Abstract
Plantar fibromatosis (Ledderhose disease) is a rare, benign, hyperproliferative fibrous tissue disorder resulting in the formation of nodules along the plantar fascia. This condition can be locally aggressive, and often results in pain, functional disability, and decreased quality of life. Diagnosis is primarily clinical, but MRI and ultrasound are useful confirmatory adjuncts. Given the benign nature of this condition, treatment has historically involved symptomatic management. A multitude of conservative treatment strategies supported by varying levels of evidence have been described mostly in small-scale trials. These therapies include steroid injections, verapamil, radiation therapy, extracorporeal shock wave therapy, tamoxifen, and collagenase. When conservative measures fail, surgical removal of fibromas and adjacent plantar fascia is often done, although recurrence is common. This review aims to provide a broad overview of the clinical features of this disease as well as the current treatment strategies being employed in the management of this condition.
Keywords: plantar fibromatosis, plantar fascia, Ledderhose disease
Introduction
Plantar fibromatosis (PF), or Ledderhose disease as it is eponymously known, is a rare pathology of the plantar aponeurosis characterized by disordered fibrous tissue proliferation and the subsequent formation of nodules.1 Compared with Dupuytren’s disease, the upper extremity analog of PF, relatively little has been published since German physician Georg Ledderhose first described his initial observations of 50 cases in 1897.2 However, more recent literature has elucidated a framework for clinical diagnosis and optimal treatment of this condition. This review, based on available studies devoted to PF from the US National Library of Medicine and National Institutes of Health database (PubMed.gov), describes the relevant anatomy, diagnostic workup, and approach to treatment for the uncommon and challenging disorder.
Anatomy and biomechanics of the plantar fascia
The plantar fascia is a broad fibrous aponeurosis that originates from the medial and anterior aspects of the calcaneus, divides into five digital slips at the metatarsophalangeal joints, and inserts distally into the periosteum at the base of the proximal phalanges (Figure 1).3,4 It is composed of three separate bands of dense connective tissue: central, medial, and lateral.5 PF is most often present on the medial and central bands.6
Figure 1.
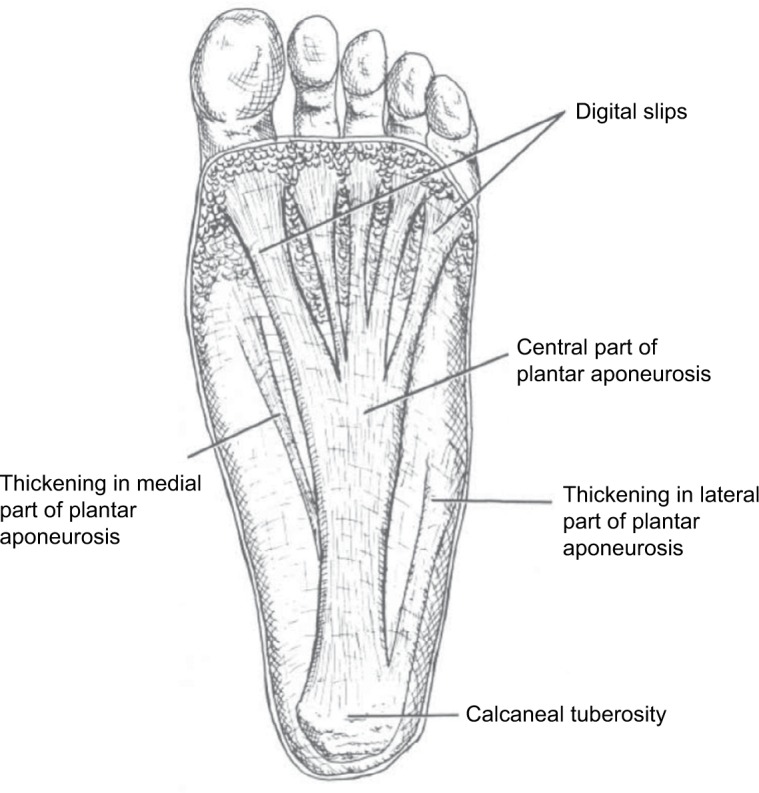
Anatomy of the plantar fascia.
