Abstract
Introduction:
Chronic Kidney Disease is a growing health burden world wide. Traditional and mutual risk factors between CVD and CKD are age, hypertension, diabetes mellitus, dyslipidem-ia, tobacco use, family history and male gender. In this review, we will focus on whether or not early CKD is an important risk factor for the presence, severity and progression of CVD. Specifically, we will examine both traditional and novel risk factors of both CKD and CVD and how they relate to each other.
Conclusion:
We will also assess if early treatment of CKD, intensive compared to standard, has an important effect on the halt of the development of CKD as well as CVD. Insights into the pathogene-sis and early recognition of CKD as well as the importance of novel kidney biomarkers will be point-ed out. Also, common pathogenetic mechanisms between CKD and CVD will be discussed
Keywords: CKD, CVD, pathogenesis, early detection, kidney biomarkers, United States
1. Introduction
Chronic Kidney Disease (CKD) is a growing health burden not only in the United Stated but also worldwide [1]. During the past years, the nomenclature for CKD included terms such as ‘’chronic renal failure”, ‘’chronic renal insufficiency”, ‘’pre-dialysis” and ‘’pre-end-stage renal disease”, and categorized mainly by cause [2]. Evaluation, classification, and risk stratification in CKD are based on clinical practice guidelines that have been published by National Kidney Foundation (NKF) in 2002 [1, 3]. The diagnosis of chronic kidney disease is settled when there is either kidney damage for ≥ 3 months, as confirmed by kidney biopsy or markers of kidney damage, with or without a decrease in Glomerular Filtration Rate (GFR), or a reduction in GFR, GFR < 60 mL / min per 1.73 m2 for ≥ 3 months, with or without kidney damage [3]. Any reduction in the GFR mirrors a functional kidney abnormality, while signs such as albuminuria, hematuria, abnormal urinary sediment or a pathologic kidney biopsy represent an anatomical or structural abnormality. These aspects have to be persistent for at least 3 months, in order to establish a diagnosis of CKD [3]. After establishing the diagnosis, the clinician should be able to define the stage of CKD in which the patient belongs to. There are 5 different stages; the degree of reduction in GFR correlates to stage and severity of disease. The first two stages may have normal GFR or only a slight reduction, but are often accompanied by positive markers of kidney damage; usually abnormal urinary albumin to creatinine ratio [1]. The disease is underdiagnosed mainly due to CKD being asymptomatic in nature; CKD is often not detected until its later stages [4]. The overall prevalence of CKD increased from 12 percent to 14 percent between 1988 and 1994 and from 1999 to 2004, but has remained relatively stable since 2004. The largest increase occurred in people with Stage 3 CKD, from 4.5 percent to 6.0 percent, since 1988. Women (15.93%) are more likely to have stages 1 to 4 CKD than men (13.52%) [5]. Chronic kidney disease frequently interrelates with cardiovascular disease [6, 7]. According to the U.S. Renal Data System report published in 2013, 43% of patients with CKD and CVD had Heart Failure (HF), and 15% had a history of Acute Myocardial Infarction (AMI); the equivalent proportions in non-CKD patients with CVD were 18.5% and 6.4%, respectively [8]. It has been acknowledged that patients with advanced kidney disease, stage 4 or 5, are at high risk of cardiovascular disease morbidity and mortality [9]. In patients requiring dialysis, CVD is being recognized as the leading cause of death. Nonetheless, it is nowadays being advocated that patients with earlier stages of chronic kidney disease also suffer a high rate of fatal and nonfatal cardiovascular events [10]. A recent, prospective population based cohort study concluded that patients at the early stages of CKD, even without manifestations of vascular disease, were associated with excess risk of subsequent coronary heart disease [6]. For this reason, CKD itself is now deliberated as an independent CVD risk factor and a Coronary Artery Disease (CAD) equivalent for all-cause mortality [6]. It is worth mentioning that CKD patients are more likely to die from cardiovascular events instead of developing kidney failure and ending up on renal replacement therapy [11].
The potential explanations for the co-existence of these two entities may be explained from the following: 1. CKD is associated with increased prevalence of traditional and non-traditional cardiovascular disease risk factors, 2. CKD is an independent risk factor for CVD, 3. many risk factors of cardiovascular disease exacerbate the progression of CKD, and 4. CVD by itself may be a risk factor for CKD [12, 13]. Particularly, CKD patients have a higher incidence of increased cardiovascular morbidity and mortality, attributable not only to the traditional CV risk factors, but also to the presence of a wide array of non-traditional risk factors that are unique to patients with CKD [12, 13]. Although it has been reported that multiple classical and novel risk factors of patients with CKD predispose to CVD morbidity and mortality, many studies examining CVD’s population have excluded patients with renal dysfunction due to the high risk for adverse outcomes, and this exclusion would likely weaken the assumption that these two diseases are strongly interrelated [14].
Nevertheless, the NKF underlies the importance of early identification and treatment of CKD and its associated co-morbid conditions, including cardiovascular disease [3]. Currently, a large observational study of patients with early CKD followed for 5.5 years concluded that 24.9% of patients died before reaching dialysis, while 3.1% progressed to requiring Renal Replacement Therapy (RRT). Most of the deaths were attributed to cardiovascular events [15].
In this review, we will focus on whether or not early CKD is an important risk factor for the presence, severity and progression of CVD. Specifically, we will examine both traditional and novel risk factors of both CKD and CVD and how they relate to each other. We will also assess if early treatment of CKD, intensive compared to standard, has an important effect on the halt of the development of CKD as well as CVD.
2. CKD, CVD and mutual risk factors
As we mentioned earlier, an international classification of CKD has identified five stages. Early CKD is depicted as stages 1-3. Structural abnormalities, presence of persistent proteinuria or hematuria are characteristics of the presence of stage 1 and 2, while estimated Glomerular Filtration Rate (eGFR) between 30 and 59ml/min/1.73 m2 on at least two occasions at a minimal interval of 3 months, defines stage 3 [3]. Stage 3 is the first stage that can be detected on serum chemistry and accounts for the vast majority of people now being detected and labelled with CKD on general practice disease registers [3]. The course of early CKD is almost always subclinical [16]. Varma et al. conducted a cross-sectional study of Army Personnel in Central India and concluded that 9.54% of healthy army personnel were found to have early stages of CKD [16]. The results were based on an investigative profile that included a routine urine exam, semi-quantitative microalbuminuria (MAU), serum creatinine, lipid profile and fasting blood glucose [16]. GFR was calculated using the Modification of Diet in Renal Diseases (MDRD) study equation [17]. Using the same calculations and equations, it is estimated that as many as 10% of the US adult population have early CKD [18, 19]. This increases with age to approximately 20% over 65 years and more than 30% over 80 years [18, 19].
