Abstract
In nearly every species examined, administration of the persistent environmental pollutant, 2,3,7,8-tetrachlorodibenzo- p-dioxin (dioxin, TCDD) causes profound immune suppression and thymic atrophy in an aryl hydrocarbon receptor (AhR) dependent manner. Moreover, TCDD alters the development and differentiation of thymocytes, resulting in decreases in the relative proportion and absolute number of double positive (DP, CD4+CD8+) thymocytes, as well as a relative enrichment in the relative proportion and absolute number of double negative (DN, CD4-CD8-) and single-positive (SP) CD4+CD8- and CD4-CD8+ thymocytes. Previous studies suggested that the target for TCDD-induced thymic atrophy resides within the hemopoietic compartment and implicated apoptosis, proliferation arrest of thymic progenitors, and emigration of DN thymocytes to the periphery as potential contributors to TCDD-induced thymic atrophy. However, the precise cellular and molecular mechanisms involved remain largely unknown. Our results show that administration of 10 μg/kg TCDD and 8 mg/kg 2-(1H-indol-3-ylcarbonyl)-4-thiazolecarboxylic acid methyl ester (ITE) induced AhR-dependent thymic atrophy in mice on day 7, whereas 100 mg/kg indole 3-carbinol (I3C) did not. Though our studies demonstrate that TCDD triggers a twofold increase in the frequency of apoptotic thymocytes, TCDD-induced thymic atrophy is not dependent on Fas-FasL interactions, and thus, enhanced apoptosis is unlikely to be a major mechanistic contributor. Finally, our results show that activation of the AhR in CD11c+ dendritic cells is directly responsible for TCDD-induced alterations in the development and differentiation of thymocytes, which results in thymic atrophy. Collectively, these results suggest that CD11c+ dendritic cells play a critical role in mediating TCDD-induced thymic atrophy and disruption of T lymphocyte development and differentiation in the thymus.
Keywords: Involution, TCDD, ITE, I3C, AhRd, Apoptosis
Introduction
The thymus is a complex, specialized organ that is responsible for the maturation and education of most peripheral T cells. Progenitor cells enter the thymus from the bloodstream after originating in the bone marrow and/or fetal liver. Once in the thymus, these cells progress through multiple developmental stages that can be delineated by cell surface markers such as CD3 (pan T cell marker), CD4, and CD8. In the earliest stage, classified as double negative (DN) thymocytes, cells do not express any of these markers. Subsequently, cell surface markers are up-regulated to give rise to CD4+CD8+ double positive (DP) cells, which then undergo a rigorous selection process eventually down-regulating either CD4 or CD8 expression to become CD4+ or CD8+ single-positive (SP) cells, which are released into the periphery. Self-reactive cells failing negative selection are removed via apoptotic pathways, and mature, self-tolerant SP thymocytes are released into the periphery as naïve Th cells (CD4) or cytotoxic T cells (CD8). Because ongoing thymopoiesis is essential for the development and maintenance of a healthy immune system, agents that trigger thymic atrophy may decrease host ability to reconstitute the peripheral T cell repertoire or respond to new antigens. Similarly, because the overall process of maturation and education of T cells is orchestrated, to a degree, by the supporting cells of the stromal network, which includes thymic epithelial cells, dendritic cells, and macrophages (Nowell et al. 2007), agents that induce thymic atrophy may be acting on a variety of cellular targets.
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor belonging to the Per-ARNT-SIM-basic helix-loop-helix (PAS/bHLH) protein family, which mediates a wide range of biological responses resulting from exposures to both natural and synthetic ligands (Gu et al. 2000; Okey 2007; Pohjanvirta 2011). It was first identified in the early 1970s and has since been shown to have dual roles as an activator of xenobiotic metabolism and as a participant in normal homeostasis, organogenesis, and immune modulation (Nebert and Gelboin 1968, 1969; Okey 2007; Wright et al. 2017). Nevertheless, details of the cellular and molecular mechanisms underlying many AhR-dependent physiological and toxicological effects are currently unknown.
Halogenated dioxins, biphenyls, and polycyclic aromatic hydrocarbons represent the best-characterized high-affinity, planar, and hydrophobic ligands of the AhR. Although numerous high-affinity AhR ligands have since been discovered, the potent and persistent environmental pollutant, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, dioxin) remains the prototypic AhR ligand for mechanistic studies (Kerkvliet 2012b; Poland et al. 1976). Exposure of laboratory rodents to TCDD and TCDD-like chemicals profoundly affects the immune system, causing immunosuppression typified by suppressed cellular immunity, inhibition of antibody production, and thymic atrophy in a variety of animal species (Faith and Luster 1979; Funatake et al. 2005; Harris et al. 1973; Kerkvliet 2002; Poland and Glover 1980; Van Loveren et al. 1991; Vecchi et al. 1980) via direct effects of AhR activation (Fernandez-Salguero et al. 1996; Harrill et al. 2016; Staples et al. 1998). C57Bl/6 mice, which harbor the AhRb allele that encodes for a receptor with a high binding affinity for agonists, exhibit a decrease in thymic weight and cellularity as early as 3 days after exposure to a single 30 μg/kg dose of TCDD (Laiosa et al. 2003; Poland and Glover 1990; Thigpen et al. 1975). Maximal decline in thymocyte cellularity emerges 10 days after TCDD treatment, yet recovery to near baseline levels does not occur until approximately 30 days after exposure (Laiosa et al. 2003; Thigpen et al. 1975).
During TCDD-induced thymic atrophy, the thymus under goes a marked reduction in the frequency of double positive (DP, CD4+CD8+) thymocytes, as well as a relative increase in the frequency of double negative (DN, CD4-CD8-) and single-positive (CD4+ SP and CD8+ SP) thymocytes. Moreover, based upon the observed values for thymic cellularity, these shifts in thymocyte subset frequencies correspond to significant decreases in the absolute number of thymocytes in each of the four subpopulations (Fine et al. 1990; Kamath et al. 1997, 1998; Lundberg et al. 1990). Although the precise cellular and molecular mechanisms involved remain largely unknown, previous studies demonstrated that the target for TCDD-induced thymic atrophy resides within the hematopoietic compartment and implicated reduced proliferation of DN precursor thymocytes (Lai et al. 1994), enhanced apoptosis via Fas:FasL interactions at the DP stage (Camacho et al. 2005b), and enhanced emigration of thymocytes (Temchura et al. 2005), or a combination of these possible mechanisms. Therefore, it was of great interest to evaluate whether an endogenous, non-toxic AhR ligand 2-(1H-indol-3-ylcarbonyl)-4-thiazolecarboxylic acid methyl ester (ITE) (Abron et al. 2018; Benson and Shepherd 2011b; Song et al. 2002) and a dietary AhR ligand, indole 3-carbinol (I3C) (Benson and Shepherd 2011b; Bjeldanes et al. 1991; Connor and Finley 2003) exhibited thymotoxic effects similar to TCDD. Furthermore, we assessed naive wild-type mice (C57Bl/6), mice expressing the low affinity AhR (AhRd), and AhR conditional knock out mice (LyzMCreAhRfx, CD11cCreAhRfx, RORcCreAhRfx, and FoxN1CreAhRfx) to identify the target(s) specifically responsible for mediating TCDD-induced thymic atrophy. The findings presented here extend our understanding of how activation of the AhR contributes to thymic atrophy and mediates immunomodulation.
Materials and methods
Chemicals
2,3,7,8,Tetrachlorodibenzo-p-dioxin (dioxin, TCDD) was obtained from Cambridge Isotopes (Cambridge, MA). A 1 mg/mL stock solution of TCDD in anisole/peanut oil was diluted to yield treatment solutions containing 1 or 10 μg/ mL in peanut oil. 2-(1H-Indol-3-ylcarbonyl)-4-thiazolecar- boxylic acid methyl ester (ITE) was obtained from Tocris (Bio-techne brand, Minneapolis, MN). A 20 mg/mL stock solution of ITE in DMSO was diluted to yield a treatment solution containing 0.8 mg/mL in peanut oil. Indole 3-car- binol (I3C), obtained from Sigma-Aldrich (St. Louis, MO), was suspended in peanut oil (10 mg/mL). The estimated half-life of TCDD is approximately 10 days in mice (Birn-baum 1986; Gasiewicz et al. 1983), whereas the in vivo absorption, metabolism, distribution, and excretion rates of ITE and I3C in mice remain largely undetermined. There fore, the selected doses and routes of exposure for these AhR ligands were based upon previous reports, thus integrating our results with previous findings in other model systems (Abron et al. 2018; Benson and Shepherd 2011a; Boule et al. 2018b; Nugent et al. 2013; Quintana et al. 2010b; Singh et al. 2016).
