Abstract
Sarcopenia, the progressive and generalised loss of muscle mass and strength/function, is a major health Issue in older adults given its high prevalence and burdensome clinical implications. Over the years, this condition has been endorsed as a marker for discriminating biological from chronological age. However, the absence of a unified operational definition has hampered its full appreciation by healthcare providers, researchers and policymakers. In addition to this unsolved debate, the complexity of musculoskeletal ageing represents a major challenge to the identification of clinically meaningful biomarkers. Here, we illustrate the advantages of biomarker discovery procedures in muscle ageing based on multivariate methodologies as an alternative approach to traditional single-marker strategies. The rationale, design and methods of the “BIOmarkers associated with Sarcopenia and PHysical frailly in EldeRly pErsons” (BIOSPHERE) study are described as an application of a multi-marker strategy for the development of biomarkers for die newly operationalised Physical Frailly & Sarcopenia condition.
Keywords: Muscle atrophy, Inflammation, Cytokine, Neuromuscular junction, Multivariate analysis, Disability
1. Introduction
Over the last decades, Western countries have experienced a dramatic demographic transition. On a positive note, this is the successful result of advances in medicine and improved socioeconomic conditions. On the other hand, population ageing carries the downsides of challenging the societal structure, social security and healthcare systems [1].
As a matter of fact, existing healthcare systems conceived around the traditional paradigm of patients suffering from a single acute illness are unprepared to deal with the medical needs of older, multimorbid and functionally impaired people [2]. In this context, sarcopenia and physical frailty are increasingly recognised as prototype conditions around which current models of care may be re-shaped [3]. Indeed, the two conditions are based on a theoretical construct that surpasses the disease paradigm, thereby shifting the medical focus from the traditional concept of “healing through treating a single illness” toward a function-centred approach [4].
In the late ‘80s, Irwin Rosenberg [5], starting from the assumption that “there is probably no decline in structure and function more dramatic than the decline in lean body mass or muscle mass over the decades of life”, coined the term “sarcopenia” to refer to the loss of muscle mass that accompanies the ageing process. From its original description as purely an age-dependent loss of muscle mass, the concept of sarcopenia has evolved into a more complex construct encompassing both quantitative (i.e., mass) and qualitative (i.e., strength and/or function) declines of skeletal muscle [6]. Though, depending on the cutpoints used to distinguish “normal” from “abnormal” muscle-related parameters, the resulting phenotypes and risk profiles are only partly overlapping [7]. Despite this significant drawback, all of the existing, definitions of sarcopenia predict negative health-related events in older people [8]. Indeed, sarcopenia has recently gained the dignity of a “disease entity” with the recognition of a dedicated ICD-10-CM code in September 2016 [9] This landmark achievement, while adding further impetus to the study of muscle ageing, may leverage the agreement on a unique operational definition of sarcopenia. This, in turn, will facilitate the identification of sarcopenia determinants, promote the discovery of meaningful biological targets for treatment, and faster the incorporation of the condition in every-day practice [10].
Frailty is the term used to refer to a geriatric syndrome char-acterised by reduced homeostatic reserves, which exposes individuals at increased risk of negative health-related events [11,12]. A multitude of operational definitions of frailty have been proposed, each of them capturing specific aspects of the condition and identifying different risk profiles [13]. With the notable exception of the frailty index proposed by Rock wood and Mitnitski [14], the vast majority of available frailty scales point to physical function impairment as the central determinant of vulnerable health status [15]. When focused on the physical domain, the clinical picture of frailty shows remarkable overlap with sarcopenia [16]. This observation has led to envision muscle wasting as the biological substrate for the development of physical frailty (PF) and the pathway through which the negative health-related outcomes of PF ensue [17], In other words, sarcopenia may be considered to be the “organ failure” underlying the clinical manifestations of PF (Fig. 1) [17].
Fig. 1.
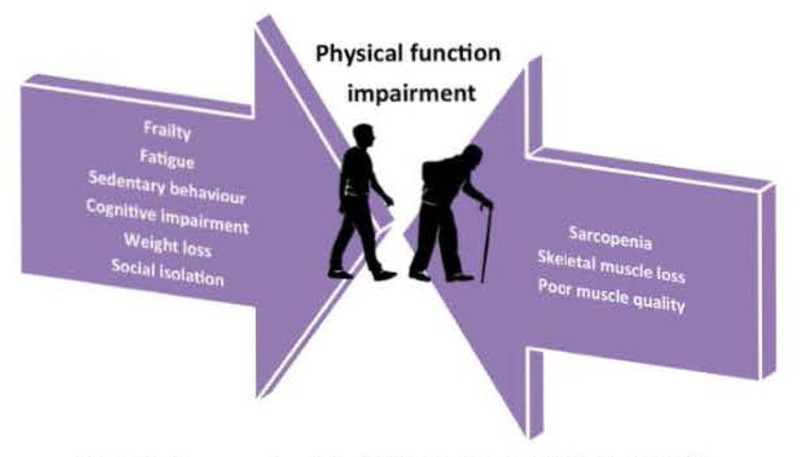
Frailty, sarcopenia and physical function impairment: a tight relationship.
The two conditions have therefore been merged into a new entity (i.e., PF & sarcopenia; PF&S) [18], defined by the following distinctive parameters:
-
(1)
Low muscle mass, as determined by dual X-ray absorptiometry (DXA) using the cut-points recommended by the Foundation for the National Institutes of Health (FNI11) sarcopenia project [19];
-
(2)
Low physical performance, defined as a summary score on the Short Physical Performance Battery (SPPB) [20] between 3 and 9; and
-
(3)
Absence of major mobility disability, operationalised as inability to walk 400 m in 15 min without sitting, the use of a walker, help from another person or stopping to rest for more the 60 s at a time [21].