Screening and detection of early stages of CKD can help institute interventions that may delay the progression of the disease [18]. Indeed, based on the aforementioned data, the emphasis for treatment and intervention should be shifted towards the earlier stages of chronic kidney disease, since early identification through instituting screening programs may essentially improve the impact of CKD and delay or even halt its progression [18, 19].
3. Mutual risk factors
Traditional and mutual risk factors between CVD and CKD are age, hypertension, diabetes mellitus, dyslipidemia, tobacco use, family history and male gender. High blood pressure, glucose, and lipid levels, as well as tobacco use can aggressively be modified. However, toxic metabolites produced by uremia in chronic kidney disease as well as conditions that alter the metabolism of chemical elements, such as calcium and phosphorus, account for the excess CVD in patients with CKD, and are known as non-traditional risk factors [19, 20].
4. The role of microalbuminuria
A common finding in hypertension, diabetes and dyslipidemia is microalbuminuria, which is an essential predictor of identifying those at risk of kidney disease progression [18]. Observing patients with diabetic nephropathy, there is a tremendously increased incidence in mortality with the onset of proteinuria; thus, trials aiming to reduce the incidence and progression of proteinuria through the blockade of the Renin-Angiotensin-Aldosterone System (RAAS) have been successful [21]. However, once nephropathy has been established, the beneficial effect of RAAS blockade is not observed, so early intervention is crucial for halting the disease progression [21]. Also, hypertension is essentially linked with CKD, establishing a vicious cycle. Hypertensive subjects with increased pulse pressure and proteinuria have increased incidence of all-cause mortality, CV mortality, acute myocardial infarction and heart failure [22].
Interestingly, cigarette smoking, the most preventable cause for CVD, has been implicated in the pathogenesis of CKD. Specifically, the stimulation of postganglionic sympathetic nerve endings by nicotine, results in increased levels of epinephrine and norepinephrine, with subsequent increase in blood pressure levels, activation of the RAAS, increase in GFR and finally in intra-glomerular pressure [23]. Additionally, an association of the severity of albuminuria with the number of cigarettes smoked has been observed [23].
There have been a few studies that examined proteinuria as a predictor of cardiovascular mortality and some studies acknowledged a statistically significant association for at least one gender [24-28]. Microalbuminuria, often an early sign of diabetic kidney disease, defined as urinary albumin excretion between 30 mg and 300 mg/24 hours, is strongly associated with CVD in cross-sectional analysis [18]. Subjects with microalbuminuria, with or without diabetes, have a higher prevalence of cardiovascular disease risk factors, including hyperlipidemia, HTN, BMI, insulin resistance, and a history of smoking, compared to subjects without microalbuminuria [26-28]. Jager et al. reported a positive association of microalbuminuria and increased intima-media thickness of the carotid artery in hypertensive subjects, while Dell’Omo et al. noted more frequent concentric LVH in hypertensive men with microalbuminuria [22, 24]. Other surrogates of CVD that are robustly correlated with the presence of microalbuminuria are any electrocardiographic evidence of myocardial ischemia, as well as the abnormal left ventricular geometry and mass in subjects with hypertension and Left Ventricular Hypertrophy (LVH) [24-26].
Despite the fact that the prevalence of microalbuminuria is low among young adults, it accelerates with age; 8-10% in the general elderly population and even greater in the presence of hypertension and or diabetes: 20% and 30% respectively [26-28]. Distinct studies have demonstrated that microalbuminuria can be theorized as an independent factor for cardiovascular disease development and there are certain clues which support this [26-28]. Initially, the traditional threshold of urinary albumin-to-creatinine ratio that correlates microalbuminuria with CVD has been lowered from 2.5 mg/mmol in men and 3.5 mg/mmol in women to 1 mg/mmol or even less [18]. Also, it has been indicated that progression of microalbuminuria in subjects with type II diabetes mellitus, is independently associated with a further increased risk for cardiovascular disease [27-30]. Remarkably, the findings of the Losartan Intervention For Endpoint reduction in hypertension (LIFE) study suggested that during treatment any reduction in the risk for the primary outcome; myocardial infarction, stroke and cardiovascular mortality, was linked to a proportional reduction of urinary albumin excretion [31]. The favorable outcomes were not explained and subsequently were not attributed to the Losartan group [31]. Matsushita et al., conducted a collaborative meta-analysis of general population cohorts and examined any association that exists between albuminuria and all-cause mortality as well as cardiovascular [32]. There was a linear association between albuminuria and mortality on the log-log scale, independently of eGFR and conventional risk factors. This may further suggest that microalbuminuria provides additional prognostic information beyond eGFR alone [32]. Albeit the association between urinary albumin excretion and risk for cardiovascular disease is under investigation, the pathophysiology of this process is still under question, as there is no clear explanation how microalbuminuria is linked to cardiovascular risk [32, 33]. It is uncertain whether microalbuminuria causes atherothrombosis or vice versa. The fact that they share common risk factors with identical pathophysiological processes may underlie this association [32, 33]. It is mainly hypothesized that the existence of microalbuminuria may demonstrate the severity of end-organ damage and an inflammatory status where generalized endothelial dysfunction and abnormalities in the coagulation cascade system are incorporated [32, 33]. This feature may introduce proteinuria as a novel risk factor for CVD, which plays a direct causal role in the progression of the vascular disease [33, 34].
5. CKD and pathogenetic mechanisms: Inflammation and Endothelial dysfunction
5.1. The Role of NO
Dysregulation of intrinsic mechanisms that maintain the homeostasis of the vascular endothelium has been described. This disarrangement of endothelial function is considered to play an important role in both the initiation and the progression of atherosclerosis [34]. Levels of Nitric Oxide (NO), a principally important endothelium-derived mediator, have been examined [34]. Nitric oxide encompasses several properties, notably potent vasodilation, along with antiplatelet, anti-proliferative, anti-adhesive, permeability-decreasing, and anti-inflammatory characteristics [34]. In a large, population-based study of 645 participants, comparing subject with normal and abnormal glucose metabolism as well as type 2 diabetes, the endothelial NO synthesis was significantly impaired in groups with abnormal glucose metabolism and type 2 diabetes mellitus [34]. The NO synthesis was evaluated by ultrasonically measurement of the endothelium-dependent, flow-mediated dilation of the brachial artery. Interestingly, it was described that in subjects with type 2 diabetes and in subjects with microalbuminuria, irrespectively of glycemic status, there was a significantly impaired endothelium-dependent, flow-mediated dilation of the brachial artery [34]. Thus, defective endothelial NO synthesis is an essential characteristic that underlies the interconnection of microalbuminuria with cardiovascular disease risk [34, 35]. However, the causal-relationship still needs to be clarified, as it remains unclear whether microalbuminuria causes endothelial dysfunction or vice versa [34, 35].