Notes: Adapted from Gramatikoff.42
The primary function of the plantar fascia is to maintain the longitudinal plantar arch.7 This is accomplished in part due to the plantar fascia’s great tensile strength, especially with weight bearing. When an individual dorsiflexes his or her toes, the plantar fascia tightens, the distance between calcaneus and metatarsals is decreased, and the medial longitudinal arch is elevated. This dynamic mechanism has been described as the windlass mechanism, reflecting its similarity to tightening a cable in an efficient, predictable manner. This series of events is critical for maintenance of the gait cycle, where even minor arch collapse can cause great inefficiency in ambulation.3
Epidemiology
The prevalence and etiology of PF is not currently understood.8,9 Though the number of people afflicted has not been precisely assessed, the disease continues to appear on the National Institute of Health’s list of rare diseases affecting <200,000 people.10 Furthermore, multiple studies have suggested that for those affected, this condition negatively affects quality of life and causes great functional disability.8
PF affects primarily those in their middle age, although several cases have been described in children <16 years of age, and even as young as 9 months.11 Men are more often affected than women.12,13 Bilateral disease is present ~25% of the time.9 It often appears concomitantly with hyperproliferative fibromatosis of other appendages, such as Dupuytren’s disease in the hands, Peyronie’s disease in the penis, or keloid formation more generally. Other associated conditions include frozen shoulder, alcohol addiction, diabetes, epilepsy, smoking, repeated trauma, and long-term phenobarbital use.9,14 One recent genome-wide association study has suggested a possible genetic predisposition to plantar fascial disorders, including PF.15
Clinical presentation
The characteristic nodule in PF is approximately 0.5–3.0 cm in diameter, slow-growing, and located in the medial or central plantar aponeurosis.16,17 These nodules typically do not affect smooth muscle tissue or skin, and thus, do not usually result in the contractions commonly seen in the palmar fascia with Dupuytren’s disease.16 However, contracture of the toes, including the great toe, has been reported in some instances with severe proliferation and infiltration of the nodule.12,18 In general, symptoms range from local pressure and distention, to painless nodules, to tender, erythematous lesions that can affect the patient’s ability to bear weight.16,19 The primary symptom most patients experience is a slow-growing lump along the medial longitudinal arch. While the mass is initially painless, it becomes painful as it enlarges. Restrictive shoes, direct pressure on the mass, walking barefoot, and standing for long periods of time may exacerbate one’s discomfort. Multiple fibromas may develop over time and can contribute to an exacerbation in symptoms.16,19
Physical examination is of paramount importance in the diagnosis of plantar fascia fibromatosis. The practitioner must perform a visual assessment of the foot, which can identify swelling, skin breakdown, bruising, or deformity. Bony prominences must be palpated along with tendinous insertions along the heel and midfoot. The presence of an Achilles tendon or gastrocnemius contracture must also be noted, as it can contribute to one’s symptoms. Ankle and hindfoot motion must also be documented, as well as the patient’s gait. The differential diagnosis must include calcaneal stress fracture, tarsal tunnel syndrome, and plantar fasciitis.
Although the presence of a single (or multiple) well-defined nodules along the plantar fascia is pathognomonic for fibromatosis, other pathology may be present concurrently. The squeeze test can identify a calcaneal stress fracture, and is performed with the examiner performing medial and lateral heel compression along the posterior tuberosity of the calcaneus. Swelling and warmth may also be present. Tarsal tunnel is identified by the presence of pain and numbness that radiate to the plantar heel with percussion of the tibial nerve in the tarsal tunnel. Plantar fasciitis presents with tenderness over the medial aspect of the calcaneal tuberosity.
The natural history of the disease has been described as three distinct phases.1 Initially, there is a proliferative phase characterized by increased fibroblastic activity and cellular proliferation. An active phase follows, in which nodule formation occurs. Finally, there is a residual phase marked by collagen maturation, scar formation, and tissue contracture.
Though diagnosis of PF is based on history and physical examination, imaging is useful in confirmation and, in some cases, a biopsy may be indicated to rule out malignancies.19
Imaging
PF is easily distinguished on imaging from other lesions affecting the plantar fascia. Ultrasound and MRI are both acceptable imaging modalities to aid in the diagnosis of plantar fibromas. On an MRI, plantar fibromas appear as focal, oval-shaped areas of disorganization embedded in the plantar fascia; however, larger, more lobulated lesions continuous with the plantar fascia are also recognized. Oftentimes, these lobulated lesions are of low signal intensity due to their fibrous nature although signal isointense with the muscle can also be observed (Figure 2).20 There is variable enhancement with gadolinium administration.21
Figure 2.