Biohazard precaution
TCDD is highly toxic and a probable human carcinogen. All personnel were instructed in safe handling procedures. Proper personal protective equipment (e.g., lab coats, gloves and masks) were worn at all times, and contaminated materials were collected separately for hazardous waste disposal. TCDD-treated mice were housed separately, and their carcasses and bedding regarded as contaminated materials.
Mice
Breeding pairs of mice were originally purchased from the Jackson Laboratory (Bar Harbor, ME) and maintained as both individual colonies and crossed strains to yield AhR conditional knockouts (supplemental Table 1). Tail snips were digested in direct PCR lysis reagent according to the manufacturer’s protocol (Viagen Biotech, Los Angeles, CA). Mice of the correct genotype were identified according to PCR conditions furnished by the Jackson Laboratory, and littermates were used as controls. Because conditional Cre negative × AhRfx/fx mice carry the low affinity AhRd allele (Poland and Glover 1980; Walisser et al. 2005), AhRd mice were used interchangeably with Cre-AhRfx as controls for experiments involving AhR conditional knockout mice. All mice were maintained in microisolator cages in the University of Montana specific pathogen-free (SPF) laboratory animal facility, and provided breeder (Teklad 2019, Envigo, Denver, CO) or standard rodent chow (Teklad 2020x, Envigo) and tap water ad libitum. All animal use procedures were approved by the University of Montana IACUC, and in accordance with NIH guidelines.
In vivo exposure
Naïve, adult (6–10-week-old) mice received 10 or 100 μg TCDD/kg, 1–8 mg/kg ITE, or 100 mg/kg I3C (Camacho et al. 2005b; Nguyen and Bradfield 2007; Quintana et al. 2010a; Singh et al. 2014) via oral gavage (p.o.) or intraperitoneal injection (i.p.) as described in the appropriate results sections. Control mice received solvent/peanut oil or peanut oil only vehicle control. Each experiment was performed in duplicate or triplicate with age-matched littermates (± 7 days), containing a minimum of n = 5–6 per treatment group. Mice were weighed daily, tissues collected 7 days later, and analyzed individually.
Cell isolation
Following euthanasia by CO2 asphyxiation, body weights were recorded and thymi removed free of lymph nodes and blood vessels. Individual thymi were weighed and pressed through a 70-μm sterile cell strainer with the flat end of a 1-mL syringe plunger to release the cells into cold complete RPMI (cRPMI) media supplemented with 10% FBS (Atlanta Biologicals, Atlanta, GA), 50 μM β-mercaptoethanol, 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 10 mM sodium pyruvate, and 50 μg/ml gentamicin (Gibco, Grand Island, NY) (Corning, Manassas, VA). Thymocytes were pelleted by centrifugation at 1500 rpm for 5 min, resuspended in fresh cRPMI, and maintained on ice.
Flow cytometry
Single cell suspensions of thymocytes were washed once with cRPMI and re-suspended in 100 μl of purified rat anti-mouse CD16/CD32 (2.4G2, Tonbo, San Diego, CA) diluted 1:100 in PBS containing 1% bovine serum albumin and 0.1% sodium azide (PAB) to block Fc receptors. 2×106 thymocytes were immunostained for 30 min on ice with titrated monoclonal Abs specific to CD3ε PerCPCy5.5 (145–2C11, Tonbo), CD4 FITC or redfluor 710 (RM4–5, Tonbo), and CD8 PE-Cy7 (53–6.7, Tonbo) to identify live, propidium iodide (PI, Tonbo) negative singlet thymocytes. Another 2×106 thymocytes were immunostained for 30 min on ice with monoclonal Abs specific to CD45 v450 (30- F11, Tonbo), CD4 FITC (RM4–5, Tonbo), CD8α PE-Cy7 (53–6.7, Tonbo), CD11c PE (HL3, BD Pharmingen, San Diego, CA), Fas Alexa Fluor 647 (Jo2, BD Pharmingen), FasL PerCP Cy5.5 (MFL3, BD Pharmingen) to identify cell surface expression of Fas and FasL. Alternatively, 2×106 thymocytes were immunostained with monoclonal Abs specific to CD4 FITC (RM4–5, Tonbo), CD8a PE-Cy7 (53–6.7, Tonbo), in addition to Annexin V and 7-AAD staining solution according to the manufacturer’s instructions (Tonbo). Unstained, single stained, and fluorescence minus one controls were used to set positive/negative gating. Cells were washed twice with 1 mL PAB and re-suspended in 350 μL of PAB and acquisition was performed on a FACS Aria II flow cytometer using FACS Diva software (v 6.1.2, Becton Dickinson, Franklin Lakes, NJ). Compensation of the spectral overlap was performed using One Comp compensation control beads (BD Biosciences, San Diego, CA) in combination with single stained controls where appropriate. Data files were exported as FCS 3.1 files and analyzed by Flow Logic (v 4.0, Miltenyi, Auburn, CA).
RNA isolation and RT-qPCR
Total RNA was extracted from 5×106 thymocytes using TRIzol reagents (Invitrogen) or RNeasy mini kit (Qiagen, Germantown, MD) according to the manufacturer protocols. Two-step qRT-PCR was performed by synthesizing cDNA using iScript Reverse Transcription Supermix (BioRad, Hercules, CA) followed by RT-qPCR relative quantification of 50 ng cDNA per reaction using CFX Connect, SSO Advanced Universal SYBR Green Master Mix, and Prime- PCR validated primers for murine GAPDH, Hprt, Tbp, Fas, and FasL (BioRad, Hercules, CA). The data were normalized to the reference genes: Hprt, Tbp, and GAPDH.
Statistical analysis
For each parameter, the values for individual experiments were averaged and the standard error calculated. Student’s t tests, one-way or two-way ANOVA were performed using Prism 7 (GraphPad, La Jolla, CA). A p value of ≤ 0.05 was considered significant.
Results
ITE, but not I3C, induces thymic atrophy
Although AhR activation promotes immunoregulatory responses in a ligand-dependent manner (Ehrlich et al. 2018), little is known about the thymotoxic effects of other AhR ligand chemotypes. To test the hypothesis that activation of the AhR induces thymic atrophy in a ligand-dependent manner, C57Bl/6 mice were gavaged with an endogenous ligand (ITE) (Song et al. 2002) and a dietary ligand (I3C) (Connor and Finley 2003), with chemical structures that are distinctly different from TCDD (Fig. 1a). The dose and route of exposure to each chemical were selected based on prior reports, thus simplifying integration of our results with previous findings (Benson et al. 2012b; Boule et al. 2018b; Quintana et al. 2010b). Consistent with the published literature, administration of 10 μg/kg TCDD to C57Bl/6 mice resulted in extensive thymic atrophy as evidenced by a significant decline in organ weight (65% decrease) and a dramatic reduction in thymic cellularity (86% decrease) relative to vehicle control on day 7. Similarly, daily administration of 8 mg/kg ITE to C57Bl/6 mice resulted in extensive thymic atrophy as evidenced by a significant loss in organ weight (52% decrease) and a dramatic reduction in thymic cellularity (73% decrease) relative to vehicle control on day 7. In contrast, administration of 100 mg/kg I3C to C57Bl/6 mice every other day resulted in a trend towards increased thymic weight and a significant increase in cellularity (30% increase) relative to vehicle control on day 7 (Fig. 1b, c). No sex specific effects were observed with regards to the capacity of TCDD, ITE, or I3C to induce thymic atrophy in male vs female mice (data not shown).
Fig. 1.