5.2. The Glycocalyx and the Endothelium
Recent findings suggest that vascular endothelium has a crucial role in determining the permeability of albumin, through a molecule called glycocalyx [36]. Glycocalyx fills the endothelial fenestrae and plays a key-role in terms of permeability, specifically glomerular size and charge selectivity [36]. Defects in endothelial glycocalyx have been associated not only to microalbuminuria, but also to the pathogenesis of atherosclerosis. It is speculated that the penetrance of albumin through the glomerulus reflects a generalized trans-vascular leakage of albumin and possibly other lipoprotein particles into the arterial wall, thus contributing to atherosclerosis [36].
5.3. Inflammation and Inflammatory Markers
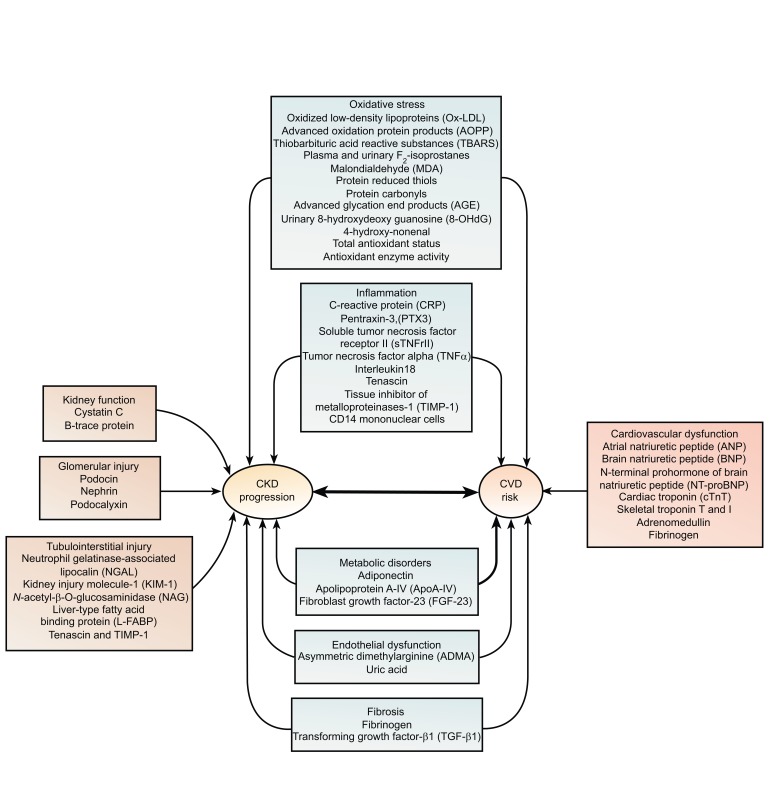
Cellular processes, such as inflammation and insulin resistance, through oxidative stress mechanisms have been implicated in the pathogenesis of atherogenesis. The Heart Outcomes and Protection (HOPE) study, a post-hoc analysis of patients with elevated creatinine, did not find a benefit of Vitamin E on cardiovascular disease outcomes [37]. Nevertheless, a reduction in cardiovascular disease events with vitamin E has been demonstrated by the Secondary Prevention with Disease (SPACE) trial [38]. Furthermore, another antioxidant agent, known as acetyl-cysteine has been shown to lower cardiovascular disease progression in patients with kidney failure [39]. Chronic, low-grade inflammation can be determined by measurement of markers that are markedly related to microalbuminuria, such as plasma levels of C-reactive protein and cytokines such as IL-6 and TNF-a [40, 41]. C-reactive Protein (CRP) may not only be a red flag for inflammatory conditions, but may be the mediator for the initiation and the progression of atherosclerosis, as it is implicated in plaque initiation, formation, and rupture [40, 41]. In a longitudinal analysis, CRP measured at baseline in the Modification of Diet in Renal Disease (MDRD) Study was an independent predictor of all-cause and cardiovascular disease mortality [17]. Other pro- and anti-inflammatory cytokines, including IL-10 and TNF-a, may also play a role in the development of cardiovascular disease in chronic kidney disease [39-41]. Biomarkers of CKD progression and renal cardiovascular disease are shown in Fig. (1).
Fig. (1).
Biomarkers of Chronic Kidney Disease (CKD) progression and renal Cardiovascular Disease (CVD).
However, further molecular and clinical studies need to be performed in order to identify if these three conditions: low-grade inflammation, endothelial dysfunction and microalbuminuria are tightly linked together or are a consequence of one another. Current studies have not been able to clarify if microalbuminuria independently enhances endothelial dysfunction and low-grade inflammation [40, 41]. Urine Albumin-to-Creatinine Ratio (ACR) has been recommended as the preferred measure of albuminuria for the definition as well as for staging of CKD, by clinical and laboratory guidelines [3]. In terms of cost, the urine dipstick is often used for initial screening, because despite the fact that is less expensive, can easily be performed at the point of care [3]. However, findings from a recent meta-analysis that assessed the independent role of albuminuria with mortality, displayed that even a trace urine protein on dipstick test was associated with an increase in mortality risk, consistent with the fact that 60% of individuals with trace on dipstick had microalbuminuria [42]. According to these findings, it is suggested that even though dipstick being a less precise measure of albuminuria is quiet useful for risk stratification [42].
5.4. Adiponectin and CKD
Adiponectin is a biomarker for metabolic syndrome, which is closely correlated to kidney disease [39]. Adiponectin levels are inversely related to several metabolic parameters, such as body mass index and glucose levels [39, 43]. In a cohort study of chronic hemodialysis patients, it has been postulated that, decreased plasma levels of adiponectin are associated with increased risk of cardiovascular disease mortality [43]. Thus, the role of adiponectin and its association to mild and moderate kidney disease may be of interest, as mentioned in Fig. (1).
5.5. Anemia and CKD
Anemia due to decreased Erythropoietin Production (EPO) is commonly seen in CKD [39]. It is crucial to realize that CKD patients need EPO replacement therapy, to increase the hematocrit levels to a normal level. The use of intravenous iron dextran and also inadequate dialysis has been linked with adverse cardiovascular outcomes in hemodialysis patients with preexisting heart failure and ischemic heart disease [39]. Iron generates not only oxygen-derived free radicals, which cause damage to coronaries, but induce LVH, as well. Anemia in CKD patients is rarely macrocytic, but this finding may be associated with impaired flow-mediated dilation due to endothelial dysfunction [39]. Hence, this macrocytic anemia in CKD may be an independent risk factor for an increase in CV events.
