Abstract
Chemotherapeutic agents, either in the form of systemically injected free drug or encapsulated in nanoparticles transport vehicles, must overcome three main obstacles prior to reaching and interacting with their intended target inside tumor cells. Drugs must leave the circulation, overcome the tissue–tumor barrier and penetrate the cell's plasma membrane. Since, many agents enter the cell by endocytosis, they must avoid entrapment and degradation by the intracellular endolysosome complex. Ultrasound has demonstrated potential to enhance the efficacy of chemotherapy by reducing these barriers. The purpose of this review is to highlight the potential of ultrasound in combination with sonosensitizers to enhance the efficacy of chemotherapy by optimizing the anticancer agent's intracellular ability to engage and interact with its target.
Keywords: : chemotherapy, endosomal escape, sonochemical internalization, sonodynamic therapy, sonosensitizers, tumor spheroids, ultrasonic activation
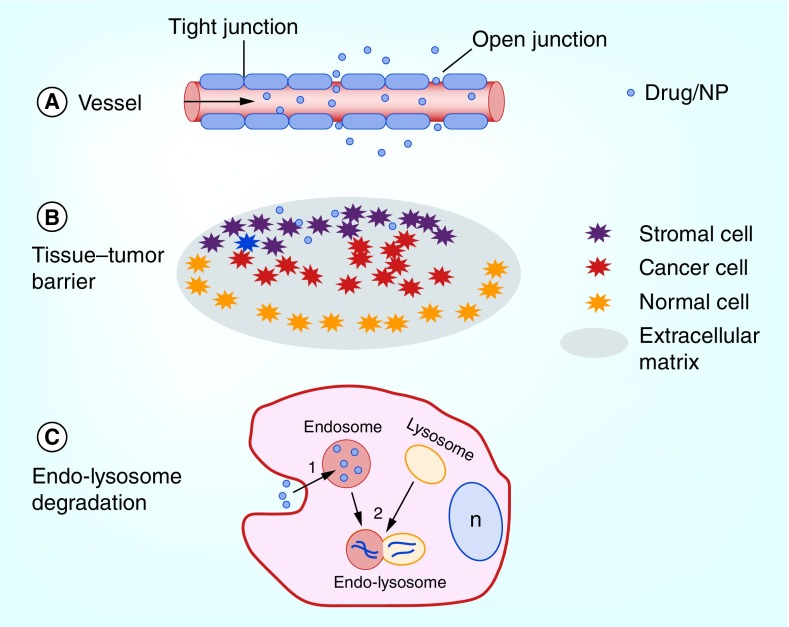
Chemotherapeutic agents, either in the form of systemically injected free drug or encapsulated in nanoparticles (NPs) transport vehicles, must overcome three main obstacles prior to reaching their intended target inside tumor cells, as illustrated in Figure 1. Agents must be transported through the endothelial cells lining the tumor capillaries or by passing through the junctions between them (Figure 1A). Once outside the circulation, the agent must overcome the extracellular tissue–tumor barrier. This barrier limits migration of drug or NPs throughout the tumor due to the presence of other cell types, such as, stromal-, immune-infiltrating and normal cells as well as a dense extracellular matrix (Figure 1B).
Figure 1. . Barriers limiting efficacy of chemotherapy.
(A) Vascular barrier; (B) Tissue–tumor barrier; (C) Endolysosomal entrapment. The cell nucleus is denoted by n.
An increasing interest in ultrasound (US) to overcome these two limitations has resulted in over 8000 publications with more than 350 in the brain alone. By far it is the use of microbubbles for vasculature opening and triggered controlled release of therapeutic agents from nanosystems, including the microbubbles themselves, that has garnered the most attention. The ability of US to enhance the efficacy of chemotherapy and gene transfection has also been extensively investigated. Since several excellent reviews [1–6] of the use of US to facilitate the above-mentioned topics have recently been published, these topics will not be repeated in this review. The focus of this review therefore, is to highlight the potential of US in combination with sonosensitizing compounds, defined as sonodynamic therapy (SDT), to enhance the efficacy of anticancer agents and in particular to overcome a significant barrier, intracellular endolysosome entrapment and drug degradation as illustrated in Figure 1C.
Sonodynamic therapy
US has been proposed and explored as a means of activating sensitizing agents in a manner similar to that of light-based photodynamic therapy (PDT). Although PDT has shown efficacy in a great many experimental and clinical studies, a significant drawback of light-based therapies is the limited penetration depth of light in biological tissues at the wavelengths required to activate most photosensitizers [7].
The term ‘sonodynamic’ was coined by Yumita et al. in 1989 [8] who demonstrated US-induced activation of a commonly used photosensitizer (hematoporphyrin) in vitro. SDT is generally considered to be nontoxic since it employs: sensitizers commonly used as photosensitizers in PDT and low-intensity (nonthermal) US. SDT has therefore been defined as requiring three components: US, a sonosensitizer and oxygen [9].
A considerable advantage of SDT over PDT is the reduced tissue attenuation of US compared with visible and near-IR light, facilitating the use of SDT to tumor sites buried deep within tissues or through the intact skull. Although acoustic impedance mismatches and the increased US attenuation in bone pose significant challenges for neurological applications, the recent use of highly focused US for the noninvasive treatment of movement disorders has demonstrated the potential of this technology.
The use of sonosensitizers in SDT distinguishes it from US-only therapies for enhancing the toxicity of macromolecules, such as, chemotherapeutic agents. In this type of therapy, the enhanced effects of the macromolecule are primarily due to the ability of US to porate cell membranes (sonoporation) and/or US-induced dispersion of the agent through poorly vascularized tissues in tumors [10].
Sonosensitizers for SDT
A variety of sensitizers have been used in SDT studies including porphyrins, such as, hematoporphyrin, Photofrin®, protoporphyrin IX (PPIX) and its precursor, 5-aminolevulinic acid (5-ALA). Xanthene dyes (e.g., rose bengal and its derivatives) have also been investigated in a number of in vitro studies [11,12], however, these dyes are ill suited for in vivo applications due to their rapid localization in the liver and subsequent clearance [13]. Although most sensitizers demonstrate preferential uptake in tumor tissues, their distribution in normal tissues could be problematic especially if the sensitizer is found in tissues located between the US source and the tumor tissue. The use of US-mediated SDT may provide a solution to this problem as the US beam can be focused (and hence the acoustic energy concentrated) to the tissue depth matching the location of the tumor. Following exposure to acoustic fields, sonosensitizers generate reactive oxygen species (ROS) which are likely responsible for the cytotoxic effects observed in SDT [9]. The types of ROS produced are dependent on the nature of the photosensitizer, however, singlet oxygen and hydroxyl radicals appear to be the most common.
