Abstract
Aim:
Previous studies suggest telomere shortening represses PGC1A and PGC1B expression leading to mitochondrial dysfunction. Methylation of CpG sites within these genes may interact with these factors to affect cancer risk.
Materials & methods:
Among 385 men, we identified 84 incidents of cancers (predominantly prostate and nonmelanoma skin). We examined associations between leukocyte DNA methylation of 41 CpGs from PGC1A and PGC1B with telomere length, mitochondrial 8-OHdG lesions, mitochondrial abundance and cancer incidence.
Results:
Methylation of five and eight CpG sites were significantly associated with telomere length and mitochondrial abundance at p < 0.05. Two CpG sites were independently associated with cancer risk: cg27514608 (PGC1A, TSS1500; HR: 1.55, 95% CI: 1.19–2.03, FDR = 0.02), and cg15219393 (PGC1B, first exon/5′UTR; HR: 3.71, 95% CI: 1.82–7.58, FDR < 0.01). Associations with cg15219393 were observed primarily among men with shorter leukocyte telomeres.
Conclusion:
PGC1A and PGC1B methylation may serve as early biomarkers of cancer risk.
Keywords: : cancer biomarkers, cancer epigenetics, DNA methylation
Background
Cancer is an age-associated disease, primarily affecting elderly individuals; nearly half of all malignancies occur in individuals aged over 70 years [1]. Prostate and skin cancers are two of the five most commonly diagnosed tumors among males, with particularly pronounced incidences among older individuals [2]. While carcinogenesis is multifaceted, exploration of biological pathways associated with aging processes may elucidate common events in tumor formation.
Aging and cancer development have common biological foundations [3]. Aging is marked by increases in cellular oxidative stress which may reduce mitochondrial function [4–6] and induce telomere shortening [7–10]. Mitochondrial dysfunction may influence cancer risk by facilitating the generation of reactive oxygen species resulting in increased cellular oxidative stress; the feedback cycle between oxidative stress and mitochondrial dysfunction is believed to lead to cellular alterations that increase cancer risk [11–14]. Telomere shortening is additionally hypothesized to be a marker of aging [15] and may play an important role in the progression to cancer [16–19]. As such, telomere shortening and mitochondrial dysfunction have been independently observed to be dysregulated during the formation of various tumor types.
Epigenetic regulation of PGC1A and PGC1B may be a possible link between telomere shortening and mitochondrial dysfunction [20,21]. Telomere reverse transcriptase (TERT) and telomerase RNA component (TERC) knockout mice serve as a model to examine the effects of telomere shortening on various physiologic functions. Using this model, researchers observed profound repression of PGC1A and PGC1B expression leading to subsequent mitochondrial dysfunction; moreover, these functions were restored after enforced TERC or PGC1A expression [20].
Telomere shortening and mitochondrial dysfunction may interact with epigenetic repression of PGC1A and PGC1B leading to increased cancer risk. However, previous investigations examining association with cancer incidence remain largely limited to individual components rather than the interrelations of these factors. We aimed to test whether DNA methylation of PGC1A and PGC1B link telomere shortening with mitochondrial function in humans and if methylation of these genes can serve as a biomarker of future cancer development. We further examined potential modification of PGC1A and PGC1B methylation associations with cancer development by telomere length and mitochondrial function.
Materials & methods
Sample characteristics
The US Department of Veteran Affairs established the Normative Aging Study (NAS) in 1963 with a total of 2280 healthy men initially enrolled [22]. The NAS is a research component of the Massachusetts Veterans Epidemiology Research and Information Center. Eligibility criteria included veteran status, Boston metro area residence, aged 21–80 years and no history of cancer or other chronic conditions. Participants were recalled every 3–5 years for clinical examinations where they were evaluated for anthropometric measurements using standardized medical examinations and responded to questionnaires about medical history and lifestyle. Information on age, smoking status and body mass index (BMI) were collected at each follow-up examination. Starting in 1999, follow-up examinations included drawing a 7-mL blood sample for epigenetic analyses; of the 829 remaining participants, 802 (97%) consented to these blood donations. We restricted our study to white participants as nonwhite individuals made up fewer than 2% of the total sample. The current study focuses on 541 observations from 385 participants who were cancer free at the first blood draw and had information on telomere length, methylation of PGC1A and PGC1B, and measures of mitochondrial function. Overall, 230 (60%) participants had one medical visit prior to cancer diagnosis or censorship, 154 (40%) had two medical visits and one participant had three medical visits. A flow chart of the participant selection criteria beginning at first blood draw is shown in Supplementary Figure 1. The NAS was conducted per guidelines laid down in the Declaration of Helsinki and the Institutional Review Board of Harvard University and all participating institutions approved all procedures involving human subjects. All study participants provided informed written consent.
Telomere length, PGC1A & PGC1B methylation & markers of mitochondrial function
Telomere length, DNA methylation of PGC1A and PGC1B, and mitochondrial functional markers were measured in circulating blood leukocytes.
To determine telomere length, we used quantitative real-time polymerase chain reactions (qPCR) [23]. Relative telomere length was estimated by taking the ratio of the telomere (T) repeat copy number to a single-copy gene (S) copy number (T:S ratio) in a given sample and reported in relative units expressing the ratio between test DNA telomere length and reference pooled telomere length. The reference data were created using DNA from 475 randomly selected participants and used to generate a standard curve used in every T and S qPCR run. A detailed explanation of these methods is described elsewhere [24]. The intra-assay coefficient of variation for the T/S ratio was 8.1%. The average coefficient of variation for the T reaction was 8%, and for the S reaction was 5.6%. Measures were repeated when the coefficient of variation for the T or S reactions was greater than 15% [25].