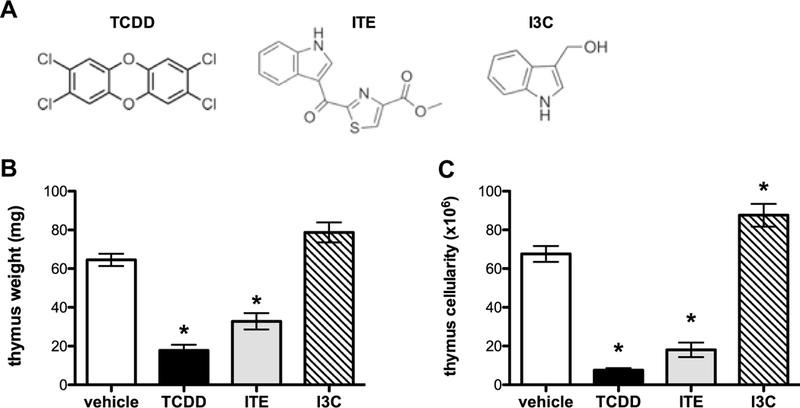
ITE, but not I3C, induces thymic atrophy. Naïve wild-type mice (C57Bl/6) were gavaged with three chemically distinct AhR ligands (a): TCDD (10 μg/kg), ITE (8 mg/kg), or I3C (100 mg/kg). Three indicators of toxicity: body weight (data not shown), thymus weight (b), and thymus cell number (c) were measured on day 7 toevaluate toxicity and thymic atrophy after administration of TCDD, ITE, and I3C. Data represents one of two independent experiments, n = 6–10 per treatment group, mean ± SEM; one-way ANOVA, *p < 0.05 vs. vehicle
Previous studies demonstrated that administration of sublethal levels of TCDD to mice alters the development and differentiation of thymocytes (Esser and Welzel 1993; Holladay et al. 1991; Lai et al. 1994; Lundberg et al. 1990), and led us to investigate the effects of ITE and I3C on intrathymic differentiation by assessing the expression of the cell surface markers CD3, CD4, and CD8 via multicolor flow cytometric analysis (Fig. 2). Representative dot plots gating on live thymocytes from wild-type C57Bl/6 mice revealed a significant decline in the frequency of DP thymocytes, as well as a relative enrichment in the percent of DN and CD4+CD8- and CD4-CD8+. SP thymocytes in 10 μg/kg TCDD-treated mice compared to vehicle control. Although administration of ITE revealed a trend similar to TCDD with regards to a significant reduction in the frequency of DP and increase in the frequency of DN and CD4+ SP thymocytes, no difference was observed in the frequency of CD8+ SP thymocytes compared to vehicle control. Administration of I3C to C57Bl/6 mice resulted in no change in the development and/or differentiation of any CD4/CD8 thymocyte subsets examined (Figs. 1, 2). Finally, based upon the presented values for thymic cellularity, these shifts in the frequency of CD4/CD8 thymocyte subsets correspond with a significant decrease in the absolute number of thymocytes in each of the four subpopulations—an effect that was not observed in mice exposed to I3C (data not shown). Together, these data show that activation of the AhR induces thymic atrophy in a ligand-dependent manner.
Fig. 2.
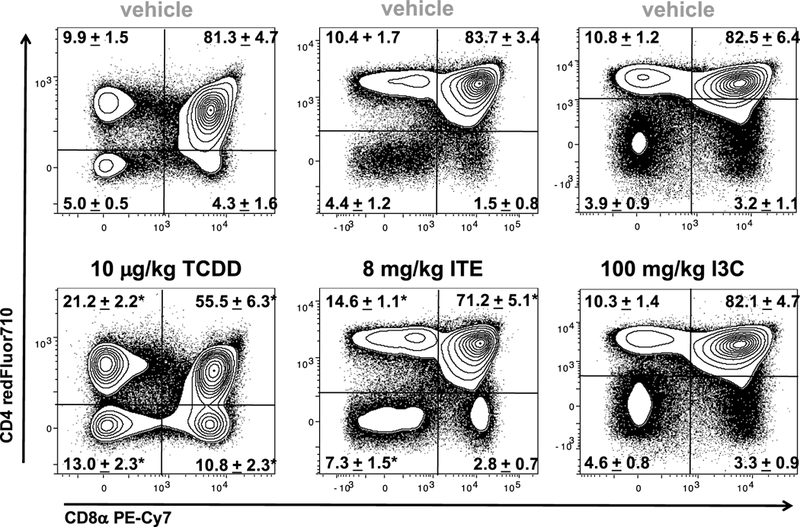
Comparison of CD4/CD8 thymocyte subsets from vehicle, TCDD, ITE, and I3C exposed mice. C57Bl/6 mice were gavaged with the appropriate vehicle control, TCDD (10 μg/kg, once), ITE (8 mg/ kg daily), or I3C (100 mg/kg every other day). Representative contour plots gating on live thymocytes revealed a significant decline in the frequency of CD4+ CD8+ DP thymocytes, as well as a relative enrichment in the percent of CD4− CD8− DN and CD4+ CD8− and CD4− CD8+ SP thymocytes in 10 μg/kg TCDD- and 8 mg/kg ITEtreated mice compared to vehicle controls on day 7. These shifts in CD4/CD8 thymocyte subsets were not observed in mice treated with 100 mg/kg I3C. The mean percentages of the CD4/CD8 thymocyte subsets ± SEM are indicated in the plots. Data represents one of two independent experiments, n = 6–10 per treatment group; one-way ANOVA, *p < 0.05 vs. vehicle
ITE induces thymic atrophy in a dose- and an AhR-dependent manner
To validate the use of mice expressing the low affinity AhRd allele as a model system in which to study aryl hydrocarbon receptor (AhR) dependent thymic atrophy, AhRd and wild type C57Bl/6 (AhRb) mice were exposed to TCDD and thymi analyzed 7 days later. As expected, AhRd mice were unresponsive to 10 μg/kg TCDD, whereas administration of a tenfold higher dose of TCDD (e.g., 100 μg/kg) resulted in the archetypal thymic atrophy (Supplemental Fig. 1) and alterations in thymocyte development and differentiation (Supplemental Fig. 2). To test the hypothesis that ITE induces thymic atrophy in an AhR-dependent manner, C57Bl/6 and low affinity AhRd mice were gavaged daily with a previously described immunosuppressive dose of ITE (Abron et al. 2018; Henry et al. 2006; Nugent et al. 2013; Quintana et al. 2010b) and thymi analyzed 7 days later. ITE exposure to naive C57Bl/6 mice resulted in thymic atrophy as evidenced by a significant decline in organ weight (49% decrease) and a reduction in thymic cellularity (73% decrease) relative to vehicle control. In contrast, AhRd mice were refractory to the same 8 mg/kg dose of ITE with regards to thymic weight (Fig. 3a) and cellularity (Fig. 3b). Likewise, daily administration of 1, 2, 4, and 8 mg/ kg ITE (p.o.) resulted in dose-dependent thymic atrophy as evidenced by a decline in organ weight of 13%, 25%, 30% and 35%, respectively (Fig. 3c) and a dramatic decrease in thymic cellularity of 25%, 25%, 40% and 50%, (Fig. 3d) respectively, in C57Bl/6 mice relative to vehicle controls on day 7. Finally, comparable levels of thymic atrophy were observed following daily administration of 1 mg/kg ITE i.p. and 4 mg/kg ITE p.o. (data not shown).
Fig. 3.

ITE-induced thymic atrophy in an AhR- and a dose-dependent manner. Naïve wild-type mice (C57Bl/6) and mice expressing the low affinity receptor ( AhRd mice) were gavaged with vehicle (anisole/ peanut oil) or ITE (8 mg/kg). Three indicators of toxicity: body weight (data not shown), thymus weight (a), and thymus cell number (b) were measured on day 7 to evaluate toxicity and thymic atrophy. n = 5–6 mice per treatment group, mean ± SEM; one-way ANOVA *p < 0.05 vehicle. An additional cohort of C57Bl/6 mice was gavaged with vehicle (anisole/peanut oil) or increasing doses of ITE (1, 2, 4, or 8 mg/kg). Three indicators of toxicity: body weight (data not shown), thymus weight (c), and thymus cell number (d) were measured on day 7 to evaluate toxicity and thymic atrophy. n = 5–6 mice per treatment group, mean ± SEM; one-way ANOVA, *p < 0.05 vs. vehicle
Dioxin-mediated thymic atrophy is not dependent on apoptosis via Fas:FasL interactions
Since TCDD has been reported to initiate apoptosis in immature thymocytes (Fisher et al. 2004; Rhile et al. 1996) and Fas:FasL interactions have been implicated in this process (Dencker et al. 1985; Kamath et al. 1999a), C57Bl/6 mice were treated with 10 μg/kg TCDD and the degree of apoptosis measured by assessing the expression of 7AAD and Annexin V, as well as Fas and FasL on thymocytes via flow cytometry. Representative dot plots gating on singlet thymocytes revealed a slight, but statistically significant increase in the frequency of Annexin V+7AAD- apoptotic thymocytes in 10 μg/kg TCDD-treated mice (6.5% ± 0.8 of thymocytes) compared to vehicle (3.8% ± 0.6 of thymo cytes) on day 7 (Fig. 4a). However, this did not correspond to a significant increase in the absolute number of apoptotic cells—perhaps due to the massive decline in thymus cellularity (data not shown). Moreover, representative dot plots gating on Annexin V+7AAD- apoptotic thymocytes revealed that the majority of apoptotic cells were DP thymocytes, followed by DN and CD4+ SP thymocytes, with very few apoptotic cells observed in the CD8+ SP subset. In contrast, TCDD exposed animals exhibited a distribution shift at day 7—resulting in an increased frequency of Annexin V+7AAD- apoptotic thymocytes within the DN, CD4+, and CD8+ SP thymocytes and a decreased frequency of Annexin V+7AAD- apoptotic thymocytes being of the DP thymocyte subset (Fig. 4a, b). Additionally, C57Bl/6 mice were treated with 10 μg/kg TCDD and Fas-FasL gene expression was analyzed on freshly isolated thymocytes on days 3, 7, and 14 post exposure. TCDD resulted in no change in either Fas or FasL gene expression as measured by quantitative RT-PCR (data not shown). Furthermore, 3 days after administration of TCDD to C57Bl/6 mice, there were no observable effects on the frequency or the relative expression (MFI) of Fas or FasL on live CD45+ thymocytes relative to vehicle controls (Fig. 5a). Because previous studies reported that dioxin-induced FasL-dependent thymocyte cell death (Camacho et al. 2005a; Kamath et al. 1999b), we further examined the role of Fas-FasL signaling in TCDD- induced thymic atrophy using FasL-deficient (gld/gld) mice. FasL-deficient mice were exposed to 10 μg/kg TCDD (p.o.) and their thymic weight and cellularity measured on day 7. Contrary to a previous report (Kamath et al. 1999b), FasL- deficient mice in our experiments were not protected against TCDD-induced thymic atrophy and exhibited significant reductions in thymic weight (60% decrease) and cellularity (70% decrease) comparable to wild type C57Bl/6 mice (Fig. 5b). Similarly, representative dot plots (gating on live thymocytes) revealed a significant decline in the frequency of DP thymocytes, as well as a relative enrichment in the percent of DN and CD4+ and CD8+ SP thymocytes in 10 μg/kg TCDD-treated mice compared to vehicle control (Fig. 5c).
