Abstract
Background:
Suicidal ideation (SI) is an important predictor of suicide attempt, yet SI is difficult to predict. Given that SI begins in adolescence when brain networks are maturing, it is important to understand associations between network functioning and changes in severity of SI.
Methods:
Thirty-three depressed adolescents were administered the Columbia-Suicide Severity Rating Scale to assess SI and completed resting-state fMRI at baseline (T1) and 6 months later (T2). We computed coherence in the executive control (ECN), default mode (DMN), salience (SN), and non-relevant noise networks and then examined the association between changes in brain network coherence and changes in SI severity from T1 to T2.
Results:
A greater reduction in severity of SI was associated with a stronger increase in SN coherence from T1 to T2. There were no associations between the other networks and SI.
Limitations:
We cannot generalize our findings to more psychiatrically diverse samples. More time-points are necessary to understand the trajectory of SI and SN coherence change.
Conclusions:
Our finding that reductions in SI are associated with increases in SN coherence extends previous cross-sectional results documenting a negative association between SI severity and SN coherence. The SN is involved in coordinating activation of ECN and DMN in response to salient information. Given this regulatory role of the SN, the association between SN coherence and SI suggests that adolescents with reduced SN coherence might more easily engage in harmful thoughts. Thus, the SN may be particularly relevant as a target for treatment applications in depressed adolescents.
Keywords: resting-state functional connectivity, network coherence, suicidal ideation, adolescence, salience network, longitudinal
Introduction
Suicide is the second leading cause of death in adolescents in the United States (CDC Injury Prevention and Control, 2016). An important predictor of suicide attempt is suicidal ideation (SI; Reinherz et al., 2006), which are thoughts that can range from passive death wishes (“I wish I was never born”) to making more detailed and concrete plans; indeed, 60% of suicide attempts occur within first year of the onset of SI (Kessler et al., 1999). Given the rising prevalence of SI beginning around age 13 (CDC, 2018; Nock et al., 2013) and the fact that SI and suicidal behaviors are symptoms that are found in depression, bipolar disorder, PTSD, substance abuse, and borderline personality disorder (Hoertel et al., 2015), identifying and elucidating the psychological and biological mechanisms that contribute to SI in adolescence could have a wide-ranging clinical impact.
In this context, identifying neural correlates of SI may inform novel neuroscience-based models of suicide risk and guide interventions for this vulnerable age group. In particular, elucidating neural correlates of SI using a method that is independent of task performance and that reliably elicits patterns of network connectivity that are associated with specific cognitive processes is important for understanding the neural basis of SI in patient populations. MRI research using resting-state functional connectivity (RSFC) in individuals with mental health difficulties has consistently identified aberrations in co-activated brain regions, or brain networks (Cullen et al., 2009; Menon, 2011). These networks include the default mode network (DMN)—including the medial prefrontal cortex, posterior cingulate, and hippocampus—which has been found to be involved in ruminative, negative self-referential processes (Hamilton et al., 2015); the executive control network (ECN)—including the lateral prefrontal cortex and the posterior parietal cortex—has been involved in initiating goal-directed responses, including regulating emotions (Menon, 2011). Finally, the salience network (SN)—including the dorsal anterior cingulate cortex (dACC) and the insula—has been posited to guide the ECN and DMN to achieve externally or internally directed goals and to be involved in emotional attention and rumination (Hamilton et al., 2012; Ordaz et al., 2017; Sadaghiani and D’Esposito, 2015; Uddin, 2015). Given that these networks have been implicated in maladaptive emotional behaviors, elucidating their contribution to SI may advance our understanding of the complex mechanisms that underlie suicidality.
A small number of studies have used RSFC to examine neural correlates of SI in depressed adults. Du and colleagues (Du et al., 2017) found compared to individuals without SI, individuals with SI have decreased RSFC between the rostral anterior cingulate cortex (rACC), a region implicated in emotional processing in MDD and suicide attempts (Etkin et al., 2011; Van Heeringen and Mann, 2014), and the orbitofrontal cortex (OFC), a region involved in emotional processing and decision-making (Van Heeringen and Mann, 2014; Zhang et al., 2014). Researchers have also documented differences between adults with and without SI in their connectivity between the dACC (part of the SN) and subregions of the posterior cingulate cortex, part of the DMN (Chase et al., 2017). Thus, altered connectivity between regions of the SN, which are involved in attention to threat, and regions of the DMN, which are implicated in self-referential processing, may contribute to the development and persistence of SI. It is important to note, however, that these two studies assessed SI in adults. Given the maturation of the brain throughout adolescence, it is not clear whether these findings generalize to younger individuals.
Only three studies to date have examined the association between SI and RSFC in depressed adolescents. Cullen et al. (2014) used a seed-to-whole brain approach focusing on the amygdala and found evidence that amygdala-hippocampal and parahippocampal RSFC is related to depressive symptoms; however, they did not find evidence that amygdala RSFC is related to suicidality (Cullen et al., 2014). Schreiner et al. (2018) used a seed-to-whole-brain approach focusing on the bilateral precuneus/PCC in the DMN and found that suicidal thoughts and self-harm were associated with increased precuneus functional connectivity (FC) and decreased PCC FC (Schreiner et al., 2018). In contrast, Ordaz et al. (2018) used a data-driven approach—independent components analysis (ICA)—to examine resting-state networks associated with SI (Ordaz et al., 2018). These investigators found that decreased network coherence, indexed by the temporal correlations among brain regions, in the left ECN, anterior DMN, and SN was associated with severity of lifetime SI and, further, that the left ECN was the network that was associated most strongly with severity of SI (i.e., above and beyond the effects of DMN and SN). Together, these studies document RSFC patterns that are associated with suicidal thoughts in adolescents. Because these data are cross-sectional, however, it is difficult to draw conclusions about the temporal nature of the association between patterns of RSFC and SI. The brain is maturing rapidly over adolescence, making it critical to examine changes in neural function and connectivity that are associated with changes in pathology (Gotlib and Ordaz, 2016).