PGC1A and PGC1B methylation was quantified using the Illumina HumanMethylation450 BeadChip. We selected a total of 41 CpG sites located upstream of the gene transcription state site (TSS) through the 3′-untranslated region (UTR) as determined by Illumina annotation (18 sites for PGC1A and 23 sites for PGC1B). Additional information on chromosomal position and gene region location can be found in Supplementary Table 1. Most of the tested sites were near the promoter region (TSS1500, TSS200 or first exon/5′ UTR) as designed by the assay manufacturer (6 of 18 for PGC1A, 33%; 7 of 23 for PGC1B, 30%). CpG site methylation was converted from β-values to M-values via logit transformation. β-values derived from highly methylated or unmethylated sites have severe statistical heteroscedasticity due to value constraints between zero and one. A logit transformation of the β-values to M-values reduces this heteroscedasticity. While we recognize the more direct biological interpretation of using β-values, we elected to use M-values in statistical analysis in order to minimize heteroscedasticity in regression models which allows for a more precise and valid measurement of associations [26].
Mitochondrial function was estimated by measuring mitochondrial DNA (mtDNA) copy number abundance (i.e., greater mtDNA abundance is hypothesized to represent impaired mitochondrial function) and mtDNA 8-hydroxy-2′-deoxyguanosine (8-OHdG) lesions caused by oxidative stress [27]. Briefly, mitochondrial abundance was measured using a well-established protocol which examines the ratio of mtDNA copy number to nuclear copy number using qPCR [28–30]. To measure mitochondrial abundance, we used the mtDNA 12S ribosomal ribonucleic acid TaqMan (Applied Biosystems, MA, USA) probe (6FAM-5′ TGCCAGCCACCGCG 3′-MGB). The sequences of primers used for amplification in mtDNA were mtF805 (5′ CCACGGGAAACAGCAGTGATT 3′) and mtR927 (5′ CTATTGACTTGGGTTAATCGTGTGA 3′). Samples were run in triplicate. Detailed laboratory methodology for mtDNA copy number quantification can be found elsewhere [27]; the intra- and interassay coefficients of variation were 3.4 and 3.3%.
Mitochondrial DNA 8-OHdG lesions were measured using a qPCR method previously described elsewhere [31,32]. Briefly, when mitochondrial DNA contains 8-OHdG, qPCR efficiency decreases after exogenous digestion with 8-oxoguanine DNA glycosylase. Therefore, the degree of 8-OHdG levels in mtDNA is presented as the ratio of the starting quantity of treated sample over the starting quantity of untreated sample. Prior to qPCR, 10 μl of mixture with and without 8-oxoguanine DNA glycosylase (New England Biolabs, MA, USA) were added to samples containing 40 ng mtDNA, 1× NE buffer (50 mM NaCl, 10 mM Tris-HCl, 10 mM MgCl2, 1 mM DTT, pH 7.9) and 100 μg/ml of bovine serum albumin. Samples were incubated at 37°C for 1 h followed by 65°C for 20 min. qPCR was applied for determination of the starting quantity of treated and untreated DNA samples using iQ SYBR Green Supermix (Bio-Rad, CA, USA). The primers used for qPCR for mtDNA were as follows: mtF3212 (5′CACCCAAGAACAGGGTTTGT3′) and mtR3319 (5′TGGCCATGGGTATGTTGTTA3′). qPCR conditions were 50°C for 2 min and 95°C for 13 min, followed by 40 cycles of 15 s at 95°C for denaturisation and 10 min of annealing at 62°C. qPCR was performed using Bio-Rad CFX384 Touch™ Real-Time PCR Detection System (Bio-Rad). All samples were run in triplicates.
Identification of cancer cases
Cancer diagnosis information was obtained through self-report and confirmed via review of medical records. Of the 385 participants, 84 were diagnosed with cancer after their first blood draw allowing for the assessment of prediagnostic measurements for patients with a subsequent cancer diagnosis. Cancer cases were followed-up for an average of 4.2 years between first blood draw and cancer diagnosis; the participants were generally diagnosed with tumors affecting older individuals, namely skin (33 cases: six melanomas, 27 unspecified; 9% of total sample) and prostate (22; 6%), as well as other tumors from the following systems: respiratory (8; 2%), gastrointestinal (8; 2%) and unclassified (13; 3%). Participants who remained cancer-free for the full duration of the study were censored at 31 December 2013 (average cancer-free follow-up between first blood draw and censorship was 8.7 years).
Statistical analysis
We first examined associations between single site PGC1A and PGC1B methylation and telomere length and markers of mitochondrial function (mitochondrial abundance and mtDNA 8-OHdG lesions). Using mixed-effect linear regression models with random intercepts to account for repeated measures, we treated telomere length as an independent variable and methylation at each CpG site as separate dependent variables to examine associations of telomere length on PGC1A and PGC1B CpG methylation. We dichotomized telomere length about the median value of all measurements to create equal sized groups representing longer and shorter relative telomere lengths. We then examined relationships between PGC1A and PGC1B CpG methylation and logit-transformed markers of mitochondrial function by applying mixed-effect linear regression models with random intercepts, treating methylation at each CpG site as an independent variable (in separate models) and logit-transformed mitochondrial abundance or mtDNA 8-OHdG lesions as the dependent variable. We report associations with p ≤ 0.05, although we additionally calculated the Benjamini-Hochberg false discovery rate (FDR) to examine the effects of multiple testing [33].
To explore relationships between methylation at the 41 measured PGC1A and PGC1B CpGs and cancer incidence, we employed Cox regression models with discrete time-varying covariates to estimate hazard ratios (HRs). We considered statistical significance using a traditional cut-point of FDR (q) ≤ 0.05 to account for multiple testing. We then examined potential interaction of the CpG site-cancer incidence relationship by telomere length and mitochondrial functional markers; interaction was determined by the inclusion of an interaction term in the model using a p ≤ 0.10 cut-point. We further explored associations between logit-transformed mitochondrial functional markers (mitochondrial abundance and mtDNA 8-OHdG lesions) and cancer incidence overall and stratified by telomere length using the p ≤ 0.05 threshold. All statistical models were adjusted for age, smoking status and BMI as well as estimated blood cell type composition and processing batch. Due to the small sample size, we were unable to include additional adjustment variables and therefore focused on the most relevant potential confounders. Because cancer is epigenetically heterogenous, we additionally conducted a sensitivity analyses examining PGC1A and PGC1B CpG methylation associations with cancer risk stratified by tumor type (skin and prostate only due to sample size constraints). All analyses were conducted using Stata version 14.2 (Stata Corp., TX, USA).