Fig. 4.
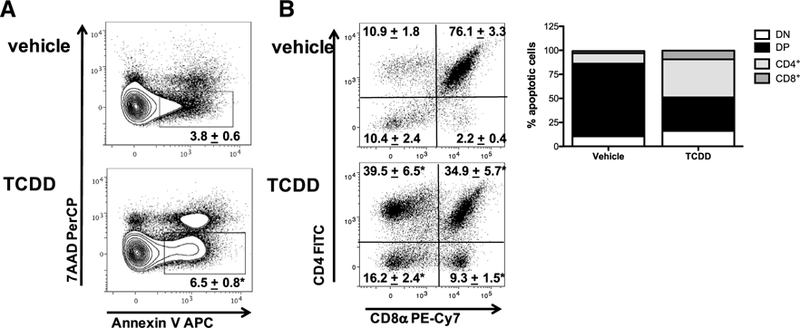
The effect of TCDD on apoptotic thymocytes. Naïve wildtype mice (C57Bl/6) were gavaged with vehicle (anisole/peanut oil) or TCDD (10 μg/kg). Representative contour plots gating on 7-AAD+Annexin V+ thymocytes revealed a significant increase in the frequency of apoptotic thymocytes (a), as well as a dramatic shift in the distribution of thymocytes which were undergoing apoptosis 7 days following exposure to TCDD (b). Data represent one of two independent experiments, n = 6–10 mice per treatment group, mean ± SEM; one-way ANOVA, *p < 0.05 vs. vehicle
Fig. 5.
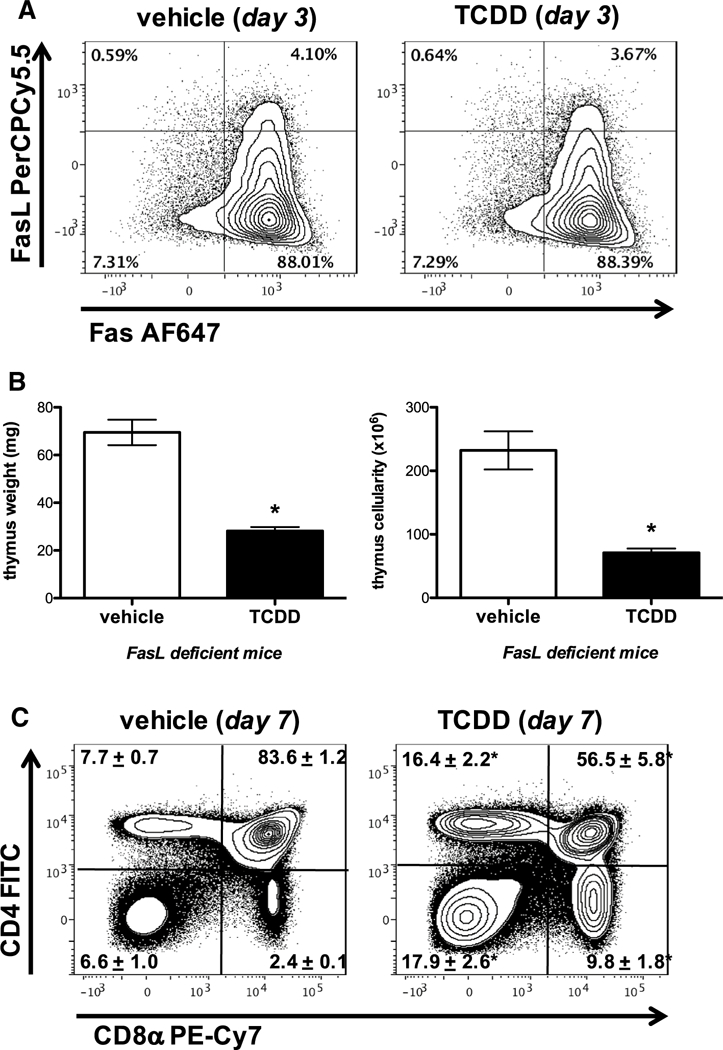
TCDD-mediated thymic atrophy is not dependent on Fas–FasL interactions. Naïve wild-type mice (C57Bl/6) were gavaged with vehicle (anisole/ peanut oil) or TCDD (10 μg/ kg). Representative contour plots gating on singlet thymocytes revealed that 3 days after administration of vehicle or 10 μg/kg TCDD to C57Bl/6 mice, there were no observable effects on the frequency of Fas and FasL expression on CD45+ Thymocytes relative to vehicle control (a). FasL-deficient (gld/gld) mice were exposed to 10 μg/kg TCDD and their thymic weight and cellularity measured on day 7 (b). Similarly, representative dot plots (gating on live thymocytes) revealed a significant decline in the frequency of DP thymocytes, as well as a relative enrichment in the percent of DN and CD4+ CD8− and CD4− CD8+ SP thymocytes in 10 μg/kg TCDD-treated FasL-deficient (gld/gld) mice compared to vehicle control (c). n = 5 mice per treatment group, mean ± SEM; t test, *p < 0.05 vs. vehicle
Targeted deletion of the AhR in CD11c+ dendritic cells protects against dioxin-induced thymic atrophy
Previous studies revealed that the target(s) for TCDD- induced thymic atrophy resides within the hematopoietic cells, and not stromal tissues (Staples et al. 1998); however, it is uncertain which hematopoietically derived cells contribute to TCDD-induced thymic atrophy. To determine how AhR signaling in specific immune cells mediates TCDD-induced thymic atrophy, we generated AhR conditional knockout mice for myeloid derived cells (LyzMCreAhRfx), CD11c+ dendritic cells (CD11cCreAhRfx), RORγt+ DP and SP thymocytes (RORcCreAhRfx), and thymic epithelial cells (FoxN1CreAhRfx). Mice were gavaged with vehicle or 100 μ/kg TCDD—a dose of dioxin necessary to elicit thymo-toxic endpoints in conditional AhRfx/fx mice which carry the low affinity AhRd allele (Poland and Glover 1980; Walisser et al. 2005). Seven days later, Cre-AhRfx/fx and AhRd mice, as well as LyzMCreAhRfx, RORcCreAhRfx, and FoxN1CreAhRfx exhibited extensive thymic atrophy as evidenced by a decline in organ weight (68%, 63%, and 63% decrease, respectively) (Fig. 6a) and a dramatic reduction in thymic cellularity (89%, 89%, and 82% decrease, respectively) (Fig. 6b) relative to vehicle controls. In contrast, CD11cCreAhRfx mice were protected from TCDD-induced thymic atrophy and showed no significant difference from vehicle-treated controls in either thymic weight (Fig. 6a) or cellularity (Fig. 6b). These findings led us to further investigate whether targeted deletion of the AhR in specific immune cell types also facilitates TCDD-induced effects on intra-thymic development and differentiation. Representative dot plots from AhRd, LyzMCreAhRfx, RORcCreAhRfx, and FoxN1CreAhRfx mice administered 100 μg/kg TCDD mirrored the typical alterations in thymocyte subsets which are consistently observed in wild-type mice following exposure to TCDD: decreases in the frequency of DP thymocytes and increases in the frequency of DN, CD4+, and CD8+ SP thymocytes compared to vehicle controls (data not shown). Importantly, representative dot plots gating on live thymocytes from CD11cCreAhRfx mice treated with 100 μg/kg TCDD exhibited protection from TCDD-induced alterations in thymocyte subsets (Fig. 7). Because conditional Cre negative x AhRfx/fx mice carry the low affinity AhRd allele (Poland and Glover 1980; Walisser et al. 2005), this strain can be utilized inter changeably with Cre-AhRfx as controls for experiments involving AhR conditional knockout mice (supplemental Fig. 3). Collectively, these data show that targeted deletion of the AhR in CD11c+ dendritic cells protects against TCDD-induced thymic atrophy.