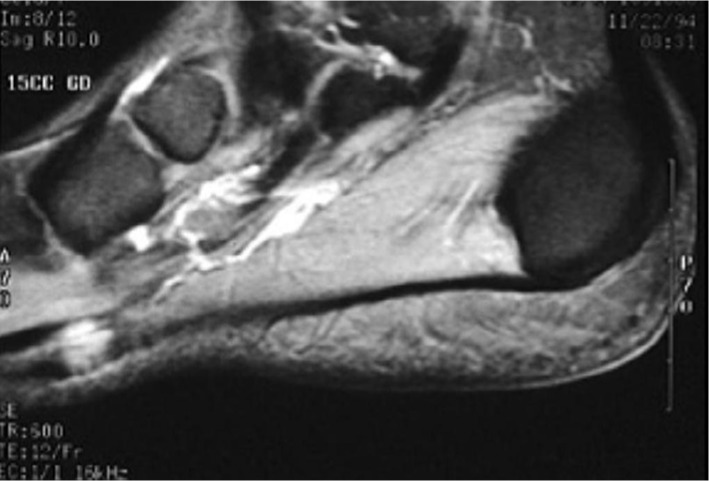
Sagittal T2 MRI demonstrating a plantar fascia fibroma.
Note: The fibroma has low-to-intermediate signal relative to muscle. (Reproduced with permission; Case courtesy of Radswiki, Radiopaedia.org, rID: 11776).43
On ultrasound, characteristic presentation of PF involves multiple lesions embedded on the plantar fascia, with sharp juxtaposition between the less reflective fibroma and the much brighter plantar fascia surrounding it (Figure 3).20 Doppler ultrasound rarely shows vascular flow inside the lesion.22 Unlike MRI, ultrasound allows the physician to better differentiate small lesions from the plantar fascia, as the contrast is more pronounced between the two structures.20 It also allows the physician to examine both feet simultaneously in a time- and cost-efficient manner without reducing in-plane resolution.22
Figure 3.
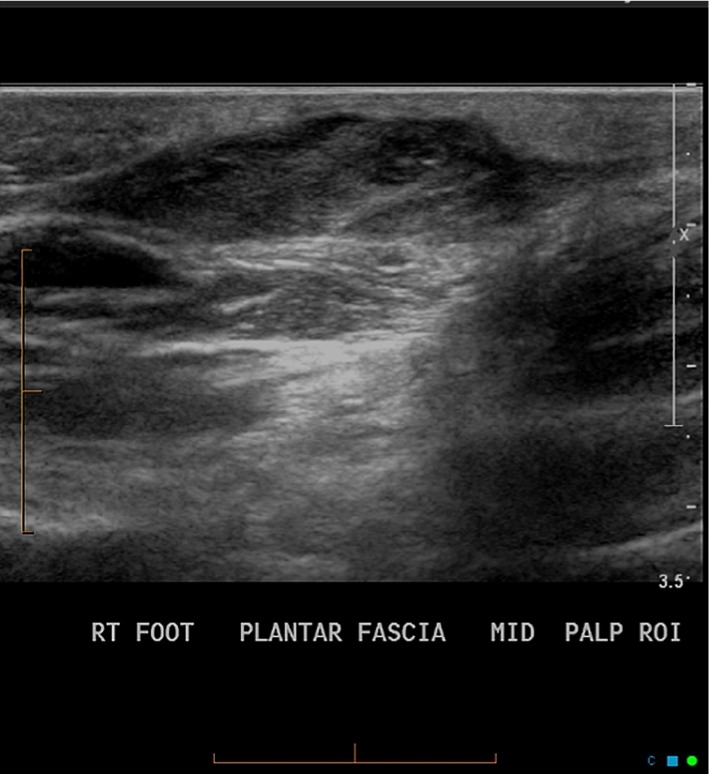
Plantar fibroma as seen on ultrasound.
Note: Reproduced with permission; Case courtesy of Dr Chris O’Donnell, Radiopaedia.org, rID: 30471.44
Abbreviation: RT, right.
