Abstract
A recombinant humanized anticocaine monoclonal antibody, h2E2, has shown potential in the preclinical phases for the treatment of cocaine abuse. The standard tests for cocaine usage are the detection of benzoylecgonine (BE) and cocaine in the urine. This includes workplace drug screens as well as in clinical trials for potential treatments of cocaine abuse. By sequestering cocaine into the plasma compartment, h2E2 prevents cocaine from entering the brain. Due to the altered disposition of cocaine in the presence of h2E2, we investigated the effects of h2E2 on cocaine and metabolite levels in the urine of rats to clarify the use of BE as an endpoint measurement for effectiveness in future clinical trials. The urine concentrations of cocaine and metabolites were considerably altered in the presence of h2E2. After a single injection of h2E2 (120 mg/kg) and cocaine hydrochloride (0.56 mg/kg), the concentration of cocaine and BE excreted into the urine of rats decreased by 92% and 91%, respectively, from vehicle controls. Due to the significant decrease in urinary excretion, BE is not an appropriate indicator of cocaine usage in the presence of h2E2. Another endpoint measurement must be selected for the measurement of cocaine usage in the upcoming clinical trials of h2E2. In contrast to the effects on cocaine and BE urinary excretion, there was a 3-fold increase in ecgonine methyl ester (EME) in the presence of h2E2. Therefore, we conclude that EME is a more appropriate measurement of cocaine intake in the presence of h2E2.
Introduction
After decades of research, there is still no Food and Drug Administration approved pharmacological treatment for cocaine abuse. Immunotherapies have been on the rise for the past decade and show potential as treatments for cocaine abuse (Kosten and Owens, 2005). Antibodies targeting small molecule drugs are known to alter the distribution of their targeted drug. Anticocaine antibodies have been shown to bind to cocaine and prevent its entry into the brain (Norman and Ball, 2012). A monoclonal antimethamphetamine antibody has also been shown to decrease brain concentrations of methamphetamine, while increasing plasma concentrations (Laurenzana et al., 2003). By altering the disposition of a drug of abuse and preventing it from reaching its target in the brain, antibodies could prove to be an effective treatment for drugs of abuse.
A novel recombinant humanized anticocaine monoclonal antibody (mAb), designated h2E2, is at an advanced stage of preclinical development for the treatment of cocaine abuse. This novel mAb has a high sequence homology with the human IgG subtype 1 isotype, a molecular weight of about 150 kDa, and a high affinity for cocaine (Kd = 2.2 ± 0.3 nM) (Norman et al., 2014, Wetzel et al., 2017). The mAb, h2E2, has been shown to antagonize the entry of cocaine into the brains of both rats and mice (Norman et al., 2007, 2014), and decrease the probability of cocaine-induced relapse in a self-administration rat model (Wetzel et al., 2016). These characteristics predict that h2E2 has the potential to be an effective immunotherapy for decreasing the probability of relapse in cocaine addicts.
The measurements of cocaine and its metabolite benzoylecgonine (BE) in urine are standard tests for cocaine intake. This includes workplace drug screens and clinical trials of a cocaine vaccine as an indicator of effectiveness (Martell et al., 2009, Kosten et al., 2014). Similarly, the effectiveness of h2E2 in clinical trials would likely be determined by urine levels of cocaine and/or BE. However, h2E2’s binding to cocaine may prevent its metabolism into BE and/or prevent urinary clearance, which could confound the results of a clinical trial.
To our knowledge, the effects of an antidrug antibody on urine excretion of the target drug and its metabolites have never been reported. Therefore, we investigated the effects of the anticocaine mAb, h2E2, on cocaine and metabolite levels in the urine of rats to clarify the use of these measures in determining the effectiveness of h2E2 in clinical trials.
Materials and Methods
Animals.