Fig. 6.
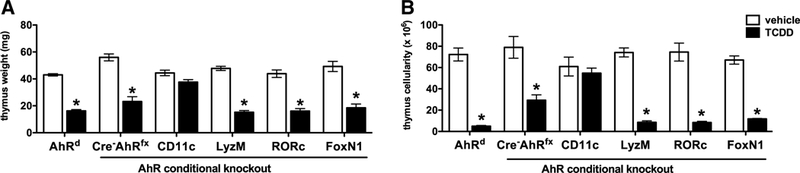
Deletion of the AhR in CD11c+ dendritic cells protects against dioxin-induced thymic atrophy. AhR conditional knockout mice were generated for myeloid derived cells ( LyzMCreAhRfx), CD11c+ dendritic cells (CD11cCreAhRfx), RORγt+ DP and SP thymocytes (RORcCreAhRfx), and thymic epithelial cells ( FoxN1CreAhRfx). Mice were exposed to either solvent/peanut oil vehicle or 100 μg/kg TCDD. Three indicators of toxicity: body weight (data not shown), thymus weight (a), and thymus cell number (b) were measured on day 7. Data represent one of three independent experiments, n = 6–8 mice per treatment group, mean ± SEM; two-way ANOVA, *p < 0.05 vs. vehicle
Fig. 7.
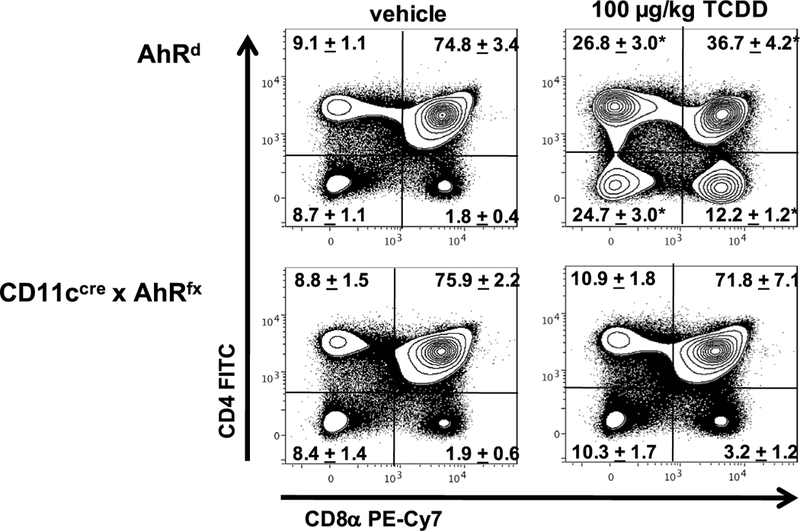
Comparison of CD4/CD8 thymocyte subsets from CD11cCreAhRfx and AhRd mice exposed to vehicle or TCDD. AhR conditional knockout and AhRd control mice were exposed to either solvent/peanut oil vehicle or 100 μg/kg TCDD. Representative contour plots gating on live thymocytes revealed a significant decline in the frequency of CD4+ CD8+ DP thymocytes, as well as a relative enrichment in the percent of CD4− CD8− DN and CD4+ CD8− and CD4− CD8+ SP thymocytes in AhRd mice exposed to 100 μg/kg TCDD compared to vehicle controls on day 7. These shifts in CD4/ CD8 thymocyte subsets were not observed in CD11cCreAhRfx mice treated with TCDD. The mean percentages of the CD4/CD8 thymocyte subsets ± SEM are indicated in the plots. Data represents one of three independent n = 6–8 per treatment group; two-wayANOVA, *p < 0.05 vs. vehicle
Discussion
Administration of dioxin (TCDD) and dioxin-like chemicals to laboratory rodents dramatically affects the immune system, triggering immunosuppression characterized by suppressed cellular immunity, inhibition of antibody production, and thymic atrophy (Silkworth and Antrim 1985; Silkworth et al. 1986)—effects which are dependent on the AhR (Fernandez-Salguero et al. 1996; Harrill et al. 2016; Staples et al. 1998). Unlike many nuclear receptors, the AhR is a highly promiscuous receptor, directly binding a wide variety of structurally diverse natural and synthetic compounds, thereby promoting the up- or down-regulation of a multitude of target genes in different tissues. The spectrum of biological effects produced by the ligand/AhR/ARNT signaling complex is dependent on the physicochemical characteristics and persistence of the ligand (Ehrlich et al. 2018). To date, most analyses exploring AhR-induced immune modulation have taken advantage of TCDD’s specificity and high affinity for the AhR (6 pM-2.4 nM), as well as its long half-life (~ 11 days in mice; 8–10 years in humans) (Miniero et al. 2001). However, the same properties that make TCDD an excellent tool also contribute to its profound toxicity. While other ligands such as ITE and indole-3-carbinol (I3C) can activate the AhR and have been evaluated as potential therapeutics (Abron et al. 2018; Quintana et al. 2010b; Yeste et al. 2012), little is known about their potential thy- motoxic effects. Thus, the present study investigated the effects of an endogenous (ITE) and a dietary (I3C) ligand on thymic development and differentiation.
Although previous studies suggested that sex-specific effects exist with select AhR ligands in a murine colitis model (Benson et al. 2012a), no sex-specific effects were observed with regards to thymic weight or cellularity following exposure to TCDD, ITE, or I3C. This discrepancy may be due to enhanced bioavailability and the presence of active metabolites of I3C in the gastrointestinal tract vs the thymus (Benson et al. 2012a; Bjeldanes et al. 1991; Hubbard et al. 2015). Likewise, because AhR activation by TCDD and TCDD-like compounds results in thymic atrophy and alterations in the frequency of thymocyte subsets, we investigated whether ITE and I3C similarly affect thymic development and differentiation. Our results demonstrate for the first time that administration of 8 mg/kg ITE to C57Bl/6 mice resulted in a dramatic reduction in organ weight and thymic cellularity, whereas administration of 100 mg/kg I3C conversely resulted in a slight increase in thymic weight and a significant increase in cellularity on day 7. ITE-induced thymic atrophy, as well as reduced the frequency of DP thymocytes and increased the frequency of DN, CD4+ and CD8+ SP thymocytes—albeit to a slightly lesser degree than TCDD. The ITE data presented are in contrast to a previous report (Henry et al. 2006), which failed to detect changes in thymus weight 12 days following a single (i.v.) delivery of 5.6 mg/kg ITE. It is likely that the discrepancy between our results and the study by Henry et al. (2006) reside in the differences between dose (acute vs sub-acute) and route of delivery (i.v. vs p.o.) (Boule et al. 2018b; Ehrlich et al. 2018). Recent advances in AhR biology suggest that binding affinity and ligand metabolism may be better predictors of immunosuppressive outcome than the ligand source (Boule et al. 2018a). Interestingly, administration of I3C resulted in no change in thymopoiesis in mice. Therefore, future studies to determine the immunosuppressive characteristics of I3C should encompass not only varying doses of I3C, but also purified metabolites. Together, this is the first study to compare AhR activation by different chemotypes on thymocyte development and differentiation; thus, extending our knowledge of ligand-specific, AhR-mediated immunotoxic effects.
Because we observed gross thymic atrophy with ITE, we more extensively characterized the ability of ITE to induce thymic atrophy. Following previously published dosing regimens (Nugent et al. 2013; Quintana et al. 2010b), our data clearly demonstrate that daily administration of 8 mg/kg ITE p.o. induced significant thymic atrophy in an AhR-dependent manner. In addition, ITE-induced noticeable decreases in both thymic weight and cellularity at doses as low as 1 mg/ kg. Moreover, 4 m/kg ITE p.o. and 1 mg/kg i.p. induced comparable levels of thymic atrophy in C57Bl/6 mice (data not shown), demonstrating that ITE causes thymic atrophy regardless of the route of systemic exposure. This point is particularly important given the growing interest in AhR research on identifying novel classes of potent, non-toxic AhR ligands for use in various therapeutic settings (Stockinger 2009). Moreover, these observations raise concerns about potential off target immune toxicities associated with the use of ITE based immune therapies (Abron et al. 2018; Dolciami et al. 2018; Hao and Whitelaw 2013; Nugent et al. 2013; Quintana et al. 2010b).