Recent advances in spatial and contrast resolution in musculoskeletal ultrasounds have allowed physicians to better characterize plantar fibromas. Cohen et al described a novel morphologic appearance of plantar fibromas for which they coined the term “Comb Sign”; this was visible in 51% of the cases studied. 23 This sign is defined as alternating linear bands of hypoechogenicity and isoechogenicity relative to the plantar fascia, similar to the alternations between teeth on a comb. This sign likely demonstrates the hyperechoic, fibrous regions of the fibroma on a background of hypoechogenic cellular matrix.23
Non-operative management
Several non-surgical options exist for the symptomatic management of PF, with varying degrees of scientific evidence to support their use. Many of these modalities have been used with differing degrees of success for other hyperproliferative fibrous tissue disorders. Given the low morbidity associated with many of these measures, it is prudent for the physician and the patient to use conservative measures prior to recommending surgery.
Steroid injections
Steroid injections are common as an initial treatment strategy in the management of PF. The objective of the treatment is to shrink the size of the nodules or fibromas, thus decreasing the associated pain experienced with ambulation. Steroid injections work by decreasing the expression of VCAM1 and altering the production of pro-inflammatory cytokines TGF-β and bFGF.9 These biochemical changes decrease inflammation, contracture rates, and growth rates, which result in smaller, less painful nodules. Prior studies have shown that these results may be brief, as recurrence of the nodule to its original size has been observed within the first 3 years after treatment.9,19,24 For this reason, many patients elect to have multiple rounds of injections for continued symptomatic management. Current recommendations for intralesional steroid injections call for a total of 3–5 injections administered approximately 4–6 weeks apart at a concentration of 15–30 mg per nodule.9 Exact dosage for optimal results has yet to be determined. Patients should be counseled that the use of multiple injections has been associated with an increased risk of fascial or tendon rupture.25
Interestingly, the pathophysiology of hyperkeratotic scars and fibroma formation is quite similar as both are highly influenced by the expression of TGF-β and bFGF. Thus, certain treatments for hyperkeratotic scars have been employed on fibromas. One recent study demonstrated that using steroid injections along with verapamil on hypertrophic scars was more effective at reducing the size of the scar than either treatment alone.26 This synergistic effect demonstrates the overlap in management between different hyperproliferative, fibrous tissue disorders. Verapamil as an independent treatment modality will be subsequently discussed.
Verapamil
Verapamil is a calcium channel blocker typically used for blood pressure management, but it also plays a vital role in the metabolism of the extracellular matrix. It inhibits collagen production and increases the activity of collagenase, which, in turn, decreases the contractile function of fibroblasts and myofibroblasts. Verapamil has also been shown to exhibit anti-inflammatory properties by altering the release of pro-inflammatory cytokines interleukin (IL)-6 and IL-8.9
Anecdotally, verapamil has been used as a first-line treatment in the conservative management of PF; however, there is little published data assessing its efficacy.9 One study has shown that 15% transdermal verapamil cream and intralesional verapamil can decrease plaque size in Peyronie’s by up to 55%–85%, with the only adverse effect observed being contact dermatitis.9 Treatment recommendations for Peyronie’s include using the transdermal cream twice a day for 9 months, or one intralesional injection every other week.9,27 Based on the similar pathophysiology of Peyronie’s and PF, it is reasonable to consider verapamil as an initial primary or adjunct treatment in conservative management of the latter.
Radiation therapy
Radiation therapy is another non-surgical modality, which has been employed for the treatment of PF; however, there is little published data on the efficacy of this modality and its direct mechanism of action is not fully understood.9,25 Ionizing radiation is believed to reduce the proliferative activity of fibroblasts via disruption of TGF-β production by those cells.9,28 This affects cellular development by slowing cellular growth, which causes slowing of the disease progression. Hence, radiation therapy has been shown to be most effective in the early stages of the disease.9,25,28 Current treatment guidelines suggest weekly doses of 3.0 Gy for 5 weeks followed by one additional session after 6 weeks, for a total of 30.0 Gy.9 Documented adverse effects include red/dry skin, lethargy, local edema, and local pain.19,28,29 As with any form of radiation therapy, there is a minor increase in cancer risk, with studies demonstrating an approximately 0.5%–1.0% risk of soft tissue sarcoma or skin cancer after latency periods of 8–30 years.9,29
One recent study has demonstrated that after treatment with radiation, one-third of patients with PF had complete remission of their nodules and slightly more than half of the patients had partial remission. Nearly two-thirds of patients reported pain remission and gait improvement as well.9,28 Another study evaluating patient outcomes demonstrated that 94% of patients reported minimal toxicity and a high rate of satisfaction with radiation treatment.30 Radiation treatment in PF has been shown to be an effective modality for decreasing the size of the nodules and alleviating associated symptoms and can be a possible treatment option for those patients pursuing conservative treatment measures.