The PF&S operational definition, elaborated in the context of the “Sarcopenia and Physical fRailty IN older people: multi-componenT Treatment strategies” (SPRINTT) project (IMI-JU # 115621) [22], frames a pre-disability condition that can be diagnosed and monitored in an objective manner. At the same time, the recognition of a clear biological substrate (i.e., muscle atrophy) allows for the search of novel biomarkers which can be subsequently used for detecting and tracking the condition of interest, obtaining information about the underlying pathophysiology, and identifying meaningful targets for preventive or therapeutic interventions [23]. This has set the momentum for the conception of the “BIOmarkers associated with Sarcopenia and PHysical frailty in EldeRly pErsons” (BIOSPHERE) study.
2. Rationale of BIOSPHERE
With the intent of pushing forward the search for biological markers associated with PF&S, the BIOSPHERE study was designed to determine and validate a panel of biomarkers able to integrate specific biochemical measurements into the assessment of PF&S both in clinical and research settings.
The identification of PF&S relies on the assessment of parameters pertaining to different domains (i.e.. clinical, functional and imaging). Although specific circulating markers have previously been associated with single domains of the PF&S condition, none of them has yet been incorporated into standard practice [24], This is partly due to the existence of a heterogeneous (i.e., muscle-specific and non-muscle specific) set of candidate mediators and the lack of a “gold standard” biomarker for the prediction of clinically meaningful outcomes (Fig. 2) [25],
Fig. 2.
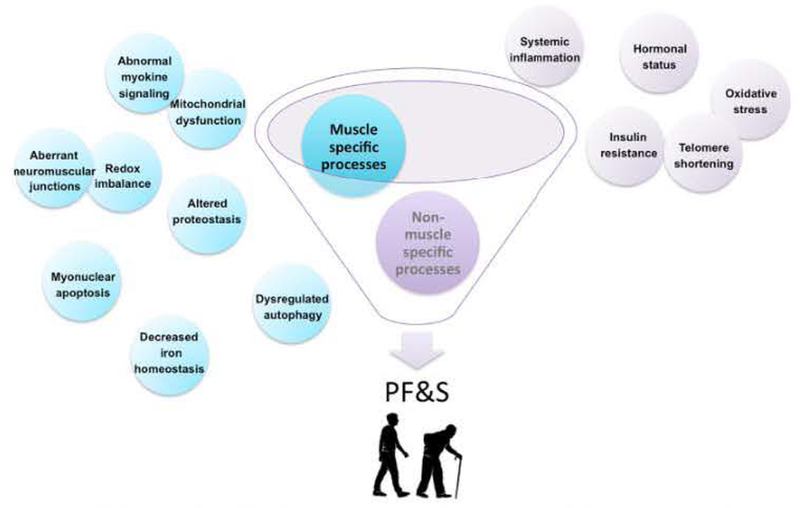
Contribution of muscle-specific and non muscle-specific pathogenic factors to physical frailty & sarcopenia (PF&S).
Given the complexity of PF&S, BIOSPHERE will apply multivariate modelling of an array of circulating mediators as a strategy to identify a set of biomarkers specific for the condition of interest This task will be pursued through (a) the analysis of multiple circulating biomarkers that reflect specific pathophysiological processes directly and/or indirectly linked to muscle ageing and its clinical correlates, and (b) the development and validation of multivariate statistical models to identify specific biomarkers of PF&S.
3. Methods
3.1. Study design and population
BIOSPHERE has been conceived as a cross-sectional, case-control study, aimed at analysing a panel of candidate biomarkers for PF&S through multivariate statistical models. The study protocol was approved by the Ethics Committee of the Catholic University of the Sacred Heart (Rome, Italy). After obtaining written informed consent, 200 older persons, 100 cases (individuals with PF&S) and 100 controls (elderly non-sarcopenic persons with no functional impairment), aged 70+ have been enrolled. Recruitment strategies included the use of newspapers, radio and television advertisements. The study was also advertised via flyers and brochures available in patient waiting areas throughout the Teaching Hospital “Agostino Gemelli” of the Catholic University of the Sacred Heart. Finally, the study was presented in the, senior centres within the catching area. Inclusion and exclusion criteria are summarised in Table 1. The rationale that drove the choice of eligibility criteria is thoroughly explained elsewhere [26].
Table 1.
Eligibility criteria in BIOSPHERE.