Results
At first blood draw, the sample participants’ mean age was 72.3 (standard deviation: 6.7). Participants were generally overweight or obese (81%) and predominately former smokers (66%) (Table 1). There were no significant differences in these characteristics between those who went on to be diagnosed with cancer and those who did not. Moreover, at first blood draw prior to any cancer diagnosis, no significant differences existed in telomere length (p = 0.51), mtDNA 8-OHdG lesions (p = 0.53) or mitochondrial abundance (p = 0.53).
Table 1. . Sample characteristics at first blood draw by cancer development .
| Characteristic | Overall sample (n = 385) | No cancer (n = 301) | Developed cancer (n = 84) | p-value† | |||
|---|---|---|---|---|---|---|---|
| N | % | n | % | n | % | ||
| Age: | 0.17 | ||||||
| – <70 | 137 | 36 | 103 | 34 | 34 | 41 | |
| – 70–74 | 109 | 28 | 82 | 27 | 27 | 32 | |
| – 75+ | 139 | 36 | 116 | 39 | 23 | 27 | |
| Body mass index: | 0.47 | ||||||
| – Under/normal (<25) | 74 | 19 | 55 | 18 | 19 | 23 | |
| – Overweight (25–29.9) | 216 | 56 | 168 | 56 | 48 | 57 | |
| – Obese (30+) | 95 | 25 | 78 | 26 | 17 | 20 | |
| Smoking status: | 0.84 | ||||||
| – Never | 110 | 29 | 85 | 28 | 25 | 30 | |
| – Former | 256 | 66 | 202 | 67 | 54 | 64 | |
| – Current | 19 | 5 | 14 | 5 | 5 | 6 | |
| Telomere length (median [IQR]) | 1.21 | 0.9–1.5 | 1.21 | 1.0–1.5 | 1.20 | 0.9–1.5 | 0.51‡ |
| mtDNA 8OH-dG (median [IQR]) | 0.69 | 0.6–0.8 | 0.70 | 0.6–0.8 | 0.69 | 0.6–0.8 | 0.53‡ |
| mtDNA abundance (median [IQR]) | 1.01 | 0.9–1.2 | 1.01 | 0.9–1.2 | 0.99 | 0.9–1.2 | 0.53‡ |
†Derived from χ2 test.
‡Derived from Wilcoxon-rank sum test (nonparametric test for differences).
In total, methylation at 13 (32%) distinct PGC1A and PGC1B CpG sites were associated with either telomere length or mitochondrial abundance at p ≤ 0.05 (Table 2). No CpG sites were associated with both telomere length and mitochondrial function. Five CpGs were associated with telomere length, although no sites reached statistical significance after accounting for multiple testing (all q > 0.05). Generally longer telomere length was associated with lower gene body methylation (PGC1A, cg07744449: β = −0.11, 95% CI: -0.20, -0.01; q = 0.38; PGC1A, cg24716152: β = -0.07, 95% CI: -0.13, -0.00; q = 0.38) and higher promoter methylation (PGC1A, cg20723350: β = 0.07, 95% CI: 0.01–0.12; q = 0.38; PGC1B, cg16786458: β = 0.06, 95% CI: 0.00–0.12; q = 0.38; PGC1B, cg20563759: β = 0.07, 95% CI: 0.00–0.14; q = 0.38). Only gene body methylation of PGC1B was associated with mitochondrial abundance after accounting for multiple testing; higher methylation of these sites was generally associated with greater mitochondrial abundance with one exception (cg26876242: β = 0.08, 95% CI: 0.04–0.13; q = 0.01; cg10500461: β = -0.10, 95% CI: -0.16, -0.05; q = 0.01; cg17645677: β = 0.13, 95% CI: 0.06–0.20; q = 0.01; cg18604199: β = 0.07, 95% CI: 0.03–0.10; q = 0.01). One CpG site from the 3′UTR region of PGC1A was additionally associated with increased mitochondrial abundance, but it did not reach significance after accounting for multiple testing (cg06772578: β = 0.04, 95% CI: 0.00–0.07; q = 0.14). Complete results exploring PGC1A and PGC1B CpG methylation associations with telomere length and mitochondrial functional markers can be found in Supplementary Tables 2–4.
Table 2. . CpGs associated with telomere length and mitochondrial abundance at p < 0.05.
| Illumina ID | β | 95% CI | p-value | FDR | Gene | Region |
|---|---|---|---|---|---|---|
| Telomere length† | ||||||
| cg20723350 | 0.07 | 0.01, 0.12 | 0.02 | 0.38 | PGC1A | First exon; 5′UTR |
| cg07744449 | -0.11 | -0.20, -0.01 | 0.03 | 0.38 | PGC1A | Body |
| cg24716152 | -0.07 | -0.13, -0.00 | 0.05 | 0.38 | PGC1A | Body |
| cg16786458 | 0.06 | 0.00, 0.12 | 0.05 | 0.38 | PGC1B | TSS1500 |
| cg20563759 | 0.07 | 0.00, 0.14 | 0.05 | 0.38 | PGC1B | TSS1500 |
| Mitochondrial abundance‡ | ||||||
| cg26876242 | 0.08 | 0.04, 0.13 | <0.01 | 0.01 | PGC1B | Body |
| cg10500461 | -0.10 | -0.16, -0.05 | <0.01 | 0.01 | PGC1B | Body |
| cg17645677 | 0.13 | 0.06, 0.20 | <0.01 | 0.01 | PGC1B | Body |
| cg18604199 | 0.07 | 0.03, 0.10 | <0.01 | 0.01 | PGC1B | Body |
| cg06051582 | -0.05 | -0.09, -0.01 | 0.01 | 0.10 | PGC1B | Body |
| cg07226964 | -0.04 | -0.08, -0.01 | 0.03 | 0.12 | PGC1B | Body |
| cg08928958 | 0.10 | 0.02, 0.18 | 0.02 | 0.12 | PGC1B | Body |
| cg06772578 | 0.04 | 0.00, 0.07 | 0.03 | 0.14 | PGC1A | 3′UTR |
No PGC1A or PGC1B sites were associated with mitochondrial 8-OHdG at p < 0.05. All models adjusted for age, BMI, smoking status, blood cell composition and processing batch.