Extracorporeal shock wave therapy
Extracorporeal shock wave therapy (ESWT) is a relatively new type of treatment for many musculoskeletal disorders.31,32 Shock waves mimic various mechanical loading conditions and cause a biochemical response in tendon fibroblasts. It is thought that ESWT plays a role in tendon metabolism by stimulating the biosynthesis of the extracellular matrix in tenocytes. After treatment with ESWT, biochemical signals like TGF-β and insulin-like growth factor 1 become overexpressed, which suggests that tendon tissue can convert shock wave stimulation into biochemical signals.31 It is this increased production of extracellular matrix components that helps in counteracting the maturation process of myofibroblasts and leads to decreased tissue contraction.31,33
Like many of the other conservative therapies, ESWT has been shown to be effective in Peyronie’s disease and Dupuytren’s contracture, but there is limited published data supporting its use in PF. ESWT has not been shown to change the physical size of the nodules, but has been able to reduce pain and soften the fascia and nodules as early as 2 weeks after initiation of treatment.9,19,31,32,34 Although there is variability in the protocol for the treatment in terms of devices, energy, and frequency, focused shock waves can be considered a valid therapeutic option and an effective tool for pain relief and improved functionality.19,31,32
Tamoxifen
Estrogen plays many roles in the body, including that of increasing the contractile properties of certain cell types. For this reason, antiestrogen therapy has been proposed as a treatment for PF.9 Although there have been no in vivo studies assessing its efficacy, tamoxifen, a selective estrogen-receptor modulator, has been successfully studied in vitro.9,19 Fibroblasts were isolated from patients with Dupuytren’s and exposed to tamoxifen for 5 days. After the treatment period, those cells showed decreased rates of contractures compared with cells not treated with an antiestrogen.9,19 Studies have also shown that tamoxifen is effective in inhibiting the release of TGF-β, which, in turn, reduces the proliferative activity of fibroblasts.19
The decreases in both contracture rates and proliferative activity of fibroblasts show that antiestrogen therapy has promise as a conservative treatment for PF. Another study involving Dupuytren’s patients showed that 15%–20% reported nodule size regression and 25%–30% reported no further increase in nodule growth after treatment with an antiestrogen.35 Thus, using antiestrogens like tamoxifen for patients with PF may help to prevent the progression of the disease.
Collagenase
Collagenase is a matrix metalloprotease that breaks down peptide bonds in order to dissolve interstitial collagen. Collagenase Clostridium histolyticum (CCH) is a mixture of two collagenases (AUX-1 and AUX-2) that has been shown to decrease contractions in Peyronie’s and Dupuytren’s and is currently being studied as a treatment option for PF.9,36 A recent study tested the effectiveness of CCH by injecting it into a nodule once a month for 3 months; this failed to improve nodule size, softening, or pain with ambulation.36 It is likely that the anatomical nature of PF (nodules) compared with that of Peyronie’s and Dupuytren’s (plaques and cords) plays a role in the ineffectiveness of CCH for treatment of the former. The only documented adverse effects of this treatment are erythema, ecchymosis, and pain at the injection site.9 More studies are necessary to further evaluate CCH as an effective treatment modality.
