests (< 2%) confirms that Malawians do not often receive the recommended malaria care in the informal sector. Furthermore, all fever, treatment-seeking, diagnostic, and antimalarial drug variables were based on self-report and/or caregiver reports for children, so mild episodes of fever were likely to be under-recognized and underreported, particularly for SAC compared with younger children. If the symptoms of malaria were less severe in SAC and adults, or less recognized and reported by the caregivers, fever would be disproportionately underreported in these groups. The proportion of febrile SAC and adults seeking treatment would correspondingly be overestimated. We also lacked specific details about the quality of the health facilities and resources available, which may have influenced treatment source decisions. If local health facilities are known to be undersupplied and understaffed, people may more readily use shops, despite the theoretical promise of free, government-provided malaria care. Future research should further examine the reasons for treatment-seeking choices to guide intervention policies.
CONCLUSION
When febrile, > 70% of people in this cross-sectional study in southern Malawi chose to seek treatment. However, among those who sought treatment, SAC and adults were more likely to do so in retail shops outside the formal health sector and were subsequently less likely than people who used the formal health-care system to receive the diagnostic testing and ACTs recommended by the Malawian Ministry of Health. Provision of subsidized RDTs and ACTs through retail shops may improve access to prompt and appropriate malaria diagnosis and treatment for SAC and adults, although education campaigns may also be needed to encourage appropriate treatment of clinical malaria in these age groups. Such improvements in treatment delivery could help to enhance the health of these children and adults, as well as reducing persistent sources of parasite transmission in this and other malaria-endemic settings.
Supplementary Material
Acknowledgments:
Our sincere thanks to the field team, staff coordinators, laboratory technicians, and study participants from the cross-sectional studies, without whom this analysis would not be possible, including Nelson Chimbiya, Jacqueline Fiore, Kondwani Nkanaunena, and Alick Sixpence. Additional thanks to Nicole Dear for assistance with ArcGIS.
Note: Supplemental figure and table appears at www.ajtmh.org.
REFERENCES
- 1.World Health Organization , 2017. World Malaria Report 2017. Geneva, Switzerland: WHO. [Google Scholar]
- 2.World Health Organization Global Malaria Programme , 2015. Global Technical Strategy for Malaria 2016–2030. Geneva, Switzerland: WHO. [Google Scholar]
- 3.World Health Organization , 2009. WHO Country Cooperation Strategy 2008–2013: Malawi. Brazzaville, Republic of Congo: WHO Regional Office for Africa. [Google Scholar]
- 4.Government of Malawi Ministry of Health , 2013. Guidelines for the Treatment of Malaria in Malawi, 4th edition Lilongwe, Malawi: National Malaria Control Programme, Community Health Sciences Unit. [Google Scholar]
- 5.Malawi Ministry of Health , 2009. Malawi Standard Treatment Guidelines, 4th edition Lilongwe, Malawi: Malawi Ministry of Health. [Google Scholar]
- 6.Ewing VL, Lalloo DG, Phiri KS, Roca-Feltrer A, Mangham LJ, SanJoaquin MA, 2011. Seasonal and geographic differences in treatment-seeking and household cost of febrile illness among children in Malawi. Malar J 10: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galactionova K, Tediosi F, De Savigny D, Smith T, Tanner M, 2015. Effective coverage and systems effectiveness for malaria case management in sub-Saharan African countries. PLoS One 10: e0127818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansson EW, Gething PW, Hildenwall H, Mappin B, Petzold M, Peterson SS, Selling KE, 2014. Diagnostic testing of pediatric fevers: meta-analysis of 13 national surveys assessing influences of malaria endemicity and source of care on test uptake for febrile children under five years. PLoS One 9: e95483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walldorf JA, et al. 2015. School-age children are a reservoir of malaria infection in Malawi. PLoS One 10: e0134061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchwald A, et al. 2016. Bed net use among school-aged children after a universal bed net campaign in Malawi. Malar J 15: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coalson JE, Cohee LM, Buchwald AG, Nyambalo A, Kubale J, Seydel KB, Mathanga D, Taylor TE, Laufer MK, Wilson ML, 2018. Simulation models predict that school-age children are responsible for most human-to-mosquito P. falciparum transmission in southern Malawi. Malar J 17: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molyneux CS, Mung’ala-Odera V, Harpham T, Snow RW, 1999. Maternal responses to childhood fevers: a comparison of rural and urban residents in coastal Kenya. Trop Med Int Health 4: 836–845. [DOI] [PubMed] [Google Scholar]
- 13.Mujica Mota RE, Lara AM, Kunkwenzu ED, Lalloo DG, 2009. Health seeking behavior after fever onset in a malaria-endemic area of Malawi. Am J Trop Med Hyg 81: 935–943. [DOI] [PubMed] [Google Scholar]
- 14.Chuma J, Okungu V, Molyneux C, 2010. Barriers to prompt and effective malaria treatment among the poorest population in Kenya. Malar J 9: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vialle-Valentin CE, LeCates RF, Zhang F, Ross-Degnan D, 2015. Treatment of febrile illness with artemisinin combination therapy: prevalence and predictors in five African household surveys. J Pharm Policy Pract 8: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guyatt HL, Snow RW, 2004. The management of fevers in Kenyan children and adults in an area of seasonal malaria transmission. Trans R Soc Trop Med Hyg 98: 111–115. [DOI] [PubMed] [Google Scholar]