The present study extends the previous cross-sectional investigation of the relation between most severe lifetime SI and network coherence (Ordaz et al., 2018) by using follow-up data from the same sample of participants Ordaz et al. (2018) recruited. In this present study, we examine the longitudinal association between change in the most severe SI since the baseline assessment, herein termed ‘change in SI’, and change in network coherence. Because measurements of current SI may not reflect participants’ most severe occurrence of ideation, at T1 we assessed lifetime severity of SI and at T2 we assessed severity of SI since T1. Given the previous one-time point findings that higher levels of SI are associated with decreased network coherence of the SN, the anterior DMN, and the left ECN (Ordaz et al., 2018), we hypothesized that there would be a similar inverse longitudinal association between change in severity of SI and change in coherence of these networks.
Methods
Participants
Participants in this study were 40 depressed adolescents (30 female) ages 14–17 years recruited through the Pediatric Mood Disorders Program at Stanford School of Medicine, community mental health clinics, media advertisements, and flyers posted throughout the San Francisco Bay Area. Inclusion criteria included having a current episode of MDD according to DSM-IV criteria, assessed with the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS-PL) (Kaufman et al., 1997) and the Child Depression Rating Scale (Jain et al., 2007). Exclusion criteria included meeting DSM-IV criteria for Bipolar disorder, an eating disorder, a psychotic disorder, alcohol/substance dependence, contraindications for scanning (e.g., metal implants), a lifetime history of neurological, cardiovascular, or any other major medical problems. This study was approved by the Stanford University Institutional Review Board and all participants and their parents provided informed written assent/consent. At the baseline (Time 1; T1) assessment, adolescents were interviewed for inclusion/exclusion criteria and, if eligible to participate in the study, they completed questionnaires assessing psychopathology and a resting-state scan. Participants returned to the laboratory approximately 6 months (Time 2; T2) later to complete clinical assessments and a second resting-state scan. In the present study we include data from the T2 assessment of the Ordaz et al. (2018) sample of depressed adolescents in order to examine within-individual differences in the highest reported severity of SI and the associated changes in brain network coherence. Of the original 40 adolescents recruited at T1, 5 did not return for their second scan; two of the remaining participants did not have usable scan data, leaving a final sample of 33 adolescents (25 female). There were no baseline clinical differences between adolescents who completed the first time point only and those who completed both time points (See Supplementary Table 1).
Behavioral Measures
At each laboratory session, participants were administered the KSADS-PL to assess the presence of an MDD diagnosis as well as suicidal behaviors. Participants were also administered the Columbia-Suicide Severity Rating Scale (C-SSRS; Posner et al., 2011) through a clinical interview to assess, at T1, the most severe SI in the adolescent’s lifetime and, at T2, the most severe SI since T1. The C-SSRS rates severity of ideation from 1–5 (1=a wish to be dead; 5=active suicidal ideation with a specific plan and intent to end one’s life); a score of 0 indicates no endorsement of SI. Participants’ highest endorsement rating on the C-SSRS was used as their most severe level of SI (See Supplementary Table 2 for count of adolescents’ C-SSRS scores from T1 to T2). We also used the C-SSRS to assess current SI (i.e., within the past 2 weeks). The C-SSRS has established high convergent and divergent validity with other measures of SI, high sensitivity and specificity, as well as high internal consistency (Posner et al., 2011). Adolescents also completed a slightly modified Patient Health Questionnaire-9 (PHQ-9; Kroenke and Spitzer, 2001) to assess severity of depression (we omitted Question 9 concerning suicide given that we had more comprehensive data about suicide from the C-SSRS), and the Multidimensional Anxiety Scale for Children (MASC) to assess severity of anxiety. The PHQ-9 has demonstrated high sensitivity and specificity for adolescents (Richardson et al., 2010) and the MASC has demonstrated high reliability and validity (March et al., 1997).
fMRI Data Acquisition and Preprocessing
See Supplementary Materials and Methods for details on data acquisition. Data were preprocessed using conservative motion correction procedures and regression of physiological noise based on tools from Freesurfer (Fischl et al., 2004), FSL (Smith et al., 2004) and AFNI (Cox, 1996) and according to well-validated (Yan et al., 2013), previously published protocols (Ordaz et al., 2017). See Supplementary Materials and Methods for details on preprocessing.
Resting-State Independent Component Analysis
Following the approach Ordaz et al. (2018) used to investigate the association between network coherence and lifetime severity of SI at one time point, we conducted an ICA to examine change in network coherence between two time points. Using ICA to examine resting-state brain networks allows us to examine networks of voxels and to conduct a data-driven exploration of how multiple regions are integrated to underlie behavior (in contrast to seed-based analyses that require selecting specific regions a priori); ICA also has higher test-retest reliability than do seed-based analyses (Smith et al., 2014; Zuo et al., 2010). We used the T1 resting-state data to conduct a group ICA using FSL’s MELODIC, specifying 25 components, which splits the functional data into a set of spatial maps, each with an associated time course, generating a series of networks. We used only T1 data for this step because the ICA assumes independence of the data points, and thus cannot use clustered data. Using T1 data also allows for standardization of the data because at that timepoint all participants were experiencing a diagnosed depressive episode.