Operative management
Given the benign nature of this condition, surgical management has generally been reserved for pain relief. Today, indications for surgery include both pain refractory to conservative treatments as well as local aggressiveness of the lesion. Dating back to at least the 1950s, concerns have existed over possible recurrence with only partial excision. For this reason, complete plantar fasciectomy had historically been the procedure of choice in the treatment of plantar fibromas.13
More recently, three main techniques have been employed in the surgical management of plantar nodules: local excision, wide excision, and complete fasciectomy. Multiple studies have demonstrated that local excision of the nodule has the highest rate of recurrence, ranging from 57% to 100%.2,9,37 Wide excision involves the removal of a 2–3 cm margin of surrounding tissue along with the nodule. The rate of recurrence with wide excision was found to be slightly lower than local excision at approximately 8%–80%.2,9,37 Removing the entire plantar fascia has the lowest rate of recurrence, at approximately 0%–50%.2,9,37 Some surgeons have also advocated for the use of partial fasciectomy, noting the ability to remove diseased tissue along with a cuff of normal fascia with less presumed morbidity than total fasciectomy. Kadir and Chandraskar reported on 18 patients treated with partial fasciectomy, noting a recurrence rate of just 6% in this cohort.38
Prognosis and complications following surgical intervention
Overall, studies have demonstrated a 60% nodular recurrence rate when surgery is chosen as the therapeutic option for PF.19,39 This risk is increased with bilateral foot involvement, multiple nodules, and a positive family history of PF.38 Apart from recurrence, other surgical risks include impaired wound healing, skin necrosis, painful scarring, nerve entrapment, and loss of arch height.9
Adjuvant radiotherapy has been proposed as a solution to fibroma recurrence. de Bree et al noted that recurrence after excision was rarely observed following adjuvant radiotherapy.40 Others have demonstrated that a recurrence rate <50% can be expected with wide excision and adjuvant radiotherapy.2,9,37 While these results are promising, the rare but significant risks of radiation, including impaired function of the foot, impaired wound healing, lymphedema, marked fibrosis, fracture of irradiated bone, and radiation-induced malignancy must be balanced with the benefit of recurrence prevention.40,41
Summary
The optimal management of PF continues to evolve as a multitude of standard conservative therapies and emerging treatment options have been studied with varying degrees of efficacy. Given the benign nature of this condition, conservative therapies continue to be first-line options for symptomatic management; however, convincing, long-term research surrounding their use does not yet exist. Further research is needed to determine an optimal treatment algorithm. Several operative options exist for recalcitrant or particularly aggressive cases, although recurrence of the nodules is not uncommon.
Footnotes
Disclosure
All authors report no conflicts of interest in this work.
References
- 1.Lee TH, Wapner KL, Hecht PJ. Plantar fibromatosis. J Bone Joint Surg Am. 1993;75(7):1080–1084. doi: 10.2106/00004623-199307000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Dürr HR, Krödel A, Trouillier H, Lienemann A, Refior HJ. Fibromatosis of the plantar fascia: diagnosis and indications for surgical treatment. Foot Ankle Int. 1999;20(1):13–17. doi: 10.1177/107110079902000103. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbaum AJ, Dipreta JA, Misener D. Plantar heel pain. Med Clin North Am. 2014;98(2):339–352. doi: 10.1016/j.mcna.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Lareau CR, Sawyer GA, Wang JH, Digiovanni CW. Plantar and medial heel pain: diagnosis and management. J Am Acad Orthop Surg. 2014;22(6):372–380. doi: 10.5435/JAAOS-22-06-372. [DOI] [PubMed] [Google Scholar]
- 5.Neufeld SK, Cerrato R. Plantar fasciitis: evaluation and treatment. J Am Acad Orthop Surg. 2008;16(6):338–346. doi: 10.5435/00124635-200806000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Johnston FE, Collis S, Peckham NH, Rothstein AR. Plantar fibromatosis: literature review and a unique case report. J Foot Surg. 1992;31(4):400–406. [PubMed] [Google Scholar]