| PF&S older adults | Non-sarcopenic, non-frail controls |
|---|---|
| Inclusion criteria | |
| Men and women aged ≥ 70 years | Men and women aged ≥ 70 years |
| SPPB score between 3 (included) and 9 (included) | SPPB score > 9 |
| Sedentary lifestyle | Sedentary lifestyle |
| Low mittclc mass according to the cut points indicated by the FNIH sarcopenia project [19]a | Normal musclc mass according to the FNIH sarcopenia project |
| Ability to complete the 400-m walk test within 15min without sitting, use of any assistive device or help of another person [21] | Ability to complete the 400 m walk test within 15min without sitting, use of any assistive device or help of another person [21] |
| Permanent exclusion criteria for both cases and controls | |
| Inability or unwillingness to provide informed consent Nursing home residence | |
| Current diagnosis of schizophrenia, other psychotic or bipolar disorder Consumption of > 14 alcoholic drinks per week Self reported inability to walk across a room | |
| Difficulty communicating with the study personnel due to speech, language, or hearing problems | |
| MMSE < 24 [27] | |
| Severe arthritis (e.g., awaiting joint replacement) that would interfere with the ability to perform physical performance testing Cancer requiring treatment in the past three years, except for non melanoma skin cancers or cancers that have an excellent prognosis (e.g., early stage breast or prostate cancer] | |
| Lung disease requiring regular use of corticosteroids or supplemental oxygen Severe cardiovascular disease (including NY1IA class ID or IV congestive heart failure, clinically significant valvular disease, history of cardiac arrest, presence of an implantable defibrillator, or uncontrolled angina) | |
| Parkinson’s disease or other progressive neurological disorder Renal disease requiring dialysis | |
| Chest pain, severe sl>ortness of breath, or occurrence of any other safety concerns during the 400-n walk test Other medical, psychiatric, or behavioural factors that in the investigator’s judgment may interfere with study participation Other illnesses of such severity that life expectancy is < 12 months | |
| Temporary exclusion criteria for both cases and controlsb | |
| Uncontrolled hypertension (systolic blood pressure > 200 mmHg. or diastolic blood pressure > ll0mmHg) | |
| Uncontrolled diabetes with recent weight loss, diabetic coma, or frequent hypoglycaemia | |
| Stroke, hip fracture, hip or knee replacement, or spinal surgery in the past six months Serious conduction disorder (e.g., third-degree heart block) | |
| Uncontrolled arrhythmias, new Q waves within the past six months or ST segment depression (> 3 mm) on the ECG Myocardial infarction, major heart surgery (i.e., valve replacement or bypass surgery) in prior six months Deep vein thrombosis or pulmonary embolism in the past six months | |
Abbreviations: FNIH, Foundation for the National Institutes of Health; MMSE, Mini Mental State Examination; NYHA, New York Heart Association; SPPB, short physical performance battery.
Low muscle mass was defined as a crude appendicular lean mass (ALM) < 19.75 kg In men and < 15.02 kg in women or as an ALM-to-body mass index ratio < 0.789 in men and < 0.512 in women.
Participants who were excluded for one or more of the temporary medical conditions listed above could be rescreened after a period considered clinically appropriate by the study physician.
3.2. Participant recruitment and assessment
Participant recruitment took place from March 2016 through October 2017. The enrolment phase was run in parallel with the recruitment of SPRINTT trial participants. Indeed, BIOSPHERE participants with PF&S share the same characteristics as the “cases” enrolled in the SPRINTT trial [26],
Participant assessment was carried out through a multi-step process: (a) an interview to assess the general selection/non selection criteria; (b) the collection of medical history and assessment of all medical criteria; (c) the assessment of previous and concomitant pharmacological treatments; (d) an interview regarding the consumption of alcohol and recreational substances; (e) a clinical examination including weight, height, pulse rate, and sitting diastolic and systolic blood pressure measurement; (f) the assessment of cognitive function (Mini Mental State Examination, MMSE) [27] and mood (Center for Epidemiological Studies-Depression scale, CES-D) [28]; (g) an ECG; and (h) a fasting blood draw to assess standard biochemical and haematological parameters and to establish the biobank. Subsequently, candidate participants underwent a physical function assessment that included: (a) SPPB [20]; (b) handgrip strength test; (c) physical function questionnaires (basic [29] and instrumental [30] activities of daily living); and (d) the 400-m walk test. Finally, a whole-body DXA scan was obtained on a Hologic densitometer following the manufacturer’s protocols. Two visits were required to complete the assessments, each one ranging in time between one and three hours.
3.3. Blood sample collection
Blood samples were collected in the morning by venipuncture of the median cubital vein after overnight fasting, using commercial collection tubes (BD-Vacutainer). For serum collection, samples were left at room temperature for SO min and subsequently centrifuged at 1000 ×g for 10 min at 4°C. For plasma separation, blood samples were collected in EDTA tubes and immediately centrifuged at 1000 ×g for 10 min at 4° C. Aliquots of serum and plasma were subsequently stored at — 80 C.
3.4. Rationale for biomarker selection
3.4.1. Inflammatory biomarkers
Eight circulating inflammatory mediators [C-reactive protein (CRP), granulocyte-monocyte colony-stimulating factor (GM-CSF), interferon γ (IFNγ), interleukin (IL) 6 and 8, myeloperoxidase (MPO), P-selectin, tumour necrosis factor α (TNFα)] have been included in the BIOSPHERE panel given their previous association with low muscle mass and strength and physical function impairment in older adults [31,32].
3.4.2. C-terminal agrin fragment (CAF)
Agrin is a heparan sulfate proteoglycan synthesised in motor neurons, transported along axons and released into the synaptic basal lamina of the neuromuscular junction (NMJ), where it induces the assembly of the postsynaptic apparatus, including the clustering of acetylcholine receptors and the stabilisation of presynaptic structures [33]. The proteolytic cleavage of agrin at the NMJ by neurotrypsin produces a C-terminal 22-kDa fragment (CAF), which is released into the circulation and is therefore measurable. Previous studies have shown that serum CAF levels are increased in sarcopenic persons relative to peers with normal muscle mass and strength [34-37].
3.4.3. High-temperature requirement serine protease A1 (HtrA1)
HtrAl is a protease belonging to the broadly conserved family of HtrA proteins [38]. HtrAl is involved in the inflammatory process through inhibiting signalling of active transforming growth factor-β (TGF-β) protein family [39]. In addition to its effect on TGF-β signalling, HtrAl plays a role in the progression of several pathological processes, including macular degeneration, Alzheimer’s disease, osteoarthritis, preeclampsia, and periodontal diseases [40]. Recently, serum levels of HtrAl were found to be higher in frail older outpatients compared with robust controls [41] using either the criteria proposed by Fried and colleagues [42] or the index designed by Rockwood and Mitnitski [14]. A role for HtrAl in musculoskeletal disorders has also been proposed [38].