Mechanisms of action
Interactions of US with cells or tumors depend on a number of parameters including acoustic power (intensity: W cm-2), pressure amplitude (MPa), frequency (MHz), pulse repetition frequency, pulse length and mechanical index. Low-intensity US generally used for SDT is <5.0 W cm-2, corresponding to a root mean square pressure amplitude of about 0.3 MPa. The relationship between pressure amplitude and intensity is given by: I = p2/ρc, where I is the intensity, p is the root mean square pressure amplitude, ρ is the density and c is the speed of sound. The mechanical index, defined as the peak negative pressure amplitude estimated in situ divided by the square root of the frequency, is a particularly useful metric in US applications as it provides an estimate of the nonthermal bioeffects, that is, those due to cavitation [14]. This index is commonly used as a standard for setting limits on the nonthermal bioeffects produced by US, for example, the US FDA mandates that the mechanical index be kept below 1.9 for diagnostic US applications [14].
At high-pressure amplitudes, microbubbles can form from small gas pockets in the tissue. The formation and interaction of these gas bubbles with the US field is referred to as acoustic cavitation. Cavitation is typically classified as either stable or inertial. In stable cavitation, bubbles oscillate about an equilibrium radius over many acoustic cycles. The oscillations result in the streaming of surrounding fluid thereby inducing mechanical stresses. Although stable cavitation can cause various bioeffects, permanent tissue damage is typically not observed [15]. Higher pressure amplitudes may result in violent bubble collapse accompanied by shock waves with high pressures and shear forces that can cause significant mechanical damage to tissues: a phenomenon known as inertial cavitation. The temperature and pressure within the imploding cavities can reach 5000 K and 800 atm, respectively [1]. These extreme temperatures and pressures may cause a number of chemical reactions within and surrounding the bubble, including light generation: a phenomenon known as sonoluminescence [16]. Cavitation also induces chemical reactions resulting in the production of free radicals similar to those produced in radiation chemistry [17]. These free radicals play a role in the biological effects induced by SDT. Taken together, these observations provide the rationale for the use of focused-ultrasound (FUS)-mediated cavitation effects for therapeutic applications.
Although there is general consensus that ROS are involved in SDT-mediated cytotoxic effects, it is not entirely clear how they are created. One theory posits that ROS are produced via the direct action of US at high-pressure amplitudes resulting in inertial cavitation effects including violent microbubble collapse which is accompanied by extremes in both temperature and pressure resulting in the release of heat and, in some cases, light (sonoluminescence). Therefore, the collapsing bubble may be thought of as a sonochemical reactor that results in the production of free radicals [18] either directly, via cavitation-induced pyrolysis or indirectly by cavitation-induced pyrolysis of water. In this theory, the presence of a sonosensitizer is not required for the production of ROS, however, if a sonosensitizer is in close proximity to a collapsing cavitation bubble, the formation of sensitizer-derived free radicals via direct or indirect effects (described above) has been suggested [19].
A role for sonoluminescence in SDT has been suggested by a number of investigators [20,21]. Although the exact mechanism by which light is produced from cavitating bubbles is unknown, it likely results from inertial cavitation events associated with bubble implosion as discussed previously. However, it is important to note that this light phenomenon has been observed both in vitro [22] and in vivo [21] during stable cavitation at low acoustic pressures (0.10–0.14 MPa). Since most sonosensitizers used in SDT are also photosensitizers, it is likely that US-induced sonoluminescence causes sono/photosensitizer activation resulting in the production of ROS. Since many of the sonosensitizers are porphyrin based, it is not surprising that singlet oxygen features prominently in the cytotoxic effects of SDT. Sonoluminescence emissions occur over wavelength ranges of approximately 350–550 nm [21,23] – a region where most porphyrins have strong absorption.
It has been suggested that SDT may be associated with sonomechanical mechanisms leading to sensitizer-dependent cell membrane damage [24]. This seems plausible since it is known that porphyrins interact with cell membranes [25]. Although the interaction of hydrophobic molecules (e.g., porphyrins), with cell membranes might induce membrane sensitivity to US, other studies suggest that US exposure of sonosensitizers results in chemical changes of membrane lipids [26]. It has been postulated that gas molecules trapped in lipid bilayers can serve as centers of bubble formation [27]. In this scenario, it is likely that membrane-localizing sensitizers would be in close proximity to bubbles. If subjected to cavitation at low-US intensities, the bubbles could produce sonoluminescence which would explain the SDT-based membrane lipid peroxidation observed in some studies [9]. From the preceding discussions, it appears that both stable and inertial cavitations are implicated in SDT.
In vitro & in vivo SDT studies
The majority of SDT studies have employed US frequencies centered around 1 MHz (0.4–3 MHz) and US intensities ranging from 0.5 to 4.0 W cm-2. Of particular relevance is that the sonodynamic effects observed in the vast majority of SDT studies have been elicited using US-delivering mechanical indices significantly below the limit of 1.9 for diagnostic US devices.
SDT has demonstrated efficacy in a wide variety of cancer cell lines, including glioblastoma [28], lung [29], breast [30] and leukemia [31]. As with PDT, SDT has been shown to induce apoptosis [32] and autophagy [33]: the exact mode of cell death being dependent on the sonosensitizer, US exposure parameters and target type.
In vivo SDT studies have been confined exclusively to rodent models employing either mice or rats. SDT efficacy has been demonstrated in a variety of tumor types and, as observed with PDT, a number of different types of responses have been observed including direct cytotoxic effects on tumor cells, secondary effects on the tumor vasculature and immune responses. The first in vivo SDT study was reported by Yumita et al. who used a gallium–porphyrin complex (ATX-70) in an ectopic murine colon adenocarcinoma model [34]. Twenty four hours following intravenous administration (2.5 mgkg-1) of ATX-70, 2 MHz US at an intensity of 3 W cm-2 resulted in 50% tumor reduction 3 days following treatment. In a subsequent study using identical drug doses and US irradiation parameters, this group demonstrated significant tumor regression in a rat mammary tumor model [35]. Ohmura et al. used very high US intensities (10 W cm-2) in combination with 5-ALA in an orthotopic rat glioma model [36]. Significant tumor regression was reported and, in spite of the high intensities employed, no damage to normal brain was observed. These results suggest that SDT has potential for the treatment of deep-seated lesions in the brain.