- 7.Jeswani T, Morlese J, Mcnally EG. Getting to the heel of the problem: plantar fascia lesions. Clin Radiol. 2009;64(9):931–939. doi: 10.1016/j.crad.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Gudmundsson KG, Jónsson T, Arngrímsson R. Association of Morbus Ledderhose with Dupuytren’s contracture. Foot Ankle Int. 2013;34(6):841–845. doi: 10.1177/1071100713475352. [DOI] [PubMed] [Google Scholar]
- 9.Carroll P, Henshaw RM, Garwood C, Raspovic K, Kumar D. Plantar fibromatosis: pathophysiology, surgical and nonsurgical therapies: an evidence-based review. Foot Ankle Spec. 2018;11(2):168–176. doi: 10.1177/1938640017751184. [DOI] [PubMed] [Google Scholar]
- 10.Ledderhose Disease. Gaithersburg: National institutes of health genetic and rare diseases information center; c2017-18. [Accessed April 23, 2018]. (updated 2018 April 1 cited 2018 April 23). Available from: https://rarediseases.info.nih.gov/diseases/6873/ledderhose-disease. [Google Scholar]
- 11.Godette GA, O’Sullivan M, Menelaus MB. Plantar fibromatosis of the heel in children: a report of 14 cases. J Pediatr Orthop. 1997;17(1):16–17. [PubMed] [Google Scholar]
- 12.Fausto de Souza D, Micaelo L, Cuzzi T, Ramos-E-Silva M. Ledderhose disease: an unusual presentation. J Clin Aesthet Dermatol. 2010;3(9):45–47. [PMC free article] [PubMed] [Google Scholar]
- 13.Pickren JW, Smith AG, Stevenson TW, Stout AP. Fibromatosis of the plantar fascia. Cancer. 1951;4(4):846–856. doi: 10.1002/1097-0142(195107)4:4<846::aid-cncr2820040422>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 14.Strzelczyk A, Vogt H, Hamer HM, Krämer G. Continuous phenobarbital treatment leads to recurrent plantar fibromatosis. Epilepsia. 2008;49(11):1965–1968. doi: 10.1111/j.1528-1167.2008.01684.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim SK, Ioannidis JPA, Ahmed MA, et al. Two genetic variants associated with plantar fascial disorders. Int J Sports Med. 2018;39(4):314–321. doi: 10.1055/s-0044-100280. [DOI] [PubMed] [Google Scholar]
- 16.Espert M, Anderson MR, Baumhauer JF. Current concepts review: plantar fibromatosis. Foot Ankle Int. 2018;39(6):751–757. doi: 10.1177/1071100718768051. [DOI] [PubMed] [Google Scholar]
- 17.English C, Coughlan R, Carey J, Bergin D. Plantar and palmar fibromatosis: characteristic imaging features and role of MRI in clinical management. Rheumatology. 2012;51(6):1134–1136. doi: 10.1093/rheumatology/ker522. [DOI] [PubMed] [Google Scholar]
- 18.Yasui Y, Takao M, Miyamoto W, Matsushita T. Plantar fibromatosis with flexion contracture and valgus deformity of the great toe. J Orthop Sci. 2016;21(3):395–398. doi: 10.1016/j.jos.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Veith NT, Tschernig T, Histing T, Madry H. Plantar fibromatosis–topical review. Foot Ankle Int. 2013;34(12):1742–1746. doi: 10.1177/1071100713505535. [DOI] [PubMed] [Google Scholar]
- 20.Mcnally EG, Shetty S. Plantar fascia: imaging diagnosis and guided treatment. Semin Musculoskelet Radiol. 2010;14(3):334–343. doi: 10.1055/s-0030-1254522. [DOI] [PubMed] [Google Scholar]
- 21.Woertler K. Soft tissue masses in the foot and ankle: characteristics on MR Imaging. Semin Musculoskelet Radiol. 2005;9(3):227–242. doi: 10.1055/s-2005-921942. [DOI] [PubMed] [Google Scholar]
- 22.Draghi F, Gitto S, Bortolotto C, Draghi AG, Ori Belometti G. Imaging of plantar fascia disorders: findings on plain radiography, ultrasound and magnetic resonance imaging. Insights Imaging. 2017;8(1):69–78. doi: 10.1007/s13244-016-0533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen BE, Murthy NS, Mckenzie GA. Ultrasonography of plantar fibromatosis: updated case series, review of the literature, and a novel descriptive appearance termed the “Comb Sign”. J Ultrasound Med. 2018;37(11):2725–2731. doi: 10.1002/jum.14615. [DOI] [PubMed] [Google Scholar]
- 24.Plantar Fibroma. Chicago: American college of foot and ankle surgeons; c2018. [Accessed April 23, 2018]. [cited 2018 April 23]. Available from: https://www.acfas.org/footankleinfo/Plantar_Fibroma.htm. [Google Scholar]
- 25.Ledderhose disease treatment United Kingdom: British Dupuytren’s society. 2018. [Accessed cited 2018 April 23April 23, 2018]. Available from: http://dupuytrens-society.org.uk/treatment/ledderhose-disease/