Twelve male, Sprague-Dawley cocaine-naive rats (250–325 g) were used during the course of this study (Harlan Laboratories, Indianapolis, IN). Rats were housed individually on a 14/10-hour light/dark (6 AM to 8 PM on/8 PM to 6 AM off) cycle with unrestricted access to food and water. All studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council (U.S.) Committee for the Update of the Guide for the Care and Use of Laboratory Animals, 2011) and under a protocol approved by the Institutional Animal Care and Use Committee at the University of Cincinnati.
Catheter Implantation.
Rats were implanted with an indwelling catheter into the right jugular vein under isoflurane anesthesia. Buprenorphine (0.03 mg/rat, s.c.) was administered postsurgery for pain control and gentamycin (25 mg/rat, s.c.) was used for 3 days to prevent infection following surgery. Rats were allowed 5 days to recover before the study began.
Baseline Urine Collections.
After the 5-day recovery period from catheter implantation surgery, rats were placed individually in metabolic cages and allowed a 48-hour acclimation period. The urine accumulated during this time was discarded. After the acclimation period, urine was collected every 6 hours in a 24-hour time period to provide baseline measurements for urine output and kidney function. Food and water intake was also measured and recorded during this 24-hour baseline period.
h2E2 Infusions.
After the 24-hour baseline urine collection period, a dose of 123 mg/kg of h2E2 [(n = 6), 18.15 mg/ml in 10 mM phosphate-buffered saline, pH 7] or an equivalent volume of vehicle [(n = 6), phosphate-buffered saline, pH 7] was infused intravenously over 2 minutes. One hour after h2E2 or vehicle infusion, an equimolar dose to h2E2 binding sites of cocaine hydrochloride (0.56 mg/kg) was rapidly injected via the jugular catheter. Due to the existence of two complimentary determining regions on h2E2, it is assumed that two molecules of cocaine can bind to h2E2 at the same time. The binding of one cocaine molecule to h2E2 is assumed not to inhibit or promote the binding of a second cocaine molecule. Rats were returned to their metabolic chambers, and urine was collected every 6 hours for 24 hours with food, water, and urine output also measured during this period.
Cocaine and Metabolite Quantification by Liquid-Chromatography/Electrospray Ionization–Mass Spectrometry.
Cocaine, BE, ecgonine methyl ester (EME), and norcocaine were quantified using liquid-chromatography/electrospray ionization–mass spectrometry essentially as described previously for cocaine and BE (Lin et al., 2001). Conditions for including the metabolites EME and norcocaine were applied, with the exception that atmospheric pressure chemical ionization was changed to electrospray ionization (Lin et al., 2003). Plasma samples were not collected and quantified in this study due to the difficulty of collecting plasma without losing urine. Our previously published studies of the effects of the same dose of h2E2 and cocaine hydrochloride revealed that the total cocaine concentration in the plasma of rats after 1 hour in the presence and absence of h2E2 is negligible (Norman et al., 2014). Therefore, due to the lengthy collection intervals (6 hours), collecting plasma concentrations to correlate with the urinary excretion of cocaine likely would have not added to the value and conclusions of this study.
Urinary Creatinine, Electrolyte, Osmolality, and Ammonium Quantification.
Urine creatinine concentrations were determined using the Biovision Colorimetric/Fluorometric Assay Kit (Biovision Inc., Milpitas, CA) according to the manufacturer’s instructions. Urine concentrations of Na+, K+, and Cl− were measured using the Medica EasyLytePLUS analyzer (Medica Corporation, Bedford, MA). Urine osmolality was measured by freezing point–based osmometry with the Advanced Micro-Osmometer (Advanced Instruments, Inc., Norwood, MA). Ammonium excretion was measured with a phenol/sodium hypochlorite method described by Berthelot (1859) and used by other investigators (Amlal et al., 2006). In some cases, there was not enough urine to perform the full battery of tests. The value of n was always at least five except for the 24-hour time point, which in some cases was only performed on one rat.
Statistics.