As has been observed with PDT, SDT can also act at the level of the tumor vasculature resulting in vascular shutdown and subsequent tumor regression. Following administration of 5-ALA, Guo et al. [37] used 1.1 MHz US at an intensity of 2 W cm-2 in a human tongue squamous cell carcinoma xenograft model in mice. The treatment had a significant effect on the tumor vasculature as well as inhibiting the expression of VEGF. Based on these results, it seems highly plausible that SDT is capable of exhibiting an antiangiogenic effect in this model.
In contrast to conventional cancer treatments (radiation therapy and chemotherapy) which are typically immunosuppressive, SDT has been shown to stimulate the host immune system [38]. Using 1 MHz US (1.1 W cm-2) combined with a porphyrin sensitizer (SF1) in S-180 murine tumors, Wang et al. noted significant tumor growth inhibition as well as an inflammatory response around the irradiated area following treatment [39]. This type of response has also been observed by other investigators [9], however, it is not clear whether this could result in an antitumor immunological effect, such as, limiting tumor recurrence or inhibiting metastasis.
Combinations of sonosensitizers & drugs
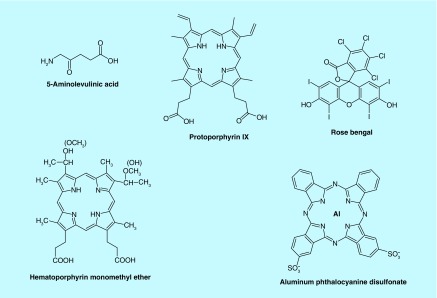
Although many sonosensitizers have been evaluated and proven effective for SDT [5], only a handful has been studied (Figure 2) combined with the chemotherapeutic agents, bleomycin (BLM), doxorubicin (DOX) and 5-FU.
Figure 2. . Structure of sonosensitizers evaluated for combined sonodynamic therapy + drug therapy.
SDT–BLM: sonochemical internalization
Endosomal entrapment
Drug or drug-loaded NPs must enter tumor cells through the cell's plasma membrane. This barrier limits the agents that are in clinical use to mostly lipophilic or low-MW compounds which passively diffuse into the cell cytoplasm, for example, 5-FU and temozolomide (TMZ). Although a number of small molecule drugs can readily enter cells, they have relatively low therapeutic specificity primarily due to their structural limitations. In contrast, large macromolecular drugs can easily be coupled to targeting ligands, which are able to bind to specific receptors on target cells, thus they have the potential advantage of exerting a higher therapeutic specificity compared with small molecule drugs. Unfortunately, since many highly effective chemotherapeutic agents, like BLM, immune-conjugates or drug-carrying NPs are large and/or water soluble, they are actively transported into cells by endocytosis and end up trapped in intracellular organelles, such as, endosomes and lysosomes. The limited ability of endocytosed agents to escape from the resulting intracellular endosomes leads to their degradation by powerful lysosomal enzymes, following lysosome–endosome fusion as illustrated in Figure 1C. Sonochemical internalization (SCI) is designed to favor the delivery of therapeutic agents without inactivating the agent or lethally damaging the cell. The cytotoxic effect of SCI is primarily due to the toxicity of the ‘escaped’ therapeutic molecule, rather than from SDT toxicity directly. SCI therefore allows effective treatment to take place at lower US intensities than those required for SDT.
The concept of SCI is based on the use of amphiphilic photosensitizers, which localize in the cell membrane and are carried into the cell during the endocytotic event and remain in the endosome and lysosome membranes. Upon US exposure, the sonosensitizer interacts with ambient oxygen to produce singlet oxygen. Since singlet oxygen has a very short range of action (<20 nm), only the area of the endosomal or lysosome membrane where the sonosensitizer is localized, will be damaged by singlet oxygen mediated reactions with amino acids, unsaturated fatty acids and cholesterol in the membrane bilayer. The released agent can therefore exert its full biological activity, in contrast to being degraded by lysosomal hydrolases.
Previous studies have established a number of sensitizing agents suitable for SCI including the amphiphilic photosensitizer, aluminum phthalocyanine disulfonate (AlPcS2a) [40,41]. AlPcS2a is a phthalocyanine derivative containing two charged sulfonate groups linked to phthalic subunits in adjacent positions and an aluminum metal ion incorporated at its center (Figure 2). The most important property of AlPcS2a is its amphiphilicity, that is, it has both hydrophilic and lipophilic properties and, as such, it localizes in cellular membranes. The lipophilic phthalocyanine skeleton of AlPcS2a localizes in the lipophilic interior of the cellular membrane while the sulfonate groups dissolve in the hydrophilic outer layer of the membrane. AlPcS2a molecules localize in the cell membrane following systemic administration. During endocytosis, a partial cell membrane with previously localized AlPcS2a molecules pinches inward to form an endocytic vesicle and, via the vesicle membrane, the attached AlPcS2a molecules are subsequently transported into the cell. The overall damage induced by SDT is closely related to the precise subcellular localization of the sonosensitizer.
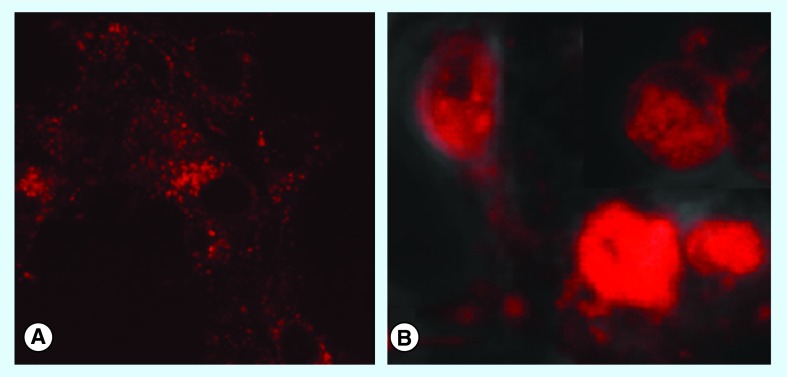
Madsen et al. have determined the effects of US on the intracellular distribution of AlPcS2a which are shown in Figure 3 [42]. Fluorescence microscopy was used to verify the uptake and intracellular localization of AlPcS2a in the absence or presence of FUS. The F98 rat glioma cells were incubated with AlPcS2a for 18 h, followed by 4-h incubation in sonosensitizer-free medium prior to microscopy. The photosensitizer (red) is taken up in the F98 cells and localized in granular organelles representing endosomes and lysosomes (Figure 3A), as also previously observed for other cell types. One hour post US exposure, a diffuse red fluorescence throughout the cell cytosol was observed (Figure 3B), indicating endosomal membrane escape of the photosensitizer. Other amphiphilic photosensitizers, such as, disulfonate tetraphenyl porphyrin (TPPS2a) and its chlorin derivative, disulfonated meso-tetraphenyl chlorin (TPCS2a) have also proven to be efficient photosensitizers for PDT-based photochemical internalization (PCI) but have not yet been evaluated as sonosensitizers for SCI [43,44].