- 26.Ahuja RB, Chatterjee P. Comparative efficacy of intralesional verapamil hydrochloride and triamcinolone acetonide in hypertrophic scars and keloids. Burns. 2014;40(4):583–588. doi: 10.1016/j.burns.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 27.Jordan GH, Carson CC, Lipshultz LI. Minimally invasive treatment of Peyronie’s disease: evidence-based progress. BJU Int. 2014;114(1):16–24. doi: 10.1111/bju.12634. [DOI] [PubMed] [Google Scholar]
- 28.Grenfell S, Borg M. Radiotherapy in fascial fibromatosis: a case series, literature review and considerations for treatment of early-stage disease. J Med Imaging Radiat Oncol. 2014;58(5):641–647. doi: 10.1111/1754-9485.12178. [DOI] [PubMed] [Google Scholar]
- 29.Heyd R, Dorn AP, Herkströter M, Rödel C, Müller-Schimpfle M, Fraunholz I. Radiation therapy for early stages of morbus Ledderhose. Strahlenther Onkol. 2010;186(1):24–29. doi: 10.1007/s00066-009-2049-x. [DOI] [PubMed] [Google Scholar]
- 30.Schuster J, Saraiya S, Tennyson N, Nedelka M, Mukhopadhyay N, Weiss E. Patient-reported outcomes after electron radiation treatment for early-stage palmar and plantar fibromatosis. Pract Radiat Oncol. 2015;5(6):e651–e658. doi: 10.1016/j.prro.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Frizziero A, Barazzuol M, Vittadini F, Bellon G, Masiero S, Meneghini A. Plantar fascial fibromatosis: two cases treated with low-energy focused shock waves. J Clin Rheumatol. 2017;23(1):63–65. doi: 10.1097/RHU.0000000000000462. [DOI] [PubMed] [Google Scholar]
- 32.Yin MC, Ye J, Yao M, et al. Is extracorporeal shock wave therapy clinical efficacy for relief of chronic, recalcitrant plantar fasciitis? A systematic review and meta-analysis of randomized placebo or active-treatment controlled trials. Arch Phys Med Rehabil. 2014;95(8):1585–1593. doi: 10.1016/j.apmr.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 33.Frairia R, Berta L. Biological effects of extracorporeal shock waves on fibroblasts. A review. Muscles Ligaments Tendons J. 2011;1(4):138–147. [PMC free article] [PubMed] [Google Scholar]
- 34.Knobloch K, Vogt PM. High-energy focussed extracorporeal shockwave therapy reduces pain in plantar fibromatosis (Ledderhose’s disease) BMC Res Notes. 2012;5:542. doi: 10.1186/1756-0500-5-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel SR, Benjamin RS. Desmoid tumors respond to chemotherapy: defying the dogma in oncology. J Clin Oncol. 2006;24(1):11–12. doi: 10.1200/JCO.2005.03.6566. [DOI] [PubMed] [Google Scholar]
- 36.Hammoudeh ZS. Collagenase Clostridium histolyticum injection for plantar fibromatosis (Ledderhose disease) Plast Reconstr Surg. 2014;134(3):497e–499. doi: 10.1097/PRS.0000000000000433. [DOI] [PubMed] [Google Scholar]
- 37.Aluisio FV, Mair SD, Hall RL. Plantar fibromatosis: treatment of primary and recurrent lesions and factors associated with recurrence. Foot Ankle Int. 1996;17(11):672–678. doi: 10.1177/107110079601701105. [DOI] [PubMed] [Google Scholar]
- 38.Kadir HKA, Chandrasekar CR. Partial fasciectomy is a useful treatment option for symptomatic plantar fibromatosis. Foot. 2017;31:31–34. doi: 10.1016/j.foot.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 39.van der Veer WM, Hamburg SM, de Gast A, Niessen FB. Recurrence of plantar fibromatosis after plantar fasciectomy: single-center long-term results. Plast Reconstr Surg. 2008;122(2):486–491. doi: 10.1097/PRS.0b013e31817d61ab. [DOI] [PubMed] [Google Scholar]
- 40.de Bree E, Zoetmulder FA, Keus RB, Peterse HL, van Coevorden F. Incidence and treatment of recurrent plantar fibromatosis by surgery and postoperative radiotherapy. Am J Surg. 2004;187(1):33–38. doi: 10.1016/j.amjsurg.2002.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Lui TH. Endoscopic Subtotal Fasciectomy of the Foot. Arthrosc Tech. 2016;5(6):e1387–e1393. doi: 10.1016/j.eats.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kosi Gramatikoff PF-PlantarDesign.jpg. [Accessed December 12, 2018]. Available from: https://en.wikipedia.org/wiki/File:PF-PlantarDesign.jpg.
- 43.Radiopaedia Plantar fibromatosis. [AccessedDecember 12, 2018]. Available from: https://radiopaedia.org/cases/plantar-fibromatosis.
- 44.Radiopaedia Plantar fibroma. [Accessed December 12, 2018]. Available from: https://radiopaedia.org/cases/plantar-fibroma-2.


