SUMMARY
The angiotensin II (AngII) type 1 receptor (AT1R) is a critical regulator of cardiovascular and renal function and is an important model for studies of G-protein-coupled receptor (GPCR) signaling. By stabilizing the receptor with a single-domain antibody fragment (“nanobody”) discovered using a synthetic yeast-displayed library, we determined the crystal structure of active-state human AT1R bound to an AngII analog with partial agonist activity. The nanobody binds to the receptor’s intracellular transducer pocket, stabilizing the large conformational changes characteristic of activated GPCRs. The peptide engages the AT1R through an extensive interface spanning from the receptor core to its extracellular face and N terminus, remodeling the ligand-binding cavity. Remarkably, the mechanism used to propagate conformational changes through the receptor diverges from other GPCRs at several key sites, highlighting the diversity of allosteric mechanisms among GPCRs. Our structure provides insight into how AngII and its analogs stimulate full or biased signaling, respectively.
In Brief
Structural analysis of the interactions between the angiotensin receptor and a partial agonist shows how peptide ligands induce receptor activation.
Graphical Abstract

INTRODUCTION
G-protein-coupled receptors (GPCRs) are the largest family of transmembrane proteins in humans, and these receptors serve as important regulators of almost every aspect of human physiology. The angiotensin II type 1 receptor (AT1R) is a prototypical GPCR best known for its central role in maintaining cardiovascular and renal homeostasis (Karnik et al., 2015; Kawai et al., 2017). Binding of the AT1R’s endogenous agonist, the eight-residue peptide angiotensin II (AngII), triggers AT1R activation of the heterotrimeric G protein Gq, which in turn activates calcium signaling that culminates in vasoconstriction. The AT1R’s contribution to hypertension and related pathologies has made it a prominent therapeutic target, and non-peptide antagonists called angiotensin receptor blockers (ARBs) are used by over 5% of the adult population in the United States (Kantor et al., 2015). In addition to signaling through Gq, the AT1R also interacts with other G protein isoforms as well as GPCR kinases (GRKs) and β-arrestins (Kawai et al., 2017). β-arrestins terminate G protein stimulation both by competing with G proteins for the same binding pocket on the receptor’s intracellular side and by recruiting endocytic machinery, but they can additionally initiate signaling pathways in their own right (Peterson and Luttrell, 2017).
In recent years, high-resolution structures of several GPCRs in both inactive, antagonist-bound, and activated, agonist-bound conformations have revealed that binding of chemically diverse agonists to the extracellular “orthosteric” pocket of their cognate receptors culminates in highly conserved conformational changes at the intracellular surface of the receptor. However, given the very small number of such matched pairs of inactive and active structures available, it has been difficult to ascertain the generality of the molecular mechanisms used to propagate conformational changes from the ligand-binding pocket to the intracellular regions. Another intriguing aspect of GPCR activation is that certain “biased agonists” selectively promote the coupling of particular transducers (e.g., G proteins versus β-arrestins). Elucidation of the mechanisms by which biased agonists achieve these distinct functional profiles will require the structural characterization of receptor-ligand pairs, which have clear structure-activity relationships.
Notably, mutation of even one or two residues in the AngII peptide can have profound effects on signaling efficacy and bias (Domazet et al., 2015; Holloway et al., 2002; Wei et al., 2003). Since β-arrestin-mediated signaling downstream of the AT1R results in increased cardiomyocyte inotropy and anti-apoptotic effects (Ahn et al., 2009; Rajagopal et al., 2006), β-arrestin-biased AT1R agonists (deficient in coupling to Gq but retaining β-arrestin coupling) have been pursued as alternatives to ARBs for certain heart failure patients (Cotter et al., 2018; Ryba et al., 2017). In our accompanying manuscript, we use double electron-electron resonance (DEER) spectroscopy to demonstrate that AngII and biased agonists differentially modulate the intra-cellular conformational ensemble of the AT1R and to map key features of the multiple agonist-stabilized conformations observed (Wingler et al., 2019, in this issue of Cell). While DEER and other spectroscopic techniques provide powerful insights into conformational dynamics, they can only probe specific labeled sites. High-resolution structures are essential to provide a global view of the receptor’s conformation and to define the structural states of regions that are inaccessible to DEER spectroscopy, including the core of the receptor. This is of critical importance as the receptor’s core region propagates allosteric changes from the ligand binding site to the intracellular surface.
Receptors bound to peptide ligands are difficult to crystallize, and only a single rhodopsin-like peptide receptor (the μ opioid receptor) has been characterized in both inactive and active, peptide-bound states (Koehl et al., 2018; Manglik et al., 2012). Currently, the only crystal structures of the AT1R reported are those of an inactive conformation of the receptor bound to small-molecule antagonists, which have no appreciable chemical similarity to peptide agonists (Zhang et al., 2015a; Zhang et al., 2015b). Since these antagonists engage the receptor in a fundamentally different binding mode from that of peptides (Unal et al., 2010), our understanding of how peptide agonists activate the AT1R is based exclusively on models developed from extensive mutagenesis, biochemical, and pharmacological data (Fillion et al., 2013; Karnik et al., 2015).
Conformation-stabilizing antibody fragments have proved to be powerful tools for addressing the challenges of crystallizing agonist-bound GPCRs (Manglik et al., 2017). Using a recently developed synthetic camelid antibody fragment (“nanobody”) discovery platform (McMahon et al., 2018), we obtained a high-affinity, conformationally specific nanobody that enabled us to determine the 2.9-Å resolution crystal structure of active-state AT1R bound to the peptide Sarcosine1,Isoleucine8-AngII (S1I8), an AngII analog with partial agonist activity. This structure provides a basis for understanding how AngII and its analogs interact with the AT1R and a framework for characterizing the conformational changes that result from agonist binding.
RESULTS
Discovery of a Conformationally Specific AT1R Nanobody
GPCRs are highly dynamic, and our DEER study shows the AT1R exhibits striking conformational heterogeneity that is amplified by the binding of AngII and its derivatives (Wingler et al., 2019). Due to this inherent plasticity, crystallization of most GPCRs in their activated conformations is only possible in the presence of a stabilizing reagent (Kang et al., 2015; Rasmussen et al., 2011b). Nanobodies, the isolated VHH domains from camelid heavy chain-only antibodies, are a privileged scaffold for this application. This has been attributed to their long complementarity-determining region (CDR) loops, their small size, and their biochemical tractability (Manglik et al., 2017). However, there are technical and logistical difficulties inherent to immunization-based approaches. Challenges include the poor stability of many antigens (such as GPCRs) in serum, the inability to target conserved epitopes due to immunological tolerance, and the long turnaround time and high costs associated with large animal experiments. In the case of the AT1R, repeated immunization attempts did not yield conformationally selective nanobodies. To address such bottlenecks, we recently developed a library of synthetic nanobodies displayed on the surface of Saccharomyces cerevisiae (McMahon et al., 2018).
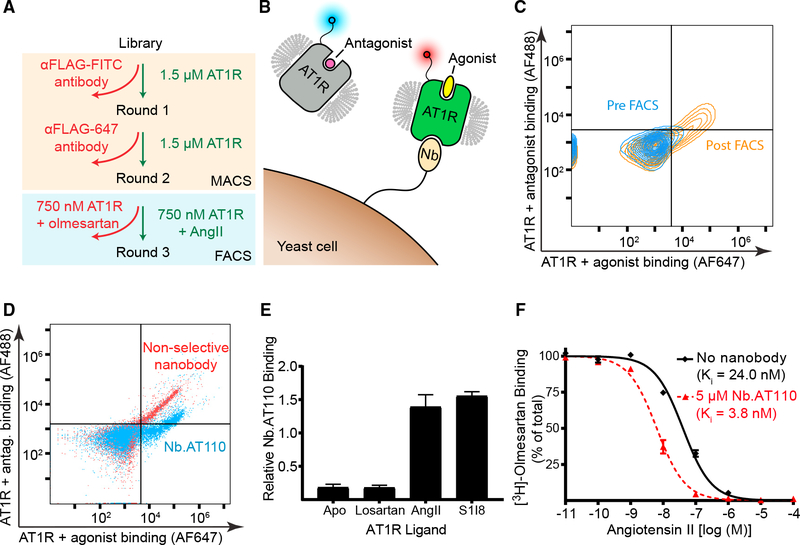
To enrich for AT1R-binding nanobodies from the naive library’s 5 × 108 clones, we performed two rounds of magnetic-activated cell sorting (MACS) using as an antigen FLAG-tagged full-length AT1R labeled with a fluorophore-conjugated anti-FLAG antibody (Figure 1A). We then performed fluorescence-activated cell sorting (FACS) to enrich for yeast displaying conformationally selective nanobodies. To do this, we simultaneously stained the MACS-enriched nanobody yeast with two preparations of the AT1R, one bound to the inverse agonist olmesartan and the other bound to the endogenous agonist AngII (Figure 1B). After FACS enrichment, a number of clones exhibited selectivity for the agonist-bound receptor (Figures 1C, 1D, and S1A). Clone Nb.AT110 exhibited the highest degree of specificity for agonist-bound AT1R, and it retains this exquisite specificity as a purified protein, co-immunoprecipitating with the AT1R only in the presence of agonists such as AngII (Figure 1E). Furthermore, Nb.AT110 increased the affinity of AngII for the AT1R approximately 6-fold, displaying the reciprocal cooperativity expected for an allosteric interaction (Figure 1F; Table S1) (De Lean et al., 1980). Despite these favorable properties, preliminary crystallographic experiments using Nb.AT110 in complex with AT1R were unsuccessful.
Figure 1. Discovery of Synthetic Nanobodies that Selectively Target Agonist-Bound AT1R Using Yeast Display.
(A) Flowchart of selection process. Binders to AT1R from the naive yeast-displayed nanobody library were enriched over two rounds of selection by performing magnetic-activated cell sorting (MACS) on yeast that were stained with purified unliganded AT1R. For round 3, yeast were simultaneously stained with agonist-bound and antagonist-bound AT1R, and fluorescence-activated cell sorting (FACS) was performed to enrich for yeast clones that preferentially interacted with agonist-bound AT1R.
(B) Cartoon representation of FACS selection for yeast displaying agonist-bound AT1R specific nanobodies. Yeast were stained with antagonist (olmesartan)-bound or agonist (AngII)-bound AT1R, each labeled (through dye-conjugated anti-FLAG antibody) with a different fluorophore.
(C) Flow cytometry plot indicating that an increased proportion of yeast interact with agonist (AngII)-bound AT1R following FACS selection.
(D) Flow analysis demonstrating the selective binding of yeast displaying the nanobody Nb.AT110 (blue) to agonist-bound (x axis) versus antagonist-bound (y axis) AT1R. A non-conformationally selective AT1R clone (red) is shown for comparison.
(E) Co-immunoprecipitation of purified Nb.AT110 with purified FLAG-AT1R shows its specificity for receptor bound to peptide agonists (the endogenous ligand AngII and the partial agonist S1I8) compared to unliganded (Apo) or antagonist (losartan)-bound AT1R. Data represent the pixel intensity of the nanobody band in a Coomassie-blue-stained gel relative to the co-eluted receptor. Error bars represent the SEM from three independent experiments performed as single replicates.
(F) Nb.AT110 allosterically increases the binding of AngII to the AT1R by competition radioligand binding. Ki values are provided in Table S1. Error bars represent the SE from at least three independent experiments performed as single replicates.