Multiple mechanisms for TCDD-induced thymic hypocellularity have been suggested including reduced proliferation of DN precursor thymocytes (Lai et al. 1994), enhanced apoptosis at the DP stage (Camacho et al. 2005b), and enhanced emigration of thymocytes (Poland et al. 1994; Temchura et al. 2005), or a combination of these possible mechanisms. Although our results demonstrate that TCDD- induced thymic atrophy corresponded with an almost doubling of the frequency of Annexin V+7-AAD- apoptotic thymocytes, this did not correspond to a significant increase in the absolute number of apoptotic cells—likely due to the massive decline in overall thymus cellularity. Analysis of Fas-FasL gene and protein expression across multiple time points also failed to substantiate TCDD-mediated induction of apoptosis in the thymus. Moreover, FasL-deficient (gld/gld) mice experience the same degree of thymic atrophy as control mice when exposed to 10 |Jg/kg TCDD. While earlier studies suggested that the timing and pathway of apoptosis was important to AhR-mediated thymic atrophy (Camacho et al. 2005a; Kamath et al. 1997, 1998) and that thymic and peripheral T cells are highly sensitive to TCDD- induced apoptosis in vitro (Camacho et al. 2004), our work and that of others (Comment et al. 1992; De Heer et al. 1994; Silverstone et al. 1994a, b; Staples et al. 1998) have failed to support this mechanism as playing a prominent role in vivo. The difference between our study and previous reports likely resides in disparities of dose (10 vs 30–50 μg/kg TCDD), route of delivery (p.o. vs i.p.), and timing of exposure (3–14 days vs 6–24 h) (Camacho et al. 2002; Kamath et al. 1999b; Rhile et al. 1996). Interestingly, assessment of an apoptosis and survival RT-PCR array generated four genes which showed a significant increase in expression: BAD (seven fold change vs vehicle), Akt1 (fourfold change vs vehicle), Pik3cd (2.5-fold change vs vehicle), and Ppp3ca (2.5-fold change vs vehicle) in thymocytes on day 7 following administration of 10 μg/kg TCDD and no interrogated genes which were significantly reduced in expression. Collectively, our results fail to support apoptosis and Fas-FasL interactions as a prominent mechanism of TCDD-induced thymic atrophy.
Previous studies identified AhR signaling as the pivotal event in TCDD-induced thymic atrophy (Laiosa et al. 2003; Staples et al. 1998) and the hematopoietic compartment as the critical target for TCDD-induced thymic atrophy (Staples et al. 1998). However, until now, it was unclear which hematopoietically derived cell(s) specifically mediated TCDD-induced thymic atrophy. To investigate the importance of cell-specific AhR signaling in TCDD-induced thymic atrophy, mice expressing the AhR floxed allele (AhRfx) were crossed to mice expressing Cre transgenes driven by LyzM (myeloid derived cells), CD11c (dendritic cells), RORyt (DP and SP thymocytes), and FoxNl (thymic epithelial cells) specific promoters. Our results show, for the first time, that deletion of the AhR in CD11c+ dendritic cells prevents TCDD-induced thymic atrophy, a previously unreported phenomenon. Therefore, the immunotoxic responses of TCDD on the thymus are dependent on AhR activation in CD11c+ dendritic cells (DCs). Dendritic cells make up a small percentage of the thymic stroma (Wu and Shortman 2005) and are located mainly in the medulla and corticome- dullary region. Thymic CD8a- DCs cross-present self-antigens to developing thymocytes, facilitate the generation of regulatory T cells, and act as gatekeepers of lymphocyte trafficking (Bonasio et al. 2006; Hubert et al. 2011; Lei et al. 2011; Proietto et al. 2008). Dendritic cells are thus poised to exert control over thymic output in response to environmental conditions. Unfortunately, the solubility limitations of ITE (~ 30 mg/mL DMSO) and I3C (~ 10 mg/mL in ethanol) prevent the use of either of these ligands in AhR conditional knockout mice at doses that would be expected to induce thymic atrophy. Together, the current study significantly advances our understanding of how the AhR modulates the immune system and demonstrates for the first time that TCDD-induced thymic atrophy occurs as a result of activation of the AhR in CD11c+ dendritic cells. The results presented herein lead us to postulate that alterations in thymic CD11c+ DCs and/or their function underlie TCDD-induced thymic atrophy in mice. Indeed, recent studies from our laboratory indicate that administration of TCDD shifts the ratio of DC subsets in the thymus from CD8α+ to CD8α- DCs, an effect that may be responsible for an observed AhR-mediated induction of thymic FoxP3+ Tregs (manuscript in preparation). Moreover, TCDD induces systemic immune suppression and promotes the differentiation and activity of peripheral FoxP3+ Tregs (Kerkvliet 2012a; Kerkvliet et al. 2009; Vos and Moore 1974)—a response which is abrogated in thymectomized mice (manuscript in preparation). Therefore, identification of CD11c+ dendritic cells as the direct target of TCDD-induced thymic atrophy offers insights into novel pathways to further understand mechanisms of AhR-mediated immune regulation. Collectively, this study emphasizes the importance of research examining the contribution of cell- and tissue-specific consequences of chemical exposures on the immune system.
Supplementary Material
Acknowledgements
The authors wish to thank the following scientists: Pam Shaw (Fluorescence Cytometry Core) and Britten Postma (Animal Core) for the shared expertise needed to conduct and/or analyze the experiments described in this manuscript.
Funding
Research reported in this publication was supported by the National Institute of Environmental Health Sciences and the National Institute of General Medical Sciences of the National Institutes of Health under Grant numbers R01-ES013784 (DMS), P30-GM103338, P20-GM103546. JMK was supported by the American Association of Immunologists through Careers in Immunology Fellowship. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Electronic supplementary material
The online version of this article (https://doi.org/10.1007/s00204–018-2366-x) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- Abron JD, Singh NP, Mishra MK, Price RL, Nagarkatti M, Nagarkatti PS, Singh UP (2018) An endogenous aryl hydrocarbon receptor ligand, ITE, induces regulatory T cells and ameliorates experimental colitis. Am J Physiol Gastrointest Liver Physiol 315(2):G220–G230. 10.1152/ajpgi.00413.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson JM, Shepherd DM (2011a) Aryl hydrocarbon receptor activation by TCDD reduces inflammation associated with Crohn’s disease. Toxicol Sci 120(1):68–78. 10.1093/toxsci/kfq360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson JM, Shepherd DM (2011b) Dietary ligands of the aryl hydrocarbon receptor induce anti-inflammatory and immunoregulatory effects on murine dendritic cells. Toxicol Sci 124(2):327–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson J, Beamer C, Seaver B, Shepherd D (2012a) Indole-3-carbinol exerts sex-specific effects in murine colitis. Eur J Inflamm 10(3):335–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson JM, Beamer CA, Seaver BP, Shepherd DM (2012b) Indole- 3-carbinol exerts sex-specific effects in murine colitis. Eur J Inflamm 10(3):335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum LS (1986) Distribution and excretion of 2,3,7,8-tetrachlorodibenzo-p-dioxin in congenic strains of mice which differ at the Ah locus. Drug Metab Dispos Biol Fate Chem 14(1):34–40 [PubMed] [Google Scholar]
- Bjeldanes LF, Kim J-Y, Grose KR, Bartholomew JC, Bradfield CA (1991) Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc Natl Acad Sci 88(21):9543–9547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH (2006) Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol 7(10):1092– 1100. 10.1038/ni1385 [DOI] [PubMed] [Google Scholar]