3.4.4. Extracellular heat shock protein 72 (eHsp72)
eHSP72 is a conserved protein expressed both constitutively and under stressful conditions. In a cohort of 665 Japanese community- dwellers aged 65-96 years, higher circulating eHSP72 levels were found to be associated with lower muscle mass, weaker grip strength, and slower walking speed [43]. Although the exact mechanisms whereby eHsp72 impacts muscle homeostasis are not fully understood, it is believed that this mediator may act through promoting inflammatory signalling and motor neuron apoptosis [25].
3.4.5. Procollagen III N-terminal peptide (P3NP)
P3NP is released during collagen synthesis and has been proposed as a marker of muscle growth, repair and remodelling [44]. In a recent cross-sectional study, linear regression analyses were used to estimate the association between circulating P3NP levels and muscle mass and strength in 687 men and women from the Framingham Offspring Study [44], Plasma P3NP concentrations were found to be inversely associated with total and appendicular muscle mass in postmenopausal women, thus suggesting its possible use as a biomarker for muscle mass at least in women [44].
In summary, we have examined 12 candidate biomarkers for PF&S pertaining to different pathways and processes linked directly or indirectly to the condition of interest (i.e., inflammation, muscle remodelling, neuromuscular junction damage, and muscle growth signalling). Analytical methods applied in BIOSPHERE are reported in Table 2.
Table 2.
Biomarkcrs selected for the BIOSPHERE panel and related analytical methods.
| Biomarker | Biological pathway in PF&S | Analytical methoda |
|---|---|---|
| CRP | Inflammation | ELLA™ |
| GM-CSF | Inflammation | Multiplex |
| HtrAl | Inflammation | ELISA |
| IFNγ | Inflammation | Multiplex |
| IL6 | Inflammation | Multiplex |
| IL8 | Inflammation | Multiplex |
| MPO | Inflammation | ELLA™ |
| P-selectin | Inflammation | ELISA |
| TNFα | Inflammation | Multiplex |
| CAF | NMJ dysfunction | ELISA |
| eHSP72 | Inflammation/motor neuron apoptosis | ELISA |
| P3NP | Muscle remodelling | ELISA |
Abbreviations: CAF, C-terminal agrin fragment; CRP, C-reactive protein; eHSP72. extracellular heal shock protein 72; GM-CSF, granulocytc-monocyte colony-stimulating factor, HtrAl, high-temperature requirement serine protease A1; IFNγ, interferon γ: IL interleukin; MPO, myeloperoxidase; P3NP, procollagen III N-terminal peptide; TNFα, tumour necrosis factor α.
Manufacturers; ELLA™, R&D Systems Inc., Minneapolis, MN; CAF ELISA, Neurotune AG, Schlieren-Zurich, Switzerland; multiplex, Bio-Rad, Hercules, CA; eHSP72, HtrAl. P3NP, and P-selectin ELISAs, MyBiosource, San Diego, CA.
3.5. Statistical analysis
In studies analysing multiple co-linear biomarkers simultaneously, a basic question is how many participants and which experiments are required for an adequately powered study. Power analysis quantifies the relationship between the number of participants and the statistical significance of the effect and, hence, it represents a way to address this optimisation prcblem. Furthermore, it can be used to decide which experiments to perform and what type of design to use for a study.
In BIOSPHERE, the analytical strategy is geared toward assessing the relationship between circulating levels of multiple biomolecules and PF&S in a relative small cohort of individuals. Moreover, since some of the assayed biomarkers are rooted into the same (patho)phy-siological pathways, their abundances are expected to be highly correlated. This makes power calculation based on the standard multivariate Hotelling’s (T2) statistics somehow problematic. Although there is no explicit formula for sample size estimation in this kind of studies, previous work from our group indicates that a sample of 200 enrolees should provide sufficient power to detect differences in serum biomarker profiles between cases and controls [45].
The analytical strategy that will be pursued in the present study could be summarised as follows: (a) preliminary evaluation of the cohort and estimation of proper sample size/diversity (assisted by experimental design techniques); (b) identification of the training set/test set; (c) exploratory data analysis to understand the characteristics of the cohort and detect anomalous observations; (d) building of a classification model; (e) validation of the model.
Principal component analysis (PCA) will be used to identify subsets and groupings of participants [46]. The evaluation of candidate biomarkers for PF&S will then be performed by constructing and validating a predictive classification model. The approach chosen in BIOSPHERE will be based on partial least squares-discriminant analysis (PLS-DA) [47], due to its versatility and ability to deal with highly correlated predictors.
The first 25 cases and 25 controls will be used as the training set for model building and the remaining participants (75 cases and 75 controls) as the validation set In addition, re sampling strategies will be used. In particular, the so-called double cross-validation strategy will be adopted [48], To rule out any possibility of chance correlation, the average results obtained from the double cross-validation procedure will be further compared with the results of permutation tests. These tests are used to obtain an empirical distribution of the classification figures of merit under the null hypothesis, and are carried out by repeating the whole modelling stage on datasets for which the class labels are randomly permuted. In BIOSPHERE permutation tests will involve 1000 randomisations. Three figures of merit will be considered: (a) number of misclassifications ( NMC), (b) the area under the receiver operating characteristic (ROC) curve (AUROC), and (c) the value of the discriminant Q2 (DQ2) under their respective null hypothesis [49,50].