We then visually inspected the networks based on their neuroanatomical components. For this longitudinal analysis we focused on the same six networks that Ordaz et al. (2018) used in their one-timepoint analysis: the anterior and ventral portions of the DMN; the bilateral ECN; the SN; as well as a noise network as a control (Kelly et al., 2010; Zuo et al., 2010). These networks are presented in Figure 1.
Figure 1.
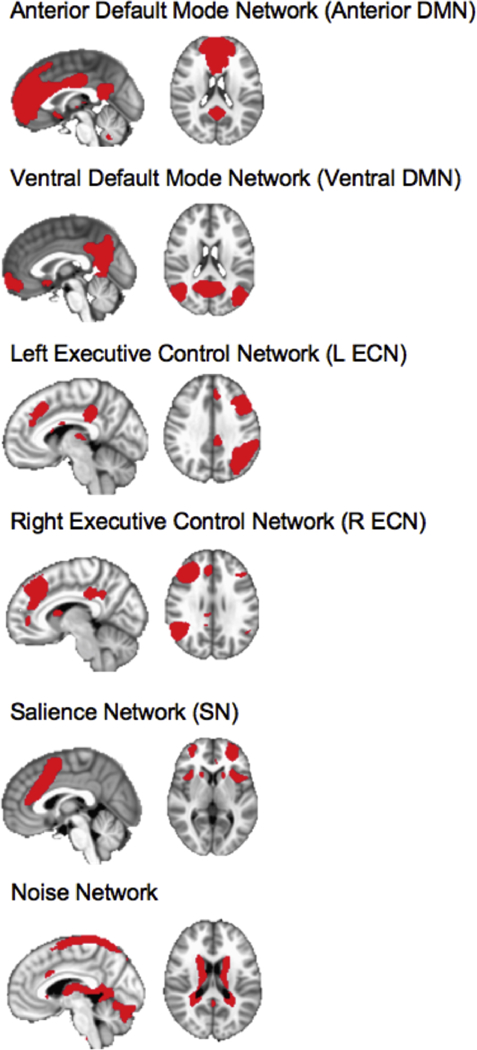
Networks of Interest, including a non-relevant noise network, identified through an independent components analysis. Images are shown in RAI orientation, with sagittal slice (on the left) and axial slice (on the right).
After generating group-averaged spatial maps of each network, we related these maps to individual participants. To do this, we conducted a dual regression analysis (Beckmann et al., 2005; Filippini et al., 2009), which first regresses the group-averaged spatial maps into each individual’s resting-state data (both at T1 and at T2). This first regression creates a timeseries for each individual, associated with each group-averaged spatial map. Second, the individuals’ timeseries were regressed into their own resting-state data to create a spatial map for each individual. Each individual’s spatial map is composed of regression weights that represent the functional connectivity (i.e., correlations) between voxels in the network (which was defined by the group of subjects), regressing out shared variance with other networks (Smith et al., 2014). We then normalized the correlation coefficients.
After applying the group masks to the individual spatial maps, we averaged the normalized correlation values within each mask to produce a metric of network coherence for each individual (van Duijvenvoorde et al., 2015) at T1 and T2. Thus, network coherence in our study is an average normalized correlation between the timecourse of each voxel and all other voxels within the group-identified network.
Calculating Change Scores
All statistical analyses were conducted using R (version 1.1.383) (R Core Team, 2014). We examined the association between change in brain network coherence from T1 to T2 and the difference between the most severe SI in the child’s lifetime, assessed at T1, and the most severe SI since T1, assessed at T2. To examine the association between change in SI and the change in their brain network coherence, we first calculated the difference between T1 and T2 SI and the difference between T1 and T2 coherence for each of the six brain networks (see also Supplementary Table 3 for intra-class correlations (ICCs) for each network). Second, we calculated the time interval between T1 and T2 for each participant and included that interval in the model by dividing both the SI and the network coherence change scores by the interval. To elucidate which network is most strongly associated with the change in SI, we included the six network coherence change scores in a linear regression model. In addition, we controlled for the same covariates as Ordaz et al. (Ordaz et al., 2018), including centered covariates of T1 lifetime severity of SI, T1 severity of depression, T1 age, T1 anxiety, and relative motion estimates at T2. Following Ordaz et al. (2018), we also controlled for age of initial depression onset; including this covariate, however, did not improve model fit.
Results
Sample Characteristics
Sample demographic and psychiatric characteristics are presented in Tables 1 and 2. All participants were in a depressive episode at T1. At T2, an average of 5.7 months after T1, 15 participants were still in episode. Given these data, it is not surprising that, as a group, participants reported significantly lower scores on the PHQ-9 at T2 than at T1 (see Table 2).
Table 1.
Sample Characteristics
| Depressed Adolescents (N=33) | |
|---|---|
| T1 Age: M (SD) | 16.33 years (1.03) |
| T2 Age: M (SD) | 16.80 years (1.06) |
| Average time between T1 and T2 | 5.7 months |
| Time range between T1 and T2 | 4.17 – 13.30 months |
| Sex (F) | 25 (75.76%) |
| Ethnicity (%) | |
| Hispanic/Latino | 51.51 |
| Not Hispanic/Latino | 48.49 |
| Race (%) | |
| African American | 3.03 |
| Hispanic | 9.09 |
| Asian | 6.06 |
| Biracial | 24.24 |
| Other | 9.09 |
Note. T1 = Time 1; T2 = Time 2
One subject had an average time interval exceeding 6 months. Analyses were also done with the removal of this participant.