†Coefficients derived from models with telomere length serving as the independent variable.
‡Coefficients derived from models with individual PGC1A and PGC1B CpG methylation serving as the independent variables.
FDR: False discovery rate.
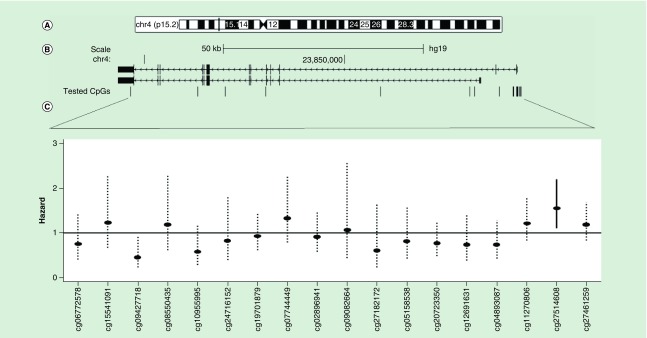
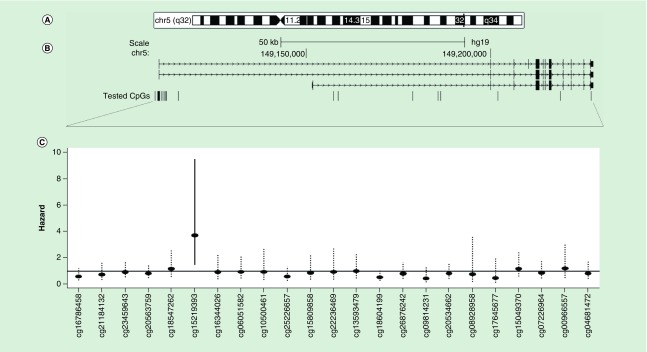
Figures 1 & 2 (panels A & B) depict the genomic, RefSeq and tested CpG site locations for PGC1A and PGC1B. Panel C of the figures additionally shows the HR point estimates and 99% confidence intervals for the relationships between CpG site methylation and cancer incidence after adjustment for age, BMI, smoking status, blood cell composition and processing batch. We elected to show the 99% confidence interval to visually account for multiple testing. Solid confidence interval denote significance at q ≤ 0.05. Figure 1 (panel C) shows one CpG site from the promoter proximal region (TSS1500) of PGC1A was significantly associated with cancer incidence (cg27514608: HR: 1.55, 95% CI: 1.19–2.03; q = 0.01). Figure 2 (panel C) shows that cg15219393, from the promoter proximal region of PGC1B (first exon/5′ UTR), was significantly associated with cancer incidence (HR: 3.71, 95% CI: 1.82–7.58; q = 0.003). Results for all tested CpG loci are shown in Table 3. We generally identified the same strength and direction of associations for cg15219393 and cg27514608 in both prostate (cg15219393: HR: 4.43, 95% CI: 1.39–14.14, q = 0.18; cg27514608: HR: 1.72, 95% CI: 1.12–2.62; q = 0.18) and skin cancers (cg15219393: HR: 3.93, 95% CI: 0.98–15.74; q = 0.51; cg27514608: HR: 2.08, 95% CI: 1.22–3.56; q = 0.29), although these sites were not significantly associated with cancer incidence after accounting for multiple testing (Supplementary Table 5).
Figure 1. . PGC1A methylation associations with cancer risk.
Genomic (A), RefSeq and tested CpG site locations for PGC1A (B). Arrows denote direction of transcription (right to left). (C) shows the resulting hazard point estimates and 99% confidence intervals for each individual CpG site. Dotted confidence intervals represent FDR > 0.05.
FDR: False discovery rate.
Figure 2. . PGC1B methylation associations with cancer risk.
Genomic (A), RefSeq and tested CpG site locations for PGC1B (B). Arrows denote direction of transcription (left to right). (C) shows the resulting hazard point estimates and 99% confidence intervals for each individual CpG site. Dotted confidence intervals represent FDR > 0.05.
FDR: False discovery rate.
Table 3. . Associations between PGC1A and PGC1B CpG sites and cancer incidence.