Figure 3. . Ultrasound-induced endosomal escape of aluminum phthalocyanine disulfonate.
Fluorescence microscopy verified the uptake and intracellular localization of the sonosensitizers (FOV: 40 μm). (A) Photosensitizer (red) was taken up in F98 cells and localized in granular organelles, that is, endosomes/lysosomes; (B) One hour post ultrasound exposure. The diffuse AlPcS2a fluorescence throughout the cytosol indicates an induced endosomal escape of the photosensitizer by sonication.
AlPcS2a: Aluminum phthalocyanine disulfonate.
Reproduced with permission from [42].
In SCI experiments, Gonzales et al. studied AlPcS2a in combination with the anticancer drug, BLM [45]. BLM has had limited clinical use since it must be given in relative high concentrations and has a small therapeutic window. Due to its hydrophilic nature and large size, once internalized via endocytosis, BLM is trapped inside endosomes and rapidly degraded following endolysosome fusion. In contrast, if released into the cell cytosol, BLM rapidly diffuses to the nucleus where it has a significant toxic effect resulting in single- and double strand DNA breaks. Dramatically, a single molecule of BLM is capable of yielding 15 DNA-strand breaks making it one of the most efficient chemotherapeutic agents known.
Effects of SDT & BLM-SCI on glioma tumor spheroids
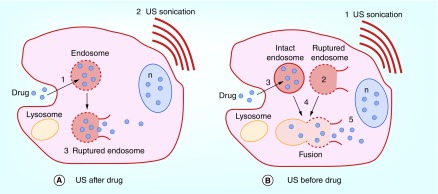
Since SCI is a combined treatment modality, SDT + drug, the sequence of application of sonication can be performed either after or before the cells are exposed to the drug. Figure 4 depicts the possible mechanism for the two different SDT + BLM sequences.
Figure 4. . Basic concept of sonochemical internalization of bleomycin.
(A) ‘Sonication after:’ cell membranes are loaded with an amphiphilic photosensitizer: (1) drug binds to the plasma membrane and it enters the cell together with the photosensitizer by endocytosis. The photosensitizer and the drug colocalize in the endosome, with the photosensitizer localized in the membrane and the drug in the lumen, (2) Exposure to FUS leads to, (3) sono-induced rupture of the endosome leading to the sequestered drug being released into the cell cytosol, entering the nucleus and thus inhibiting cell division; (B) ‘Sonication before:’ cell membranes are loaded with an amphiphilic photosensitizer: (1) FUS sonication, (2) FUS-induced disruption of endosome membrane containing photosensitizer, (3) Drug endocytosis and localization in intact endosomes, (4) Fusion of intact drug-containing and FUS-disrupted endosomes resulting in the sequestered drug being released into the cell cytosol, entering the nucleus and thus inhibiting cell division.
FUS: xxx; n: Nucleus.
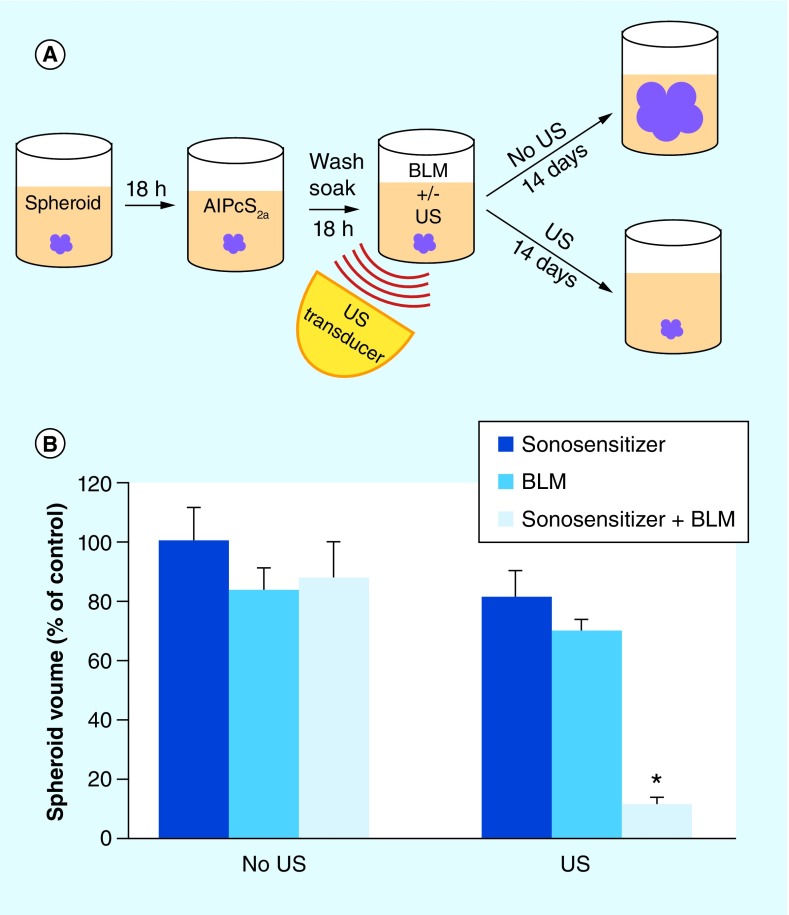
The growth inhibitory potential of US + sonosensitizer (SDT), in the absence or presence of BLM (SCI) was assayed by the ability of the treatment to inhibit the growth of rat F98 multicell glioma spheroids. The experimental set up is shown in cartoon form in Figure 5A.
Figure 5. . Inhibition of spheroid growth by bleomycin sonochemical internalization.
(A) Experimental protocol: spheroid formation, 24 h post-formation, sonosensitizers AlPcS2a added, incubated together for 18 h, wash incubated for 24 h in fresh medium to allow the sonosensitizers to redistribute from the cell membrane to the endosome–lysosome membranes, BLM added before or after sonication, spheroid growth monitored for 14 days. (B) Spheroid growth following treatment: FUS before BLM sequence, 0.4 W/cm2, 3 min. exposure, BLM concentration of 0.5 μg/ml, AlPcS2a for 18 h. Continuous wave ultrasound at 1 MHz was used, in all exposures. Each data point represents spheroid volume as a percentage of untreated control spheroid volume 3 weeks following exposure.