We reasoned that Nb.AT110 did not have sufficiently high affinity for agonist-bound AT1R to permit crystallization of the complex, and so we used yeast display to affinity mature a more suitable clone. Nb.AT110 DNA was mutagenized by error-prone PCR and then transformed into yeast to create a new library of1.5 × 108 clones. High-affinity clones were enriched by performing two rounds of FACS, selecting for cells that robustly bound progressively lower concentrations of AngII-AT1R while maintaining high nanobody expression (Figure 2A). Following this selection, 50 clones were sequenced, revealing a set of strongly enriched mutations (Figure 2B). These four mutations were introduced into Nb.AT110 to create a consensus variant called Nb.AT110i1 (Figure S1B). Nb.AT110i1 showed a strong affinity enhancement relative to Nb.AT110, with an approximately 10-fold improvement measured by on-yeast binding (Figure 2C) and a greater than 100-fold increase when assessed in recombinant form through an allosteric shift assay (Figures 2D, S1C, and S1D; Table S1).
Figure 2. Affinity Maturation of Nb.AT110.
(A) A yeast display library generated by error-prone PCR of Nb.AT110 demonstrated increased binding to AngII-bound AT1R following two FACS selection rounds by staining with 50 nM AT1R (round 1) and then 10 nM AT1R (round 2).
(B) Sequence analysis of 50 randomly picked yeast colonies following FACS affinity enrichment reveals mutations enriched in the library. Thirty-six unique sequences were obtained from the 50 sequenced yeast colonies. Four of the most prevalent mutations, A31V, N58D, I98V, and Y113N, were combined to generate Nb.AT110i1.
(C) Compared to Nb.AT110 (red), affinity-matured variant Nb.AT110i1 (blue) has a higher on-yeast affinity for AngII-bound AT1R. Error bars represent the SEM from three concurrent measurements for each concentration of antigen.
(D) Compared to Nb.AT110, purified Nb.AT110i1 shows a greater allosteric enhancement of the affinity of the agonist TRV055 at lower nanobody concentrations. The lower-affinity agonist TRV055 was used rather than AngII to better visualize the dynamic range of the shift, which is limited by the radioligand tracer’s affinity. Error bars represent the SE from at least three independent experiments performed as single replicates. Ki values are provided in Table S1.
Nanobody-Stabilized Structure of the AT1R
Previously reported structures of the AT1R bound to antagonist were obtained with a receptor construct containing a C-terminal truncation at the end of helix 8, a deletion of the N terminus from residues 7–16, and an N-terminal insertion of thermostabilized apocytochrome b562RIL (BRIL) (Zhang et al., 2015a, 2015b). However, we found that these modifications decrease the AT1R’s affinity for AngII by over two orders of magnitude (Figure S2A; Table S2). Therefore, we developed an alternative crystallization construct without the N-terminal deletion, where BRIL was instead inserted into the third intracellular loop (ICL3), as described for other GPCRs(Rosenbaum et al., 2007) (Figure S2B). This new crystallization construct has a 2-fold increase in AngII affinity compared to wild-type receptor (Figure S2A; Table S2), and it retains strong binding to Nb.AT110i1 (Figures S2C–S2E).
Since ligand affinity is often a critical determinant for obtaining diffraction-quality crystals of GPCRs (Ring et al., 2013), complexes between AT1R and Nb.AT110i1 were formed in the presence of the AngII analog S1I8. This high-affinity peptide ligand is a partial agonist of both Gq- and β-arrestin-mediated activities (Figures S2F–S2H) (Domazet et al., 2015; Miura and Karnik, 1999), and it promotes binding of our agonist-selective nanobodies (Figure 1E). The purified AT1R crystallization construct was incubated with S1I8 peptide, followed by the addition of Nb.AT110i1. The resulting complex was purified by size exclusion chromatography (Figures S2D and S2E) and yielded crystals via the lipidic mesophase method (Caffrey, 2009).
Diffraction data from 5 crystals were merged to compile a dataset at 2.9Å resolution, and the structure was solved by molecular replacement using the structures of ZD7155-bound AT1R (Zhang et al., 2015b) and a nanobody (Rasmussen et al., 2011a) as search models (Figures 3A and S3A–S3C; Table S3). Nb.AT110i1 binds to the intracellular side of the AT1R, inserting its unusually long CDR3 into the transducer-binding pocket (Figure 3A), a binding mode highly similar to that of nanobodies stabilizing the active conformations of other GPCRs (Figure S3D) (Kruse et al., 2013; Rasmussen et al., 2011a). The asymmetric unit consists of two copies of the AT1R and four nanobodies. The two additional nanobodies in each unit cell are poorly resolved and do not interact with the receptor, but each forms a parallel dimer with a receptor-bound nanobody through a salt bridge and β sheet contacts. The two receptor copies make contacts at the extracellular ends of transmembrane domains (TMs) 2 and 3 and the intracellular end of TM1 (Figure S3A). The inactive structures of the AT1R do not exhibit such an interface (Zhang et al., 2015a, 2015b), but evidence for AT1R dimerization has been found in a cellular context, where homo-dimers are reported to carry out β-arrestin-mediated but not Gq-mediated functions (Hansen et al., 2004; Porrello et al., 2011). As has been observed in other GPCR structures (Rasmussen et al., 2011a), density for the BRIL in ICL3 could not be resolved, suggesting that it remains flexible in the crystal lattice and does not contribute to packing.
Figure 3. Conformational Changes in the Nb.AT110i1-Stabilized AT1R.
(A) Overall structure of the active-state AT1R (orange) in complex with partial agonist S1I8 (magenta) and Nb.AT110i1 (green).
(B) Conformational changes in active AT1R (orange) compared to the inactive structure of inverse agonist (ZD7155)-bound AT1R (blue, PDB: 4YAY). Extracellular changes include the inward movements of TMs 5 and 7, and intracellular changes include the outward movement of TMs 5 and 6, the inward movement of TM7, and the repositioning of helix 8 parallel to the membrane.
See also Figures S2 and S3 and Tables S2 and S3.
Alignment of the active and inactive structures of AT1R demonstrate conformational changes in regions that are characteristic of activated GPCRs (Figure 3B) (Manglik and Kruse, 2017). On the intracellular side, most notable is an 11Å outward displacement of TM6, accompanied by the rotation of TM5 away from the transducer binding pocket and an inward rotation of TM7. Additional intracellular changes in the active AT1R include reorganization of the second intracellular loop (ICL2) to form a short α helix and a substantial repositioning of helix 8. In the inactive AT1R structures, helix 8 adopts an unusual conformation bent away from the membrane plane, whereas the active structure reported here shows helix 8 lying in a conventional position parallel to the membrane near the interface between TM1 and TM7 (Figure 3B). Since the AT1R lacks the palmitoylation site that tethers the C-terminal end of helix 8 to the membrane in most GPCRs (Escribá et al., 2007), this discrepancy could reflect either intrinsic flexibility of helix 8 or the effects of crystallization conditions and lattice packing. Molecular dynamics simulations and DEER spectroscopy indicate helix 8 is likely intrinsically dynamic in the AT1R (Wingler et al., 2019), supporting the former possibility.
Rearrangements of highly conserved structural motifs accompany these large-scale conformational changes. Y3027.53 (superscripts indicate Ballesteros-Weinstein numbering for conserved GPCR residues [Ballesteros and Weinstein, 1995]), located at the end of the NPxxY motif at the intracellular base of TM7, has rotated 10Å inward, as measured from the phenolic hydroxyl, which likely hydrogen bonds to Y2155.58 in TM5 via a bridging water molecule (Figure 4A). This interaction is believed to play a key role in stabilizing the structural rearrangements in activated GPCRs (Fritze et al., 2003). In the inactive state of GPCRs, R3.50 of the DRY motif at the end of TM3 typically forms an “ionic lock” with E/D6.30 to stabilize the inward conformation of TM6 (Ballesteros et al., 2001). While the AT1R lacks an acidic residue at position 6.30, in the inactive structures R1263.50 is positioned near N2356.30, suggesting a polar interaction with the unresolved side chain. In the active structure, R3.50 participates in a hydrogen bonding network that also involves D1253.49, R137ICL2, R140ICL2, and D112 in CDR3 of Nb.AT110i1 (Figure 4B). At the center of CDR3, the nanobody residue D108 engages in a network of polar interactions with backbone amides in the rest of the loop (Figure 4C). N113 of the nanobody, which was introduced during affinity maturation, also interacts with D108 and may serve to further rigidify the large CDR3 loop. In addition to this interaction, several aromatic residues in CDR3 face into the receptor core, forming extensive interactions with receptor hydrophobic residues (Figure 4C). Another residue introduced in affinity maturation, D58 in CDR2, interacts with K135ICL2 (Figure S4) and forms a salt bridge with R45 in the adjacent nanobody, potentially contributing to the dimerization of Nb.AT110i1.
Figure 4. Rearrangement and Nanobody Stabilization of Conserved Intracellular Activation Motifs.
(A) Compared to inactive AT1R (PDB: 4YAY), Y3027.53 of the NPxxY motif rotates to a position likely capable of forming a hydrogen bond to Y2155.58 via a bridging water molecule.
(B) Following the outward displacement of TM6, D112CDR3 stabilizes the DRY motif of TM3 through an extensive ionic network also involving two arginine residues from ICL2.
(C) The side Nb.110i1 (green) side chains D108CDR3 and N113CDR3, a mutation introduced during affinity maturation, establish a network of polar interactions that stabilize the conformation of Nb.AT110i1’s long CDR3. Several aromatic residues in CDR3 point into an exposed groove in the receptor core (orange).
See also Figure S4.
Importantly, the active-state AT1R structure shows good accordance with an AT1R conformation mapped by DEER spectroscopy—specifically, a conformation that is populated detectably only upon binding to agonists with efficacy through the Gq pathway (Wingler et al., 2019). The DEER studies employed receptor in solution bound only to orthosteric ligands, without stabilizing antibody fragments. The high degree of concordance between these two methods thus argues, first, that neither Nb binding nor crystallization introduced major artifacts in our structure and, second, that our structure reveals a physiologically relevant conformation important in establishing biased signaling at the AT1R.
Peptide Binding Mode
S1I8 interacts with the AT1R through a series of polar and nonpolar contacts across its length (Figure 5A). The peptide binds in an extended conformation, with its N terminus facing the solvent through a narrow opening at the extracellular face of the receptor and its C terminus buried deep within the receptor core (Figures 5B and 5C). The binding pocket is shaped by essential contributions from both the transmembrane helices and the highly structured extracellular loops (ECLs). The inactive AT1R structures previously showed that ECL2 forms a β-hairpin (Zhang et al., 2015b), but the active AT1R structure reveals a far more intricate ordering of the extracellular surface of the receptor (Figure 5C). The receptor N terminus wraps across the top of the receptor and is secured to the top of TM7 by a non-canonical disulfide bridge (C18-C2747.25). The N terminus adds a third strand to ECL2’s two anti-parallel β strands. The first three residues of the peptide ligand provide a fourth strand that creates a half-β-barrel. The intimate engagement of the peptide with the receptor’s N terminus is consistent with previous cross-linking studies (Fillion et al., 2010) and likely accounts for the severely attenuated peptide binding in the previously reported N-terminal BRIL fusion (Zhang et al., 2015a). It also explains the lesser impact this modification has on TRV055, an AngII analog with the first residue deleted (Figure S2A; Table S2), since this residue has the most contacts with the receptor N terminus. The side chain of H183ECL2 is positioned to hydrogen bond with the peptide’s N terminus, and in AngII the aspartate in the first position may engage in a charge-charge interaction with H183ECL2, in accordance with previous mutagenesis data (Feng et al., 1995). Several acidic residues also surround the opening to the ligand-binding pocket, and the high density of negative charge likely accounts for the overall increase in ligand affinity that results from substituting sarcosine for D1 in AngII analogs (Fillion et al., 2010).
Figure 5. Binding Mode of S1I8.