6. CKD, CVD and pathogenetic mechanisms: Molecular Paths
6.1. Dimethylarginines
Other biochemical molecules known as dimethylarginines, which are derivatives of methylated amino acid, are implicated in the pathogenesis of CKD [44]. They are usually excreted into the urine as products of protein degradation, and subsequently their urinary levels are raised in clinical conditions associated with increased protein breakdown, such as tumor growth and neurodegenerative diseases [45]. Post-translational methylation of arginine residues results in the formation of two isomers: asymmetric and symmetric dimethylarginines. The circulating levels of asymmetric dimethylarginine (ADMA) are regulated by its release from methylated proteins, glomerular filtration, and enzymatic degradation by Dimethylarginine Dimethylaminohydrolase (DDAH) [46]. ADMA acts as an endogenous inhibitor of Nitric Oxide (NO) synthesis, and thus counter-regulates its favorable properties [46, 47]. Interestingly, it was observed that the levels of dimethylarginine were substantially increased in subjects with CKD, where the loss of DDAH activity contributes more to elevated ADMA concentrations than does the reduced glomerular filtration. However, the isomer form of ADMA, Symmetric Dimethylarginine, (SDMA), although elevated in renal disease, has no inhibitory effect on nitric oxide synthase in vivo [46, 47]. Nonetheless, SDMA is more abundant than ADMA, and has recently emerged as an endogenous marker of renal function, due to its exclusive renal excretion [46, 47]. ADMA is now being considered a marker of endothelial dysfunction in humans given its inhibition of NO [46, 47]. Studies that were investigating the pathophysiological mechanisms of atherogenesis in animal models suggested that ADMA levels were noted to rise early in the atherogenesis formation, indicating that this may be a possible causative factor [48-51]. Interestingly, Schwedhelm et al. noted that ADMA levels were elevated independently of renal function in patients with incipient CKD, suggesting that mechanisms other than impaired glomerular filtration might contribute to the accumulation of ADMA in this setting [47]. Consequently, it is being assumed that not only genetic polymorphisms in DDAH1 have been described that modify the activity of DDAH-1, but DDAH-2 activity is affected by redox balance as well [46, 47]. Upregulation of ADMA levels results in reduced nitric oxide generation, which underlies many of the clinical symptoms related to renal diseases and their vascular complications [46, 47]. With reference to SDMA, its potential pathogenic role in vascular disease is probably mediated through generation of reactive oxygen species in monocytes and activation of store-operated calcium channels [46, 47]. Whereas several studies documented a strong connection of cardiovascular and cerebrovascular diseases with the ADMA and SDMA plasma levels, further experimental and clinical studies need to be done in order to clarify and understand the role of these methylated amino acid derivatives in the development and progression of cardiovascular diseases [46, 47].
6.2. Bufadienolides
A group of steroid hormones, called Bufadienolides, may act as a bridge between chronic kidney disease and cardiovascular disease [48]. Bufadienolides along with cardenolides (of which Digoxin is a derivative), belong to a class of circulating substances known as cardiac glycosides [48]. These hormones possess different structural and physiological characteristics, but their main mechanism of action is quite similar. Bufadienolides have the ability to inhibit the adenosine triphosphatase sodium-potassium pump (Na-K-ATPase), thus inducing natriuresis, vasoconstriction and subsequently increase in blood pressure, as well as cardiac inotrope activity [48, 49]. They are synthesized in the adrenal cortex and placenta, and experimental observations in rats have shown that, in situations characterized by volume expansion, increased secretion has been noted in the brain and plasma levels [48, 49]. At present, marinobufagenin is the most actively studied compound of bufadienolides that has been isolated from mammals [49, 50]. So far, it has been demonstrated, through experimental studies, that the development of kidney failure is accompanied by chronic volume overload and increased levels of marinobufagenin [50-53]. Additionally, its role has been implicated in the development of fibrosis in experimental uremic cardiomyopathy [52]. This was confirmed by observing that after immunization against marinobufagenin, the rate of cardiac hypertrophy and diastolic dysfunction significantly weakened [52]. Comparing these data with the recent evidence that spironolactone attenuates cardiac fibrosis and cardiomyopathy in both partial nephrectomy and marinobufagenin infusion models, researchers suggested that this compound might be responsible for some of the cardiac injury, which has been attributed to aldosterone and that mineralocorticoid antagonists may produce some of their beneficial effects by antagonism of marinobufagenin signaling through Na-K- ATPase [52]. Furthermore, the concept of cardiac fibrosis induced by marinobufagenin, lead to the extension of the experiments, involving other organs that are interrelated to the cardiac muscle, such as the kidney. Peri-glomerular and peri-tubular deposition of collagen was described, as well as kidney fibrosis and kidney disease progression. Hence, it is of outmost importance for researchers and clinicians to conduct a series of in vitro and in vivo studies for bufadienolides in humans and determine an association between CKD and CVD [53].
6.3. Homocysteine, Phosphorus and FGF-23
Conditions such as hyper-homocysteinemia, which are associated with increased cardiovascular disease risk in the general population, have been proven to be further detrimental in patients with renal failure undergoing hemodialysis [39]. Nonetheless, there is limited data between the relationship of homocysteine levels and cardiovascular disease risk in patients in the early stages of chronic kidney disease [39]. A recent analysis of a cohort of United States veterans with CKD stage 3 demonstrated that serum phosphorus levels>3.5 mg/dL were independent predictors of all-cause mortality [39].
Similarly, any abnormalities in the calcium phosphate metabolism, which are highly prevalent in patients undergoing hemodialysis need to be investigated early in the course of CKD [39]. Mineral metabolism promotes vascular calcification and stiffness, thus leading to increased pulse pressure, decreased coronary perfusion pressure, and LVH [39].