Table 2.
Difference between sample psychiatric characteristics and T1 and T2.
| Time 1 (N=33) | Time 2 (N=33) | Difference | |
|---|---|---|---|
| C-SSRS (M, SD) | |||
| Lifetime SI Severity | (3.30, 1.69) | --- | |
| Since Last Visit SI Severity | --- | (1.73, 1.72) | t(32)=5.87, p<.001* |
| Current SI Severity | (1.36, 1.52) | (.76, 1.44) | t(32)=1.80, p=.08 |
| PHQ-9 (M, SD) | (14.86, 4.21) | (12.52, 4.74) | t(32)=2.23, p=.033 |
| MASC (M, SD) | (52.88,16.87) | (47.21, 18.03) | t(31)=3.01, p=.005 |
| Currently on psychotropic medication |
10 (30.30%) | 11 (33.33%) | χ2(1)=0.11, p=.739 |
| Currently in psychotherapy (individual, family, or group) |
26 (78.79%) | 24 (72.73%) | χ2(1)=2.78, p=.096 |
| KSADS Suicidal Behaviors # children with attempt history (%) |
10 (30.30%) | 3 (9.09%) | χ2(1)=7.00, p=.008 |
Note. One participant did not complete the MASC at T1.
Difference between Lifetime SI at T1 and “Since Last Visit” SI at T2
Decreases in Suicidal Ideation from T1 to T2
Adolescents reported a significant decrease in SI from the T1 (lifetime) to the T2 (since T1) assessments (see Fig. 2A for individual trajectories); there was also a marginally significant decrease in current level of SI from T1 to T2 (see Table 2).
Figure 2A,B.
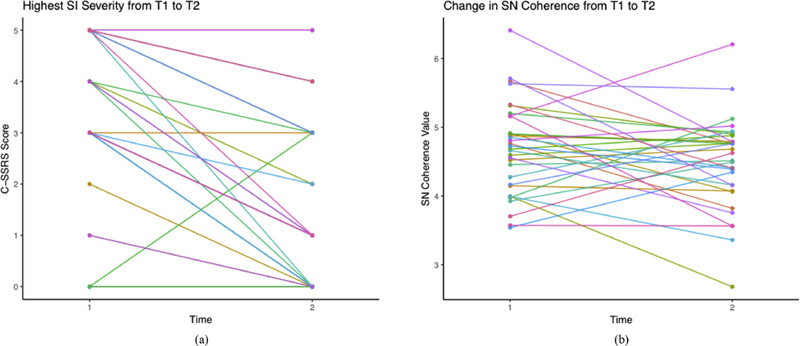
(A) Individual trajectories of SI severity from T1 to T2. (B) Individual trajectories of SN coherence from T1 to T2.
Change in Network Coherence is Associated with Change in Severity of Suicidal Ideation
A greater reduction in severity of SI was associated with a stronger increase in SN coherence from T1 to T2 (see Fig. 2B individual trajectories of SN coherence from T1 to T2.). More specifically, change in SN coherence explains change in SI above and beyond changes in coherence of the other networks, T1 age, T1-assessed lifetime SI, T1 depression, T1 anxiety, and relative motion estimates at T2, β=−.50, t=−3.05, p=.006 (see Fig. 3 and Table 3). The association between SN coherence and SI remained when controlling for age of initial onset of depression and current medication status (dummy coded) at T1 and T2. We also examined whether change in network coherence was associated with change in current severity of SI. Although there were no significant associations between change in network coherence and change in current SI (β =−.27, t=−1.41, p=.174), the direction of association between change in SN coherence and change in current SI was consistent with our primary finding. Standardized beta coefficients for each network coherence are presented in Table 3.
Figure 3.
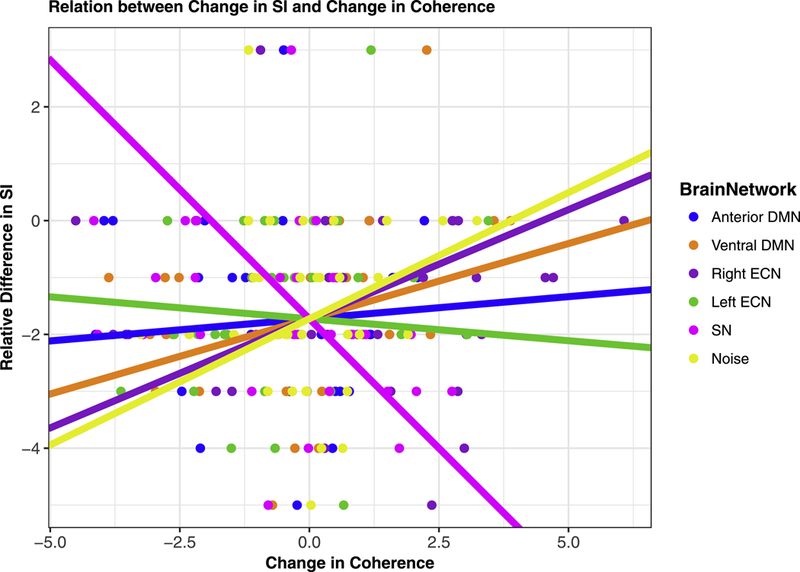
The association between the change in SI (between lifetime SI and past 6 months SI) and change in brain network coherence (between Time 1 and Time 2). Note. Association between change in SI and change in SN coherence remains significant with the removal of the participant showing an increase in SI.
Table 3.