This analytical strategy will serve to identify specific biomarkers for PF&S in the study participants. The validation and implementation of these biomarkers will be instrumental for adequately framing PF&S, supporting its clinical detection, enhancing the existence of its biological background, and providing a novel metric for participant follow-up over time as well as in response to specific interventions. To relate the levels of circulating analytes with clinical data, a key role will be played by so-called multi-block methods, which are a family of techniques aiming at integrating in a single model the information from multiple matrices (the “blocks” of data) collected on the same set of individuals. The idea behind multi-block approaches is to look for components (latent variables, LVs) that account for the common structure between the different matrices (and, in the case of more re cently developed methods, also for block-specific information which is relevant for the prediction). Examples of such methods are, for instance, multi-block PLS-DA, where the classification algorithm described above is applied to a “supermatrix” resulting from the concatenation of the individual data blocks after suitable scaling, or SO-PLS(DA), in which the model is built by sequentially including the discriminant information in the different blocks not yet accounted for by the preceding matrices.
4. Discussion
Over the last years, geriatrics and gerontology researchers have centred their interests in deconstructing the foundations of the “twin” conditions of PF and sarcopenia to focus on their clinical features. This approach may help in (a) defining a unique target for PF&S, (b) simplifying the operational definition, and (c) promoting the implementation of the condition in both clinical and research settings.
This scenario has led to the conception of the SPRINTT project, a multicentre randomised clinical trial testing multicomponent strategies for PF&S, approved in the context of the 9th call for proposals by the Innovative Medicines Initiative Joint Undertaking. A major output of SPRINTT has been the definition of a conceptual framework of PF&S [18], the implementation of which is expected to identify a specific “nosographical entity” for healthcare professionals, research activities, pharmaceutical industry, regulators, and policy-makers.
In parallel to the SPRINTT project, the BIOSPHERE study has been designed to enrol participants with the clinical characteristics of the SPRINTT “cases” (age ≥ 70 years, SPPB score between 3 and 9, low muscle mass, absence of major mobility disability) as well as non-sarcopenic non-frail controls. The multi-marker approach adopted in BIOSPHERE deeps its roots in previous studies from our group. An analytical strategy similar to the one here described has been applied to study the association between a set of inflammatory markers and physical performance in older adults [45], In this previous investigation, community-dwelling older persons were categorised as “normal walkers” (NWs; n = 27) or “slow walkers” (SWs; n = 11) using 0.8 m/s as the 4-m gait speed cut-off. A panel of 14 inflammatory markers, growth factors, and vascular adhesion molccules, related to systemic and/or vascular inflammation were measured via a multiplex, magnetic bead-based immunoassay. Subsequently, PLS-DA was applied to identify patterns of inflammatory mediators associated with gait speed categories. The optimal complexity of the PLS-DA model was found to be five LVs. The proportion of correct classification was 88.9% for NW participants (74.1% in cross-validation) and 90.9% for SW individuals (81.8% in cross-validation). Six discriminant biomarkers were identified by the model. Among them GM-CSF, INFγ, and P-selectin were higher in the NVV group, whilst IL8, MPO, and TNFα were higher in SW participants. The distribution of NMC and AUROC, and the DQ2 value under their respective null hypothesis, as estimated by permutation tests, showed that the results of the PLS-DA classification model were statistically significant
In a subsequent study from our group, multi-block PLS-DA was employed to explore the relationship among inflammatory profiles and functional and imaging parameters in persons of different ages and varying levels of physical performance [51]. The optimal complexity of the PLS-DA model was found to be two LVs. The proportion of correct classification was 92.3% in calibration, 84.6% in internal validation, and 82.6% in external validation. Compared with young control participants (mean age: 23.4 ± 3.9 years; n = 17), older individuals (mean age: 78.1 ± 5.9 years; n = 35) were characterised by smaller thigh muscle volume, greater intermuscular fat volume, lower muscle strength, and higher levels of MPO, P-selectin, soluble intercellular adhesion molecule 1, and vascular cell adhesion molecule 1. The model was also able to discriminate between older adults scoring > 8 on the SPPB from those with poorer physical performance. In particular, older participants with SPPB score ≤ 8 were characterised by smaller thigh muscle volume, greater subcutaneous adipose tissue volume, lower muscle strength, and higher circulating levels of GM-CSF, IL1β, 6, 10, 12. 13, and TNFα [51].
Taken as a whole, these preliminary findings indicate that specific patterns of circulating biomarkers characterise older people with different body composition and varying levels of physical performance. What is more, the multivariate analytical strategy adopted allowed overcoming the “one mediator fits all” paradigm and identified robust relationships between biomolecule clusters and physical function levels.
Although pruposing an innovative biomarker discovery methodology, BIOSPHERE has some limitations that need to be acknowledged. The cross-sectional design will not allow for inferring about the time course of changes in biomarkers and the progression of PF&S over time or in response to specific interventions. Furthermore, the study is associative in nature and no definite cause-effect relationship may be implied between the investigated biomarkers and PF&S pathophysiology. Finally, since the purpose of BIOSPHERE is to identify possible biomarkers for PF&S, eligibility criteria were somewhat restrictive. This approach will not allow for extending the results to severely ill, multimorbid older persons. These considerations extend to the proposed statistical plan. Indeed, the models that will be built for individual blocks and those obtained after data fusion will capture the variation spanned by the study design, with all the limits described above. On the other hand, the presence of a wider spectrum of phenotypical/pathological conditions would call for different and arguably more complicated integration approaches.