(A) Schematic diagram of interactions between the S1I8 peptide (pink) and the AT1R (black). Hydrophobic interactions are indicated in yellow, and polar contacts are shown with dashed black lines. Note that some interactions could not be shown due to the limitations of two-dimensional representation of the three-dimensional binding site.
(B) Extended conformation of S1I8 (magenta) in the ligand-binding pocket of the AT1R (orange), with the N terminus facing outward from the extracellular face of the receptor and the C terminus located deep within the receptor core.
(C) The peptide backbone of S1I8 (magenta) joins with three beta strands from AT1R, one contributed by the N terminus (yellow) and two from the ECL2 β-hairpin (orange), to form a half β-barrel. Two disulfide bonds (green) stabilize the N terminus and ECL2.
(D) Electron density of S1I8 (pink mesh) and surrounding residues (blue mesh), shown as a 2Fo-Fc simulated annealing composite omit map contoured at 1.2 σ.
(E) Key hydrogen bonding networks between S1I8 (pink) side chains and the AT1R. Polar residues are shown in cyan and non-polar in yellow.
The clear and continuous electron density of S1I8 allows its interactions with the receptor to be modeled unambiguously (Figure 5D). The peptide-receptor interaction shows striking asymmetry, with a clear division of polar and non-polar interactions on opposite sides of the binding pocket (Figures 5A, 5D, and 5E). W842.60, V1083.32, L1123.36, and I2887.38 sit at the bottom of the orthosteric binding pocket near the center of the receptor, interacting with P7 and I8 of S1I8 (F8 in AngII). I8 of the peptide also engages in a more limited hydrophobic contact with another residue critical for AT1R activation, H2566.51 (Noda et al., 1995). Moving upward, the peptide’s I5 and V3 side chains point in the same direction and engage in hydrophobic interactions with Y92ECL1, I172ECL2, V179ECL2, and A181ECL2. In contrast, peptide residues R2, Y4, H6, and the terminal carboxylate are pointed toward the opposite side of the binding pocket and interact with the receptor through a network of polar interactions (Figures 5A, 5D, and 5E). S1I8’s R2 guanidinium moiety engages in polar contacts with D2817.32 and D2636.58 as previously predicted (Feng et al., 1995; Fillion et al., 2013), but this network further incorporates H6 of S1I8. At the base of the ligand-binding pocket, the terminal carboxylate of residue 8 in S1I8 interacts with K1995.42, shown to be a key contact involved in AT1R activation by mutagenesis studies (Fillion et al., 2010; Noda et al., 1995). The carboxylate also interacts with the phenolic hydroxyl of Y4 in the ligand.
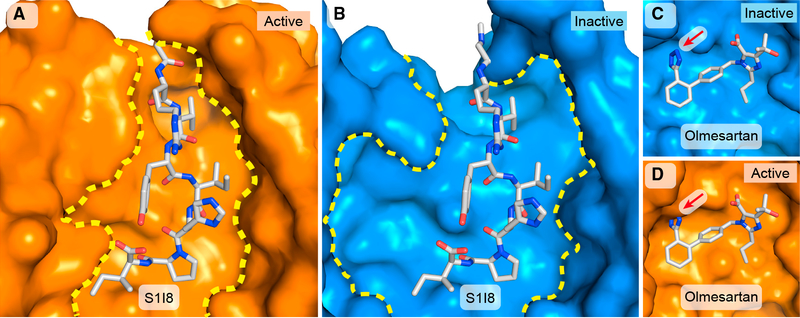
Relative to the inactive structures, the ligand-binding pocket in the active-state receptor is substantially constricted around the peptide (Figures 6A and 6B). This is primarily due to inward shifts of TMs 5 and 7 (Figure 3B) as well as changes in ECL2. The peptide binding mode contrasts starkly with that of ARBs, which occupy a smaller volume at the bottom of the ligand-binding groove. The pocket’s remodeling in the active structure creates clear incompatibilities with ARB binding (Figures 6C and 6D), which may account for the inverse agonism displayed by many ARBs (Van Liefde and Vauquelin, 2009). In particular, the position of the F182ECL2 side chain in the active state would lead to a steric clash with the crystallographically observed position of the tetrazole pharmacophore present in most AT1R antagonists (Figure 6D).
Figure 6. Remodeling of the AT1R’s Ligand-Binding Pocket by S1I8.
(A and B) Constriction of the AT1R ligand-binding pocket (yellow dotted-line) around S1I8 (gray sticks). The same view of the aligned structures of (A) active AT1R (orange surface) bound to S1I8 and (B) inactive AT1R (blue surface, PDB:4YAY) with S1I8 superimposed are shown with TM6, TM7, and Lys199 removed for clarity.
(C and D) Incompatibility of the active AT1R with ARB binding. The tetrazole moiety (red arrow) of olmesartan (gray sticks) fits neatly into the larger pocket of(C) inactive AT1R (blue surface), but, when superimposed at the same position in (D) active AT1R (orange surface), the tetrazole moiety clashes with the constricted pocket. The same view of aligned active and inactive (PDB: 4ZUD) AT1R are shown with olmesartan in both panels for reference.
Mechanism of AT1R Activation
Interactions between the AT1R and S1I8—particularly I8—initiate a cascade of conformational changes that are propagated through the receptor core to the intracellular regions (Figure 7A). Compared to the inactive AT1R structures, K1995.42 moves in toward the carboxylate of I8, while the aliphatic I8 side chain forces W2536.48 and Y2927.43 downward to avoid a steric clash (Figure 7B). These changes are linked to a conformational lock immediately below, causing F2496.44 and F2506.45 to ratchet past F2085.51 (Figure 7C). This rotation creates a void that N2957.46 fills by moving inward, breaking a hydrogen bond with N1113.35 present in the inactive structure (Figure 7D). Finally, the outward movement of TM6 and inward movement of TM7 are stabilized by the hydrogen bond between Y2155.58 and Y3027.53 (of the NPXXY motif, Figure 4A).
Figure 7. Activation Mechanism of AT1R.
(A) Active AT1R (orange) superimposed on the structure of inactive AT1R (blue, PDB: 4ZUD), highlighting the cascade of conformational changes induced by the peptide ligand.
(B) Initiation of AT1R activation. I8 of S1I8 (magenta) forces nearby residues to move to avoid a steric clash. Major conformational changes are indicated with arrows.
(C) Phenylalanine ratchet rearrangement in active AT1R defines discrete inactive and active conformational states.
(D) Outward rotation of N2947.45 and N2957.46 upon activation of AT1R. The side-chain rotamer in N2957.46 in the inactive conformation (PDB: 4ZUD) is flipped to account for hydrogen bonding.
(E) The Y292F mutation (orange) enhances the affinity of AngII compared to wild-type AT1R (black), likely because the mutation eliminates or reduces potential steric clashes with the peptide F8. Error bars represent the SE from three independent experiments performed in duplicate using membranes from Expi293F cells overexpressing Flag-tagged AT1R constructs. Ki values are provided in Table S4.
This model of AT1R activation is quite distinct from the mechanisms observed in other active-state GPCRs but is supported by many lines of evidence in addition to the structure reported here. First, the angiotensin II type 2 receptor (AT2R), the GPCR with the most homology to the AT1R, lacks many of these key conformational locks and assumes an active-like conformation when crystallized with either small molecule ligands or the S1I8 peptide (Asada et al., 2018; Zhang et al., 2017). The AT2R is highly unusual in that it does not appear to signal through typical G-protein- and b-arrestin-mediated pathways, potentially due to steric blockade of the transducer-binding pocket by helix 8 (Zhang et al., 2017). Y2927.43 is substituted by F3087.43 in the AT2R, and an AT1R Y292F mutation increases the affinity of AngII while decreasing the affinity of antagonists (Figures 7E and S5A; Table S4), indicative of a mutation-induced stabilization of an active or active-like state. AT2R also lacks the TM5/TM6 phenylalanine ratchet (AT1R F2085.51/F2496.44/F2506.45) due to aliphatic substitutions at two of these sites (AT2R L2245.51/F2656.44/I2666.45) that would not create a kinetic barrier to rotation. In both the small-molecule and S1I8-bound AT2R structures, these residues align with active rather than inactive AT1R. The N1113.35-N2957.46 hydrogen bond stabilizing the AT1R inactive state is not present in the AT2R structures, which instead have a serine at this position (S3117.46), while N3.35 is rotated away from the receptor core. Interestingly, N3.35 and S7.46 are well-conserved residues among family A GPCRs that form a sodium-binding site, allowing this ion to allosterically stabilize the inactive receptor state (Katritch et al., 2014). The replacement of S7.46 with Asn in the AT1R suggests an alternative mechanism that obviates the need for sodium regulation entirely. In fact, sodium does not appear to allosterically modulate AngII binding to wild-type AT1R, but disruption of the N1113.35-N2957.46 hydrogen bond with an N111A substitution makes AngII binding sodium sensitive (Zhang et al., 2015a). Conversely, in the dopamine D2 receptor, mutation of S4207.46 to Asn abrogates sodium regulation of ligand binding while enhancing the affinity of antagonists and decreasing the affinity of agonists (Neve et al., 2001). The importance of N1113.35 in stabilizing the inactive conformation of the AT1R is further underscored by the fact that N111G and N111A mutants are well-characterized constitutively active mutants (Noda et al., 1996). Taken together, these results define a model in which the side chain of N7.46 functionally substitutes for a sodium ion, serving as a conformational stabilizer and rendering the receptor sodium insensitive.
Our model demonstrates the pivotal role of I8 in initiating conformational changes, which provides insights into the functional profile of biased AngII analogs. Altering the size of the side chain of this residue has profound effects on the relative strength of signaling through the β-arrestin and G protein pathways (Domazet et al., 2015; Rajagopal et al., 2011; Zimmerman et al., 2012). The I8 substitution of S1I8 converts it into a partial agonist for Gq signaling, while AngII and other full agonists that efficiently activate Gq end with F8. The most strongly β-arrestin-biased ligands (deficient in Gq-mediated functions but retaining β-arrestin-mediated functions) have side chains even smaller than I8 (e.g., Ala) or even deletions of position 8 (Rajagopal et al., 2011; Strachan et al., 2014). Our companion DEER study further shows that AT1R ligands with F8 stabilize a conformation that matches well with our active-state structure at all sites probed, but β-arrestin-biased ligands stabilize conformations that lack the full complement of changes seen in the canonical GPCR active state (Wingler et al., 2019). In our structure the I8 side chain fits closely into a cavity primarily shaped by TM3 (Figure S5B). The cavity is slightly too small to accommodate the side chain of F8 of AngII (Figure S5C), suggesting the phenyl ring would induce additional disruption of the AT1R’s core (Cabana et al., 2013). Conversely, smaller side chains or deletions of residue 8 would not clash with the inactive-state position of Y2927.43 that plays a key role in initiating the cascade of conformational changes.
DISCUSSION
Conformation-specific nanobodies have become invaluable tools in GPCR biology (Manglik et al., 2017) as well as many other areas of research (De Meyer et al., 2014). However, obtaining these reagents via llama or alpaca immunization is difficult and often unsuccessful. Our failure to obtain selective AT1R nanobodies despite several years of immunization efforts may reflect the instability of purified AT1R in blood, tolerance to self antigens (AT1R sequence is 96% identical between human and alpaca), or the presence of immunodominant epitopes that prevent development of conformation-specific antibody clones. Irrespective of the cause, our lack of success with animal immunization in this and other research projects motivated us to create a synthetic yeast surface-displayed library for in vitro nanobody discovery (McMahon et al., 2018). Using this platform, we were able to obtain multiple nanobodies that recognize the AT1R, including the intracellular active-state stabilizing clone Nb.AT110. An affinity-matured derivative of this nanobody quickly led to crystals of the activated receptor and enabled structure determination. This overall approach may be broadly useful in determining structures of GPCRs and other dynamic proteins in defined conformations.