Several studies have demonstrated an index, which could be used to interpret the impact of chronic kidney disease on the cardiovascular system in the presence of Left Ventricular Hypertrophy (LVH) [54]. LVH is the most frequent structural cardiac abnormality in patients with CKD and a strong and independent predictor of survival and CV events in dialysis patients [54]. Conditions such as hypertension, anemia, fluid and electrolyte imbalance, as well as oxidation stress, inflammation, and activation of collagen and muscle cell growth factors may have a critical role in the development of left ventricular hypertrophy and diastolic dysfunction [54, 55]. Various studies have shown that the prevalence of LVH ranges from 34% to 78%, with increasing values correlating with the deterioration in renal function [54, 55]. Dialysis patients have a 75% occurrence rate of LVH, with an association with poorer CV outcomes [56]. Specifically, a study with 455 nondiabetic hypertensive patients, free of CV diseases, all with e GFR > 30ml/min per 1.73 m2, examined the relationship between Left Ventricular Mass (LVM) and mild-moderate reduction of kidney function [56]. It was concluded that there was a progressive increase of LVH prevalence with decreasing renal function, and an inverse association between GFR and LVM, independent of potential confounders, i.e. 24-hour BP load [56]. Also, in the ARIC study, among African Americans with chronic kidney disease stage 3, lower GFR and lower hemoglobin were associated with LVH [57]. Additionally, it has been postulated that even subclinical renal damage has been associated with impaired coronary flow reserve due to increased myocardial fibrosis [57]. The fibrosis is enhanced by the raised levels of fibroblast growth factor-23 (FGF-23), which are observed in CKD patients [58]. FGF-23 is a hormone secreted by osteoblasts and osteocytes, which helps maintain the phosphate homeostasis in CKD patients [59]. Myocardial fibrosis is detected either in post mortem analysis or by means of endocardial biopsy. Hence, the early detection of LVH and LV dysfunction in CKD patients can yield an improvement in the adverse cardiovascular outcomes of CKD patients [60, 61]. However, larger prospective randomized trials need to be conducted with early stages of CKD in order to assess the role of early aggressive treatment in this subpopulation group.
6.4. Cystatin C, eGFR Equations and NT-proBNP
Aston et al., documented that Cystatin C and b2-microglobulin performed better than hs-CRP for CVD prediction in the entire CKD population they had examined [62]. They concluded that their findings are in line with an established approach to use eGFRCreatinine-based, eGFRCystatin-based and ACR to identify the high-risk CKD population [62]. Their research suggests that cardiac-specific markers, such as cTnT, NT-proBNP and Cystatin C would be more useful to predict CVD outcomes in CKD population [62].
6.5. CKD, CVD and Major Trials
Summarizing, CKD has been recognized as an independent CVD risk equivalent, and it is strongly recommended that the CKD population be considered the highest risk group for subsequent development of CVD [1]. Interestingly, mild to moderate CKD is robustly associated with increased cardiovascular morbidity and mortality, independently from classical cardiovascular risk factors, such as hypertension and diabetes. Novel risk factors such as inflammation, oxidative stress, bone and mineral disorders; hyperphosphatemia, hypercalcemia, secondary hyperparathyroidism - that are attributed to compromised renal function-, are highly associated with elevated cardiovascular risk in patients with kidney disease [39]. Accounting for these various risk factors, we may be able to postulate that the pathophysiology involved in the development of CVD is common in the CKD and non-CKD population [39]. However, renal dysfunction alone, through novel risk factors, plays an important role in the worsening of CVD. Hence, treatment should target not only in slowing the progression of renal disease, but in modifying the additional CV factors early in the course of the disease, as well [39]. Lifestyle modifications are known to counteract the development of CVD, such as decreased consumption of salt, glucose, protein, and free fatty acids, in addition to non-dietary factors like, smoking cessation, regular exercise, and weight loss. However, adequate control of diabetes, hypertension, and hyperlipidemia has been robustly investigated in CKD population [39]. Specifically, based on the results of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, it has been acknowledged that intensive glycemic control is associated with higher cardiovascular and all-cause mortality rates compared with standard therapy (ACCORD). Findings from other large trials, that observed no reduction in cardiovascular events with intensive glycemic therapy, further support the findings in ACCORD [63]. Papademetriou et al. hypothesized that the cardiovascular risk was increased by the presence of mild-to-moderate CKD at baseline, in addition to intensive glycemic control [64]. A subgroup analysis of the ACCORD data found that CKD patients had worse outcomes with intensive therapy, compared to standard treatment, in terms of increased all-cause and CV mortality, by two-fold, while no significant effects were found on patients without CKD. This may be attributable to the more frequent use of insulin and oral hypoglycemic agents in CKD patients [64]. It is well established, that these adverse drug events are more common and more severe in CKD population. Additionally, in another post hoc analysis of Outcome Reduction with an Initial Glargine Intervention (ORIGIN) trial, there was an adverse effect of mild to moderate CKD on macrovascular outcomes, similar to findings in the ACCORD trial [64, 65]. However, both the baseline glycemic status and glycemic management goals in these trials were significantly different [64, 65].
Hypertension may result in cardiovascular events, which can deteriorate renal function, while further reduction in renal function can lead to worsening hypertension, and consequently contribute to a vicious cycle [39]. Optimal blood pressure levels for reno-protection and prevention of harmful cardiovascular outcomes, is believed to be at 130mmHg systolic and at 80mmHg diastolic [21]. While the common rationale is to achieve well-controlled blood pressure levels in CKD subpopulation, only a few studies can uphold this assumption, such as the SPRINT Study [66].
Dyslipidemia is another important risk factor for both CVD and CKD [39]. Several trials have examined the effects of different statins in the progression of CKD. Data suggest that not all statins have the same effect on kidney function. Trials involving Pravastatin showed that there was a significant improvement in the decline of kidney function and the same was noted with the Heart Protection Study that examined the effects of Simvastatin in kidney function in diabetic and non-diabetic population [67]. Pravastatin and Simvastatin did not demonstrate a significant improvement in eGFR, compared to atorvastatin, due to their lesser effect on LDL cholesterol. Atorvastatin treatment at high doses (80mg/day) was significantly associated with increases in eGFR and reductions in CVD events. Interestingly, Papademetriou et al., in a subgroup analysis of ACCORD population, examined the effects of fenofibrate therapy in addition to statin therapy. Surprisingly, it was concluded that for patients with type 2 diabetes at high CV risk but no CKD, fenofibrate therapy added to statin reduced the CV mortality and the rate of fatal and non-fatal CHF (Table 1) [68].
Table 1.