| Illumina ID | HR | 95% CI | p-value | FDR | Gene | Region |
|---|---|---|---|---|---|---|
| cg15219393 | 3.71 | 1.82–7.58 | <0.01 | <0.01 | PGC1B | First exon; 5′UTR |
| cg27514608 | 1.55 | 1.19–2.03 | <0.01 | 0.02 | PGC1A | TSS1500 |
| cg09427718 | 0.46 | 0.20–0.79 | 0.01 | 0.07 | PGC1A | Body |
| cg18604199 | 0.53 | 0.31–0.89 | 0.02 | 0.17 | PGC1B | Body |
| cg10955995 | 0.58 | 0.34–1.00 | 0.05 | 0.34 | PGC1A | Body |
| cg09814231 | 0.43 | 0.19–1.00 | 0.05 | 0.34 | PGC1B | Body |
| cg16786458 | 0.60 | 0.35–1.02 | 0.06 | 0.35 | PGC1B | TSS1500 |
| cg25226657 | 0.59 | 0.32–1.09 | 0.09 | 0.48 | PGC1B | Body |
| cg04893087 | 0.74 | 0.48–1.13 | 0.16 | 0.58 | PGC1A | TSS1500 |
| cg07744449 | 1.33 | 0.89–2.00 | 0.17 | 0.58 | PGC1A | Body |
| cg20723350 | 0.77 | 0.53–1.12 | 0.17 | 0.58 | PGC1A | First exon; 5′UTR |
| cg17645677 | 0.48 | 0.16–1.40 | 0.18 | 0.58 | PGC1B | Body |
| cg11270806 | 1.21 | 0.91–1.62 | 0.19 | 0.58 | PGC1A | TSS1500 |
| cg27182172 | 0.61 | 0.28–1.30 | 0.20 | 0.58 | PGC1A | Body |
| cg27461259 | 1.18 | 0.90–1.56 | 0.23 | 0.59 | PGC1A | TSS1500 |
| cg12691631 | 0.74 | 0.45–1.22 | 0.23 | 0.59 | PGC1A | TSS200 |
| cg06772578 | 0.75 | 0.47–1.22 | 0.25 | 0.59 | PGC1A | 3′UTR |
| cg21184132 | 0.74 | 0.40–1.40 | 0.36 | 0.81 | PGC1B | TSS1500 |
| cg15541091 | 1.23 | 0.76–1.98 | 0.40 | 0.82 | PGC1A | Body |
| cg05158538 | 0.81 | 0.49–1.35 | 0.42 | 0.82 | PGC1A | Body |
| cg20563759 | 0.83 | 0.52–1.32 | 0.42 | 0.82 | PGC1B | TSS1500 |
| cg26876242 | 0.81 | 0.47–1.39 | 0.44 | 0.82 | PGC1B | Body |
| cg20534662 | 0.82 | 0.48–1.40 | 0.47 | 0.82 | PGC1B | Body |
| cg08550435 | 1.19 | 0.71–1.97 | 0.51 | 0.82 | PGC1A | Body |
| cg04681472 | 0.84 | 0.49–1.44 | 0.53 | 0.82 | PGC1B | 3′UTR |
| cg24716152 | 0.83 | 0.46–1.50 | 0.54 | 0.82 | PGC1A | Body |
| cg18547262 | 1.19 | 0.66–2.14 | 0.56 | 0.82 | PGC1B | TSS200 |
| cg15049370 | 1.18 | 0.68–2.06 | 0.56 | 0.82 | PGC1B | Body |
| cg02896941 | 0.91 | 0.64–1.30 | 0.61 | 0.82 | PGC1A | Body |
| cg00966557 | 1.19 | 0.58–2.44 | 0.63 | 0.82 | PGC1B | Body |
| cg07226964 | 0.88 | 0.51–1.51 | 0.63 | 0.82 | PGC1B | Body |
| cg08928958 | 0.76 | 0.23–2.49 | 0.65 | 0.82 | PGC1B | Body |
| cg19701879 | 0.93 | 0.67–1.29 | 0.66 | 0.82 | PGC1A | Body |
| cg15809858 | 0.87 | 0.42–1.79 | 0.70 | 0.85 | PGC1B | Body |
| cg23459643 | 0.93 | 0.59–1.48 | 0.77 | 0.90 | PGC1B | TSS1500 |
| cg16344026 | 0.93 | 0.47–1.84 | 0.84 | 0.93 | PGC1B | First exon |
| cg09082664 | 1.06 | 0.54–2.09 | 0.86 | 0.93 | PGC1A | Body |
| cg06051582 | 0.96 | 0.52–1.74 | 0.88 | 0.93 | PGC1B | Body |
| cg10500461 | 0.94 | 0.41–2.15 | 0.89 | 0.93 | PGC1B | Body |
| cg22236469 | 0.96 | 0.43–2.11 | 0.91 | 0.94 | PGC1B | Body |
| cg13593479 | 1.01 | 0.54–1.89 | 0.98 | 0.98 | PGC1B | Body |
We further examined potential effect modification by telomere length and mitochondrial function on associations between PGC1A and PGC1B CpG site methylation and cancer incidence. Interaction was detected by telomere length for the association between cg15219393 methylation and cancer incidence (p-interaction = 0.096). Upon stratification by telomere length, cg15219393 was significantly associated with cancer incidence, but only among those with shorter telomeres (HR: 10.09, 95% CI: 3.56–28.63; q = 0.003) (Supplementary Table 6). When stratified by markers of mitochondrial function, we did not detect any significant statistical interaction with mitochondrial 8-OHdG lesions (cg27514608 p-interaction = 0.14, cg15219393 p-interaction = 0.60), although associations between cg27514608 and cancer incidence were mainly observed among those with higher amounts of 8-OHdG lesions (HR: 1.84, 95% CI: 1.26–2.67; q = 0.04) (Supplementary Table 7). We additionally detected significant statistical interaction by mitochondrial abundance for associations with cg24716152 and cancer incidence (p-interaction = 0.004). This novel site is located in the gene body of PGC1A; methylation of this CpG showed protective associations with cancer incidence among those with lower mitochondrial abundance (HR: 0.22, 95% CI: 0.09–0.56; q = 0.03) (Supplementary Table 8).
Finally, we examined associations between mitochondrial functional markers and cancer incidence overall and stratified by telomere length. Mitochondrial abundance was not independently associated with cancer incidence in either situation, although notably, a higher number of mtDNA 8-OHdG lesions was inversely associated with cancer incidence (HR: 0.28, 95% CI: 0.07–1.02; p = 0.06), particularly among those with longer telomeres (p-interaction = 0.087, HR: 0.05, 95% CI: 0.01–0.35; p = 0.003) (Supplementary Table 9).
Discussion
This is the first study to examine a possible role of DNA methylation of PGC1A and PGC1B in relation to telomere length, mitochondrial function and cancer risk. While we identified associations between PGC1B gene body methylation and mitochondrial abundance, we did not observe methylation at any CpG site which had overlapping associations with both telomere length and mitochondrial function. We additionally observed that higher methylation at cg15219393 and cg27514608, from the promoter proximal regions of PGC1B and PGC1A, was associated with increased risk of future cancer diagnosis. Moreover, we showed that telomere length modified the relationship with the PGC1B site, such that the effects were mainly observed among those with shorter telomeres.