The AT1R is a critically important receptor not only for its physiological and therapeutic roles but also as a model for understanding GPCR signaling, trafficking, and biased agonism. Due to the size and flexibility of AT1R peptide ligands and the major conformational changes they induce in the orthosteric pocket, it has not been possible to develop a reliable model from inactive-state structures of how peptides bind to and activate the AT1R. While previous efforts to map the receptor-peptide binding interface by site-directed mutagenesis were quite successful in predicting most key interactions (reviewed by Balakumar and Jagadeesh, 2014), we observe features that could not have been readily predicted in mutagenesis studies. One example is the formation of a partial β-barrel comprising the receptor N terminus, the ECL2 β-hairpin, and the N terminus of the peptide ligand itself. This particular binding mode used by the AT1R has not yet been observed in other peptide-bound GPCRs, though several other peptides were shown to add a β strand to receptors’ extracellular regions (Liu et al., 2018; Ma et al., 2017), pointing to β strand joining as a commonly used strategy for ligand engagement among peptidergic receptors.
Together with previously published inactive AT1R structures, our structure provides the first view of both inactive and active conformations of a receptor from an important subfamily of rhodopsin-like GPCRs that also includes chemokine receptors. Unexpectedly, several of the conformational locks that stabilize the active and inactive states of the AT1R have not been observed in other active-state GPCRs, including the phenylala-nine ratchet between TMs 5 and 6 and profound alterations in the sodium-binding site. This points to the diversity of mechanisms used to propagate information from the ligand-binding site to the intracellular regions across the broader GPCR family and underscores the fact that not all features identified in the well-studied biogenic amine receptors and their relatives are generalizable across the GPCR family.
The AT1R is a particularly informative model GPCR for studies of biased signaling due to the fact that simple mutations within the short, easily synthesized AngII octapeptide have clear effects on the balance of signaling pathways. Our structure shows that the eighth, C-terminal residue—which has the strongest influence on signaling bias—makes key contacts that initiate a cascade of conformational changes through the receptor, providing a molecular rationale for the functional profiles of AT1R biased ligands. We anticipate additional active-state AT1R structures and molecular dynamic simulations will further clarify how various ligands alter the structure and dynamics of the ligand-binding site. Notably, our DEER analysis demonstrates the AT1R exhibits a high degree of conformational plasticity in the intracellular regions and samples several distinct activated states that contribute to biased agonists’ functional profiles. The AT1R conformation observed in this crystal structure best accords with the conformation observed in our DEER studies that is detectable only upon binding to agonists that promote Gq coupling (Wingler et al., 2019). A key priority for future research will be to characterize other ligand-stabilized conformations—particularly those stabilized by β-arrestin-biased agonists—structurally and functionally and to better understand the mechanisms by which changes in ligand structure alter the AT1R’s conformational ensemble and coupling to transducers. We anticipate the approach presented here, discovering nanobodies stabilizing defined receptor conformations through in vitro selection, will aid this endeavor not only for the AT1R but also for other dynamic proteins.
STAR★METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Requests for further information or reagents may be directed to the Lead Contact, Andrew C. Kruse (andrew_kruse@hms.harvard.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Expi293F cells and their derivatives were maintained at 37°C and 8% CO2 in a humidified atmosphere, with shaking at 110 rpm in Expi293F expression media. Lines with stably integrated tetracycline repressor (pcDNA/TR) were maintained in the presence of 10 μg/mL blasticidin, and lines with stably integrated pcDNA-Zeo-tetO-AT1R constructs were maintained with 5 μg/mL zeocin. The PathHunter U2OS EA β-Arrestin parental cell line was grown at 37°C in a humidified environment with 5% CO2 in MEM media supplemented with 10% FBS, 1% penicillin-streptomycin, 300 ug/mL hygromycin, and 2 mM glutamine. The stable rat AT1aR/HEK293 cell line was grown at 37°C in a humidified environment with 5% CO2 in MEM media supplemented with 10% FBS, 1% penicillin-streptomycin, and 100 μg/mL zeocin. All mammalian cell lines used (Expi293F, U2OS, and HEK293) are genetically female. Yeast cells of strain BJ5465 were cultured at 30°C in tryptophan dropout medium with glucose, except during induction when they were instead incubated at 25°C in tryptophan dropout medium with galactose. For isolation of clonal populations yeast were grown on agar plates of the same medium.
METHOD DETAILS
Isolation of AT1R-binding nanobodies
For the first round of magnetic-activated cell sorting (MACS), 1 × 1010 S. cerevisiae, cells expressing a surface displayed library of synthetic nanobodies (McMahon et al., 2018), were centrifuged, resuspended in binding buffer (20 mM HEPES pH 7.5, 150 mM NaCl, 2.8 mM CaCl2, 0.05% MNG, 0.005% CHS, 0.1% BSA, 0.2% maltose) and then incubated with anti-fluorescein isothiocyanate (FITC) microbeads (Miltenyi) and FITC-labeled anti-FLAG antibody for 40 min at 4°C. These yeast were then passed through an LD column (Miltenyi) to remove any yeast expressing nanobodies which interacted with the microbeads or anti-FLAG antibody. Remaining yeast that flowed through the column were centrifuged, resuspended in binding buffer, and incubated with 1 μM of FITC-labeled anti-FLAG antibody and 1.5 μM of FLAG-tagged AT1R for 1 h at 4°C. Yeast were then centrifuged, resuspended in binding buffer with anti-FITC microbeads, and incubated for 15 min at 4°C before passing them into an LS column (Miltenyi) and collecting the eluate enriched for AT1R-binding nanobodies. The eluted yeast were expanded and used in a subsequent round of MACS to further enrich for AT1R-binding nanobodies. The second round was performed similarly to the first, but beginning with 4 × 108 yeast and substituting FITC-labeled anti-FLAG antibody with AlexaFluor647-labeled anti-FLAG antibody and anti-FITC microbeads with anti-AlexaFluor647 microbeads.
In order to obtain conformationally specific binders to AT1R, 1 × 108 yeast, enriched after the second round of MACS, were stained for 20 min simultaneously with 750 nM each of AT1R bound to the antagonist olmesartan and AT1R bound to the agonist angiotensin II, labeled with anti-FLAG AlexaFluor647 and anti-FLAG AlexaFluor488 antibodies, respectively. Yeast that selectively interacted with angiotensin II-bound AT1R were isolated with FACS. Individual clones were then grown, stained in a 96-well plate, and assessed via flow cytometry for binding specificity to agonist-bound AT1R. From these clones, the highly selective nanobody, Nb.AT110, was isolated and chosen for further characterization.
Affinity maturation of Nb.AT110
Error-prone PCR was performed on Nb.AT110 DNA using the GeneMorph II Random Mutagenesis Kit (Agilent) and the resulting library was scaled up with a second PCR using Q5 High-Fidelity DNA Polymerase (New England Biolabs). One hundred mL of BJ5465 S. cerevisiae cells were grown to OD600 = 1.5 and transformed with 55 μg of the error prone library and 17 μg of linearized pYDS649 plasmid (McMahon et al., 2018) using an ECM 830 Electroporator (BTX-Harvard Apparatus). The resulting yeast display library of Nb.AT110 mutants was comprised of ~1.5 × 108 transformants which had a mean mutation rate of about 2 amino acid changes per nanobody clone.
To obtain high-affinity AT1R binders, 3 × 108 yeast from the error prone library were stained with 50 nM of AT1R labeled with anti-FLAG AlexaFluor488 antibody as well as anti-HA antibody, to assess nanobody expression levels, in binding buffer (20 mM HEPES pH 7.5, 150 mM NaCl, 3 mM CaCl2, 5 mM AngII, 0.1% MNG, 0.01% CHS, 0.1% BSA, 0.2% maltose). FACS was performed on these stained yeast with 32,832 cells collected from a total of 7 × 107 cells sorted. Yeast isolated from the first round of FACS were expanded and used for a second round to further enrich for high-affinity nanobodies. For the second FACS experiment, 1 × 108 yeast were stained iteratively with binding buffer containing 10 nM AT1R for 50 min at 4°C and then with 200 nM FITC-labeled anti-FLAG antibody for 20 min at 4°C. 2,025 cells were collected and then expanded.
In order to generate the high-affinity clone Nb.AT110i1, two approaches to sequencing were taken. Fifty colonies from the affinity maturation were randomly sequenced, revealing consensus mutations contributing to affinity. In parallel, nine clones appearing to have the highest affinity for AT1R from a 96-well staining of individual clones were sequenced, revealing the mutations associated with these high-affinity clones. These data were then used to design gBlock (Integrated DNA Technologies) DNA oligonucleotides for Nb.AT110i1 which differs from the original Nb.AT110 by four mutations (A31V, N58D, I98V, Y113N).
Nanobody purification
Nanobodies were amplified from the yeast display vector or gBlocks and ligated into pET26b, adding a C-terminal 6xHis tag to the nanobody sequence. Sequence-verified clones were transformed into T7 Express lysY BL21 E. coli. Bacteria were grown in Terrific Broth containing 1 mM MgCl2 and 0.01% glucose to an OD600 = 0.7 before induction with 1 mM IPTG. Cells were harvested after an overnight incubation at 27°C. Following osmotic shock, nanobodies were purified from the periplasmic fraction by Ni-NTA chromatography (Gold Biotechnology) and dialyzed against 20 mM HEPES pH 7.4, 100 mM NaCl to remove imidazole. For crystallography, Nb.AT110i1 was treated with a 1:100 ratio of carboxypeptidase A (Sigma) overnight at 4°C, then incubated with 100 μL of TALON cobalt affinity resin (Clontech) for 1 h and subjected to size exclusion chromatography.
AT1R expression and purification
Human AT1R with an N-terminal hemagglutinin signal sequence and Flag tag (Strachan et al., 2014) was ligated into pcDNA-Zeo-tetO (Staus et al., 2018) and stably (Fugene 6) or transiently (Expifectamine) transfected into Expi293F cells containing a stably integrated tetracycline repressor (pcDNA/TR) (Staus et al., 2018). For transient transfection, two days post-transfection, receptor expression was induced with 4 mg/mL doxycycline, 5 mM sodium butyrate, and 1 μM losartan, and cells were harvested 30 to 36 h thereafter. For stable integrants, cells were grown in the presence of 1 μM losartan to a density of 4–5 × 106 cells/mL, treated with enhancers from the Expifectamine Transfection kit, induced with doxycycline and sodium butyrate the following day, and harvested after an additional 48 h of growth. For crystallography, the thermostabilized apocytochrome b562RIL (BRIL) from E. coli (Chun et al., 2012) was inserted into the third intracellular loop between AT1R residues 226 and 227, residue I320 was mutated to a stop codon (Quikchange Lightning Site-Directed Mutagenesis kit), and cells were grown in the presence of 5 μM kifunensine.