Significance for urinary excretion of cocaine and its metabolites was determined by paired t tests comparing vehicle and treated animals across time. All other urine analysis tests for significance were performed by two-way repeated measures analysis of variance comparing vehicle and treated animals across time. If the data were non-normal, a Friedman repeated measures analysis of variance on ranks was applied. If the P value was less than 0.05, the difference was considered significant. All statistics were analyzed using SigmaPlot software version 13 (Systat Software Inc., San Jose, CA).
Materials.
(−) Cocaine hydrochloride was provided by the Research Triangle Institute (Chapel Hill, NC) under the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). Recombinant h2E2 was produced from stably transfected Chinese hamster ovary cell lines by Catalent PharmaSolutions (Madison, WI) using their proprietary GPEx technology (Bleck, 2012). Buprenorphine, gentamycin, isoflurane, and heparin were purchased from Henry Schein Animal Health (Dublin, OH). Creatinine bioassay kits were purchased from Biovision Inc.
Results
The Effects of h2E2 on Urinary Excretion of Cocaine and Metabolites in Rats.
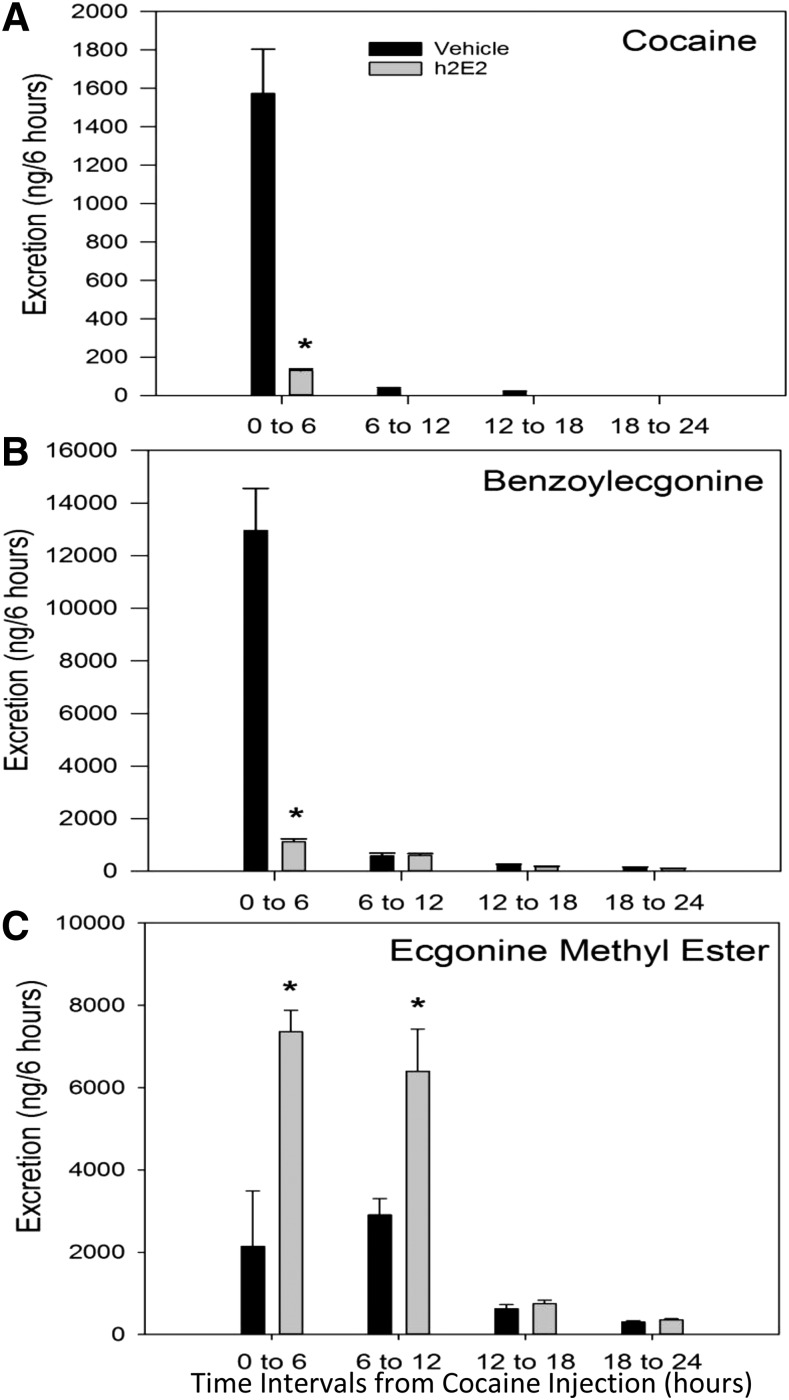
In h2E2-treated animals, cocaine excretion was significantly decreased by 92% compared with vehicle controls (0–6 hours; P = 0.002) (Fig. 1A). Cocaine was detectable until the 12–18 hour collection interval in vehicle control animals, but was not detectable past 6 hours in h2E2-treated animals. BE excretion was significantly decreased in the presence of h2E2 by 91% in the first collection interval (0–6 hours; P = 0.002) (Fig. 1B). Conversely, EME excretion was significantly increased by 3.4-fold in the presence of h2E2 at both 0–6 (P = 0.02) and 6–12 hour collection intervals (P = 0.01) (Fig. 1C). In both vehicle and h2E2 groups, EME excretion was detectable throughout all collection periods. Norcocaine was below the limits of detection in all samples.