Telomere shortening has been linked to mitochondrial function through repression of PGC1A and PGC1B [20,21]. In vitro and in vivo models found telomere shortening represses expression of these genes and leads to mitochondrial functional decline [34,35]. We found little evidence to support our hypothesis that epigenetic regulation of these genes may be associated with these cellular factors. Transcriptional control of gene expression is a complex process involving many biological mechanisms including heterochromatin, histone modifications and other epigenetic factors as well as activation of transcription factors [36]. It is possible that repression of PGC1A and PGC1B is primarily controlled by activation of the transcription factor p53, rather than DNA methylation [37]. This potential explanation is supported by the finding that TERT and TERC knockout mice show activation of p53 and repression of PGC1A and PGC1B as well as other p53 targets such as ERRα, PPARγ and TFAM [20]. As data on p53 expression were unavailable in our source cohort, we are unable to test this hypothesis, which suggests an important direction for future research.
To our knowledge, this is the first study to identify strong, positive associations between PGC1A and PGC1B proximal promoter methylation and future cancer risk. Previous studies have shown mutations in these genes affect the development of common cancers such as breast, ovarian and colorectal [38–42]. Specifically, prior evidence showed that single nucleotide polymorphisms located in the 5′UTR of PGC1A were associated with an increased risk of epithelial ovarian tumor formation [38], while other studies identified cancer associations with single nucleotide polymorphisms in nonpromoter gene regions [39,40] or modification of cancer risk by PGC1A or PGC1B genotype [41,42]. This study builds upon this literature by showing that epigenetic regulation of the promoter regions of these genes may additionally play a role in cancer development.
We also examined the relationships between methylation of PGC1A and PGC1B with cancer incidence in the context of telomere length and mitochondrial function. We found proximal promoter methylation of PGC1B among participants with shorter telomeres was associated with increased cancer risk. We additionally observed higher methylation at the promoter of PGC1A was associated with increased cancer risk among participants with higher mtDNA 8-OHdG lesions. Similarly, gene body methylation of PGC1A among participants with lower mitochondrial abundance was associated with decreased cancer risk. These findings align when considering the region of DNA methylation; gene body methylation is commonly associated with increased gene expression while promotor methylation is generally associated with reduced expression [43]. Previous research has identified inconsistent findings regarding the role of telomere length and cancer risk; studies have identified associations with cancer risk for shorter [16–19] as well as longer telomeres [44–47]. These discordant findings could be a result of interactions between telomere length and epigenetic regulation of PGC1A and PGC1B. Moving forward, researchers investigating the role of telomere length on cancer risk may consider the contexts of PGC1A and PGC1B gene methylation as our findings suggest these factors modify these relationships.
While this is the first study to identify interactions between telomere length and PGC1A and PGC1B gene regulation with cancer risk, it is subject to limitations. Most importantly, we did not have PGC1A or PGC1B expression data available. We therefore cannot confirm promoter DNA methylation is associated with decreased gene expression, although evidence from the ENCODE database and studies examining other genes suggests these associations hold true [48–50]. Another limitation was that our cohort was comprised of a mix of tumor types; while a majority of the tumors were either skin or prostate, a considerable proportion were found in other tissues. As mentioned previously, upon stratification by tumor location, we identified the same strength and direction of association between cg15219393 and cg27514608 methylation with skin and prostate cancer risk, albeit with less precision due to the decreased sample size. This leads us to believe our findings are not specific to one tumor type, rather DNA methylation of these loci is associated with cancer risk in general. Notably, we did not identify any associations between telomere length or markers of mitochondrial function with cancer status at the time of first blood draw. As these factors were measured in circulating blood leukocytes over 4 years prior to any cancer diagnosis, it is not implausible that we did not observe signals for these factors in our small study population. Similarly, we did not have tumor tissue available; we therefore used blood leukocyte methylation as a surrogate marker of tumor DNA methylation. Blood leukocyte DNA methylation, telomere length and mitochondrial function were used as proxies for tumor characteristics due to the less invasive collection procedures and sample availability. Therefore, at a minimum, blood leukocyte DNA methylation of PGC1A and PGC1B may serve as a biomarker of cancer risk – additional studies using tumor tissue are needed to examine whether these changes are truly mechanistic. Additionally, due to the limited sample size, we may be overfitting our models such that we were predicting random error; we used the simplest models available that would balance adjusting for confounding and avoiding overfitting. Finally, our study participants were elderly, male veterans; we were therefore unable to examine modification of associations by participant characteristics. Future confirmation of our findings in more diverse populations are required.
Conclusion & future perspective
To our knowledge, we are the first to show that methylation of CpG sites within the promoter region of PGC1A and PGC1B was associated with cancer incidence. Therefore, blood leukocyte DNA methylation of these genes may serve as a biomarker of cancer risk; collection of blood leukocyte DNA methylation is relatively noninvasive and could be employed on a population level. Moreover, we observed evidence that regulation of PGC1A and PGC1B is associated with all-cancer incidence suggesting dysregulation of these genes may serve as a common event, early in tumor formation. We also identified interactions between epigenetic regulation of these genes by both telomere length and mitochondrial abundance. These findings together may aid in the development of cancer screening modalities, add to the current knowledge of pan-cancer etiology and facilitate future studies of these mechanisms in the context of cancer risk.
Summary points.
Aging and cancer development have common biological foundations such as telomere shortening and reductions in mitochondrial function.
Epigenetic regulation of PGC1A and PGC1B is hypothesized to be a possible link between these factors.
Epigenetic regulation of PGC1A and PGC1B and telomere length may additionally interact in predicting cancer risk.
We used mixed-effect linear regression models to examine whether methylation of PGC1A and PGC1B CpGs linked telomere shortening with mitochondrial dysfunction.
We used Cox regression models with discrete time-varying covariates and to examine associations between PGC1A and PGC1B CpG methylation and cancer incidence; we additionally examined interactions with telomere length and mitochondrial function.