Frozen cell pellets were lysed under hypotonic conditions in room-temperature buffer (10 mL per 1 g pellet mass) comprised of 10 mM Tris pH 7.4, 2 mM EDTA, 10 mM MgCl2, 5 units/mL benzonase, benzamidine, leupeptin, and 5 μM losartan. Cell membranes were harvested by centrifugation at 30,000 × g for 15 min and resuspended in cold solubilization buffer (10 mL/g original cell pellet mass) comprised of 20 mM HEPES pH 7.4, 500 mM NaCl, 0.5% lauryl maltose neopentyl glycol (MNG), 0.05% cholesterol hemisuccinate (CHS), 10 mM MgCl2, 5 units/mL benzonase, benzamidine, leupeptin, and 5 μM losartan. Following dounce homogenization, solubilizing membrane was stirred for a total of 2 h (at room temperature until the buffer warmed, and then at 4°C). Insoluble debris was removed by centrifugation at 30,000 × g for 30 min, and the supernatant loaded onto M1-FLAG resin with 2 mM CaCl2 over the course of 1–2 h. The resin was washed with 20 column volumes of wash buffer (20 mM HEPES pH 7.4, 500 mM NaCl, 0.01% MNG,0.01% CHS, 2 mM CaCl2, benzamidine, leupeptin), and AT1R was eluted with 20 mM HEPES pH 7.4, 500 mM NaCl, 0.01% MNG,0.01% CHS, 0.2 mg/mL FLAG-peptide, and 5 mM EDTA. Monomeric AT1R was isolated and exchanged into HNM buffer (20 mM HEPES pH 7.4, 100 mM NaCl, 0.01% MNG, and 0.001% CHS) via size exclusion chromatography (SEC) on a Superdex 200 Increase column (GE). For crystallography experiments, the receptor was treated with a 1:20 mass/mass ratio of EndoH for 90 min at room temperature prior to SEC.
AT1R-Nb.AT110i1 complex crystallization
Monomeric AT1R (30 μM) was incubated with 250 μM S1I8 at room temperature for 15 min. A two-fold molar excess of carboxypeptidase-treated Nb.AT110i1 was added, and complex formation was allowed to proceed at 4°C overnight. The AT1R-Nb complex was isolated by SEC, and additional S1I8 (200 μM) was added to fractions. The complex was concentrated to A280 = 50 and flash frozen in small aliquots.
The complex was reconstituted into lipid cubic phase by mixing at room temperature for 40 min with a 1.5-fold excess by mass of 10:1 (w:w) mixture of monoolein and cholesterol. A Gryphon LCP robot (Art Robbins Instruments) was used to dispense 27 nL drops of the mesophase onto 96-well glass plates and to overlay 800 nL of precipitant solution. Crystals that yielded optimal diffraction were obtained from wells containing 100 mM Tris pH 8.0, 15–25 mM MgCl2, and 28%–29% PEG 300. Crystals were harvested after 6–11 days of growth at 20°C with mesh grid loops (MiTeGen) and flash frozen in liquid nitrogen.
Data collection and structure determination
Diffraction data were collected at Advanced Photon Source GM/CA beamline 23ID-B using raster grid scanning to locate crystals. Data were collected with a 10 mm beam using a 0.2° per frame oscillation angle with a 5-fold attenuation setting. Data were processed with XDS (Kabsch, 2010), and a complete dataset at 2.9 Å resolution was compiled from five crystals processed in spacegroup P21212 (Table S3).
Analysis of the diffraction data in phenix.xtriage (Adams et al., 2010) indicated the presence of a strong translational non-crystal-lographic symmetry element, and initial attempts to solve the structure by molecular replacement were unsuccessful. The data were expanded to spacegroup P1 in Phenix, which allowed successful molecular replacement with MolRep (Vagin and Teplyakov, 2010) using the inactive state structure of the angiotensin II type 1 receptor as a search model (PDB ID: 4YAY, (Zhang et al., 2015b)). Manual inspection of the lattice packing showed a non-crystallographic two-fold rotational symmetry element parallel to the b axis, which results in a pseudotranslation when combined with the crystallographic 2-fold rotational symmetry (Figures S3A and S3B). The solution in spacegroup P1 was converted to the correct P21212 spacegroup, and a second molecular replacement search was run in Phaser (McCoy et al., 2007) to locate the receptor-bound nanobody. In this search, the two correctly placed receptor molecules were used as a fixed partial solution, and the structure of Nanobody80 (PDB ID: 3P0G, (Rasmussen et al., 2011a)) was used as the search model. Two copies of the nanobody could be located, each showing CDR3 interacting with the intracellular face of a receptor molecule as expected. Initial refinement showed electron density indicative of two additional partially ordered nanobody molecules. These were placed manually in Coot (Emsley et al., 2010) and subjected to rigid body refinement prior to further refinement.
The model was refined through iterative reciprocal space least-squares refinement in phenix.refine and manual rebuilding in Coot. Electron density for the receptor was quite clear (Figure S3C), and major structural changes relative to the search model were immediately apparent and could be easily built. The bound peptide was placed following refinement of the receptor itself, and then nanobody CDR loops were built. Electron density for the nanobody was heterogeneous, with the two receptor-bound molecules showing clear and continuous density throughout, including CDR3, while their dimerization partners showed very poor electron density in many regions. These two nanobody molecules engage in few lattice contacts and do not directly interact with the receptor, likely accounting for their poor electron density. Data reduction and refinement statistics are summarized in Table S3. Note that the presence of translational non-crystallographic symmetry results in systematic modulation of diffraction intensities in ways that are poorly modeled with existing software, and so some statistics (e.g., Rfree) are poorer than might otherwise be expected at this resolution.
Radioligand binding experiments
For radioligand binding experiments with AT1R-overexpressing membranes, crude membranes were prepared as previously described (Strachan et al., 2014) from transiently transfected tetracycline-inducible Expi293F cells with the indicated pcDNA-ZeotetO-AT1R construct. The Y292F mutation was introduced in this construct by site-directed mutagenesis (Quikchange Lightning Site-Directed Mutagenesis kit). Membranes were incubated with varying concentrations of cold competitor ligands and [3H]-olmesartan in assay buffer (20 mM HEPES pH 7.4, 100 mM NaCl, 0.2% BSA, leupeptin, benzamidine) for 90 min at room temperature before being harvested onto glass-fiber filters (GF/B) using a 96-well Brandel harvester, washing with cold 20 mM HEPES pH 7.4, 100 mM NaCl. For radioligand binding experiments with purified AT1R, ~50 ng of receptor were incubated with varying concentrations of cold competitor ligand and nanobody, and [3H]-olmesartan in HNM buffer containing 0.2% bovine serum albumin (BSA) in 200 μL reactions. After a 90-minute equilibration at room temperature, binding reactions were harvested onto GF/B filters and rapidly washed with cold 20 mM HEPES pH 7.4, 100 mM NaCl.
Co-immunoprecipitation experiments
To measure agonist-dependent binding of Nb.AT110, FLAG-AT1R (3 μM) was incubated with ligand (10 μM) in HNM buffer for 15 min at room temperature before addition of Nb.AT110 (10 μM) for 1 h. FLAG-AT1R was captured using M1-FLAG resin, washed three times with HNM buffer, and eluted with HNM buffer supplemented with 0.4 mg/mL FLAG-peptide and 10 mM EDTA. Co-immuno-precipitation of Nb.AT110 with AT1R was assessed by SDS-PAGE and quantified by dividing the mean pixel intensity (ImageJ, (Schneider et al., 2012)) of Nb.AT110 by AT1R for three independent experiments.
Cellular signaling assays
Cellular activation of Gq protein by AT1R was indirectly measured using two assays, by quantifying inositol monophosphate (IP1) levels using the IP-One Gq Kit (Cisbio) and by measuring cellular calcium levels using the Fluo-4 direct calcium assay kit (ThermoFisher). In brief, tetracycline-inducible Expi293F cells stably expressing wild-type human FLAG-AT1R were induced at a cell density of 2 × 106 cells/mL, and 24 h thereafter 10,000–20,000 cells were seeded into a low volume 96-well dish. Cells were stimulated with AT1R ligands at 37°C and 5% CO2 for 1 h, and IP1 was quantified as per the manufacturer protocol using a CLARIOstar plate reader (BMG Labtech).
Gq-dependent increases in cellular calcium levels following AT1R activation were measured using the Fluo-4 direct calcium assay kit (ThermoFisher). HEK293 cells stably expressing 2 pmol/mg wild-type rat HA-AT1aR (Xiao et al., 2007) were plated at a density of 40,000–60,000 cells/mL in 96-well plates and 24 h thereafter incubated with Fluo-4-direct calcium reagent for 30 min at 30°C with 5% CO2. Cells were stimulated with the indicated ligand for 2 min, and fluorescence was measured on a NOVOstar plate reader (BMG Labtech).
To measure β-Arrestin recruitment using the DiscoverX assay, pCMV-AT1R (human)-Prolink was transiently transfected into U2OS cells stably expressing β-arrestin2 with an Enzyme Acceptor tag. On the following day, transfected cells were plated at a density of 30,000 cells per well. Two days post-transfection, cells were treated with a serial dilution of ligand in duplicate for 90 min at 37°C. PathHunter Detection kit (DiscoverX) reagents were added, and reactions were incubated for another hour at 27°C. Chemiluminescence resulting from the complementation of β-galactosidase fragments (Enzyme Acceptor and ProLink) was detected on a CLARIOstar plate reader (BMG Labtech).
QUANTIFICATION AND STATISTICAL ANALYSIS
Radioligand binding data were fit to a one-site model in GraphPad Prism. Error is reported or shown as the standard error of the mean of the values calculated in at least three independent experiments. IP1, calcium signaling and β-Arrestin recruitment data were normalized to 100% of the maximum AngII signal as determined by a sigmoidal dose response curve in GraphPad Prism; the standard error of the mean of the values calculated in at least three independent experiments is shown.
DATA AND SOFTWARE AVAILABILITY
Coordinates and structure factors for the AT1R–S1I8-Nb.AT110i1 complex are deposited in the Protein Data Bank under accession code PDB: 6DO1.
Supplementary Material
(A) Flow analysis of yeast clones expressing Nb.AT109 or Nb.AT111 (blue) which selectively interact with agonist-bound AT1R. Yeast were stained with antagonist (olmesartan)- and agonist (AngII)-bound AT1R, each labeled with a different fluorophore. Binding of a non-conformationally selective AT1R clone (red) is shown for comparison.
(B) Sequences of Nb.AT110 and affinity matured Nb.AT110i1. Residues that were introduced to generate Nb.AT110i1 are shown in red.
(C) Nb.AT110 and Nb.AT110i1 allosterically increase the binding of AngII to the AT1R by competition radioligand binding. Error bars represent the standard error from three independent experiments performed with single replicates.
(D) Plot of the change in the log IC50 of the agonist TRV055 (as shown in Figure 2D) with increasing concentrations of Nb.AT110 (log EC50 (M) = −6.6 ± 0.2) or Nb.AT110i1 (log EC50 too low to be accurately determined). Error bars represent the standard error from three independent experiments performed as single replicates.
(A) Competition radioligand binding with Expi293F membranes expressing wild-type AT1R, the inactive-state AT1R crystallization construct (BRIL-ΔN(7–16)-Δ320, containing an amino-terminal BRIL insertion, amino-terminal truncation, and carboxy-terminal truncation) (Zhang et al., 2015b), and the active-state AT1R crystallization construct (BRIL(227)-Δ320, see Figure S2B). TRV055 is an AngII analog with deletion of D1 and the substitution R2G. Losartan is a small-molecule AT1R antagonist (ARB). Ki values are provided in Table S2. Error bars represent standard error of the mean from at least three independent experiments performed as single replicates.
(B) Design of the AT1R construct used for crystallography with Nb.AT110i1, BRIL(227)-Δ320. This construct has an amino-terminal FLAG tag, an insertion of BRIL between residues 226 and 227 in ICL3, and a carboxy-terminal truncation at residue 320.
(C) Allosteric increase in AngII binding to purified BRIL(227)-D320 by Nb.AT110i1 (log IC50(No nanobody) (M) = −7.82 ± 0.04, log IC50(Nb.AT110i1) (M) = −8.33 ± 0.05). Note that the apparent increase in AngII is limited by the low nanomolar affinity of the radioligand [3H]-olmesartan. Error represents standard error from at least three independent experiments performed as single replicates.