AlPcS2a: Aluminum phthalocyanine disulfonate; BLM: Bleomycin; FUS: Focused ultrasound.
In vitro tumor models employing multicell tumor spheroids can be considered a bridge between monolayer cultures and animal experiments. Spheroids are three-dimensional aggregates of cells that mimic microtumors and metastases. In comparison to monolayer cultures, a significant advantage of this model is that their microenvironment more closely mimics the in vivo situation with gene expression and the biological behavior of the cells similar to that encountered in tumors. The varying oxygen gradients inside these spheroids produce a heterogeneous population of cells that differ in their response to oxygen-dependent therapies, such as, ionizing radiation, SDT, chemotherapy and, in all probability, SCI.
The results shown in Figure 5B, employing this model, demonstrated that SDT potentiated the cytotoxic effects of BLM to a significant degree. This was the case regardless of the sequence of SDT and BLM application, although SDT before drug was more effective [42,45]. This synergistic effect is postulated to be due to endosomal escape, in a similar manner to that seen for light-based PCI [46,47]. An additional effect might be due to the toxic action of SDT on lysosome membranes resulting in cell autophagy as has been demonstrated for PDT [48–51]. US also enhanced the efficacy of BLM in the absence of sonosensitizers compared with drug alone but to a lesser degree (Figure 5B). The increased efficacy of BLM caused by US in the absence of sonosensitizer might be due to sonoporation of the cell membrane allowing BLM to enter the cell cytosol directly or an increased US-induced endocytosis. Previous experiments have shown that US can enhance delivery of DNA and drugs in the presence or absence of exogenous air bubbles [52–55].
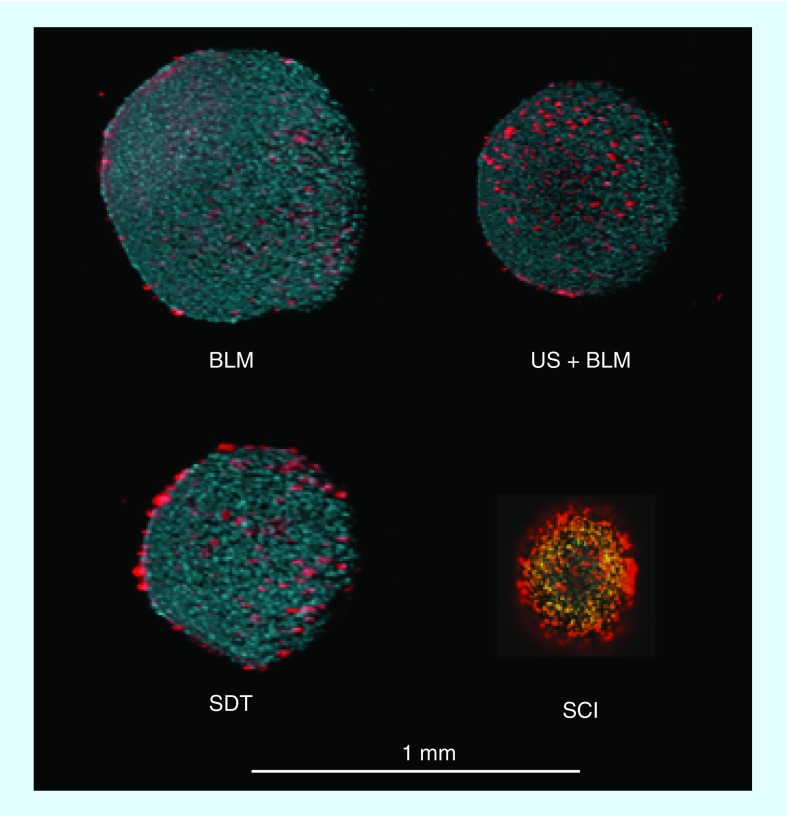
The effects of BLM, US + BLM, SDT and SDT + BLM (SCI) were also assessed by live/dead fluorescence microscopy (Figure 6). The assays, employing two-photon fluorescence images, demonstrated enhanced cell death in SCI-treated spheroids compared with BLM or SDT alone or US + BLM applied as single treatments. SDT at the sonication energy used (0.2 W cm-2) showed that the dead cells clustered mostly around the surface of the spheroid. In the spheroids receiving US + BLM, dead (red) cells were more equally distributed throughout the spheroid. In contrast, SCI-treated spheroids were much smaller and consisted mostly of dead (red) cells.
Figure 6. . Live/dead assay of control and sonochemical internalization treated spheroids.
Two-photon fluorescence microscopy images of F98 spheroids stained with Hoechst 33342 (blue: live) and ethidium homodimer (red: dead). Spheroids were stained 14 days after treatment: (A) BLM; (B) US + BLM; (C) SDT; (D) SCI (SDT + BLM).
BLM: Bleomycin; SCI: Sonochemical internalization; SDT: Sonodynamic therapy US: Ultrasound.
Recently published results by Osaki et al. evaluated the therapeutic potential of AlPcS2a SDT with BLM on the colon-26 cell line both in vitro and in vivo [56]. These authors concluded that the combination of AlPcS2a-SDT and BLM was more effective compared with BLM or SDT acting alone both in vitro and in a superficial tumor model. In their in vivo animal model, AlPcS2a (20 mg/kg) SDT combined with BLM, inhibited tumor growth and was more effective than SDT or BLM alone or US + BLM. Histological sections showed ruptured blood vessels surrounded by pyknotic nuclei in tumors treated with SDT and BLM, likely caused by vascular shutdown. Their in vitro results using the colon-26 model are in good agreement with our findings employing F98 glioma cells although the experimental procedures in these two studies differed in several respects. The US parameters were different, and these authors explored only the US after BLM exposure treatment sequence.
Osaki et al. has also evaluated the second-generation photosensitizer, 5-ALA in SDT studies to enhance the efficacy of BLM on the EMT-60 cell line both in vitro and in vivo [57]. In this study, US frequencies of 1 and 3 MHz were used. The in vitro results showed that at 1 MHz, SDT + BLM was significantly more cytotoxic than either SDT or BLM + US. Paradoxically the observed cytotoxicity of SDT or SDT + BLM was inversely proportional to the US intensity.