Five and eight distinct PGC1A and PGC1B CpG sites were associated with telomere length and mitochondrial abundance, although we did not identify any overlapping sites.
cg27514608 from the TSS1500 of PGC1A, and cg15219393 from the first exon/5′ UTR of PGC1B were significantly associated with cancer incidence.
Upon stratification by telomere length, cg15219393 was significantly associated with cancer incidence among those with shorter telomeres.
This is the first study to examine a possible role of DNA methylation in PGC1A and PGC1B in relation to telomere length, mitochondrial function and cancer risk.
Blood leukocyte DNA methylation of these genes may serve as a biomarker of future cancer risk.
Supplementary Material
Footnotes
Financial & competing interests disclosure
The Epidemiology Research and Information Center of US Department of Veterans Affairs (NIEHS R01-ES015172) support the Normative Aging Study, which is a research component of the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC). L Hou, C Achenbach and R Murphy received support from the National Cancer Institute (U54-CA221205). L Hou received additional support from the Northwestern University Robert H Lurie Comprehensive Cancer Center Rosenberg Research Fund. A Baccarelli and J Schwartz received additional support from the National Institute of Environmental Health Sciences (NIEHS R01-ES021733 and NIEHS P30-ES00002). J Kresovich received additional support from the National Cancer Institute Cancer Education and Career Development Program (NIH R25-CA057699). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Navarrete-Reyes AP, Soto-Pérez-de-Celis E, Hurria A. Cancer and aging: a complex biological association. Rev. Invest. Clin. 2016;68(1):17–24. [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J. Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448(7155):767–774. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- 4.Kong Y, Trabucco SE, Zhang H. Oxidative stress, mitochondrial dysfunction and the mitochondria theory of aging. Interdiscip. Top. Gerontol. 2014;39:86–107. doi: 10.1159/000358901. [DOI] [PubMed] [Google Scholar]
- 5.Wang CH, Wu SB, Wu YT, Wei YH. Oxidative stress response elicited by mitochondrial dysfunction: implication in the pathophysiology of aging. Exp. Biol. Med. (Maywood) 2013;238(5):450–460. doi: 10.1177/1535370213493069. [DOI] [PubMed] [Google Scholar]
- 6.Mikhed Y, Daiber A, Steven S. Mitochondrial oxidative stress, mitochondrial DNA damage and their role in age-related vascular dysfunction. Int. J. Mol. Sci. 2015;16(7):15918–15953. doi: 10.3390/ijms160715918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demissie S, Levy D, Benjamin EJ, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart study. Aging Cell. 2006;5(4):325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 8.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002;27(7):339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 9.Kawanishi S, Oikawa S. Mechanism of telomere shortening by oxidative stress. Ann. NY Acad. Sci. 2004;1019:278–284. doi: 10.1196/annals.1297.047. [DOI] [PubMed] [Google Scholar]
- 10.Fouquerel E, Lormand J, Bose A, et al. Oxidative guanine base damage regulates human telomerase activity. Nat. Struct. Mol. Biol. 2016;23(12):1092–1100. doi: 10.1038/nsmb.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Luca A, Fiorillo M, Peiris-Pagès M, et al. Mitochondrial biogenesis is required for the anchorage-independent survival and propagation of stem-like cancer cells. Oncotarget. 2015;6(17):14777–14795. doi: 10.18632/oncotarget.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen J, Gopalakrishnan V, Lee JE, Fang S, Zhao H. Mitochondrial DNA copy number in peripheral blood and melanoma risk. PLoS ONE. 2015;10(6):e0131649. doi: 10.1371/journal.pone.0131649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He Y, Gong Y, Gu J, Lee JJ, Lippman SM, Wu X. Increased leukocyte mitochondrial DNA copy number is associated with oral premalignant lesions: an epidemiology study. Carcinogenesis. 2014;35(8):1760–1764. doi: 10.1093/carcin/bgu093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu E, Sun W, Gu J, Chow WH, Ajani JA, Wu X. Association of mitochondrial DNA copy number in peripheral blood leukocytes with risk of esophageal adenocarcinoma. Carcinogenesis. 2013;34(11):2521–2524. doi: 10.1093/carcin/bgt230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanders JL, Newman AB. Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiol. Rev. 2013;35:112–131. doi: 10.1093/epirev/mxs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du J, Zhu X, Xie C, et al. Telomere length, genetic variants and gastric cancer risk in a Chinese population. Carcinogenesis. 2015;36(9):963–970. doi: 10.1093/carcin/bgv075. [DOI] [PubMed] [Google Scholar]
- 17.Hurwitz LM, Heaphy CM, Joshu CE, et al. Telomere length as a risk factor for hereditary prostate cancer. Prostate. 2014;74(4):359–364. doi: 10.1002/pros.22755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen J, Terry MB, Gurvich I, Liao Y, Senie RT, Santella RM. Short telomere length and breast cancer risk: a study in sister sets. Cancer Res. 2007;67(11):5538–5544. doi: 10.1158/0008-5472.CAN-06-3490. [DOI] [PubMed] [Google Scholar]
- 19.Willeit P, Willeit J, Mayr A, et al. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304(1):69–75. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- 20.Sahin E, Colla S, Liesa M, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470(7334):359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finkel T. Telomeres and mitochondrial function. Circ. Res. 2011;108(8):903–904. doi: 10.1161/RES.0b013e31821bc2d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell B, Rose C, Damon A. The normative aging study: an interdisciplinary and longitudinal study of health and aging. Int. J. Aging Hum. Dev. 1972;3(1):5–17. [Google Scholar]