Fig. 1.
The effects of h2E2 on urinary excretion of cocaine and metabolites. Mean ± S.E.M. excretion rates (in nanograms/6 hours) in vehicle-treated (black) and h2E2-treated (gray) animals for cocaine (A), BE (B), and EME (C) are shown. Asterisks represent statistical significance P < 0.05 from paired t tests comparing h2E2-treated to vehicle control–treated animals. All data were normalized to urine volumes to yield total excretion per 6-hour collection interval. Norcocaine was not detectable in any samples.
The Effects of h2E2 and Cocaine on Measures of Kidney Function.
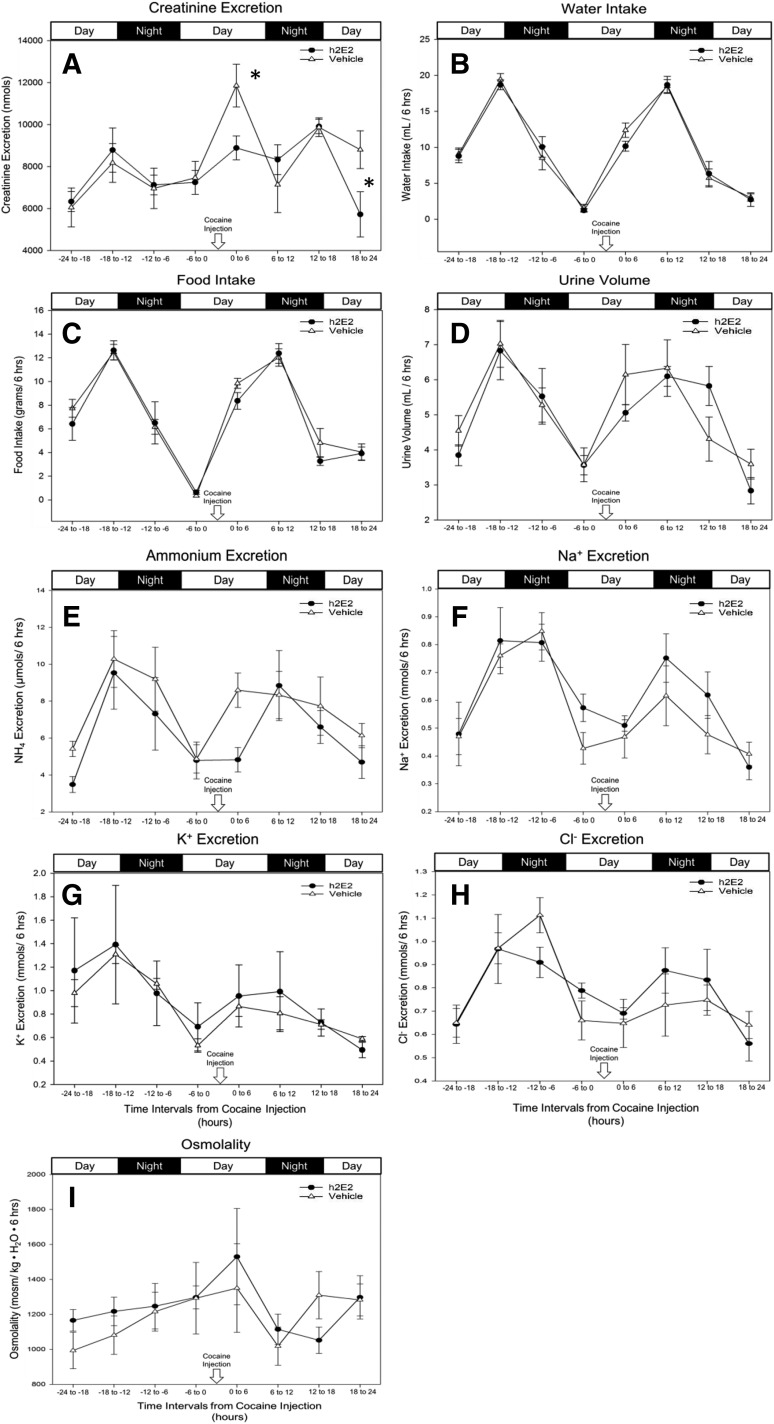
There was a significant increase in urine creatinine excretion in vehicle-treated animals compared with h2E2-treated animals immediately following cocaine injection (0–6 hour collection interval; P = 0.009) and then again during the 18–24 hour collection interval (P = 0.035) (Fig. 2A). All other measures (ion levels, food/water intake, osmolality, and urine volume) were not significantly different between treatment and vehicle groups with no observed difference following the cocaine injection. It should be noted that all measures did show expected diurnal variation following the light and dark cycles.
Fig. 2.
Cocaine’s effect on the measures of kidney function. Mean ± S.E.M. values for creatinine excretion (A), water intake (B), food intake (C), urine volume (D), ammonium excretion (E), Na+ excretion (F), K+ excretion (G), Cl− excretion (H), and osmolality (I) in vehicle-treated (open triangles) and h2E2-treated (closed circles) animals are displayed. (A) Creatinine was normalized to urine volume to yield total urine creatinine excretion (in nanomoles) per 6-hour