Relation between change in network coherence and relative difference in lifetime SI and change in current SI.
| Network | Association with Change in Lifetime SI |
Association with Change in Current SI |
|---|---|---|
| Anterior DMN | β=.07, t=.44, p=.661 | β=−.00, t=−.00, p=.997 |
| Ventral DMN | β=.10, t=.48, p=.638 | β=.20, t=.96, p=.349 |
| R ECN | β=.04, t=.22, p=.829 | β=.24, t=1.13, p=.272 |
| L ECN | β=.25, t=1.36, p=.190 | β=.03, t=.16, p=.871 |
| SN | β=−.50, t=−3.05, p=.006 | β=−.27, t=−1.41, p=.174 |
| Noise | β=.25, t=1.45, p=.162 | β=−.00, t=−.02, p=.986 |
Note. Betas are standardized coefficients from regression (N=33) controlling for baseline age, baseline PHQ-9 score, baseline MASC score, baseline C-SSRS score, and relative motion at time two (R2=.40). Associations held with the removal of a participant who did not complete the MASC at baseline and with the removal of a participant who exceeded the 6-month interval.
Exploratory Between-Network Analyses
Because some studies have reported a normative segregation between networks during adolescence (e.g., Baum et al., 2017; Cao et al., 2016; Sherman et al., 2014), we conducted exploratory analyses to examine the association between baseline between-network connectivity and baseline lifetime severity of SI, as well as the longitudinal association between-network connectivity and severity of SI. Supplementary Materials and Methods.
Exploratory Suicidal Attempt History Analyses
Ten participants reported a history of suicidal attempt (SA) at T1. Therefore, we repeated the main analyses to examine whether prior SA behaviors moderated our effects. Not surprisingly, participants with a history of SA at T1 had significantly higher SI at both time points than did participants without a history of SA; however, there was no difference between these two groups of participants in their change in SI. These analyses are presented in Supplementary Materials and Methods.
Discussion
The present study was designed to examine the longitudinal associations between changes in the severity of SI and changes in the coherence of intrinsic brain networks in adolescents. This study is important in beginning to examine RSFC as a potential biomarker for intra-individual changes in the severity of SI. Previous researchers have used task-based imaging involving emotional processing and cognitive control to identify brain-based markers of suicidality. For example, focusing on single regions of interest (ROIs), Miller et al. (2018) found that adolescents with SI had less activation in the dorsolateral prefrontal cortex while passively viewing negative emotional stimuli than did adolescents without SI, possibly reflecting a diminished ability to effectively regulate responses to negative stimuli (Miller et al., 2018). Similarly, Pan (2011) found less activation in dACC activation during an executive functioning task (a go/no go response inhibition task) in suicide attempters than in non-attempters (Pan et al., 2011). Other investigators have broadened the scope of their analyses to examine the relation between functional connectivity of brain regions and suicidality. Pan et al. (2013) found that depressed adolescents who had attempted suicide had less connectivity between the dACC and insula – two regions of the SN – when viewing angry-face stimuli than did depressed adolescents who had not attempted suicide (Pan et al., 2013). In individuals with bipolar disorder who had attempted suicide, Johnston et al. (2017) found decreased connectivity in regions of the DMN when viewing happy and neutral faces (Johnston et al., 2017). Although these are important findings, their generalizability is limited by the use of different tasks across studies. Examining intrinsic patterns of brain connectivity that are independent of task (i.e., at rest), as we did in the present study, allows researchers to identify neural patterns associated with SI that are not constrained by experimental stimuli, facilitating comparisons across studies.
In this context, Cáceda et al. (2018) examined RSFC in depressed adults who recently attempted suicide (within the past 3 days) and depressed adults with SI but no attempt within the last 6 months (Cáceda et al., 2018). Using ICA and a data-driven pattern classifier, Cáceda et al. were able to distinguish adults who recently attempted suicide from those who endorsed SI: recent suicide attempters had decreased FC between the SN and both the DMN and ECN. The authors posited that this decreased FC involving the SN reflects impaired cognitive control and emotional regulation immediately preceding suicidal actions. In contrast, in the present study we examined the same individuals over several months in order to examine how changes in neural networks are associated with changes in SI. Further, we assessed these constructs in a sample of adolescence given that it is during this developmental period that SI begins to emerge.
In support of our hypothesis, we found that change in SI over six months is associated with change in SN coherence; more specifically, reductions in SI were related to strengthened coherence of SN. This finding extends previous findings that greater severity of SI is associated with weaker coherence of the SN (Ordaz et al., 2018). Contrary to our predictions, however, we did not find associations of changes in SI with changes in coherence in the left ECN or anterior DMN; thus, the SN may be particularly relevant for treatment applications.
The SN detects and monitors salient and potentially threatening stimuli and also helps coordinate responses to these stimuli (Uddin, 2015). Indeed, in a case study, Parvizi et al. (2013) reported that electrical stimulation of the anterior midcingulate cortex of the SN led the individual to expect imminent challenges (i.e., that something negative would happen) and also to be motivated to overcome these challenges (Parvizi et al., 2013). While speculative, it is possible that the increase in SN coherence associated with reductions in SI found in this study reflects an increased attention or expectation for imminent challenges. Our findings are also consistent with other studies showing that adolescent suicide attempters show blunted physiological and endocrinological responses to social stressors (Melhem et al., 2016; O’Connor et al., 2017).