- 23.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou L, Savage SA, Blaser MJ, et al. Telomere length in peripheral leukocyte DNA and gastric cancer risk. Cancer Epidemiol. Biomarkers Prev. 2009;18(11):3103–3109. doi: 10.1158/1055-9965.EPI-09-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou L, Joyce BT, Gao T, et al. Blood telomere length attrition and cancer development in the normative aging study cohort. EBioMedicine. 2015;2(6):591–596. doi: 10.1016/j.ebiom.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du P, Zhang X, Huang CC, et al. Comparison of β-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinformatics. 2010;11:587. doi: 10.1186/1471-2105-11-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong J, Cayir A, Trevisi L, et al. Traffic-related air pollution, blood pressure, and adaptive response of mitochondrial abundance. Circulation. 2016;133(4):378–387. doi: 10.1161/CIRCULATIONAHA.115.018802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malik AN, Czajka A. Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion. 2013;13(5):481–492. doi: 10.1016/j.mito.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Malik AN, Parsade CK, Ajaz S, et al. Altered circulating mitochondrial DNA and increased inflammation in patients with diabetic retinopathy. Diabetes Res. Clin. Pract. 2015;110(3):257–265. doi: 10.1016/j.diabres.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Andreu AL, Martinez R, Marti R, García-Arumí E. Quantification of mitochondrial DNA copy number: pre-analytical factors. Mitochondrion. 2009;9(4):242–246. doi: 10.1016/j.mito.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Lin CS, Wang LS, Tsai CM, Wei YH. Low copy number and low oxidative damage of mitochondrial DNA are associated with tumor progression in lung cancer tissues after neoadjuvant chemotherapy. Interact. Cardiovasc. Thorac. Surg. 2008;7(6):954–958. doi: 10.1510/icvts.2008.177006. [DOI] [PubMed] [Google Scholar]
- 32.Lin CS, Wang LS, Chou TY, et al. Cigarette smoking and hOGG1 Ser326Cys polymorphism are associated with 8-OHdG accumulation on mitochondrial DNA in thoracic esophageal squamous cell carcinoma. Ann. Surg. Oncol. 2013;20(Suppl. 3):S379–S388. doi: 10.1245/s10434-012-2576-z. [DOI] [PubMed] [Google Scholar]
- 33.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat. Soc. Series B Stat. Methodol. 1995;57:289–300. [Google Scholar]
- 34.Moslehi J, DePinho RA, Sahin E. Telomeres and mitochondria in the aging heart. Circ. Res. 2012;110(9):1226–1237. doi: 10.1161/CIRCRESAHA.111.246868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahin E, DePinho RA. Axis of ageing: telomeres, p53 and mitochondria. Nat. Rev. Mol. Cell Biol. 2012;13(6):397–404. doi: 10.1038/nrm3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grewal SI, Moazed D. Heterochromatin and epigenetic control of gene expression. Science. 2003;301(5634):798–802. doi: 10.1126/science.1086887. [DOI] [PubMed] [Google Scholar]
- 37.Berkers CR, Maddocks OD, Cheung EC, Mor I, Vousden KH. Metabolic regulation by p53 family members. Cell Metab. 2013;18(5):617–633. doi: 10.1016/j.cmet.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Permuth-Wey J, Chen YA, Tsai YY, et al. Inherited variants in mitochondrial biogenesis genes may influence epithelial ovarian cancer risk. Cancer Epidemiol. Biomarkers Prev. 2011;20(6):1131–1145. doi: 10.1158/1055-9965.EPI-10-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wirtenberger M, Tchatchou S, Hemminki K, et al. Associations of genetic variants in the estrogen receptor coactivators PPARGC1A, PPARGC1B and EP300 with familial breast cancer. Carcinogenesis. 2006;27(11):2201–2208. doi: 10.1093/carcin/bgl067. [DOI] [PubMed] [Google Scholar]
- 40.Cho YA, Lee J, Oh JH, et al. Genetic variation in PPARGC1A may affect the role of diet-associated inflammation in colorectal carcinogenesis. Oncotarget. 2017;8(5):8550–8558. doi: 10.18632/oncotarget.14347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martínez-Nava GA, Burguete-García AI, López-Carrillo L, Hernández-Ramírez RU, Madrid-Marina V, Cebrián ME. PPARγ and PPARGC1B polymorphisms modify the association between phthalate metabolites and breast cancer risk. Biomarkers. 2013;18(6):493–501. doi: 10.3109/1354750X.2013.816776. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Wedrén S, Li G, et al. Genetic variation of ESR1 and its co-activator PPARGC1B is synergistic in augmenting the risk of estrogen receptor-positive breast cancer. Breast Cancer Res. 2011;13(1):R10. doi: 10.1186/bcr2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lou S, Lee HM, Qin H, et al. Whole-genome bisulfite sequencing of multiple individuals reveals complementary roles of promoter and gene body methylation in transcriptional regulation. Genome Biol. 2014;15(7):408. doi: 10.1186/s13059-014-0408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haycock PC, Burgess S, Nounu A, et al. Association between telomere length and risk of cancer and non-neoplastic diseases: a Mendelian randomization study. JAMA Oncol. 2017;3(5):636–651. doi: 10.1001/jamaoncol.2016.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Julin B, Shui I, Heaphy CM, et al. Circulating leukocyte telomere length and risk of overall and aggressive prostate cancer. Br. J. Cancer. 2015;112(4):769–776. doi: 10.1038/bjc.2014.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Svenson U, Roos G, Wikström P. Long leukocyte telomere length in prostate cancer patients at diagnosis is associated with poor metastasis-free and cancer-specific survival. Tumour Biol. 2017;39(2) doi: 10.1177/1010428317692236. 1010428317692236. [DOI] [PubMed] [Google Scholar]
- 47.Caini S, Raimondi S, Johansson H, et al. Telomere length and the risk of cutaneous melanoma and non-melanoma skin cancer: a review of the literature and meta-analysis. J. Dermatol. Sci. 2015;80(3):168–174. doi: 10.1016/j.jdermsci.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 48.Myers RM, Stamatoyannopoulos J, Snyder M, et al. A user's guide to the encyclopedia of DNA elements (ENCODE) PLoS Biol. 2011;9(4):e1001046. doi: 10.1371/journal.pbio.1001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012;13(7):484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 50.Rauscher GH, Kresovich JK, Poulin M, et al. Exploring DNA methylation changes in promoter, intragenic, and intergenic regions as early and late events in breast cancer formation. BMC Cancer. 2015;15:816. doi: 10.1186/s12885-015-1777-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.