collection interval. (E–H) Excretion levels were normalized to urine volume to yield ion excretion (in millimoles) per 6-hour collection interval (n = 5 to 6 animals, n = 1 for the 18–24 hour collection interval in G only due to limited urine volumes). Asterisks represent statistical significance (P < 0.05) from two-way repeated measures analysis of variance comparing h2E2-treated to vehicle control–treated animals.
Discussion
This study is the first known study to evaluate the effects of a monoclonal antibody on the urinary excretion of its target drug. By evaluating the altered disposition of cocaine and its metabolites in the urine in the presence of h2E2, appropriate endpoint measurements for effectiveness can be assessed. Proper endpoint measurements can prevent false positives and false negatives for clinical effectiveness, which can save time and money and eliminate ineffective treatments earlier in the investigative process.
In the presence of h2E2, there were significant decreases in cocaine and BE urinary excretion (Fig. 1, A and B). The differential effect of h2E2 on cocaine, BE, and EME urinary excretion can be explained by the relative affinities of h2E2 for cocaine over its metabolites; h2E2 has high affinity for cocaine, moderate affinity for BE, and low affinity for EME (Norman et al., 2014). We hypothesize that h2E2 likely binds to cocaine and BE, sequestering them in the plasma and thereby inhibiting their urinary excretion. This is consistent with our observation that in mice both BE and cocaine levels in the plasma are elevated as early as 5 minutes (Wetzel et al., 2017). The half-life of cocaine (15 minutes) is significantly shorter than the half-life of h2E2 (7.14 days) (Wetzel et al., 2017). These studies were performed in 1 day; therefore, the amount of h2E2 eliminated is considered negligible.