- Boule LA, Burke CG, Jin G-B, Lawrence BP (2018a) Aryl hydrocarbon receptor signaling modulates antiviral immune responses: ligand metabolism rather than chemical source is the stronger predictor of outcome. Sci Rep 8(1):1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boule LA, Burke CG, Jin GB, Lawrence BP (2018b) Aryl hydrocarbon receptor signaling modulates antiviral immune responses: ligand metabolism rather than chemical source is the stronger predictor of outcome. Sci Rep 8(1):1826 10.1038/s41598-018-20197-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho IA, Nagarkatti M, Nagarkatti PS (2002) 2,3,7,8-Tetrachlo rodibenzo-p-dioxin (TCDD) induces Fas-dependent activation induced cell death in superantigen-primed T cells. Arch Toxicol 76(10):570–580 [DOI] [PubMed] [Google Scholar]
- Camacho IA, Nagarkatti M, Nagarkatti PS (2004) Evidence for induction of apoptosis in T cells from murine fetal thymus following perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Toxicol Sci 78(1):96–106. [DOI] [PubMed] [Google Scholar]
- Camacho IA, Singh N, Hegde VL, Nagarkatti M, Nagarkatti PS (2005a) Treatment of mice with 2,3,7,8-tetrachlorodibenzo-p-dioxin leads to aryl hydrocarbon receptor-dependent nuclear translocation of NF-kappaB and expression of Fas ligand in thymic stromal cells and consequent apoptosis in T cells. J Immunol 175(1):90–103 [DOI] [PubMed] [Google Scholar]
- Camacho IA, Singh N, Hegde VL, Nagarkatti M, Nagarkatti PS (2005b) Treatment of mice with 2,3,7,8-tetrachlorodibenzo-p-dioxin leads to aryl hydrocarbon receptor-dependent nuclear translocation of NF-kB and expression of Fas ligand in thymic stromal cells and consequent apoptosis in T cells. J Immunol 175(1):90–103 [DOI] [PubMed] [Google Scholar]
- Comment CE, Blaylock BL, Germolec DR et al. (1992) Thymocyte injury after in vitro chemical exposure: potential mechanisms for thymic atrophy. J Pharmacol Exp Ther 262(3):1267–1273 [PubMed] [Google Scholar]
- Connor K, Finley B (2003) Naturally occurring ah-receptor agonists in foods: implications regarding dietary dioxin exposure and health risk. Hum Ecol Risk Assess 9(7):1747–1763 [Google Scholar]
- De Heer C, Verlaan AP, Penninks AH, Vos JG, Schuurman HJ, Van Loveren H (1994) Time course of 2,3,7,8-tetrachlorodibenzo-p-di- oxin (TCDD)-induced thymic atrophy in the Wistar rat. Toxicol Appl Pharmacol 128(1):97–104 [DOI] [PubMed] [Google Scholar]
- Dencker L, Hassoun E, d’Argy R, Alm G (1985) Fetal thymus organ culture as an in vitro model for the toxicity of 2,3,7,8-tetrachlorod- ibenzo-p-dioxin and its congeners. Mol Pharmacol 27(1):133–140 [PubMed] [Google Scholar]
- Dolciami D, Gargaro M, Cerra B et al. (2018) Binding mode and structure-activity relationships of ITE as an aryl hydrocarbon receptor (AhR) agonist. ChemMedChem 13(3):270–279 10.1002/cmdc.201700669 [DOI] [PubMed] [Google Scholar]
- Ehrlich AK, Pennington JM, Bisson WH, Kolluri SK, Kerkvliet NI (2018) TCDD, FICZ, and other high affinity AhR ligands dose-dependently determine the fate of CD4+ T cell differentiation. Toxicol Sci 161(2):310–320. 10.1093/toxsci/kfx215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser C, Welzel M (1993) Ontogenic development of murine fetal thymocytes is accelerated by 3,3’,4,4’-tetrachlorobiphenyl. Int J Immunopharmacol 15(8):841–852 [DOI] [PubMed] [Google Scholar]
- Faith RE, Luster MI (1979) Investigations on the effects of 2,3,7,8-tet- rachlorodibenzo-p-dioxin (TCDD) on parameters of various immune functions. Ann N Y Acad Sci 320(1):564–571 [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ (1996) Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol 140(1):173–179. 10.1006/taap.1996.0210 [DOI] [PubMed] [Google Scholar]
- Fine JS, Silverstone AE, Gasiewicz TA (1990) Impairment of prothymocyte activity by 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Immunol 144(4):1169–1176 [PubMed] [Google Scholar]
- Fisher MT, Nagarkatti M, Nagarkatti PS (2004) Combined screening of thymocytes using apoptosis-specific cDNA array and promoter analysis yields novel gene targets mediating TCDD-induced toxicity. Toxicol Sci 78(1): 116–124. 10.1093/toxsci/kfh058 [DOI] [PubMed] [Google Scholar]
- Funatake CJ, Marshall NB, Steppan LB, Mourich DV, Kerkvliet NI (2005) Cutting edge: activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells. J Immunol 175(7):4184–4188 [DOI] [PubMed] [Google Scholar]
- Gasiewicz TA, Geiger LE, Rucci G, Neal RA (1983) Distribution, excretion, and metabolism of 2,3,7,8-tetrachlorodibenzo-p-dioxin in C57BL/6J, DBA/2J, and B6D2F1/J mice. Drug Metab Dispos Biol Fate Chem 11(5):397–403 [PubMed] [Google Scholar]
- Gu Y-Z, Hogenesch JB, Bradfield CA (2000) The PAS superfamily: sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol 40(1):519–561 [DOI] [PubMed] [Google Scholar]
- Hao N, Whitelaw ML (2013) The emerging roles of AhR in physiology and immunity. Biochem Pharmacol 86(5):561–570. 10.1016/j.bcp.2013.07.004 [DOI] [PubMed] [Google Scholar]
- Harrill JA, Layko D, Nyska A et al. (2016) Aryl hydrocarbon receptor knockout rats are insensitive to the pathological effects of repeated oral exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Appl Toxicol 36(6):802–814. 10.1002/jat.3211 [DOI] [PubMed] [Google Scholar]
- Harris M, Moore J, Vos J, Gupta B (1973) General biological effects of TCDD in laboratory animals. Environ Health Perspect 5:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry EC, Bemis JC, Henry O, Kende AS, Gasiewicz TA (2006) A potential endogenous ligand for the aryl hydrocarbon receptor has potent agonist activity in vitro and in vivo. Arch Biochem Biophys 450(1):67–77. 10.1016/j.abb.2006.02.008 [DOI] [PubMed] [Google Scholar]
- Holladay S, Lindstrom P, Blaylock B et al. (1991) Perinatal thymocyte antigen expression and postnatal immune development altered by gestational exposure to tetrachlorodibenzo-p-dioxin (TCDD). Teratology 44(4):385–393 [DOI] [PubMed] [Google Scholar]
- Hubbard TD, Murray IA, Perdew GH (2015) Indole and tryptophan metabolism: endogenous and dietary routes to Ah receptor activation. Drug Metab Dispos 43(10):1522–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert FX, Kinkel SA, Davey GM et al. (2011) Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood 118(9):2462–2472. 10.1182/blood-2010-06-286393 [DOI] [PubMed] [Google Scholar]
- Kamath AB, Xu H, Nagarkatti PS, Nagarkatti M (1997) Evidence for the induction of apoptosis in thymocytes by 2,3,7,8-tetrachlorod- ibenzo-p-dioxinin vivo. Toxicol Appl Pharmacol 142(2):367–377 [DOI] [PubMed] [Google Scholar]
- Kamath AB, Nagarkatti PS, Nagarkatti M (1998) Characterization of phenotypic alterations induced by 2,3,7,8-tetrachlorodibenzo-p-di- oxin on thymocytes in vivo and its effect on apoptosis. Toxicol Appl Pharmacol 150(1):117–124 [DOI] [PubMed] [Google Scholar]
- Kamath AB, Camacho I, Nagarkatti PS, Nagarkatti M (1999a) Role of Fas-Fas ligand interactions in 2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD)-induced immunotoxicity: increased resistance of thymocytes from Fas-deficient (lpr) and Fas ligand-defective (gld) mice to TCDD-induced toxicity. Toxicol Appl Pharmacol 160(2):141–155. 10.1006/taap.1999.8753. [DOI] [PubMed] [Google Scholar]
- Kamath AB, Camacho I, Nagarkatti PS, Nagarkatti M (1999b) Role of Fas-Fas ligand interactions in 2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD)-induced immunotoxicity: increased resistance of thymocytes from Fas-deficient (lpr) and Fas ligand-defective (gld) mice to TCDD-induced toxicity. Toxicol Appl Pharmacol 160(2):141–155 [DOI] [PubMed] [Google Scholar]
- Kerkvliet NI (2002) Recent advances in understanding the mechanisms of TCDD immunotoxicity. Int Immunopharmacol 2(2–3):277–291 [DOI] [PubMed] [Google Scholar]
- Kerkvliet NI (2012a) TCDD: an environmental immunotoxicant reveals a novel pathway of immunoregulation—a 30-year odyssey. Toxicol Pathol 40(2):138–142. 10.1177/0192623311427710 [DOI] [PubMed] [Google Scholar]
- Kerkvliet NI (2012b) TCDD: an environmental immunotoxicant reveals a novel pathway of immunoregulation—a 30-year odyssey. Toxicol Pathol 40(2):138–142 [DOI] [PubMed] [Google Scholar]
- Kerkvliet NI, Steppan LB, Vorachek W et al. (2009) Activation of aryl hydrocarbon receptor by TCDD prevents diabetes in NOD mice and increases Foxp3+ T cells in pancreatic lymph nodes. Immunotherapy 1(4):539–547. 10.2217/imt.09.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai ZW, Kremer J, Gleichmann E, Esser C (1994) 3,3’,4,4’-Tetrachlorobiphenyl inhibits proliferation of immature thymocytes in fetal thymus organ culture. Scand J Immunol 39(5):480–488 [DOI] [PubMed] [Google Scholar]