Major clinical trials regarding CKD and CVD.
| Trials | Number of Participants | Outcome |
|---|---|---|
| Accord trial | 10,251 | The use of intensive therapy to target normal glycated hemoglobin levels increased mortality and did not significantly reduce major CVD events, as compared to standard treatment |
| Origin trial | 12,537 | Insulin glargine titration had neutral effects on CVD |
| Sprint trial | 9,361 | The use of intensive treatment for systolic blood pressure reduced the incidence of CVD events and death from any cause, as compared to standard treatment |
| Heart protection study | 10,001 | Intensive lipid-lowering therapy with 80 mg of atorvastatin in patients with stable CHD provides significant clinical benefit beyond that afforded by treatment with 10 mg . |
Conclusion
Consequently, there have been several studies that examined the effects of modifying the common risk factors for both cardiovascular and kidney disease, through establishing standard as well as intensive care treatment. In particular, good glycemic control as well as anti-hypertensive and hypolipidemic therapy are the cornerstone of effective treatment of both CKD and CVD. In patients with diabetes, the administration of sodium glucose co-transporter inhibitors-2 (SGLT-2 inhibitors) must be emphasized, as they have been demonstrated to exert favorable properties in both CKD and CVD [69, 70]. In addition, anti-hypertensive treatment, especially with RAAS inhibitors and hypolipidemic therapy with statins, ezetimibe or PCSK9 inhibitors have beneficial results regarding both CKD and CVD [21]. Notably, antiplatelet therapy should not be overlooked in this setting [39]. However, larger studies are needed for evaluating the relationship between CKD and CVD and defining CKD as a cardiovascular risk equivalent. Additionally, further studies are needed to investigate new early treatment measures to prevent detrimental cardiovascular outcomes. Thus, aiming to control novel risk factors in early stages of CKD, could potentially lessen the progression not only of kidney disease but also halt the continuity of cardiovascular events; the leading cause of death worldwide.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Sarnak M.J., Levey A.S., Schoolwerth A.C., et al. Kidney disease as a risk factor for development of cardiovascular disease. A Statement From the American Heart Association Councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 2.Eknoyan G. Chronic kidney disease definition and classification: The quest for refinements. Kidney Int. 2007;72:1183–1185. doi: 10.1038/sj.ki.5002576. [DOI] [PubMed] [Google Scholar]
- 3.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification and stratification. Am. J. Kidney Dis. 2002;39(2) Suppl. 1:S1–S266. [PubMed] [Google Scholar]
- 4.Stenvinkel P. Chronic kidney disease: A public health priority and harbinger of premature cardiovascular disease. J. Intern. Med. 2010;268(5):456–467. doi: 10.1111/j.1365-2796.2010.02269.x. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention 2016 https://nccd.cdc.gov
- 6.Briasoulis A., Bakris G.L. Chronic kidney disease as a coronary artery disease risk equivalent. Curr. Cardiol. Rep. 2013;15(3):340. doi: 10.1007/s11886-012-0340-4. [DOI] [PubMed] [Google Scholar]
- 7.Husain-Syed F., McCullough P.A., Birk H.W., et al. Cardio-Pulmonary-renal interactions: A multidisciplinary approach. J. Am. Coll. Cardiol. 2015;65(22):2433–2448. doi: 10.1016/j.jacc.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention 2015 http://www.cdc.gov/nchs/fastats/ leading-causes-of-death.htm
- 9.Perk J., De Backer G., Gohlke H., et al. European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur. Heart J. 2012;33(17):1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 10.Manjunath G., Tighiouart H., Ibrahim H., et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J. Am. Coll. Cardiol. 2003;41:47–55. doi: 10.1016/s0735-1097(02)02663-3. [DOI] [PubMed] [Google Scholar]
- 11.Menon V., Gul A., Sarnak M.J. Cardiovascular risk factors in chronic kidney disease. Kidney Int. 2005;68(4):1413–1418. doi: 10.1111/j.1523-1755.2005.00551.x. [DOI] [PubMed] [Google Scholar]
- 12.Subbiah A.K., Chhabra Y.K., Mahajan S. Cardiovascular disease in patients with chronic kidney disease: A neglected subgroup. Heart Asia. 2016;8:56–61. doi: 10.1136/heartasia-2016-010809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keith D.S., Nichols G.A., Gullion C.M., Brown J.B., Smith D.H. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch. Intern. Med. 2004;164(6):659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 14.Daly C. Is early chronic kidney disease an important risk factor for cardiovascular disease? A background paper prepared for the UK Consensus Conference on early chronic kidney disease. Nephrol. Dial. Transplant. 2007;22(Suppl. 9):ix19–ix25. doi: 10.1093/ndt/gfm445. [DOI] [PubMed] [Google Scholar]
- 15.Archibald G., Bartlett W., Brown A., et al. UK Consensus Conference on early chronic kidney disease. Nephrol. Dial. Transplant. 2007;22(Suppl. 9):ix4–ix5. doi: 10.1093/ndt/gfm268. [DOI] [PubMed] [Google Scholar]
- 16.Varma P.P., Raman D.K., Ramakrishnan T.S. Pragnya Singh. Prevalence of early stages of chronic kidney disease in healthy army personnel. Med J Armed Forces. 2011;67:9–14. doi: 10.1016/S0377-1237(11)80004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey A.S., Bosch J.P., Lewis J.B., Greene T., Rogers N., Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.Levey A.S., Eckardt K.U., Tsukamoto Y., et al. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 19.Culleton B.F., Larson M.G., Parfrey P.S., Kannel W.B., Levy D. Proteinuria as a risk factor for cardiovascular disease and mortality in older people: A prospective study. Am. J. Med. 2000;109:1–8. doi: 10.1016/s0002-9343(00)00444-7. [DOI] [PubMed] [Google Scholar]
- 20.Grimm R.H., Jr, Svendsen K.H., Kasiske B., Keane W.F., Wahi M.M. Proteinuria is a risk factor for mortality over 10 years of follow-up. MRFIT Research Group. Multiple risk factor intervention trial. Kidney Int. 1997;63:S10–S14. [PubMed] [Google Scholar]