There was 3-fold significant increase in EME urinary excretion that could be attributed to h2E2’s ability to sequester cocaine into the plasma compartment (Norman et al., 2007, 2014). Elevated plasma levels of cocaine have been seen in the presence of h2E2 (Norman et al., 2014), and EME is formed enzymatically in the plasma (Warner and Norman, 2000). Since h2E2 has relatively low affinity for EME, the excretion of this compound would most likely be unimpeded, consistent with our observed results (Fig. 1C). However, the presence of h2E2 could also shift the metabolic profile of cocaine. This alternative hypothesis could also explain the increase in EME urinary excretion and decrease in BE excretion compared with vehicle controls observed in this study (Fig. 1, B and C).
A complete urine analysis panel was performed to assess the function of the kidneys during the course of the experiment. During the first collection interval after cocaine administration, it appears that h2E2 had some protective effects against cocaine (Fig. 2A). In vehicle-treated animals, there was a significant increase in urine creatinine excretion compared to h2E2-treated animals at the 0–6 hour collection interval (Fig. 2A). We hypothesize that this increase in creatinine excretion is due to the sympathomimetic effects of cocaine. Cocaine is known to increase the blood pressure in cocaine addicts (Mu et al., 2018). By constricting the efferent artery in the kidney, cocaine can increase the arterial pressure on the glomerulus and increase the glomerular filtration rate. An increase in the glomerular filtration rate can result in hyperfiltration of creatinine into the urine. The hyperfiltration in the kidney appears to be a transient cocaine-induced effect that only occurs in the presence of cocaine. However, in the presence of h2E2 the urinary creatinine excretion at the 0–6 hour collection interval is comparable to pretreatment baseline values. Therefore, when cocaine is sequestered into the peripheral circulation by h2E2, its peripheral effects are antagonized as well as its central effects.
Urine electrolytes and other parameters were also analyzed to assess the function of the kidneys. There were no significant differences between vehicle- and h2E2-treated animals across all collection intervals (Fig. 2 E-I). Therefore, we conclude that the altered urinary excretion of cocaine and its metabolites is likely due to the binding affinities of h2E2 and is unlikely due to any adverse effects on the kidney caused by the injection of a large amount of an IgG protein.
In clinical trials of h2E2, a decrease in urine BE excretion might be interpreted as a positive indicator of the effectiveness of the mAb treatment. Given the altered urinary excretion of BE in the presence of h2E2, cocaine intake results should be interpreted with caution. We conclude that EME would be a more appropriate measurement of cocaine use, since its urinary excretion persists in the presence of h2E2 and remains detectable for the longest period of time in both groups. EME is also excreted into the urine in humans (Ambre et al., 1984). The results of this study are translatable to humans due to the similar metabolic profile of cocaine in both rats and humans. Cocaine is metabolized through cholinesterase enzymes that are present in both rats, mice, and humans. Therefore, the altered urinary clearance of cocaine and metabolites in the presence of h2E2 is expected to occur in human clinical trials. Although the current study uses a 123 mg/kg dose of h2E2, the intended clinical dose, it is important to note that the magnitude of effects of h2E2 on cocaine and metabolite urinary excretion is expected to be proportional to the dose of h2E2. Therefore, there may be an even greater effect on cocaine and metabolite urinary excretion in clinical trials if larger doses of h2E2 are needed. These results have important implications for the design and interpretation of clinical trials not only for h2E2, but for all immunotherapies for drugs of abuse. This includes the cocaine vaccine (Martell et al., 2009), a methamphetamine antibody (Laurenzana et al., 2003), a nicotine vaccine (Hatsukami et al., 2005) in clinical trials, as well as a phencyclidine antibody (Valentine and Owens, 1996), and heroin/morphine antibodies (Anton and Leff, 2006) that are currently in preclinical studies. It can be predicted that these antibodies may also alter urinary excretion of their target drugs. The differential effect of h2E2 on different metabolites based on affinity has particular importance to clinical trials of cocaine vaccines, which induce polyclonal responses. The production of variable amounts of antibodies with varying affinities for cocaine and metabolites make it difficult to predict how urine excretion of cocaine and its metabolites would be affected in different patients. This indicates that investigators should evaluate alternative endpoint measures for assessing the efficacy of vaccines that induce polyclonal responses.