5. Conclusions
So far, the identification of sarcopenia and frailty has been based on clinical, functional and imaging parameters. However, it is conceivable that specific biological markers may be used to identify/characterise these conditions. Although some circulating biomolecules have been associated with sarcopenia and/or frailty, the assessment of such mediators has not yet been incorporated into clinical practice, partly because there is not a “gold standard” biomarker that reliably predicts functional impairment in older adults. Given the multi factorial nature of sarcopenia and frailty, multivariate modelling of an array of circulating mediators may represent the optimal strategy to identify a set of biomarkers that characterise sarcopenia and frail older people. Built upon such a multi-marker paradigm. BIOSPHERE aims at determining and validating a panel of biomarkers that will serve for (a) integrating specific biochemical measurements into the clinical assessment of PF& S, (b) providing hints to the biological pathways leading to functional impairment in old age, (c) identifying novel targets for interventions, and (d) determining surrogate endpoints to be used in clinical and research settings.
Acknowledgements
This work was supported by grants from Fondazione Roma to R.B. and A.P. (NCDs Call for Proposals 2013), Innovative Medicine Initiative-Joint Undertaking to R.C., M.C, F.L, and E.M. (IMI-JU #115621), Catholic University of the Sacred Heart to M.B. and F.L. (D3.2 2013 and D3.2 2015), Istituto Banco di Napoli-Fondazione 2015 to A.M.S.L, and the Claude D. Pepper Older Americans Independence Center at the University of Florida’s Institute on Aging to C.L (NIA 1P30AG028740). The work was also partly supported by the nonprofit research foundation “Centro Studi Achille e Linda Lorenzon”.
The funders had no role in study design, data collection and analysis, preparation of the manuscript, or decision to publish.
R.B., M.C, R.C. F.L, and E.M. are partners of the SPRINTT Consortium, which is partly funded by the European Federation of Pharmaceutical Industries and Associations (EFPIA). M.C served as a consultant for and/or received honoraria for scientific presentations from Nestlé, 4D Pharma PLC, and Pfizer, he also received a research grant from Pfizer. R.C served as a consultant for Novartis. ILM. served as a consultant for Huron Consulting Group, Genactis, and Novartis.
Footnotes
Competing interests statement
References
- [1].WHO Global health and ageing. WHO; 2015. Available at http://www.who.int/ageing/publications/global_health.pdf (Accessed on March 23, 2018).
- [2].Bien B McKee KJ, Döhner H. Triantafillou J Lamura G. Doroszkiewicz H, et al. Disabled older people’s use of health and social care services and their unmet care needs in six European countries. Eur J Public Health 2013;23:1032–8. 10.1093/eurpub/cksl90. [DOI] [PubMed] [Google Scholar]
- [3].Cesari M Marzetti E. Thiem U, Pérez Zepeda MU. Abelian Van Kan G. Landi F, et al. The geriatric management of frailty as paradigm of the end of the disease era. Eur J Intern Med 2016;31:11–4. http://dx.doi.offc/10 1016/j ejim.2016.03.005. [DOI] [PubMed] [Google Scholar]
- [4].Marzetti E, Calvani R, Cesari M, Tosato M, Cherubini A, Di Ban M, et al. Operationalization of the physical frailty A sarcopcnia syndrome: rationale and clinical implementation. Transi Med. UniSa 2015;13:29–32. [PMC free article] [PubMed] [Google Scholar]
- [5].No authors listed. Epidemiologic and méthodologie problems in determining nu tritional status of older persons. Proceedings of a Conference- Albuquerque, New Mexico, October 19-21, 1988.50 1989. p. 1121–235. Am J Clin Nutr [PubMed] [Google Scholar]
- [6].Landi F, Calvani R, Cesari M, Tosato M, Martonr AM, Orlolani E, et al. Sarcopenia: an overview on current definitions, diagnosis and treatment. Cun Protein Pept Sci 2017; 18 10.2174/138920371B666I706a7U3459. [Epub ahead of print ]. [DOI] [PubMed] [Google Scholar]
- [7].Scott D, Hiyes A, Sanders KM, Aitkcn D, Ebcling PR, Joncs, Operational défini tions of sarcopcnia and their associations with 5-year changes in falls risk in com munity dwelling middle aged and older adults. Osteoporos Int 2014;25:187–93. 10.1007/s00198-013-24315. [DOI] [PubMed] [Google Scholar]
- [8].Bisrhoff Ferrari HA, Orav JE, Kanis JA, Rizzoli R, Schlogl M, Staehelin HB, et al. Comparative performance of current definitions of sarcopenia against the pro spective incidence of falls among community dwelling seniors age 65 and older. Osteoporos Int 2015;26:2793–80Z http:/dx dot org/ 10.1007/s00198-015-3194 y. [DOI] [PubMed] [Google Scholar]
- [9].Cao L, Mor ley JE. Sarcopenia is recognized as an independent condition by an international classification of disease, tenth revision, clinical modification (ICD-10- CM) code. J Am Med Dir Assoc 2016;17:675–7. http://dx doi.org/101016/j jamda. 2016.06.001. [DOI] [PubMed] [Google Scholar]