- 21.American Diabetes Association Standards of medical care in diabetes-2018. Abridged for primary care providers. Diabetes Care. 2018;41(Suppl. 1):S1–S59. doi: 10.2337/cd17-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dell’Omo G., Penno G., Giorgi D., Mariani M., Pedrinelli R. Association between high-normal albuminuria and risk factors for cardiovascular and renal disease in essential hypertensive men. Am. J. Kidney Dis. 2002;40:1–8. doi: 10.1053/ajkd.2002.33906. [DOI] [PubMed] [Google Scholar]
- 23.Orth S.R., Stein I., Hallan S.I. Smoking: A risk factor for progression of chronic kidney disease and for cardiovascular morbidity and mortality in renal patients-absence of evidence or evidence of absence? Clin. J. Am. Soc. Nephrol. 2008;3(1):1–8. doi: 10.2215/CJN.03740907. [DOI] [PubMed] [Google Scholar]
- 24.Jager A., Kostense P.J., Ruhe H.G., et al. Microalbuminuria and peripheral arterial disease are independent predictors of cardiovascular and all-cause mortality, especially among hypertensive subjects: Five-year follow-up of the Hoorn Study. Arterioscler. Thromb. Vasc. Biol. 1999;19:617–624. doi: 10.1161/01.atv.19.3.617. [DOI] [PubMed] [Google Scholar]
- 25.Diercks G.F., Hillege H.L., van Boven A. Relation between albumin in the urine and electrocardiographic markers of myocardial ischemia in patients without diabetes mellitus. Am. J. Cardiol. 2001;88:771–774. doi: 10.1016/s0002-9149(01)01849-5. [DOI] [PubMed] [Google Scholar]
- 26.Wachtell K., Olsen M.H., Dahlof B., et al. Microalbuminuria in hypertensive patients with electrocardiographic left ventricular hypertrophy: The LIFE study. J. Hypertens. 2002;20:405–412. doi: 10.1097/00004872-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 27.Smink P.A., Lambers Heerspink H.J., Gansevoort R.T., de Jong P.E., Hillege H.L., Bakker S.J.L., de Zeeuw D. Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: The PREVEND (Prevention of Renal and Vascular Endstage Disease) study. Am. J. Kidney Dis. 2012;60(5):804–811. doi: 10.1053/j.ajkd.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 28.Klausen K., Borch-Johnsen K., Feldt-Rasmussen B., et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004;110:32–35. doi: 10.1161/01.CIR.0000133312.96477.48. [DOI] [PubMed] [Google Scholar]
- 29.Spoelstra-de Man A.M.E., Brouwer T.B., Stehouwer C.D.A., Smulders Y.M. Rapid progression of albumin excretion is an independent predictor of cardiovascular mortality in patients with type 2 diabetes and microalbuminuria. Diabetes Care. 2001;24:2097–2101. doi: 10.2337/diacare.24.12.2097. [DOI] [PubMed] [Google Scholar]
- 30.Yuyun M.F., Dinneen S.F., Edwards O.M., Wood E., Wareham N.J. Absolute level and rate of change of albuminuria over 1 year independently predict mortality and cardiovascular events in patients with diabetic nephropathy. Diabet. Med. 2003;20:277–282. doi: 10.1046/j.1464-5491.2003.00940.x. [DOI] [PubMed] [Google Scholar]
- 31.Ibsen H., Olsen M.H., Wachtell K., et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension. 2005;45:198–202. doi: 10.1161/01.HYP.0000154082.72286.2a. [DOI] [PubMed] [Google Scholar]
- 32.Matsushita K., van der Velde M., Astor B.C., et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality: A collaborative meta-analysis of general population cohorts. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stehouwer C.D.A., Smulders Y.M. Microalbuminuria and risk for cardiovascular disease: Analysis of potential mechanisms. J. Am. Soc. Nephrol. 2006;17:2106–2111. doi: 10.1681/ASN.2005121288. [DOI] [PubMed] [Google Scholar]
- 34.Paisley K.E., Beaman M., Tooke J.E., Vidya Mohamed-Ali V., Lowe G.D.O., Shore A.C. Endothelial dysfunction and inflammation in asymptomatic proteinuria. Kidney Int. 2003;63:624–633. doi: 10.1046/j.1523-1755.2003.00768.x. [DOI] [PubMed] [Google Scholar]
- 35.Henry R.M.A., Ferreira I., Kostense P.J., et al. Type 2 diabetes is associated with impaired endothelium-dependent, flow-mediated dilation, but impaired glucose metabolism is not. The Hoorn Study. Atherosclerosis. 2004;174:49–56. doi: 10.1016/j.atherosclerosis.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Nieuwdorp M., Meuwese M.C., Vink H., Hoekstra J.B., Kastelein J.J.P., Stroes E.S. The endothelial glycocalix: A potential barrier between health and vascular disease. Curr. Opin. Lipidol. 2005;16:507–511. doi: 10.1097/01.mol.0000181325.08926.9c. [DOI] [PubMed] [Google Scholar]
- 37.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Heart Outcomes Prevention Evaluation Study Investigators. Vitamin E supplementation and cardiovascular events in high-risk patients. N. Engl. J. Med. 2000;342(3):154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 38.Eidelman R.S., Hollar D., Hebert P.R., Lamas G.A., Hennekens C.H. Randomized trials of vitamin E in the treatment and prevention of cardiovascular disease. Arch. Intern. Med. 2004;164(14):1552–1556. doi: 10.1001/archinte.164.14.1552. [DOI] [PubMed] [Google Scholar]
- 39.Subbiah A.K., Chhabra Y.K., Sandeep Mahajan S. Cardiovascular disease in patients with chronic kidney disease: A neglected subgroup. Heart Asia. 2016;8(2):56–61. doi: 10.1136/heartasia-2016-010809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jager A., van Hinsbergh V.W., Kostense P.J., et al. C-reactive protein and soluble vascular cell adhesion molecule-1 are associated with elevated urinary albumin excretion but do not explain its link with cardiovascular risk. Arterioscler. Thromb. Vasc. Biol. 2002;22:593–598. doi: 10.1161/01.atv.0000013786.80104.d4. [DOI] [PubMed] [Google Scholar]
- 41.Schram M.T., Chaturvedi N., Schalkwijk C.G., Fuller J.H., Stehouwer C.D.A., EURODIAB Prospective Complications Study Group Markers of inflammation are cross-sectionally associated with microvascular complications and cardiovascular disease in type 1 diabetes: The EURODIAB Prospective Complications Study. Diabetologia. 2005;48:370–378. doi: 10.1007/s00125-004-1628-8. [DOI] [PubMed] [Google Scholar]
- 42.Miller W.G., Bruns D.E., Hortin G.L., et al. Current issues in measurement and reporting of urinary albumin excretion. Clin. Chem. 2009;55:24–38. doi: 10.1373/clinchem.2008.106567. [DOI] [PubMed] [Google Scholar]
- 43.Uchida H.A., Norii H., Hanayama Y., et al. Steroid pulse therapy impaired endothelial function while increasing plasma high molecule adiponectin concentration in patients with IgA nephropathy. Nephrol. Dial. Transplant. 2006;21:3475–3480. doi: 10.1093/ndt/gfl423. [DOI] [PubMed] [Google Scholar]
- 44.Schwedhelm E., Böger R.H. The role of asymmetric and symmetric dimethylarginines in renal disease. Nat. Rev. Nephrol. 2011;7(5):275–285. doi: 10.1038/nrneph.2011.31. [DOI] [PubMed] [Google Scholar]