(D and E) Purification of AT1R-BRIL(227)-Δ320/S1I8/Nb.AT110i1, showing (D) the size exclusion chromatography trace and (E) gel electrophoresis analysis of the size exclusion fractions (Coomassie staining). The co-elution of the receptor and nanobody demonstrates the stability of the complex under these conditions.(F) Gq-mediated increase in inositol monophosphate (IP) levels (Cisbio HTRF IP-One assay) induced by AngII (log EC50 (M) = −8.16 ± 0.06) and the partial agonist S1I8 (log EC50 (M) = −6.8 ± 0.1, Bmax = 52% ± 3% of AngII maximum) in Expi293F cells overexpressing wild-type human Flag-AT1R. Data are normalized to 100% of the AngII response; error bars represent the standard error from four independent experiments performed in duplicate.
(G) Gq-mediated increase in cellular calcium levels (ThermoFisher Fluo-4 assay) induced by AngII (log EC50 (M) = −8.36 ± 0.07) and the partial agonist S1I8 (log EC50 (M) = [C0]7.8 ± 0.2, Bmax = 41% ± 2% of AngII maximum) in HEK293 cells overexpressing wild-type rat HA-AT1aR. Data are normalized to 100% of the AngII response; error bars represent the standard error from three independent experiments performed in duplicate.
(H) β-Arrestin2 recruitment (PathHunter DiscoverX assay) induced by AngII (log EC50 (M) = −8.3 ± 0.1) and the partial agonist S1I8 (log EC50 (M) = −9.2 ± 0.2, Bmax = 31% ± 2% of AngII maximum) in U2OS cells overexpressing wild-type human AT1R fused to the C-terminal ProLink tag. Data are normalized to 100% of the AngII response; error bars represent the standard error from three independent experiments performed in duplicate.
(A) The asymmetric unit of the crystallized complex, containing two AT1R molecules and four Nb.AT110i1 molecules. Two Nb.AT110i1 molecules (green) interact with the receptor; the other two Nb.AT110i1 molecules (blue) dimerize with the receptor-interacting nanobodies and do not contact the receptor directly. These two nanobody molecules showed poor electron density and are only partially built. The two AT1R molecules in the asymmetric unit form contacts at the extracellular and intracellular ends of TMs 1, 2, and 3. Rotation of the asymmetric unit shows the non-crystallographic two-fold rotational symmetry axis parallel to the crystallographic two-fold symmetry element coincident with the b axis. The combination of parallel crystallographic and non-crystallographic two-fold symmetry axes is equivalent to translational non-crystallographic symmetry.
(B) Crystal lattice packing. The translational non-crystallographic symmetry element (tNCS) results from the combination of the 21 screw axis along b and the non-crystallographic rotational symmetry element parallel to b. The tNCS element is indicated. Disordered BRIL is expected to be located in the aqueous channels between receptor layers.
(C) Electron density of the structure. 2Fo-Fc simulated annealing composite omit map contoured at 1.2 σ is shown in blue.
(D) Comparison of the binding mode of Nb.AT110i1 and the AT1R to other nanobody/active-state GPCRs (Nb80/β2AR, PDB ID: 3P0G; Nb9–8/M2R, PDB ID: 4MQS). The nanobodies’ CDR3 regions are highlighted in red.
The N58D mutation was introduced into Nb.AT110i1 during affinity maturation. In the receptor-bound nanobody molecule (green), this residue mediates contacts with both K135ICL2 of the AT1R (orange) and R45 from a second Nb.AT110i1 molecule that does not contact the AT1R (gray).
(A) Competition radioligand binding with Expi293F membranes expressing wild-type AT1R or AT1R Y292F. The mutation decreases losartan (antagonist) affinity at the receptor, likely by stabilizing an active conformation of the binding pocket. Ki values are provided in Table S4.
(B) A cavity at the base of the AT1R’s ligand-binding pocket (orange surface) is large enough to accommodate the I8 side chain of S1I8 (gray sticks)
(C) This pocket is sterically incompatible with the F8 side chain found in the endogenous agonist AngII. A representative F8 rotamer is shown, with steric clash indicated.
Error bars representing standard error of the mean from three independent experiments performed in duplicate are smaller than the markers.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-HA mAb (Alexa Fluor 647) | Cell Signaling Tech. | Cat# 3444; RRID: AB_10693329 |
| Anti-HA mAb (Alexa Fluor 488) | Cell Signaling Tech. | Cat# 2350; RRID: AB_491023 |
| Anti-FLAG M1 | ATCC | Hybridoma ATCC HB-9259; RRID: CVCI_J730 |
| Anti-FLAG Alexa 647 M1 | This study | Hybridoma ATCC HB-9259; RRID: CVCL_J730 |
| Anti-FLAG-FITC M1 | This study | Hybridoma ATCC HB-9259; RRID: CVCL_J730 |
| Bacterial and Virus Strains | ||
| T7 Express lysY BL21 E. coli | New England Biolabs | Cat# C3010I |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Carboxypeptidase A | Sigma-Aldrich | Cat# C9268 |
| Drop-out base medium with glucose | US Biological | Cat# D9500 |
| Drop-out base with galactose | US Biological | Cat# D9502 |
| Drop-out mix synthetic, adenine-rich minus tryptophan | US Biological | Cat# D9531 |
| Anti-Alexa Fluor 647 MicroBeads | Miltenyi Biotec | Cat# 130-091-395 |
| Anti-FITC MicroBeads | Miltenyi Biotec | Cat# 130-048-701 |
| Expi293 expression media | Thermo Fisher Scientific | Cat# A1435102 |
| MEM, Minimal Essential Media | Thermo Fisher Scientific | Cat# 11095–080 |
| Penicillin-Streptomycin | Gemini | Cat# 400–109 |
| Fetal bovine serum | Sigma-Aldrich | Cat# F2442 |
| Blasticidin | Invivogen | Cat# ant-bl-1 |
| Zeocin | Invitrogen | Cat# R25001 |
| Puromycin | Thermo Fisher Scientific | Cat# A11138–03 |
| Glutamine | Thermo Fisher Scientific | Cat# 25030–081 |
| Expifectamine transfection kit | Thermo Fisher Scientific | Cat# A14525 |
| Fugene 6 | Promega | Cat# E2692 |
| Doxycycline hyclate | Sigma-Aldrich | Cat# D9891 |
| Sodium butyrate | Sigma-Aldrich | Cat# 303410 |
| Kifunensine | Toronto Research Chemicals | Cat# K450000 |
| Losartan potassium | TCI | Cat# L0232 |
| Olmesartan | Tocris | Cat# 4616 |
| Angiotensin II (Asp-Arg-Val-Tyr-Ile-His-Pro-Phe) | GenScript | N/A |
| S1I8 (N-methyl-Gly-Arg-Val-Tyr-Ile-His-Pro-Ile) | GenScript | N/A |
| TRV055 (Gly-Val-Tyr-Ile-His-Pro-Phe) | GenScript | N/A |
| Lauryl maltose neopentyl glycol (MNG) | Anatrace | Cat# NG310 |
| Cholesterol hemisuccinate (CHS) | Sigma-Aldrich | Cat# C6512 |
| Benzamidine hydrochloride hydrate | Sigma-Aldrich | Cat# B6506 |
| Leupeptin-hemisulfate | AG Scientific | Cat# L1165 |
| FLAG peptide (DYKDDDDK) | Genscript | N/A |
| Monoolein | Sigma-Aldrich | Cat# M7765 |
| Cholesterol | Sigma-Aldrich | Cat# C8667 |
| Polyethylene glycol 300 | Hampton Research | Cat# HR2–517 |
| Magnesium chloride hexahydrate | Sigma-Aldrich | Cat# M9272 |
| Tris base | Sigma-Aldrich | Cat# T1378 |
| [3H]-olmesartan | American Radiolabeled Chemicals | Cat# ART 1976 |
| Critical Commercial Assays | ||
| GeneMorph II Random Mutagenesis Kit | Agilent | Cat# 200550 |
| QuikChange Lightning Site-Directed Mutagenesis Kit | Agilent | Cat# 200519 |
| PathHunter detection kit | DiscoverX | Cat# 93–0001 |
| IP-One Gq kit | Cisbio | Cat# 62IPAPEB |
| Fluo-4 Direct Calcium Assay Kit | Thermo Fisher Scientific | Cat# F10471 |
| Deposited Data | ||
| Atomic model of human angiotensin II type 1 receptor bound to olmesartan | (Zhang et al., 2015a) | PDB: 4ZUD |
| Atomic model of human angiotensin II type 1 receptor bound to ZD7155 | (Zhang et al., 2015b) | PDB: 4YAY |
| Atomic model of human β2 adrenergic receptor bound to BI167107 and Nb80 | (Rasmussen et al., 2011a) | PDB: 3P0G |
| Atomic model of human M2 muscarinic acetylcholine receptor receptor bound to iperoxo and Nb9–8 | (Kruse et al., 2013) | PDB: 4MQS |
| Atomic model of human angiotensin II type 1 receptor bound to S1I8 and Nb.AT110i1 | This study | PDB: 6DO1 |
| Experimental Models: Cell Lines | ||
| S. cerevisiae BJ5465 | ATCC | Cat# 208289 |
| S. cerevisiae Nanobody Library | (McMahon et al., 2018) | N/A |
| Expi293F Cells | Thermo Fisher Scientific | Cat# A14527 |
| PathHunter® U2OS EA b-Arrestin Parental Cell Line | DiscoverX | Cat# 93–0166 |
| Stable rat AT1aR HEK293 cell line | (Xiao et al., 2007) | N/A |
| Oligonucleotides | ||
| pyds_e_f: GGAGAAAAAACCCCGG | Eurofins Genomics | N/A |
| Nb.AT110i1 gBlock: CAGGTGCAGCTGCAGGAAAGCGGCGGCGGCCTGGTGCAGGCGGGCGGCAGCCTGCGCCTGAGCTGCGCGGCGAGCGGCAATATTTTTGACGTTGACATCATGGGCTGGTATCGCCAGGCGCCGGGCAAAGAACGCGAACTTGTTGCCAGTATTACTGACGGTGGTAGTACCGATTATGCGGATAGCGTGAAAGGCCGCTTTACCATTAGCCGCGATAACGCGAAAAACACCGTGTATCTGCAGATGAACAGCCTGAAACCGGAAGATACCGCGGTGTATTATTGCGCGGCTGTCGCTTACCCGGACATCCCGACTTACTTCGACTACGACTCTGACAATTTCTATTGGGGCCAGGGCACCCAGGTGACCGTGAGCAGC | IDT | N/A |
| BRIL_fwd: CAAGTTATACTCTTATTTGGAAGGCCCTAAAGAAGGCTTATGACCTGGAGGATAACTGG | IDT | N/A |
| BRIL_rev: CTTAAAAATATCATCATTTCTTGGTTTGTTCTTCTGAATTTCGGCGTTGCGAGTGGTCT | IDT | N/A |
| I320stop_fwd: GGAAAAAATTTAAAAGATATTTTCTCCAGCTTCTAAAATATTAGCCCCCAAAAGCCAAATCCCAC | IDT | N/A |
| I320stop_rev: GTGGGATTTGGCTTTTGGGGGCTAATATTTTAGAAGCTGGAGAAAATATCTTTTAAAIIIIIICC | IDT | N/A |
| Y292F_fwd: GCCTATCACCATTTGTATAGCTTTTTTTAACAATTGCCTGAATCCTC | Eton Biosciences | N/A |
| Y292F_rev: GAGGATTCAGGCAATTGTTAAAAAAAGCTATACAAATGGTGATAGGC | Eton Biosciences | N/A |
| Recombinant DNA | ||
| pYDS649 | (McMahon et al., 2018) | N/A |
| pET26b | EMD Millipore | Cat# 69862 |
| pcDNA6/TR | Thermo Fisher Scientific | Cat# V102520 |
| pcDNA-Zeo-tetO | (Staus et al., 2018) | N/A |
| pCMV-ProLink 1 Vector | DiscoverX | Cat# 93–0167 |
| Software and Algorithms | ||
| Graphpad Prism 7 | Graphpad | https://www.graphpad.com/ |
| Phaser version 2.7.16 | SBGrid Consortium | https://sbgrid.org/ |
| Coot version 0.8.8 | SBGrid Consortium | https://sbgrid.org/ |
| XDS | SBGrid Consortium | https://sbgrid.org/ |
| Phenix version 1.11.1_2575 | SBGrid Consortium | https://sbgrid.org/ |
| ImageJ | (Schneider et al., 2012) | https://imagej.nih.gov/ij/index.html |
| Other | ||
| GF/B filter paper | Brandel | Cat# FPD-196 |
| Low-volume 96-well plates | Cisbio | Cat# 66PL96001 |
Highlights.