These effects also potentially apply in situations where h2E2 is administered after cocaine, since h2E2 could reduce urine cocaine and BE concentrations, confounding the urine toxicology assays used to screen for cocaine use. Again, this may also apply to other antidrug antibodies, since workplace urine testing for opiates, phencyclidine, and amphetamines are also performed (Moeller et al., 2008, Stevens et al., 2014). Therefore, the ability of antidrug antibodies to alter the disposition, metabolism, and excretion of drugs of abuse has implications beyond therapeutic interventions.
In conclusion, caution should be exercised in the interpretation of urine cocaine and BE levels as measures of the clinical effectiveness of immunotherapies for cocaine addiction. The effects of each immunotherapy with unique specificity for its target drug and metabolites should be characterized to promote more accurate clinical trial designs and interpretation of results. Additionally, the potential for immunotherapies to confound the results of routine urine drug screens should be recognized. Finally, we propose that EME in the urine be measured in place of cocaine or BE in clinical trials of h2E2 because it will increase the probability of accurately detecting cocaine use.
Acknowledgments
Liquid-chromatography/electrospray ionization–tandem mass spectrometry analyses were conducted through the National Institute on Drug Abuse (Contract N01DA-14-7788) by Dr. David Adrenyak and Dr. David Moody at the University of Utah, Salt Lake City, UT. Special thanks to Michelle Nieman for her technical assistance.
Abbreviations
- BE
benzoylecgonine
- EME
ecgonine methyl ester
- h2E2
humanized anticocaine monoclonal antibody
- mAb
monoclonal antibody
Authorship Contributions
Participated in research design: Wetzel, Marckel, Norman, H. Amlal.
Conducted experiments: Marckel, Wetzel.
Contributed new reagents or analytic tools: Marckel, S. Amlal, Wetzel, H. Amlal.
Performed data analysis: Marckel, Wetzel.
Writing or contributed to the writing of the manuscript: Marckel, Wetzel, Norman, H. Amlal.
Footnotes
This work is supported by the National Institutes of Health National Institute on Drug Abuse [Grant U01DA039550].
A.B.N. is named as co-inventor on two patents for the matter and use of the h2E2 recombinant anticocaine monoclonal antibody (US Patents 9,758, 593 and 9,957,332).
References
- Ambre J, Fischman M, Ruo TI. (1984) Urinary excretion of ecgonine methyl ester, a major metabolite of cocaine in humans. J Anal Toxicol 8:23–25. [DOI] [PubMed] [Google Scholar]
- Amlal H, Sheriff S, Faroqui S, Ma L, Barone S, Petrovic S, Soleimani M. (2006) Regulation of acid-base transporters by vasopressin in the kidney collecting duct of Brattleboro rat. Am J Nephrol 26:194–205. [DOI] [PubMed] [Google Scholar]
- Anton B, Leff P. (2006) A novel bivalent morphine/heroin vaccine that prevents relapse to heroin addiction in rodents. Vaccine 24:3232–3240. [DOI] [PubMed] [Google Scholar]
- Berthelot M. (1859) Violet d’aniline. Rep Chim App 1:284. [Google Scholar]
- Bleck GT. (2012) Consistent production of genetically stable mammalian cell lines. Biopharm Int 25:56–59. [Google Scholar]
- Hatsukami DK, Rennard S, Jorenby D, Fiore M, Koopmeiners J, de Vos A, Horwith G, Pentel PR. (2005) Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clin Pharmacol Ther 78:456–467. [DOI] [PubMed] [Google Scholar]