- 45.Kielstein J.T., Frölich J.C., Haller H., Ritz E., Fliser D. Marked increase of asymmetric dimethylarginine in patients with incipient primary chronic renal disease. J. Am. Soc. Nephrol. 2003;13:170–176. doi: 10.1681/ASN.V131170. [DOI] [PubMed] [Google Scholar]
- 46.Schepers E., Glorieux G., Dhondt A., Leybaert L., Vanholder R. Role of symmetric dimethylarginine in vascular damage by increasing ROS via store-operated calcium influx in monocytes. Nephrol. Dial. Transplant. 2009;24:1429–1435. doi: 10.1093/ndt/gfn670. [DOI] [PubMed] [Google Scholar]
- 47.Böger R.H., Darius H., Atzler D., et al. Asymmetric dimethylarginine as independent risk marker for mortality in ambulatory patients with peripheral arterial disease. J. Intern. Med. 2011;269:349–361. doi: 10.1111/j.1365-2796.2010.02322.x. [DOI] [PubMed] [Google Scholar]
- 48.Schulze F., Wesemann R., Schwedhelm E., et al. Symmetric dimethylarginine predicts all-cause mortality following ischemic stroke. Atherosclerosis. 2010;208:518–523. doi: 10.1016/j.atherosclerosis.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 49.Puschett J.B., Agunanne E., Uddin M.N. Emerging role of the bufadienolides in cardiovascular and kidney diseases. Am. J. Kidney Dis. 2010;56:359–370. doi: 10.1053/j.ajkd.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 50.Bagrov A.Y., Agalakova N.I., Kashkin V.A., Fedorova O.V. Endogenous cardiotonic steroids and differential patterns of sodium pump inhibition in NaCl-loaded salt-sensitive and normotensive rats. Am. J. Hypertens. 2009;22(5):559–563. doi: 10.1038/ajh.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meneton P., Jeunemaitre X., de Wardener H.E., MacGregor G.A. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol. Rev. 2005;85:679–715. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- 52.Elkareh J., Periyasamy S.M., Shidyak A., et al. Marinobufagenin induces increases in procollagen expression in a process involving protein kinase C and Fli-1: implications for uremic cardiomyopathy. Am. J. Physiol. Renal Physiol. 2009;296:F1219–F1226. doi: 10.1152/ajprenal.90710.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fedorova L.V., Raju V., El-Okdi N. The cardiotonic steroid hormone marinobufagenin induces renal fibrosis: Implication of epithelial-to-mesenchymal transition. Am. J. Physiol. Renal Physiol. 2009;296:F922–F934. doi: 10.1152/ajprenal.90605.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cerasola G., Nardi E., Palermo A., Mulè G., Cottone S. Epidemiology and pathophysiology of left ventricular abnormalities in chronic kidney disease: A review. J. Nephrol. 2011;24:1. doi: 10.5301/jn.2010.2030. [DOI] [PubMed] [Google Scholar]
- 55.Nardi E., Palermo A., Mulè G., Cusimano P., Cottone S., Cerasola G. Left ventricular hypertrophy and geometry in hypertensive patients with chronic kidney disease. J. Hypertens. 2009;27:633–641. doi: 10.1097/HJH.0b013e3283220ecd. [DOI] [PubMed] [Google Scholar]
- 56.Cerasola G., Nardi E., Mulè G. Left ventricular mass in hypertensive patients with mild-to-moderate reduction of renal function. Nephrology (Carlton) 2010;15:203–210. doi: 10.1111/j.1440-1797.2009.01178.x. [DOI] [PubMed] [Google Scholar]
- 57.Weiner D.E., Tighiouart H., Vlagopoulos P.T., et al. Effects of anemia and left ventricular hypertrophy on cardiovascular disease in patients with chronic kidney disease. J. Am. Soc. Nephrol. 2005;16(6):1803–1810. doi: 10.1681/ASN.2004070597. [DOI] [PubMed] [Google Scholar]
- 58.Gutierrez O., Isakova T., Rhee E. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J. Am. Soc. Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 59.Gutiérrez O.M., Januzzi J.L., Isakova T. Fibroblast growth factor-23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mirza M., Larsson A., Melhus H., Lind L., Larsson T. Serum intact FGF-23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207:546–551. doi: 10.1016/j.atherosclerosis.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 61.Aoki J., Ikari Y., Nakajima H. Clinical and pathologic characteristics of dilated cardiomyopathy in hemodialysis patients. Kidney Int. 2005;67:333–340. doi: 10.1111/j.1523-1755.2005.00086.x. [DOI] [PubMed] [Google Scholar]
- 62.Astor B.C., Shafi T., Hoogeveen R.C., et al. Novel markers of kidney function as predictors of esrd, cardiovascular disease, and mortality in the general population. Am. J. Kidney Dis. 2012;59:653–662. doi: 10.1053/j.ajkd.2011.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.ACCORD Study Group Long-term effects of intensive glucose lowering on cardiovascular outcomes. N. Engl. J. Med. 2011;364(9):818–828. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papademetriou V., Zaheer M., Doumas M., et al. Cardiovascular outcomes in action to control cardiovascular risk in diabetes: Impact of blood pressure level and presence of kidney disease. Am. J. Nephrol. 2016;43(4):271–280. doi: 10.1159/000446122. [DOI] [PubMed] [Google Scholar]
- 65.ORIGIN Trial Investigators Basal insulin and cardiovascular and other outcomes in dysglycemia. N. Engl. J. Med. 2012;367(4):319–328. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 66.Berlowitz D.R., Foy C.G., Kazis L.E., et al. Effect of intensive blood-pressure treatment on patient-reported outcomes. N. Engl. J. Med. 2017;377(8):733–744. doi: 10.1056/NEJMoa1611179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jellinger P.S., Handelsman Y., Rosenblit P.D., et al. American association of clinical endocrinologists and American college of endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr. Pract. 2017;23(Suppl. 2):1–87. doi: 10.4158/EP171764.APPGL. [DOI] [PubMed] [Google Scholar]
- 68.Papademetriou V., Lovato L., Tsioufis C., et al. Effects of high density lipoprotein raising therapies on cardiovascular outcomes in patients with type 2 diabetes mellitus, with or without renal impairment: The action to control cardiovascular risk in diabetes study. Am. J. Nephrol. 2017;45(2):136–145. doi: 10.1159/000453626. [DOI] [PubMed] [Google Scholar]
- 69.Wanner C., Inzucchi S.E., Lachin J.M., et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med. 2016;375(4):323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 70.Neal B., Perkovic V., Mahaffey K.W., et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]