Active angiotensin II type 1 receptor crystallized with angiotensin II peptide analog
Stabilized by a conformation-specific synthetic nanobody discovered by yeast display
Extensive peptide-receptor engagement remodels ligand-binding cavity
Intra- and extracellular changes give insights on GPCR activation and biased agonism
ACKNOWLEDGMENTS
We would like to thank Victoria Ronk and Quivetta Lennon for administrative assistance and Aashish Manglik, Henry Chiou, Jon Zmuda, and Stuart Kornfeld for helpful discussions. We thank staff at Advanced Photon Source GM/CA beamlines for technical assistance and support of data collection. Funding was provided by NIH grants R01HL16037 (R.J.L.) and 5DP5OD021345 (A.C.K.), the Mandel Center for Hypertension and Atherosclerosis at Duke (R.J.L.), the Vallee Foundation (A.C.K.), and the Smith Family Foundation (A.C.K.). R.J.L. is an investigator with the Howard Hughes Medical Institute.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes five figures and four tables and can be found with this article online at https://doi.org/10.1016/j.cell.2018.12.006.
DECLARATION OF INTERESTS
R.J.L. is a founder and stockholder of Trevena and is a director of Lexicon Pharmaceuticals. A.C.K. is a founder of Ab initio Biotherapeutics.
REFERENCES
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. (2010). PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta. Crystallogr. D. Biol. Crystallogr 66, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, Kim J, Hara MR, Ren XR, and Lefkowitz RJ (2009). {beta}-Arrestin-2 mediates anti-apoptotic signaling through regulation of BAD phosphorylation. J. Biol. Chem 284, 8855–8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada H, Horita S, Hirata K, Shiroishi M, Shiimura Y, Iwanari H, Hamakubo T, Shimamura T, Nomura N, Kusano-Arai O, et al. (2018). Crystal structure of the human angiotensin II type 2 receptor bound to an angiotensin II analog. Nat. Struct. Mol. Biol 25, 570–576. [DOI] [PubMed] [Google Scholar]
- Balakumar P, and Jagadeesh G (2014). Structural determinants for binding, activation, and functional selectivity of the angiotensin AT1 receptor. J. Mol. Endocrinol 53, R71–R92. [DOI] [PubMed] [Google Scholar]
- Ballesteros JA, and Weinstein H (1995). Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors In Methods in Neurosciences, Sealfon SC, ed. (Academic Press; ), pp. 366–428. [Google Scholar]
- Ballesteros JA, Jensen AD, Liapakis G, Rasmussen SG, Shi L, Gether U, and Javitch JA (2001). Activation of the beta 2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J. Biol. Chem 276, 29171–29177. [DOI] [PubMed] [Google Scholar]
- Cabana J, Holleran B, Beaulieu ME, Leduc R, Escher E, Guillemette G, and Lavigne P (2013). Critical hydrogen bond formation for activation of the angiotensin II type 1 receptor. J. Biol. Chem 288, 2593–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey M (2009). Crystallizing membrane proteins for structure determination: Use of lipidic mesophases. Annu. Rev. Biophys 38, 29–51. [DOI] [PubMed] [Google Scholar]
- Chun E, Thompson AA, Liu W, Roth CB, Griffith MT, Katritch V, Kunken J, Xu F, Cherezov V, Hanson MA, et al. (2012). Fusion partner toolchest for the stabilization and crystallization of G protein-coupled receptors. Structure 20, 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter G, Davison BA, Butler J, Collins SP, Ezekowitz JA, Felker GM, Filippatos G, Levy PD, Metra M, Ponikowski P, et al. (2018). Relationship between baseline systolic blood pressure and long-term outcomes in acute heart failure patients treated with TRV027: An exploratory subgroup analysis of BLAST-AHF. Clin. Res. Cardiol 107, 170–181. [DOI] [PubMed] [Google Scholar]
- De Lean A, Stadel JM, and Lefkowitz RJ (1980). A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. J. Biol. Chem 255, 7108–7117. [PubMed] [Google Scholar]
- De Meyer T, Muyldermans S, and Depicker A (2014). Nanobody-based products as research and diagnostic tools. Trends Biotechnol. 32, 263–270. [DOI] [PubMed] [Google Scholar]
- Domazet I, Holleran BJ, Richard A, Vandenberghe C, Lavigne P, Escher E, Leduc R, and Guillemette G (2015). Characterization of angiotensin II molecular determinants involved in AT1 receptor functional selectivity. Mol. Pharmacol 87, 982–995. [DOI] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, and Cowtan K (2010). Features and development of Coot. Acta. Crystallogr. D. Biol. Crystallogr 66, 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escribá PV, Wedegaertner PB, Goñi FM, and Vögler O (2007). Lipid-protein interactions in GPCR-associated signaling. Biochim. Biophys. Acta 1768, 836–852. [DOI] [PubMed] [Google Scholar]
- Feng YH, Noda K, Saad Y, Liu XP, Husain A, and Karnik SS (1995). The docking of Arg2 of angiotensin II with Asp281 of AT1 receptor is essential for full agonism. J. Biol. Chem 270, 12846–12850. [DOI] [PubMed] [Google Scholar]
- Fillion D, Lemieux G, Basambombo LL, Lavigne P, Guillemette G, Leduc R, and Escher E (2010). The amino-terminus of angiotensin II contacts several ectodomains of the angiotensin II receptor AT1. J. Med. Chem 53, 2063–2075. [DOI] [PubMed] [Google Scholar]
- Fillion D, Cabana J, Guillemette G, Leduc R, Lavigne P, and Escher E (2013). Structure of the human angiotensin II type 1 (AT1) receptor bound to angiotensin II from multiple chemoselective photoprobe contacts reveals a unique peptide binding mode. J. Biol. Chem 288, 8187–8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritze O, Filipek S, Kuksa V, Palczewski K, Hofmann KP, and Ernst OP (2003). Role of the conserved NPxxY(x)5,6F motif in the rhodopsin ground state and during activation. Proc. Natl. Acad. Sci. USA 100, 2290–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JL, Theilade J, Haunsø S, and Sheikh SP (2004). Oligomerization of wild type and nonfunctional mutant angiotensin II type I receptors inhibits galphaq protein signaling but not ERK activation. J. Biol. Chem 279, 24108–24115. [DOI] [PubMed] [Google Scholar]
- Holloway AC, Qian H, Pipolo L, Ziogas J, Miura S, Karnik S, Southwell BR, Lew MJ, and Thomas WG (2002). Side-chain substitutions within angiotensin II reveal different requirements for signaling, internalization, and phosphorylation of type 1A angiotensin receptors. Mol. Pharmacol 61, 768–777. [DOI] [PubMed] [Google Scholar]
- Kabsch W (2010). XDS. Acta. Crystallogr. D. Biol. Crystallogr 66, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Zhou XE, Gao X, He Y, Liu W, Ishchenko A, Barty A, White TA, Yefanov O, Han GW, et al. (2015). Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature 523, 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor ED, Rehm CD, Haas JS, Chan AT, and Giovannucci EL (2015). Trends in prescription drug use among adults in the United States from 1999–2012. JAMA 314, 1818–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik SS, Unal H, Kemp JR, Tirupula KC, Eguchi S, Vanderheyden PM, and Thomas WG (2015). International Union of Basic and Clinical Pharmacology. XCIX. Angiotensin receptors: Interpreters of pathophysiological angiotensinergic stimuli. Pharmacol. Rev 67, 754–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katritch V, Fenalti G, Abola EE, Roth BL, Cherezov V, and Stevens RC (2014). Allosteric sodium in class A GPCR signaling. Trends Biochem. Sci 39, 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Forrester SJ, O’Brien S, Baggett A, Rizzo V, and Eguchi S (2017). AT1 receptor signaling pathways in the cardiovascular system. Pharmacol. Res 125 (Pt A), 4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehl A, Hu H, Maeda S, Zhang Y, Qu Q, Paggi JM, Latorraca NR, Hilger D, Dawson R, Matile H, et al. (2018). Structure of the m-opioid receptor-Gi protein complex. Nature 558, 547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse AC, Ring AM, Manglik A, Hu J, Hu K, Eitel K, Hübner H, Pardon E, Valant C, Sexton PM, et al. (2013). Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature 504, 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kim HR, Deepak RNVK, Wang L, Chung KY, Fan H, Wei Z, and Zhang C (2018). Orthosteric and allosteric action of the C5a receptor antagonists. Nat. Struct. Mol. Biol 25, 472–481. [DOI] [PubMed] [Google Scholar]
- Ma Y, Yue Y, Ma Y, Zhang Q, Zhou Q, Song Y, Shen Y, Li X, Ma X, Li C, et al. (2017). Structural basis for apelin control of the human apelin receptor. Structure 25, 858–866. [DOI] [PubMed] [Google Scholar]
- Manglik A, and Kruse AC (2017). Structural basis for G protein-coupled receptor activation. Biochemistry 56, 5628–5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, Pardo L, Weis WI, Kobilka BK, and Granier S (2012). Crystal structure of the m-opioid receptor bound to a morphinan antagonist. Nature 485, 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglik A, Kobilka BK, and Steyaert J (2017). Nanobodies to study G protein-coupled receptor structure and function. Annu. Rev. Pharmacol. Toxicol 57, 19–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, and Read RJ (2007). Phaser crystallographic software. J. Appl. Crystallogr 40, 658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon C, Baier AS, Pascolutti R, Wegrecki M, Zheng S, Ong JX, Erlandson SC, Hilger D, Rasmussen SGF, Ring AM, et al. (2018). Yeast surface display platform for rapid discovery of conformationally selective nanobodies. Nat. Struct. Mol. Biol 25, 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S, and Karnik SS (1999). Angiotensin II type 1 and type 2 receptors bind angiotensin II through different types of epitope recognition.J. Hypertens 17, 397–404. [DOI] [PubMed] [Google Scholar]
- Neve KA, Cumbay MG, Thompson KR, Yang R, Buck DC, Watts VJ, DuRand CJ, and Teeter MM (2001). Modeling and mutational analysis of a putative sodium-binding pocket on the dopamine D2 receptor. Mol. Pharmacol 60, 373–381. [DOI] [PubMed] [Google Scholar]
- Noda K, Saad Y, and Karnik SS (1995). Interaction of Phe8 of angiotensin II with Lys199 and His256 of AT1 receptor in agonist activation. J. Biol. Chem 270, 28511–28514. [DOI] [PubMed] [Google Scholar]
- Noda K, Feng YH, Liu XP, Saad Y, Husain A, and Karnik SS (1996). The active state of the AT1 angiotensin receptor is generated by angiotensin II induction. Biochemistry 35, 16435–16442. [DOI] [PubMed] [Google Scholar]
- Peterson YK, and Luttrell LM (2017). The diverse roles of arrestin scaffolds in G protein-coupled receptor signaling. Pharmacol. Rev 69, 256–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrello ER, Pfleger KD, Seeber RM, Qian H, Oro C, Abogadie F, Delbridge LM, and Thomas WG (2011). Heteromerization of angiotensin receptors changes trafficking and arrestin recruitment profiles. Cell. Signal 23, 1767–1776. [DOI] [PubMed] [Google Scholar]