EMT-60 tumor growth in vivo was significantly inhibited in both the US + BLM and the SDT + BLM groups, although the SDT + BLM treatment was more effective. As reported in their previous study at 3 MHz, histological sections showed ruptured blood vessels surrounded by pyknotic nuclei in tumors treated with SDT and BLM.
5-ALA is a prodrug that must be metabolized within the cancer cell as part of the heme biosynthesis pathway to PPIX, an active photosensitizer. It is therefore, not an exogenous preformed amphiphilic photosensitizer like AlPcS2a or disulfonated meso-tetraphenyl chlorin. Although seldom used for PCI studies due to its relative inefficiency, 5-ALA-induced PPIX has been shown to be incorporated in endosome and lysosome membranes and effective PCI of MOC31–gelonin in the human colon adenocarcinoma cell line has been demonstrated, but only under serum-free conditions [58]. Taken together, 5-ALA does not appear to be an optimal sonosensitizer for SCI.
SDT + doxorubicin
Liang et al. reported the synergistic effects of hematoporphyrin monomethyl ether (HMME) SDT combined with the anticancer agent, DOX [59]. Results from their experiments employing (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) tetrazolium (MTT) cell viability assay, flow cytometer, Hoechst staining and cell arrest analysis demonstrated that the combination of HMME-SDT and DOX could significantly inhibit the proliferation of human cholangiocarcinoma QBC939 cells in vitro. The cell proliferation inhibition of the combination HMME-SDT-DOX was much greater than that obtained following the application of HMME-SDT or DOX applied separately.
In addition, they found that HMME-SDT as well as HMME-SDT + DOX produced ROS but in amounts not significantly different from each other. This would indicate that it is the SDT component that produces ROS. The authors concluded that the synergistic effects on cell proliferation resulted from DNA damage as demonstrated by single-cell gel electrophoresis and DNA fragmentation. Interestingly, the inhibitory effect was independent of ROS production.
The efficacy of combined therapy consisting of DOX with PPIX-SDT was examined by Wang et al. in vitro on the DOX-resistant leukemic K562/DOX cell line [60]. A synergistic efficacy of PPIX-SDT + DOX was found in DOX cytotoxicity, cell apoptosis, DNA damage and ROS generation in K562/DOX cells. The results indicated that the molecular mechanism may be attributed to the reduced expression of P-glycoprotein which would influence the membrane drug efflux pumps leading to increased uptake and retention of DOX in multidrug resistant cancer cells like K562/DOX.
Titanium dioxide (TiO2) has been evaluated as a sonosensitizer for TiO2-SDT combined with DOX by Shen et al. [61]. These authors developed TiO2-encapsulated Fe3O4 core shell NPs (Fe3O4@TiO2 NPs). DOX was loaded onto the TiO2 shell and exhibited pH-dependent release of DOX in vitro. Cell viability of the human breast carcinoma cell line (MCF-7) was significantly inhibited in vitro by the combined chemo-SDT compared with DOX or Fe3O4@TiO2 NPs in the absence of US sonication.
Incubation of MCF-7 cells together with Fe3O4@TiO2 NPs was also shown to produce ROS following low-intensity US sonication. The ability of Fe3O4@TiO2-SDT-DOX NPs to inhibit tumor growth was also examined in vivo using S180 (mouse ascites tumor cell line) cells implanted subcutaneously into mice. The mice were intravenously injected with the NPs and a small magnet was attached over the tumor to target the Fe3O4 core of the NP. The tumors were insonated at 3-day intervals in all four-times. Biodistribution experiments, demonstrated a high in vivo tumor accumulation of Fe3O4@TiO2 NPs in the presence of the magnetic field. With the sonication parameters employed, a significant reduction in tumor volume following 14 days of growth was achieved both in the US only control group (US + NaCl) and the group that received combined treatment. (NP-SDT + DOX). The reduction in tumor volume compared with nontreated controls was 50 and 40%, respectively. Although the difference between the two groups was moderate, the concept of magnetically targeted nanocomposites for combined SDT and chemotherapy clearly motivates further investigation.
McEwan et al. recently described the use of oxygen-loaded microbubbles for pancreatic cancer treatment using combined SDT and antimetabolite therapy [62]. The microbubbles were prepared with either the sensitizer rose bengal or the anticancer agent, 5-FU, attached to their surface, respectively. The rationale for the incorporation of oxygen in the core of the microbubble was to enhance the amount of ROS generated by SDT since oxygen is the substrate for ROS production and treatment-limiting hypoxic regions are often found in tumors. The authors concluded that the microbubbles retained their oxygen until US disruption and that the combination of SDT and 5-FU conjugates provided enhanced cytotoxicity in three different pancreatic cancer cell lines cultured under anaerobic conditions. Additionally, in vivo treatment of ectopic BxPC-3 tumors, with the combined oxygen microbubble SDT and 5-FU therapy led to a statistically significant reduction in tumor volume. This is an interesting concept that will no doubt undergo further development.
Conclusion
The primary purpose of this review was to highlight the potential of SDT to enhance the efficacy of anticancer agents and in particular to overcome the significant limitation of intracellular endolysosome entrapment and drug degradation. For all three of the chemotherapeutic drugs tested, 5-FU, DOX and BLM, a significant synergistic increase in drug efficacy was demonstrated. This was also the case for all of the sonosensitizers involved. The potential of SDT to be focused at considerable depths in the body, allows for the targeted activation of chemotherapy localized to the tumor environment, greatly spearing normal tissue. The clearly demonstrated enhanced antitumor/tumor cell effects of combined SDT + drug treatment, compared with those obtained by SDT or drug acting alone, would enable effective therapy to be achieved at reduced drug dosages and US intensities.
Future perspective
Although chemotherapy has made great strides in cancer treatment, serious side effects due to toxicity toward normal tissue, leads to drug dosages that are often inadequate for complete tumor regression. The ability of SCI to enhance drug efficacy in a site- and time-specific fashion could significantly reduce this problem. Since SCI and PCI appear to have a similar underlying mechanism, many of the demonstrated advantages of PCI for cancer therapy can potentially be extended to SCI. PCI has been demonstrated to enhance the effects of a large number of macromolecules that are subject to endosome–lysosome entrapment. Among these are immunotoxins, chemotherapeutic agents and gene-encoding plasmids with both viral and nonviral carriers, on a large variety of cancer cells including treatment of therapy-resistant cancers [63–70]. The two main advantages of US over light activation greatly increased and focused depth of therapy and noninvasive treatment of other than superficial tumors, allowing for repetitive and fractionated therapy protocols. These characteristics point to an important role for SCI as a new treatment modality for cancer.