Given research indicating that executive functioning improves through adolescence (Baum et al., 2017), and that within-network connectivity increases over adolescence (Sherman et al., 2014), it is not surprising that Ordaz et al. (2018) found evidence that decreased coherence of the ECN is related to higher SI (Ordaz et al., 2018). Studies of normative development have shown that functional brain networks increasingly segregate throughout adolescence (Sherman et al., 2014; Cao et al., 2016), making it a vulnerable period for emotional problems (Somerville and Casey, 2010). Our finding that increased SN coherence is associated with decreased SI is consistent with findings of higher-within network connectivity in normal development of the SN (Solé-Padullés et al., 2016).
We should note three limitations of this study. First, we recruited adolescents who met diagnostic criteria for depression, given that depression is an important risk factor for suicidal thoughts and behaviors. It will be important to replicate and extend the present findings in individuals with other forms of psychiatric illness that are associated with SI, such as bipolar disorder, PTSD, substance abuse, and borderline personality disorder (Hoertel et al., 2015). Second, we used a 6-month interval between laboratory visits in order to limit both participant burden and attrition. This interval does not allow us to examine finer-grained changes in SI that may occur as a function of day-to-day stressful events. It is important to extend this research by use methodological approaches, such as experience sampling methodology, that are able to capture the dynamic course of SI (Ben-Zeev et al., 2012). Further, because we did not collect information about timing of participants’ most severe SI, we are limited in making interpretations about the process of change. With more time points and a larger clinical sample, we anticipate using latent change modeling, which will allow us to examine slope-intercept relations as well as how individual patterns of trajectories cluster. Finally, we took an a priori approach by examining the same networks as Ordaz et al. (2018). It is possible, of course, that other intrinsic networks are associated with changes in SI over time, and this should be examined in future studies.
In summary, we extended cross-sectional findings from Ordaz et al. (2018) to elucidate the longitudinal associations between changes in RSFC and changes in SI. In this study we begin to investigate how changes in neural FC track with changes in the severity of SI within individuals. We found that a reduction in SI is associated with an increase in SN coherence in depressed adolescents, a finding that has important implications for potential targets in prevention and intervention strategies.
Supplementary Material
Highlights.
Suicidal ideation (SI) decreased in six months in a sample of depressed adolescents
Decreased severity of SI is associated with increased coherence of salience network
Executive control and default mode networks are not associated with reduction in SI
The salience network may uniquely influence changes in SI in depressed adolescents
Acknowledgements
We thank Maria Catalina Camacho, Monica Ellwood, Meghan S. Goyer, and Sophie Schouboe for their help in running participants. We thank Meghan S. Goyer for help in recruitment and in running the study protocol. We thank the clinicians who referred participants to us. We also thank the participants for contributing their time to this research.
Role of Funding Source
This research was supported by the American Foundation for Suicide Prevention (T.C.H., grant number PDF-1-064-13); the Brain & Behavior Research Foundation (S.J.O., grant number 23582); the Klingenstein Third Generation Foundation (S.J.O., Fellowship Award); the National Institutes of Health (I.H.G., grant numbers R21-MH101545, R37-MH101495), (S.J.O., grant number K01-MH106805) the Stanford University Precision Health and Integrated Diagnostics Center (PHIND). These sponsors had no role in the study design, collection, analysis, or interpretation of data, or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
Declarations of interest: none
Additional Information
Supplementary Information accompanies this paper.
References
- Baum GL, Ciric R, Roalf DR, Betzel RF, Moore TM, Shinohara RT, Kahn AE, Vandekar SN, Rupert PE, Quarmley M, Cook PA, Elliott MA, Ruparel K, Gur RE, Gur RC, Bassett DS, Satterthwaite TD, 2017. Modular Segregation of Structural Brain Networks Supports the Development of Executive Function in Youth. Curr. Biol 10.1016/j.cub.2017.04.051 [DOI] [PMC free article] [PubMed]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM, 2005. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. B Biol. Sci 360, 1001–1013. 10.1098/rstb.2005.1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zeev D, Young MA, Depp CA, 2012. Real-time predictors of suicidal ideation: Mobile assessment of hospitalized depressed patients. Psychiatry Res 10.1016/j.psychres.2011.11.025 [DOI] [PubMed]
- Cáceda R, Bush K, James GA, Stowe ZN, Kilts CD, 2018. Modes of resting functional brain organization differentiate suicidal thoughts and actions: A preliminary study. J. Clin. Psychiatry 10.4088/JCP.17m11901 [DOI] [PMC free article] [PubMed]
- Cao M, Huang H, Peng Y, Dong Q, He Y, 2016. Toward Developmental Connectomics of the Human Brain. Front. Neuroanat 10.3389/fnana.2016.00025 [DOI] [PMC free article] [PubMed]
- CDC, 2018. Suicide rising across the US [WWW Document]. CDC’s Natl. Vital Stat. Syst. CDC Vital Signs URL https://www.cdc.gov/vitalsigns/suicide/index.html