- Rajagopal K, Whalen EJ, Violin JD, Stiber JA, Rosenberg PB, Pre-mont RT, Coffman TM, Rockman HA, and Lefkowitz RJ (2006). Beta-arrestin2-mediated inotropic effects of the angiotensin II type 1A receptor in isolated cardiac myocytes. Proc. Natl. Acad. Sci. USA 103, 16284–16289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal S, Ahn S, Rominger DH, Gowen-MacDonald W, Lam CM, Dewire SM, Violin JD, and Lefkowitz RJ (2011). Quantifying ligand bias at seven-transmembrane receptors. Mol. Pharmacol 80, 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SG, Choi HJ, Fung JJ, Pardon E, Casarosa P, Chae PS, Devree BT, Rosenbaum DM, Thian FS, Kobilka TS, et al. (2011a). Structure of a nanobody-stabilized active state of the b(2) adrenoceptor. Nature 469, 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, et al. (2011b). Crystal structure of the b2 adrenergic receptor-Gs protein complex. Nature 477, 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring AM, Manglik A, Kruse AC, Enos MD, Weis WI, Garcia KC, and Kobilka BK (2013). Adrenaline-activated structure of b2-adrenoceptor stabilized by an engineered nanobody. Nature 502, 575–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Yao XJ, Weis WI, Stevens RC, and Kobilka BK (2007). GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science 318, 1266–1273. [DOI] [PubMed] [Google Scholar]
- Ryba DM, Li J, Cowan CL, Russell B, Wolska BM, and Solaro RJ (2017). Long-term biased beta-arrestin signaling improves cardiac structure and function in dilated cardiomyopathy. Circulation 135, 1056–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, and Eliceiri KW (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staus DP, Wingler LM, Choi M, Pani B, Maglik A, Kruse AC, and Lefkowitz RJ (2018). Sortase ligation enables homogenous GPCR phosphorylation to reveal diversity in beta-arrestin coupling. Proc. Natl. Acad. Sci. USA 115, 3834–3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan RT, Sun JP, Rominger DH, Violin JD, Ahn S, Rojas Bie Thomsen A, Zhu X, Kleist A, Costa T, and Lefkowitz RJ (2014). Divergent transducer-specific molecular efficacies generate biased agonism at a G protein-coupled receptor (GPCR). J. Biol. Chem 289, 14211–14224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal H, Jagannathan R, Bhat MB, and Karnik SS (2010). Ligand-specific conformation of extracellular loop-2 in the angiotensin II type 1 receptor.J. Biol. Chem 285, 16341–16350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagin A, and Teplyakov A (2010). Molecular replacement with MOLREP. Acta. Crystallogr. D. Biol. Crystallogr 66, 22–25. [DOI] [PubMed] [Google Scholar]
- Van Liefde I, and Vauquelin G (2009). Sartan-AT1 receptor interactions: In vitro evidence for insurmountable antagonism and inverse agonism. Mol. Cell. Endocrinol 302, 237–243. [DOI] [PubMed] [Google Scholar]
- Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, and Lefkowitz RJ (2003). Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc. Natl. Acad. Sci. USA 100, 10782–10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler LM, Elgeti M, Hilger D, Latorraca NR, Lerch MT, Staus DP, Dror RO, Kobilka BK, Hubbell WL, and Lefkowitz RJ (2019). Angiotensin analogs with divergent bias stabilize distinct receptor conformations. Cell 176 Published online January 10, 2019. 10.1016/j.cell.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K, McClatchy DB, Shukla AK, Zhao Y, Chen M, Shenoy SK, Yates JR III, and Lefkowitz RJ (2007). Functional specialization of beta-arrestin interactions revealed by proteomic analysis. Proc. Natl. Acad. Sci. USA 104, 12011–12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Unal H, Desnoyer R, Han GW, Patel N, Katritch V, Karnik SS, Cherezov V, and Stevens RC (2015a). Structural basis for ligand recognition and functional selectivity at angiotensin receptor. J. Biol. Chem 290, 29127–29139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Unal H, Gati C, Han GW, Liu W, Zatsepin NA, James D, Wang D, Nelson G, Weierstall U, et al. (2015b). Structure of the Angio tensin receptor revealed by serial femtosecond crystallography. Cell 161, 833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Han GW, Batyuk A, Ishchenko A, White KL, Patel N, Sadybekov A, Zamlynny B, Rudd MT, Hollenstein K, et al. (2017). Structural basis for selectivity and diversity in angiotensin II receptors. Nature 544, 327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman B, Beautrait A, Aguila B, Charles R, Escher E, Claing A, Bouvier M, and Laporte SA (2012). Differential β-arrestin-dependent conformational signaling and cellular responses revealed by angiotensin analogs. Sci. Signal 5, ra33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Flow analysis of yeast clones expressing Nb.AT109 or Nb.AT111 (blue) which selectively interact with agonist-bound AT1R. Yeast were stained with antagonist (olmesartan)- and agonist (AngII)-bound AT1R, each labeled with a different fluorophore. Binding of a non-conformationally selective AT1R clone (red) is shown for comparison.
(B) Sequences of Nb.AT110 and affinity matured Nb.AT110i1. Residues that were introduced to generate Nb.AT110i1 are shown in red.
(C) Nb.AT110 and Nb.AT110i1 allosterically increase the binding of AngII to the AT1R by competition radioligand binding. Error bars represent the standard error from three independent experiments performed with single replicates.
(D) Plot of the change in the log IC50 of the agonist TRV055 (as shown in Figure 2D) with increasing concentrations of Nb.AT110 (log EC50 (M) = −6.6 ± 0.2) or Nb.AT110i1 (log EC50 too low to be accurately determined). Error bars represent the standard error from three independent experiments performed as single replicates.
(A) Competition radioligand binding with Expi293F membranes expressing wild-type AT1R, the inactive-state AT1R crystallization construct (BRIL-ΔN(7–16)-Δ320, containing an amino-terminal BRIL insertion, amino-terminal truncation, and carboxy-terminal truncation) (Zhang et al., 2015b), and the active-state AT1R crystallization construct (BRIL(227)-Δ320, see Figure S2B). TRV055 is an AngII analog with deletion of D1 and the substitution R2G. Losartan is a small-molecule AT1R antagonist (ARB). Ki values are provided in Table S2. Error bars represent standard error of the mean from at least three independent experiments performed as single replicates.
(B) Design of the AT1R construct used for crystallography with Nb.AT110i1, BRIL(227)-Δ320. This construct has an amino-terminal FLAG tag, an insertion of BRIL between residues 226 and 227 in ICL3, and a carboxy-terminal truncation at residue 320.
(C) Allosteric increase in AngII binding to purified BRIL(227)-D320 by Nb.AT110i1 (log IC50(No nanobody) (M) = −7.82 ± 0.04, log IC50(Nb.AT110i1) (M) = −8.33 ± 0.05). Note that the apparent increase in AngII is limited by the low nanomolar affinity of the radioligand [3H]-olmesartan. Error represents standard error from at least three independent experiments performed as single replicates.
(D and E) Purification of AT1R-BRIL(227)-Δ320/S1I8/Nb.AT110i1, showing (D) the size exclusion chromatography trace and (E) gel electrophoresis analysis of the size exclusion fractions (Coomassie staining). The co-elution of the receptor and nanobody demonstrates the stability of the complex under these conditions.(F) Gq-mediated increase in inositol monophosphate (IP) levels (Cisbio HTRF IP-One assay) induced by AngII (log EC50 (M) = −8.16 ± 0.06) and the partial agonist S1I8 (log EC50 (M) = −6.8 ± 0.1, Bmax = 52% ± 3% of AngII maximum) in Expi293F cells overexpressing wild-type human Flag-AT1R. Data are normalized to 100% of the AngII response; error bars represent the standard error from four independent experiments performed in duplicate.
(G) Gq-mediated increase in cellular calcium levels (ThermoFisher Fluo-4 assay) induced by AngII (log EC50 (M) = −8.36 ± 0.07) and the partial agonist S1I8 (log EC50 (M) = [C0]7.8 ± 0.2, Bmax = 41% ± 2% of AngII maximum) in HEK293 cells overexpressing wild-type rat HA-AT1aR. Data are normalized to 100% of the AngII response; error bars represent the standard error from three independent experiments performed in duplicate.
(H) β-Arrestin2 recruitment (PathHunter DiscoverX assay) induced by AngII (log EC50 (M) = −8.3 ± 0.1) and the partial agonist S1I8 (log EC50 (M) = −9.2 ± 0.2, Bmax = 31% ± 2% of AngII maximum) in U2OS cells overexpressing wild-type human AT1R fused to the C-terminal ProLink tag. Data are normalized to 100% of the AngII response; error bars represent the standard error from three independent experiments performed in duplicate.
(A) The asymmetric unit of the crystallized complex, containing two AT1R molecules and four Nb.AT110i1 molecules. Two Nb.AT110i1 molecules (green) interact with the receptor; the other two Nb.AT110i1 molecules (blue) dimerize with the receptor-interacting nanobodies and do not contact the receptor directly. These two nanobody molecules showed poor electron density and are only partially built. The two AT1R molecules in the asymmetric unit form contacts at the extracellular and intracellular ends of TMs 1, 2, and 3. Rotation of the asymmetric unit shows the non-crystallographic two-fold rotational symmetry axis parallel to the crystallographic two-fold symmetry element coincident with the b axis. The combination of parallel crystallographic and non-crystallographic two-fold symmetry axes is equivalent to translational non-crystallographic symmetry.
(B) Crystal lattice packing. The translational non-crystallographic symmetry element (tNCS) results from the combination of the 21 screw axis along b and the non-crystallographic rotational symmetry element parallel to b. The tNCS element is indicated. Disordered BRIL is expected to be located in the aqueous channels between receptor layers.
(C) Electron density of the structure. 2Fo-Fc simulated annealing composite omit map contoured at 1.2 σ is shown in blue.
(D) Comparison of the binding mode of Nb.AT110i1 and the AT1R to other nanobody/active-state GPCRs (Nb80/β2AR, PDB ID: 3P0G; Nb9–8/M2R, PDB ID: 4MQS). The nanobodies’ CDR3 regions are highlighted in red.
The N58D mutation was introduced into Nb.AT110i1 during affinity maturation. In the receptor-bound nanobody molecule (green), this residue mediates contacts with both K135ICL2 of the AT1R (orange) and R45 from a second Nb.AT110i1 molecule that does not contact the AT1R (gray).
(A) Competition radioligand binding with Expi293F membranes expressing wild-type AT1R or AT1R Y292F. The mutation decreases losartan (antagonist) affinity at the receptor, likely by stabilizing an active conformation of the binding pocket. Ki values are provided in Table S4.
(B) A cavity at the base of the AT1R’s ligand-binding pocket (orange surface) is large enough to accommodate the I8 side chain of S1I8 (gray sticks)
(C) This pocket is sterically incompatible with the F8 side chain found in the endogenous agonist AngII. A representative F8 rotamer is shown, with steric clash indicated.
Error bars representing standard error of the mean from three independent experiments performed in duplicate are smaller than the markers.
Data Availability Statement
Coordinates and structure factors for the AT1R–S1I8-Nb.AT110i1 complex are deposited in the Protein Data Bank under accession code PDB: 6DO1.