Executive summary.
Ultrasound
Ultrasound (US) induced bioeffects are classified as either thermal or mechanical.
US has significant tissue penetration, attenuated by approximately 50% in 7 cm.
US either alone or in combination with gas bubbles can temporarily open the blood–brain barrier or modify the tissue–tumor barrier.
Extreme temperatures and pressures caused by US generate light: sonoluminescence.
US can induce triggered controlled release of therapeutic agents from nanosystems.
US + sonosensitizing agents: sonodynamic therapy
FUS can activate sensitizing agents similar to light-based photodynamic therapy.
Sonodynamic therapy (SDT) requires three components: FUS, sonosensitizer and oxygen.
Reactive oxygen species and singlet oxygen are involved in SDT-mediated cytotoxic effects.
The exact nature of reactive oxygen species production by SDT is still being explored.
SDT has demonstrated efficacy in a wide variety of cancer cell lines, including glioblastoma.
SDT + drug: sonochemical internalization
Many highly efficient cytotherapeutic agents are taken up by endocytosis and are trapped in intracellular endosomes rendering them inactive.
Sonochemical internalization (SCI) is composed of suboptimal SDT + therapeutic agent; drug or nanoparticle.
The cytotoxic effect of SCI is primarily due to the toxicity of the ‘escaped’ therapeutic molecule, rather than from SDT toxicity directly.
For equivalent efficacy, SCI requires significantly lower US intensities compared with SDT.
SCI is based on the use of amphiphilic sonosensitizers, which localize in endolysosome membranes.
Endosome or lysosome membranes containing sonosensitizer will be damaged by singlet oxygen mediated US-driven reactions releasing the entrapped molecule.
Released agents can reach and interact with their target, in contrast to being degraded by lysosomal hydrolases.
Advantages of SCI over light activation are noninvasive treatment protocols, and greatly increased depth of therapy contrasted to light based approaches.
Acknowledgements
This work was supported by grants from the Norwegian Radium Hospital Research Foundation. Portions of this work were made possible through access to the Laser Microbeam and Medical Program (LAMMP) at the Beckman Laser Institute. S Madsen was supported, in part, by the Tony and Renee Marlon Charitable Foundation.
Footnotes
Financial & competing interests disclosure
Apart from those already disclosed, the authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Rosenthal I, Sostaric JZ, Riesz P. Sonodynamic therapy – a review of the synergistic effects of drugs and ultrasound. Ultrason. Sonochem. 2004;11(6):349–363. doi: 10.1016/j.ultsonch.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 2.O'Neill BE, King C, Li P. Augmentation of targeted delivery with pulsed high intensity focused ultrasound. Int. J. Hyperthermia. 2008;24(6):506–520. doi: 10.1080/02656730802093661. [DOI] [PubMed] [Google Scholar]
- 3.Husseinia GA, Pitt WG, Martinsa AM. Ultrasonically triggered drug delivery: breaking the barrier. Colloids Surf. B Biointerfaces. 2014;123:364–386. doi: 10.1016/j.colsurfb.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 4.Couture O, Foley J, Kassell NF, et al. Review of ultrasound mediated drug delivery for cancer treatment: updates from pre-clinical studies. Trans. Cancer Res. 2014;3(5):494–511. [Google Scholar]
- 5.Wood AKW, Sehgal CM. A review of low-intensity ultrasound for cancer therapy. Ultrasound Med. Biol. 2015;41(4):905–928. doi: 10.1016/j.ultrasmedbio.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hersh DS, Kim AJ, Winkles JA, et al. Emerging applications of therapeutic ultrasound in neuro-oncology: moving beyond tumor ablation. Neurosurgery. 2016;79(5):643–654. doi: 10.1227/NEU.0000000000001399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madsen SJ, Wilson BC. Optical properties of brain tissue. In: Madsen SJ, editor. Optical Methods and Instrumentation in Brain Imaging and Therapy. Springer; NY, USA: 2013. [Google Scholar]
- 8.Yumita N, Nishigaki R, Umemura K, et al. Hematoporphyrin as a sensitizer of cell-damaging effect of ultrasound. Jpn J. Cancer Res. 1989;80(3):219–222. doi: 10.1111/j.1349-7006.1989.tb02295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstration of sonodynamic therapy (SDT) effectiveness.
- 9.McHale AP, Callan JF, Nomikou N, et al. Sonodynamic therapy: concept, mechanism and application to cancer treatment. In: Escoffre JM, Bouakaz A, editors. Therapeutic Ultrasound, Advances in Experimental Medicine and Biology. Springer International Publishing; Switzerland: 2016. [DOI] [PubMed] [Google Scholar]
- 10.Nomikou N, Li YS, McHale AP. Ultrasound-enhanced drug dispersion through solid tumours and its possible role in aiding ultrasound-targeted cancer chemotherapy. Cancer Lett. 2010;288(1):94–98. doi: 10.1016/j.canlet.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 11.Umemura S, Yumita N, Umemura K, et al. Sonodynamically induced effect of rose bengal on isolated sarcoma 180 cells. Cancer Chemother. Pharmacol. 1999;43(5):389–393. doi: 10.1007/s002800050912. [DOI] [PubMed] [Google Scholar]
- 12.Sugita N, Iwase Y, Yumita N, et al. Sonodynamically induced cell damage using rose bengal derivative. Anticancer Res. 2010;30(9):3361–3366. [PubMed] [Google Scholar]
- 13.Sugita N, Kawabata K, Sasaki K, et al. Synthesis of amphiphilic derivatives of rose bengal and their tumor accumulation. Bioconjug. Chem. 2007;18(3):866–873. doi: 10.1021/bc060189p. [DOI] [PubMed] [Google Scholar]
- 14.Apfel RE, Holland CK. Gauging the likelihood of cavitation from short-pulse, low-duty cycle diagnostic ultrasound. Ultrasound Med. Biol. 1991;17(2):179–185. doi: 10.1016/0301-5629(91)90125-g. [DOI] [PubMed] [Google Scholar]
- 15.O'Reilly MA, Hynynen K. Emerging non-cancer applications of therapeutic ultrasound. Int. J. Hyperthermia. 2015;31(3):310–318. doi: 10.3109/02656736.2015.1004375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frenzel H, Schultes H. Luminescenz im ultraschallbeschickten wasser. Z. Phys. Chem. 1934;B27:421–426. [Google Scholar]
- 17.Riesz P, Berdahl D, Christman CL. Free radical generation by ultrasound in aqueous and nonaqueous solutions. Environ. Health Perpect. 1985;64:233–252. doi: 10.1289/ehp.8564233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Misik V, Riesz P. Free radical intermediates in sonodynamic therapy. Ann. NY Acad. Sci. 2000;899:335–348. doi: 10.1111/j.1749-6632.2000.tb06198.x. [DOI] [PubMed] [Google Scholar]; • Explanation of mechanism of SDT.