- CDC Injury Prevention and Control, 2016. 10 Leading Causes of Death by Age Group, United States – 2016 [WWW Document]. Web-based Inj. Stat. Query Report. Syst URL https://www.cdc.gov/injury/wisqars/pdf/leading_causes_of_death_by_age_group_2016-508.pdf
- Chase HW, Segreti AM, Keller TA, Cherkassky VL, Just MA, Pan LA, Brent DA, 2017.. Alterations of functional connectivity and intrinsic activity within the cingulate cortex of suicidal ideators. J. Affect. Disord 212, 78–85. 10.1016/j.jad.2017.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res 29, 162–73. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Cullen KR, Gee DG, Klimes-Dougan B, Gabbay V, Hulvershorn L, Mueller BA, Camchong J, Bell CJ, Houri A, Kumra S, Lim KO, Castellanos FX, Milham MP, 2009. A preliminary study of functional connectivity in comorbid adolescent depression. Neurosci. Lett 460, 227–231. 10.1016/j.neulet.2009.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KR, Westlund MK, Klimes-Dougan B, Mueller BA, Houri A, Eberly LE, Lim KO, 2014. Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry 10.1001/jamapsychiatry.2014.1087 [DOI] [PMC free article] [PubMed]
- Du L, Zeng J, Liu H, Tang D, Meng H, Li Y, Fu Y, 2017. Fronto-limbic disconnection in depressed patients with suicidal ideation: A resting-state functional connectivity study. J. Affect. Disord 215, 213–217. 10.1016/j.jad.2017.02.027 [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R, 2011. Emotional processing in anterior cingulate and medial prefrontal. Trends Cogn. Sci 15, 85–93. 10.1016/j.tics.2010.11.004.Emotional [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE, 2009. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Pnas 106, 7209–7214. 10.1073/pnas.0811879106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Van Der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM, 2004. Automatically Parcellating the Human Cerebral Cortex. Cereb. Cortex 14, 11–22. 10.1093/cercor/bhg087 [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Ordaz SJ, 2016. The importance of assessing neural trajectories in pediatric depression 73, 2018 10.1037/1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH, 2012. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am. J. Psychiatry 169, 693–703. 10.1176/appi.ajp.2012.11071105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Farmer M, Fogelman P, Gotlib IH, 2015. Depressive Rumination, the Default-Mode Network, and the Dark Matter of Clinical Neuroscience. Biol. Psychiatry 10.1016/j.biopsych.2015.02.020 [DOI] [PMC free article] [PubMed]
- Hoertel N, Franco S, Wall MM, Oquendo MA, Kerridge BT, Limosin F, Blanco C, 2015. Mental disorders and risk of suicide attempt: A national prospective study. Mol. Psychiatry 20, 718–726. 10.1038/mp.2015.19 [DOI] [PubMed] [Google Scholar]
- Jain S, Carmody TJ, Trivedi MH, Hughes C, Bernstein IH, Morris DW, Emslie GJ, Rush AJ, 2007. A psychometric evaluation of the CDRS and MADRS in assessing depressive symptoms in children. J. Am. Acad. Child Adolesc. Psychiatry 46, 1204–1212. 10.1097/chi.0b013e3180cc2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JAY, Wang F, Liu J, Blond BN, Wallace A, Liu J, Spencer L, Lippard ETC, Purves KL, Landeros-Weisenberger A, Hermes E, Pittman B, Zhang S, King R, Martin A, Oquendo MA, Blumberg HP, 2017. Multimodal neuroimaging of frontolimbic structure and function associated with suicide attempts in adolescents and young adults with bipolar disorder. Am. J. Psychiatry 174, 667–675. 10.1176/appi.ajp.2016.15050652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan ND, 1997. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry 36, 980–988. 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Kelly RE, Alexopoulos GS, Wang Z, Gunning FM, Murphy CF, Morimoto SS, Kanellopoulos D, Jia Z, Lim KO, Hoptman MJ, 2010. Visual inspection of independent components: Defining a procedure for artifact removal from fMRI data. J. Neurosci. Methods 10.1016/j.jneumeth.2010.03.028 [DOI] [PMC free article] [PubMed]
- Kessler RC, Borges G, Walters EE, 1999. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. rchives Gen. Psychiatry 56, 617–626. 10.1001/archpsyc.56.7.617 [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, 2001. The Phq-9. J. Gen. Intern … 46202, 606–613. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- March JS, Parker JDA, Sullivan K, Stallings P, Conners CK, 1997. The Multidimensional Anxiety Scale for Children (MASC): Factor structure, reliability, and validity. J. Am. Acad. Child Adolesc. Psychiatry 36, 554–565. 10.1097/00004583-199704000-00019 [DOI] [PubMed] [Google Scholar]
- Melhem NM, Keilp JG, Porta G, Oquendo MA, Burke A, Stanley B, Cooper TB, Mann JJ, Brent DA, 2016. Blunted HPA Axis Activity in Suicide Attempters Compared to those at High Risk for Suicidal Behavior. Neuropsychopharmacology 10.1038/npp.2015.309 [DOI] [PMC free article] [PubMed]
- Menon V, 2011. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn. Sci 10.1016/j.tics.2011.08.003 [DOI] [PubMed]
- Miller AB, McLaughlin KA, Busso DS, Brueck S, Peverill M, Sheridan MA, 2018. Neural Correlates of Emotion Regulation and Adolescent Suicidal Ideation. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3, 125–132. 10.1016/j.bpsc.2017.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Green JG, Hwang I, McLaughlin KA, Sampson NA, Zaslavsky AM, Kessler RC, 2013. Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: results from the National Comorbidity Survey Replication Adolescent Supplement. JAMA psychiatry 70, 300–10. 10.1001/2013.jamapsychiatry.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor DB, Green JA, Ferguson E, O’Carroll RE, O’Connor RC, 2017. Cortisol reactivity and suicidal behavior: Investigating the role of hypothalamic-pituitary-adrenal axis responses to stress in suicide attempters and ideators. Psychoneuroendocrinology 10.1016/j.psyneuen.2016.10.019 [DOI] [PubMed]