- Laiosa MD, Wyman A, Murante FG et al. (2003) Cell proliferation arrest within intrathymic lymphocyte progenitor cells causes thymic atrophy mediated by the aryl hydrocarbon receptor. J Immunol 171(9):4582–4591 [DOI] [PubMed] [Google Scholar]
- Lei Y, Ripen AM, Ishimaru N et al. (2011) Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J Exp Med 208(2):383–394. 10.1084/jem.20102327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg K, Gronvik K-O, Goldschmidt TJ, Klareskog L, Dencker L (1990) 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) alters intrathymic T-cell development in mice. Chem Biol Interact 74(1–2):179–193 [DOI] [PubMed] [Google Scholar]
- Miniero R, De Felip E, Ferri F, Di Domenico A (2001) An overview of TCDD half-life in mammals and its correlation to body weight. Chemosphere 43(4–7):839–844 [DOI] [PubMed] [Google Scholar]
- Nebert D, Gelboin H (1968) Substrate-inducible microsomal aryl hydroxylase in mammalian cell culture I. Assay and properties of induced enzyme. J Biol Chem 243(23):6242–6249 [PubMed] [Google Scholar]
- Nebert D, Gelboin H (1969) The in vivo and in vitro induction of aryl hydrocarbon hydroxylase in mammalian cells of different species, tissues, strains, and developmental and hormonal states. Arch Biochem Biophys 134(1):76–89 [DOI] [PubMed] [Google Scholar]
- Nguyen LP, Bradfield CA (2007) The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol 21(1):102–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell CS, Farley AM, Blackburn CC (2007) Thymus organogenesis and development of the thymic stroma In: Fairchild PJ (ed) Immunological tolerance. Methods in molecular biology™, vol 380 Humana Press, Totowa, pp 125–162. 10.1007/978-1-59745-395-0_8 [DOI] [PubMed] [Google Scholar]
- Nugent LF, Shi G, Vistica BP, Ogbeifun O, Hinshaw SJ, Gery I (2013) ITE, a novel endogenous nontoxic aryl hydrocarbon receptor ligand, efficiently suppresses EAU and T-cell-mediated immunity. Investig Ophthalmol Vis Sci 54(12):7463–7469. 10.1167/iovs.12-11479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okey AB (2007) An aryl hydrocarbon receptor odyssey to the shores of toxicology: the Deichmann lecture, international congress of toxicology-XI. Toxicol Sci 98(1):5–38. 10.1093/toxsci/kfm096 [DOI] [PubMed] [Google Scholar]
- Pohjanvirta R (2011) The AH receptor in biology and toxicology. Wiley, New York [Google Scholar]
- Poland A, Glover E (1980) 2,3,7,8-Tetrachlorodibenzo-p-dioxin: segregation of toxicity with the Ah locus. Mol Pharmacol 17(1):86–94 [PubMed] [Google Scholar]
- Poland A, Glover E (1990) Characterization and strain distribution pattern of the murine Ah receptor specified by the Ahd and Ahb-3 alleles. Mol Pharmacol 38(3):306–312 [PubMed] [Google Scholar]
- Poland A, Glover E, Kende A (1976) Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem 251(16):4936–4946 [PubMed] [Google Scholar]
- Poland A, Palen D, Glover E (1994) Analysis of the four alleles of the murine aryl hydrocarbon receptor. Mol Pharmacol 46(5):915–921 [PubMed] [Google Scholar]
- Proietto AI, van Dommelen S, Zhou P et al. (2008) Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc Natl Acad Sci USA 105(50):19869–19874. 10.1073/pnas.0810268105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana FJ, Murugaiyan G, Farez MF et al. (2010a) An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci 107(48):20768–20773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana FJ, Murugaiyan G, Farez MF et al. (2010b) An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis.Proc Natl Acad Sci USA 107(48):20768–20773. 10.1073/pnas.1009201107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhile MJ, Nagarkatti M, Nagarkatti PS (1996) Role of Fas apoptosis and MHC genes in 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)- induced immunotoxicity of T cells. Toxicology 110(1–3):153–167 [DOI] [PubMed] [Google Scholar]
- Silkworth J, Antrim L (1985) Relationship between Ah receptor- mediated polychlorinated biphenyl (PCB)-induced humoral immunosuppression and thymic atrophy. J Pharmacol Exp Ther 235(3):606–611 [PubMed] [Google Scholar]
- Silkworth JB, Antrim L, Sack G (1986) Ah receptor mediated suppression of the antibody response in mice is primarily dependent on the Ah phenotype of lymphoid tissue. Toxicol Appl Pharmacol 86(3):380–390 [DOI] [PubMed] [Google Scholar]
- Silverstone AE, Frazier DE Jr, Fiore NC, Soults JA, Gasiewicz TA (1994a) Dexamethasone, beta-estradiol, and 2,3,7,8-tetrachlorodibenzo-p-dioxin elicit thymic atrophy through different cellular targets. Toxicol Appl Pharmacol 126(2):248–259. 10.1006/taap.1994.1114 [DOI] [PubMed] [Google Scholar]
- Silverstone AE, Frazier DE Jr, Gasiewicz TA (1994b) Alternate immune system targets for TCDD: lymphocyte stem cells and extrathymic T-cell development. Exp Clin Immunogenet 11(2–3):94–101 [DOI] [PubMed] [Google Scholar]
- Singh U, Abron J, Singh N et al. (2014) An endogenous aryl hydrocarbon receptor (AhR) ligand, ITE induces regulatory T cells (Tregs) and ameliorates experimental colitis (IRC4P.490). J Immunol 192:60.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP, Singh UP, Rouse M et al. (2016) Dietary indoles suppress delayed-type hypersensitivity by inducing a switch from proinflammatory Th17 cells to anti-inflammatory regulatory T cells through regulation of microRNA. J Immunol 196(3):1108–1122. 10.4049/jimmunol.1501727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Clagett-Dame M, Peterson RE et al. (2002) A ligand for the aryl hydrocarbon receptor isolated from lung. Proc Natl Acad Sci 99(23):14694–14699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples JE, Murante FG, Fiore NC, Gasiewicz TA, Silverstone AE (1998) Thymic alterations induced by 2,3,7,8-tetrachlorodibenzo- p-dioxin are strictly dependent on aryl hydrocarbon receptor activation in hemopoietic cells. J Immunol 160(8):3844–3854 [PubMed] [Google Scholar]
- Stockinger B (2009) Beyond toxicity: aryl hydrocarbon receptor-mediated functions in the immune system. J Biol 8(7):61 10.1186/jbiol170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temchura VV, Frericks M, Nacken W, Esser C (2005) Role of the aryl hydrocarbon receptor in thymocyte emigration in vivo. Eur J Immunol 35(9):2738–2747 [DOI] [PubMed] [Google Scholar]
- Thigpen JE, Faith RE, McConnell EE, Moore JA (1975) Increased susceptibility to bacterial infection as a sequela of exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Infect Immun 12(6):1319–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loveren H, Schuurman H-J, Kampinga J, Vos JG (1991) Reversibility of thymic atrophy induced by 2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD) and bis (tri-n-butyltin) oxide (TBTO). Int J Immunopharmacol 13(4):369–377 [DOI] [PubMed] [Google Scholar]
- Vecchi A, Mantovani A, Sironi M, Luini W, Cairo M, Garattini S (1980) Effect of acute exposure to 2,3,7,8-tetrachlorodibenzo- p-dioxin on humoral antibody production in mice. Chem Biol Interact 30(3):337–342 [DOI] [PubMed] [Google Scholar]
- Vos JG, Moore JA (1974) Suppression of cellular immunity in rats and mice by maternal treatment with 2,3,7,8-tetrachlorodibenzo- p-dioxin. Int Arch Allergy Appl Immunol 47(5):777–794 [DOI] [PubMed] [Google Scholar]
- Walisser JA, Glover E, Pande K, Liss AL, Bradfield CA (2005) Aryl hydrocarbon receptor dependent liver development and hepato-toxicity are mediated by different cell types. Proc Natl Acad Sci USA 102(49):17858–17863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright EJ, De Castro KP, Joshi AD, Elferink CJ (2017) Canonical and non-canonical aryl hydrocarbon receptor signaling pathways. Curr Opin Toxicol 2:87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Shortman K (2005) Heterogeneity of thymic dendritic cells. Semin Immunol 17(4):304–312. https://doi.Org/10.1016/j.smim.2005.05.001 [DOI] [PubMed] [Google Scholar]
- Yeste A, Nadeau M, Burns EJ, Weiner HL, Quintana FJ (2012) Nano particle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 109(28):11270–11275. 10.1073/pnas.1120611109 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