- Kosten T, Owens SM. (2005) Immunotherapy for the treatment of drug abuse. Pharmacol Ther 108:76–85. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Domingo CB, Shorter D, Orson F, Green C, Somoza E, Sekerka R, Levin FR, Mariani JJ, Stitzer M, et al. (2014) Vaccine for cocaine dependence: a randomized double-blind placebo-controlled efficacy trial. Drug Alcohol Depend 140:42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenzana EM, Byrnes-Blake KA, Milesi-Hallé A, Gentry WB, Williams DK, Owens SM. (2003) Use of anti-(+)-methamphetamine monoclonal antibody to significantly alter (+)-methamphetamine and (+)-amphetamine disposition in rats. Drug Metab Dispos 31:1320–1326. [DOI] [PubMed] [Google Scholar]
- Lin SN, Moody DE, Bigelow GE, Foltz RL. (2001) A validated liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry method for quantitation of cocaine and benzoylecgonine in human plasma. J Anal Toxicol 25:497–503. [DOI] [PubMed] [Google Scholar]
- Lin SN, Walsh SL, Moody DE, Foltz RL. (2003) Detection and time course of cocaine N-oxide and other cocaine metabolites in human plasma by liquid chromatography/tandem mass spectrometry. Anal Chem 75:4335–4340. [DOI] [PubMed] [Google Scholar]
- Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, Kosten TR. (2009) Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry 66:1116–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller KE, Lee KC, Kissack JC. (2008) Urine drug screening: practical guide for clinicians. Mayo Clin Proc 83:66–76. [DOI] [PubMed] [Google Scholar]
- Mu S, Fantegrossi WE, Rusch NJ. (2018) Cocaine-responsive miRNA and blood pressure elevation. Hypertension 71:561–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (U.S.) Committee for the Update of the Guide for the Care and Use of Laboratory Animals (2011). Guide for the Care and Use of Laboratory Animals. National Academies Press, Washington, DC. [Google Scholar]
- Norman AB, Ball WJ., Jr (2012) Predicting the clinical efficacy and potential adverse effects of a humanized anticocaine monoclonal antibody. Immunotherapy 4:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AB, Gooden FC, Tabet MR, Ball WJ. (2014) A recombinant humanized anti-cocaine monoclonal antibody inhibits the distribution of cocaine to the brain in rats. Drug Metab Dispos 42:1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AB, Tabet MR, Norman MK, Buesing WR, Pesce AJ, Ball WJ. (2007) A chimeric human/murine anticocaine monoclonal antibody inhibits the distribution of cocaine to the brain in mice. J Pharmacol Exp Ther 320:145–153. [DOI] [PubMed] [Google Scholar]
- Stevens MW, Tawney RL, West CM, Kight AD, Henry RL, Owens SM, Gentry WB. (2014) Preclinical characterization of an anti-methamphetamine monoclonal antibody for human use. MAbs 6:547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine JL, Owens SM. (1996) Antiphencyclidine monoclonal antibody therapy significantly changes phencyclidine concentrations in brain and other tissues in rats. J Pharmacol Exp Ther 278:717–724. [PubMed] [Google Scholar]
- Warner A, Norman AB. (2000) Mechanisms of cocaine hydrolysis and metabolism in vitro and in vivo: a clarification. Ther Drug Monit 22:266–270. [DOI] [PubMed] [Google Scholar]
- Wetzel HN, Tsibulsky VL, Norman AB. (2016) The effects of a repeated dose of a recombinant humanized anti-cocaine monoclonal antibody on cocaine self-administration in rats. Drug Alcohol Depend 168:287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel HN, Webster RP, Saeed FO, Kirley TL, Ball WJ, Norman AB. (2017) Characterization of a recombinant humanized anti-cocaine monoclonal antibody produced from multiple clones for the selection of a master cell bank candidate. Biochem Biophys Res Commun 487:690–694. [DOI] [PMC free article] [PubMed] [Google Scholar]