- Ordaz SJ, Goyer MS, Ho TC, Singh MK, Gotlib IH, 2018. Network basis of suicidal ideation in depressed adolescents. J. Affect. Disord 226, 92–99. 10.1016/j.jad.2017.09.021 [DOI] [PubMed] [Google Scholar]
- Ordaz SJ, LeMoult J, Colich NL, Prasad G, Pollak M, Popolizio M, Gotlib IH, Price A, Greicius M, Gotlib IH, 2017. Ruminative brooding is associated with salience network coherence in early pubertal girls. Cogn. Affect. Behav. Neurosci 298–310. 10.1093/scan/nsw133 [DOI] [PMC free article] [PubMed]
- Pan LA, Batezati-Alves SC, Almeida JRC, Segreti A, Akkal D, Hassel S, Lakdawala S, Brent DA, Phillips ML, 2011. Dissociable patterns of neural activity during response inhibition in depressed adolescents with and without suicidal behavior. J. Am. Acad. Child Adolesc. Psychiatry 50, 602–611. 10.1016/j.jaac.2011.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan LA, Hassel S, Segreti AM, Nau SA, Brent DA, Phillips ML, 2013. Differential patterns of activity and functional connectivity in emotion processing neural circuitry to angry and happy faces in adolescents with and without suicide attempt. Psychol. Med 43, 2129–2142. 10.1017/S0033291712002966 [DOI] [PubMed] [Google Scholar]
- Parvizi J, Rangarajan V, Shirer WR, Desai N, Greicius MD, 2013. The will to persevere induced by electrical stimulation of the human cingulate gyrus. Neuron 10.1016/j.neuron.2013.10.057 [DOI] [PMC free article] [PubMed]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Currier GW, Melvin GA, Greenhill L, Shen S, Mann JJ, 2011. The Columbia-suicide severity rating scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am. J. Psychiatry 168, 1266–1277. 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2014. R Found. Stat. Comput. Vienna, Austria: 2014. R: A language and environment for statistical computing. [DOI] [Google Scholar]
- Reinherz HZ, Tanner JL, Berger SR, Beardslee WR, Fitzmaurice GM, 2006. Adolescent suicidal ideation as predictive of psychopathology, suicidal behavior, and compromised functioning at age 30. Am. J. Psychiatry 163, 1226–1232. 10.1176/ajp.2006.163.7.1226 [DOI] [PubMed] [Google Scholar]
- Richardson LP, McCauley E, Grossman DC, McCarty CA, Richards J, Russo JE, Rockhill C, Katon W, 2010. Evaluation of the Patient Health Questionnaire-9 Item for Detecting Major Depression Among Adolescents. Pediatrics 126, 1117–1123. 10.1542/peds.2010-0852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S, D’Esposito M, 2015. Functional characterization of the cingulo-opercular network in the maintenance of tonic alertness. Cereb. Cortex 25, 2763–2773. 10.1093/cercor/bhu072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner MW, Klimes-Dougan B, Cullen KR, 2018. Neural Correlates of Suicidality in Adolescents with Major Depression: Resting-State Functional Connectivity of the Precuneus and Posterior Cingulate Cortex. Suicide Life-Threatening Behav 1–15. 10.1111/sltb.12471 [DOI] [PubMed]
- Sherman LE, Rudie JD, Pfeifer JH, Masten CL, McNealy K, Dapretto M, 2014. Development of the Default Mode and Central Executive Networks across early adolescence: A longitudinal study. Dev. Cogn. Neurosci 10.1016/j.dcn.2014.08.002 [DOI] [PMC free article] [PubMed]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM, 2004. Advances in functional and structural MR image analysis and implementation as FSL, in: NeuroImage 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Smith DV, Utevsky AV, Bland AR, Clement N, Clithero JA, Harsch AEW, McKell Carter R., Huettel SA, 2014. Characterizing individual differences in functional connectivity using dual-regression and seed-based approaches. Neuroimage 95, 1–12. 10.1016/j.neuroimage.2014.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solé-Padullés C, Castro-Fornieles J, De La Serna E, Calvo R, Baeza I, Moya J, Lázaro L, Rosa M, Bargalló N, Sugranyes G, 2016. Intrinsic connectivity networks from childhood to late adolescence: Effects of age and sex. Dev. Cogn. Neurosci 10.1016/j.dcn.2015.11.004 [DOI] [PMC free article] [PubMed]
- Somerville LH, Casey BJ, 2010. Developmental neurobiology of cognitive control and motivational systems. Curr. Opin. Neurobiol 20, 236–241. 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, 2015. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci 10.1038/nrn3857 [DOI] [PubMed]
- van Duijvenvoorde ACK, Achterberg M, Braams BR, Peters S, Crone EA, 2015. Testing a dual-systems model of adolescent brain development using resting-state connectivity analyses. Neuroimage 124, 409–420. 10.1016/j.neuroimage.2015.04.069 [DOI] [PubMed] [Google Scholar]
- Van Heeringen K, Mann JJ, 2014. The neurobiology of suicide. The Lancet Psychiatry 1, 63–72. 10.1016/S2215-0366(14)70220-2 [DOI] [PubMed] [Google Scholar]
- Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo XN, Castellanos FX, Milham MP, 2013. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage 76, 183–201. 10.1016/j.neuroimage.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Chen Z, Jia Z, Gong Q, 2014. Dysfunction of neural circuitry in depressive patients with suicidal behaviors: A review of structural and functional neuroimaging studies. Prog. Neuro-Psychopharmacology Biol. Psychiatry 53, 61–66. 10.1016/j.pnpbp.2014.03.002 [DOI] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP, 2010. Reliable intrinsic connectivity networks: Test-retest evaluation using ICA and dual regression approach. Neuroimage 49, 2163–2177. 10.1016/j.neuroimage.2009.10.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