- [10].Marzctti F, Calvani R, Tosato M, Cesari M, Di Bari M, Cherubini A, et al. Sarcopenia: an overview. Aging Clin Exp Res 2017;29:11–7. http/dx doi.org/l0.l007/s40520 016-0704-5. [DOI] [PubMed] [Google Scholar]
- [11].Clegg A, Young J, Iliffe S, Rikkert MO. Rockwood K. Frailty in elderly people. Loncet 2013;381:752–62. http://dx.doi. org/10 1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rodriguez Mñas L, Feart C, Mann G, Viña J, Chatterji S, Chodzko-Zajko W, et al. Searching for an operational definition of frailty: a Delphi method based consensus statement The frailty operative definition consensus conference project. J Gerontol A Biol Sci MedSd 2013,68:62–7. 10.1093/gerona/gts119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Theou O Brothers TD, Peña FG, Mitnitski A, Rockwood K, Identifying common characteristics of frailty across seven scales. J Am Gcriatr Soc 2014;62:901–6. 10.1111/jgs.12773. [DOI] [PubMed] [Google Scholar]
- [14].Rockwood K Song X, MacKnight C, Bergman H, Hogan DB, McDowell I. et al. A global clinical neasure of fitness and frailty in elderly people. Can Med Assoc J 2005;173:489–95. 10.1503/cmaj050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dent E, Kowal P, Hoogendijk EO, Frailty measurement in research and clinical practice a review. Eur J Intern Med 2016;31:3–10. 10.1016/j.ejim.2016.03.007. [DOI] [PubMed] [Google Scholar]
- [16].Cesari M, Landi F, Vcllas B, Bemabci R, Marzctti E, Sarcopenia and physical frailty: two sides of the same coin. Front Aging Neurosci 2014;6:192 http://dx.doi.org/10 3389/fnagi.2014.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Landi F, Calvani R, Cesari M, Tosato M, Martonc AM, Bemabei R, et al. Sarcopcnia as the biological substrate of physical frailty. Clin Gcriatt Med 2015;31:367–74. http://dx.doi org/10.1016/j.cger.201 5.04.005. [DOI] [PubMed] [Google Scholar]
- [18].Cesan M, Landi F, Calvani R, Cherubini A, Di Ban M, Kortcbctn P, et al. Rationale for a preliminary operational definition of physical frailty and sarcopenia in the SPRINTT trial. Aging din Exp Res 2017:29:81–8 10.1007/s40520016-0716-1. [DOI] [PubMed] [Google Scholar]
- [19].Studenski SA, Fetcrs KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNUI car copen a project: rationale, study descripción, conference recomm en da tions. and final estimates. J Gerontol A Biol Sci Med Sci 2014;69A:547–58. 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Guralnik JM, Simonsick EM, Femicci L, Glynn RJ, Berkman IT. Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-rcponed disability and prediction of mortality and nursing home admis ston. J Gcrontcl 1994;49:M85–94. http://dx.doi org/10.l093/geronj/49.2.M85. [DOI] [PubMed] [Google Scholar]
- [21].Newman AB, Simonsick EM, Naydcck BL, Boudreau RM, Kritchevsky SB, Neviti MC, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability JAMA 2006,295:2018 10.1001/jantiL295.17.2018. [DOI] [PubMed] [Google Scholar]
- [22].Marzetti E, Calvani R, Landi F, Hoogendijk FO, Fougère B, Vellas B, et al. Innovative medicines initiative: the SPRINTT project. J Frailty Aging 2015;4:207–8. [PMC free article] [PubMed] [Google Scholar]
- [23].Calvani R Manni F, Cesari M, Tosato M, Picca A, Anker SD, et al. Biomarkers for physical frailty and sarcopenia. Aging din Exp Res 2017;29:29–34. http://dx.doi org/10 1007/s40520-016-0708-1. [DOI] [PubMed] [Google Scholar]
- [24].Calvani R Picca A, Cesari M, Tosato M, Marini F, Manes C, ravina E, et al. Biomarkers for sarcopenia: reductiomsm vs complexity. Cun. Protein Pept. Set 2017. http://dx.doi.org/l0.2174/1389203718666170516115422. [Epub ahead of print ]. [Google Scholar]
- [25].Calvani R Manni F, Cesari M, Tosato M, Anker SD von llaehling S, et al. Biomarkcrs for physical frailty and sarcopcnia: state of the science and future de vclopments. J Cachexia Sarcopenia Muscle 2015;6:278–86. http://dx.doi.org/10. 1002/jesm. 12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Landi F, Cesari M, Calvani R, Cherubini A, Di Bari M, Bejuit R, et al. The “sarcopcnia and physical ftailty IN older people: multi componenT treatment strategies” (SPRINTT) randomized controlled trial: design and methods. Aging Clin Exp Res 2017;29:89–100. http://dx.doi.org/10 1007/s40520 016-0715-2. [DOI] [PubMed] [Google Scholar]
- [27].Folstcin MF, Folstein SE, McHugh PR “Mini mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- [28].Radloff LS. The CES-D scale. Appl Psychol Mcasur 1977;1:385–401. http://dx.doi org/10.1177/014662167700100306. [Google Scholar]
- [29].Katz S Ford AB, Moskowrtz RW, Jackson BA, Jaffe MW, Studies of illness in the aged. The index of ADL a standardized measure of biological and psychosocial function JAMA 1963;185:914–9. [DOI] [PubMed] [Google Scholar]
- [30].Lawton MP. Brody EM Assessment of oklet people self maintaining and instru mental activities of daily living. Gerontologist 1969;9:179–86. [PubMed] [Google Scholar]
- [31].Centl M Penninx BWJH, Pahor M, Lauretani F, Cora AM, Rhys Williams G, et al. Inflammatory markers and physical performance in older persons: the InCIHANTl study. J Gerontol A Biol Sci Med Sci 2004:9:242–8. [DOI] [PubMed] [Google Scholar]