- 17.Roll Back Malaria Monitoring and Evaluation Reference Group, World Health Organization, United Nations Children’s Fund, MEASURE DHS, MEASURE Evaluation, US Centers for Disease Control and Prevention , 2005. Malaria Indicator Survey: Basic Documentation for Survey Design and Implementation. Calverton, MD: World Health Organization. [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG, 2009. Research electronic data capture (REDCap)—a metadata driven methodology and workflow process for providing translational research informatict support. J Biomed Inform 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filmer D, Pritchett L, 2001. Estimating wealth effects without expenditure data—or tears: an application to educational enrollments in states of India. Demography 38: 115–132. [DOI] [PubMed] [Google Scholar]
- 20.Geldsetzer P, Williams TC, Kirolos A, Mitchell S, Ratcliffe LA, Kohli-Lynch MK, Bischoff EJ, Cameron S, Campbell H, 2014. The recognition of and care seeking behaviour for childhood illness in developing countries: a systematic review. PLoS One 9: e93427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holtz TH, Kachur SP, Marum LH, Mkandala C, Chizani N, Roberts JM, Macheso A, Parise ME, 2003. Care seeking behaviour and treatment of febrile illness in children aged less than five years: a household survey in Blantyre District, Malawi. Trans R Soc Trop Med Hyg 97: 491–497. [DOI] [PubMed] [Google Scholar]
- 22.Kazembe LN, Appleton CC, Kleinschmidt I, 2007. Choice of treatment for fever at household level in Malawi: examining spatial patterns. Malar J 6: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oyekale AS, 2015. Assessment of Malawian mothers’ malaria knowledge, healthcare preferences and timeliness of seeking fever treatments for children under five. Int J Environ Res Public Health 12: 521–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weil A, 2003. Home management of fever in children in Zomba, Malawi. Malawi Med J 15: 95–98. [PMC free article] [PubMed] [Google Scholar]
- 25.Coalson JE, et al. 2016. High prevalence of Plasmodium falciparum gametocyte infections in school-age children using sensitive molecular detection: patterns and predictors of risk from a cross-sectional study in southern Malawi. Malar J 15: 527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen I, Clarke SE, Gosling R, Hamainza B, Killeen G, Magill A, O’Meara W, Price RN, Riley EM, 2016. “Asymptomatic” malaria: a chronic and debilitating infection that should be treated. PLoS Med 13: e1001942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L, 2013. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther 11: 623–639. [DOI] [PubMed] [Google Scholar]
- 28.Nankabirwa J, Brooker SJ, Clarke SE, Fernando D, Gitonga CW, Schellenberg D, Greenwood B, 2014. Malaria in school-age children in Africa: an increasingly important challenge. Trop Med Int Health 19: 1294–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonçalves BP, et al. 2017. Examining the human infectious reservoir for Plasmodium falciparum malaria in areas of differing transmission intensity. Nat Commun 8: 1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeffery GM, Eyles DE, 1954. The duration in the human host of infections with a Panama strain of Plasmodium falciparum. Am J Trop Med Hyg 3: 219–224. [DOI] [PubMed] [Google Scholar]
- 31.Abdel-Wahab A, Ali E, Suleiman S, Ahmed S, Walliker D, Babiker HA, 2002. Dynamics of gametocytes among Plasmodium falciparum clones in natural infections in an area of highly seasonal transmission. J Infect Dis 185: 1838–1842. [DOI] [PubMed] [Google Scholar]
- 32.Nassir E, Abdel-Muhsin AM, Suliaman S, Kenyon F, Kheir A, Geha H, Ferguson HM, Walliker D, Babiker HA, 2005. Impact of genetic complexity on longevity and gametocytogenesis of Plasmodium falciparum during the dry and transmission-free season of eastern Sudan. Int J Parasitol 35: 49–55. [DOI] [PubMed] [Google Scholar]
- 33.Chuma J, Abuya T, Memusi D, Juma E, Akhwale W, Ntwiga J, Nyandigisi A, Tetteh G, Shretta R, Amin A, 2009. Reviewing the literature on access to prompt and effective malaria treatment in Kenya: implications for meeting the Abuja targets. Malar J 8: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Namuyinga RJ, et al. 2017. Health worker adherence to malaria treatment guidelines at outpatient health facilities in southern Malawi following implementation of universal access to diagnostic testing. Malar J 16: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Opiyo N, Yamey G, Garner P, 2016. Subsidising artemisinin-based combination therapy in the private retail sector. Cochrane Database Syst Rev 3: CD009926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabot OJ, Mwita A, Cohen JM, Ipuge Y, Gordon M, Bishop D, Odhiambo M, Ward L, Goodman C, 2009. Piloting the global subsidy: the impact of subsidized artemisinin-based combination therapies distributed through private drug shops in rural Tanzania. PLoS One 4: e6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rutta E, et al. 2011. Increasing access to subsidized artemisinin-based combination therapy through accredited drug dispensing outlets in Tanzania. Health Res Policy Syst 9: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morris A, Ward A, Moonen B, Sabot O, Cohen JM, 2015. Price subsidies increase the use of private sector ACTs: evidence from a systematic review. Health Policy Plan 30: 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mbonye AK, Magnussen P, Lal S, Hansen KS, Cundill B, Chandler C, Clarke SE, 2015. A cluster randomised trial introducing rapid diagnostic tests into registered drug shops in Uganda: impact on appropriate treatment of malaria. PLoS One 10: e0129545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen JL, Yadav P, Moucheraud C, Alphs S, Larson PS, Arkedis J, Massaga J, Sabot O, 2013. Do price subsidies on artemisinin combination therapy for malaria increase household use? Evidence from a repeated cross-sectional study in remote regions of Tanzania. PLoS One 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.