- [32].Formed L, Peiminx BWJH, Volpato S, Harris TB, Bandeen Roche K, Balfour J. et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Gcriatr Soc 2002;50:1947–54. [DOI] [PubMed] [Google Scholar]
- [33].Stephan A, Mareos JM, Kozlov SV, Cinelli P, Kistler AD, Hettwer S, et al. Neurotrypsin cleaves agrin locally at the synapse. FASEB J 2008;22:1861–73. 10.1096/ij.07-100008. [DOI] [PubMed] [Google Scholar]
- [34].Hettwer S, Dahinden P Kucsera S, Farina C, Ahmed S. Fariello R. et al. Elevated level of a C terminal agrin fragment identifies o new subset of sarcopcnia patients. Exp Gerontol 2013;48:69–75. http://dx.doi org/10.1016/j.exger 2012.03.002. [DOI] [PubMed] [Google Scholar]
- [35].Drey M, Sicber CC, Bauer JM, Uter W, Dahinden P, Fariello RG, et al. C-tcrminal Agrin fragment as a potential marker for sarcopenia caused by degeneration of the neuromuscular j jnction. Exp Gerontol 2013;48:76–80. 10.1016/j.exger.2012.05.021. [DOI] [PubMed] [Google Scholar]
- [36].Marzetti E, Calvani R, Lorenzi M, Marini F, D’Angelo E, Martone AM, et al. Serum levels of C terminal agrin fragment (CAT) are associated with sarcopenia in older hip fractured paiients. Exp Gerontol 2014;60:79–82. http://dx.doi.0rg/lO.lOl6/j. exger.2014.10.003. [DOI] [PubMed] [Google Scholar]
- [37].Landi F, Calvani R, Lorenzi M, Martone AM, Tosato M, Drey M, et al. Serum levels of C-tcrminal agrin fragment (CAF) are associated with sarcopenia in older multi - morbid community-dwellers: results from the ilSIRENTE study. Exp Gerontol 2016;79:31–6. http://dx.doi org/10.1016/j.exger.2016.03.012. [DOI] [PubMed] [Google Scholar]
- [38].Tiaden AN, Richards PJ. The emerging roles of IITRA1 in musculoskeletal disease. Am J Pathol 2013;182:1482–8. http://dx.doi.0rg/lO.lOl6/j ajpath.2013.02.003. [DOI] [PubMed] [Google Scholar]
- [39].Oka C, Tsujimon R, Kajikawa M, Koshiba Takeuchi K, Ina J, Yano M, et al. HtrAl serine protease inhibits signaling mediated by Tgfbeta family proteins. Development 2004;131:1041–53. http://dx.doi org/10.l242/dev.00999. [DOI] [PubMed] [Google Scholar]
- [40].Skorko Glonek J. Zurawa Janicka D, Koper T, Jarzab M, Figaj D, Glaza P, et al. HtrA protease family as therapeutic targets. Curr. Pharm. Des 2013;19:977–1009. http://dx.doi.org/l0.2174/1381612811319060003. [DOI] [PubMed] [Google Scholar]
- [41].Lorenzi M, Lorenzi T, Marzetti E, Landi F, Vetrano DL, Settanni S, et al. Association of frailty with the serine protease lltrAl in older adults. Exp Gerontol 2016;81:8–12. http://dx.doi org/10.1016/j.exger.2016.03.019. [DOI] [PubMed] [Google Scholar]
- [42].Fried LP, Tangen CM, Walston J, Newman AB, llirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sti 2001;56;M146–56. 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- [43].Ogawa K, Kim H, Shimizu T, Abe S, Shiga Y, Calderwood SK. Plasma heat shock protein 72 as a biomarker of sarcopenia in elderly people. Cell Stress Chaperones 2012;17:349–59. http://dx.doi.org/10 1007/sl 2192-011 0310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Berry SD, Ranuchandran VS, Cawthon PM, Gona P, McLean RR, Cupples LA, et al. Procollagen type III N-terminal peptide (P3NP) and lean mass: a cross-sectional study. J Frailty Aging 2013;2:129–34. [PMC free article] [PubMed] [Google Scholar]
- [45].Marzetti E, Lardi F, Marini F, Cesari M, Buford TW, Manini TM, et al. Patterns of circulating inflammatory biomarkers in older persons with varying levels of phy-sical performance: a partial least squares-discriminant analysis approach. Front Med 2014;1:27.http: / dx.doi org/10.3389/fmed.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].<j />Shaffer RE. Multi and megavariate data analysis. Principles and applications. In: Eriksson I, Johansson E, Kettaneh Wold N, Wold S, editors. Umetrics Academy, Umea, 2001. 16. J Chcmom; 2002. p. 261 2. ISBN 91 973730 1 X, 533pp 10.1002/cem.713. [DOI] [Google Scholar]
- [47].Barker M, Rayens W. Partial least squares for discrimination. J Chemoractr 2003;17:166–73. http://dx.doi.org 10 10002/cem.785. [Google Scholar]
- [48].Westerhuis JA, Hoefsloot HCJ, Smit S, Vis DJ, Smilde AK, van Velzen EJJ, et al. Assessment of PLSDA cross validation. Metabolomics 2008;4:81–9. http://dx.doi. org/10.1007/sl 1306-007-0099-6. [Google Scholar]
- [49].Westerhuis JA, van Velzen EJJ, Hoefsloot HCJ, Smilde AK Discriminant Q2 (DQ2) for improved discrimination in PLSDA models. Metabolomics 2008;4:293–6. 10.1007/sll306-008-0126-2. [DOI] [Google Scholar]
- [50].Szymariska E, Saccenti E, Smilde AK, Westerhuis JA. Double check: validation of diagnostic statistics for PLS DA models in metabolomics studies. Metabolomics 2012;8:3–16. 10.1007/sl1306-011-0330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Calvani R, Marini F, Cesari M, Buford TW, Manini TM, Pahor M, et al. Systemic inflammation, sody composition, and physical performance in old community dwellers. J Cachexia Sarcopenia Muscle 2017;8:69–77. 10.1002/jcsm.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]