- 19.Myoshi N, Igarashi T, Riesz P. Evidence against singlet oxygen formation by sonolysis of aqueous oxygen-saturated solutions of hematoporphyrin and rose bengal. The mechanism of sonodynamic therapy. Ultrason. Sonochem. 2000;7(3):121–124. doi: 10.1016/s1350-4177(99)00042-5. [DOI] [PubMed] [Google Scholar]
- 20.Umemura S, Yumita N, Nishigaka R, et al. Mechanism of cell damage by ultrasound in combination with hematoporphyrin. Jpn J. Cancer Res. 1990;81(9):962–966. doi: 10.1111/j.1349-7006.1990.tb02674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He Y, Xing D, Tan S, et al. In vivo sonoluminescence imaging with the assistance of FCLA. Phys. Med. Biol. 2002;47(9):1535–1541. doi: 10.1088/0031-9155/47/9/308. [DOI] [PubMed] [Google Scholar]
- 22.Pickworth MJW, Dendy PP, Leighton TG, et al. Studies of the cavitational effects of clinical ultrasound by sonoluminescence: 2. Thresholds for sonoluminescence from a therapeutic ultrasound beam and the effect of temperature and duty cycle. Phys. Med. Biol. 1988;33(11):1249–1260. doi: 10.1088/0031-9155/34/11/004. [DOI] [PubMed] [Google Scholar]
- 23.Sazgarnia A, Shanei A, Eshighi H, et al. Detection of sonoluminescence signals in a gel phantom in the presence of protoporphyrin IX conjugated to gold nanoparticles. Ultrasonics. 2013;53(1):29–35. doi: 10.1016/j.ultras.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Hiraoka W, Honda H, Feril LB, Jr, et al. Comparison between sonodynamic effect and photodynamic effect with photosensitizers on free radical formation and cell killing. Ultrason. Sonochem. 2006;13(6):535–542. doi: 10.1016/j.ultsonch.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Stepniewski M, Kepczynski M, Jamroz D, et al. Interaction of hematoporphyrin with lipid membranes. J. Phys. Chem. B. 2012;116(16):4889–4897. doi: 10.1021/jp300899b. [DOI] [PubMed] [Google Scholar]
- 26.Tang W, Liu Q, Wang X, et al. Membrane fluidity altering and enzyme inactivating in sarcoma 180 cells post the exposure to sonoactivated hematoporphyrin in vitro . Ultrasonics. 2008;48(1):66–73. doi: 10.1016/j.ultras.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Wrenn SP, Small E, Dan N. Bubble nucleation in lipid bilayers: a mechanism for low frequency ultrasound disruption. Biochim. Biophys. Acta. 2013;1828(4):1192–1197. doi: 10.1016/j.bbamem.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Xu ZY, Wang K, Li XQ, et al. The ABCG2 transporter is a key molecular determinant of the efficacy of sonodynamic therapy with photofrin in glioma stem-like cells. Ultrasonics. 2013;53(1):232–238. doi: 10.1016/j.ultras.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Osaki T, Tajima M, Okamoto Y, et al. Sonodynamic antitumor effect of benzoporphyrin derivative monoacid ring a on KLN205 cells. J. Cancer Ther. 2011;2(2):99–104. [Google Scholar]
- 30.Li Y, Wang P, Zhao P, et al. Apoptosis induced sonodynamic treatment by protoporphyrin IX MDA-MB-231 cells. Ultrasonics. 2012;52(4):490–496. doi: 10.1016/j.ultras.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Zheng L, Sun X, Zhu X, et al. Apoptosis of THP-1 derived macrophages induced by sonodynamic therapy using a new sonosensitizer hydroxyl acetylated curcumin. PLoS ONE. 2014;9(3):e93133. doi: 10.1371/journal.pone.0093133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuroki M, Hachimine K, Abe H, et al. Sonodynamic therapy of cancer using novel sonosensitizers. Anitcancer Res. 2007;27(6A):3673–3678. [PubMed] [Google Scholar]
- 33.Wang X, Liu Q, Wang Z, et al. Role of autophagy in sonodynamic therapy-induced cytotoxicity in S180 cells. Ultrasound Med. Biol. 2010;36(11):1933–1946. doi: 10.1016/j.ultrasmedbio.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 34.Yumita N, Sasaki K, Umemura S, et al. Sonodynamically induced antitumor effect of a gallium-porphyrin complex, ATX-70. Jpn J. Cancer Res. 1996;87(3):310–316. doi: 10.1111/j.1349-7006.1996.tb00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yumita N, Okuyama N, Sasaki K, et al. Sonodynamic therapy on chemically induced mammary tumor: pharmacokinetics, tissue distribution and sonodynamically induced antitumor effect of gallium–porphyrin complex ATX-70. Cancer Chemother. Pharmacol. 2007;60(6):891–897. doi: 10.1007/s00280-007-0436-5. [DOI] [PubMed] [Google Scholar]
- 36.Ohmura T, Fukushima T, Shibaguchi H, et al. Sonodynamic therapy with 5-aminolevulinic acid and focused ultrasound for deep-seated intracranial glioma in rat. Anticancer Res. 2011;31(7):2527–2534. [PubMed] [Google Scholar]
- 37.Guo S, Sun X, Cheng J, et al. Apoptosis of THP-1 macrophages induced by protoporphyrin IX-mediated sonodynamic therapy. Int. J. Nanomedicine. 2013;8:2239–2246. doi: 10.2147/IJN.S43717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and antitumour immunity. Nat. Rev. Cancer. 2006;6(7):35–54. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Lewis TJ, Mitchell D. The tumoricidal effect of sonodynamic therapy (SDT) on S-180 sarcoma in mice. Integr. Cancer Ther. 2008;7(2):96–102. doi: 10.1177/1534735408319065. [DOI] [PubMed] [Google Scholar]
- 40.Berg K, Bommer JC, Moan J. Evaluation of sulfonated aluminum phthalocyanines for use in photochemotherapy. Cellular uptake studies. Cancer Lett. 1989;44(1):7–15. doi: 10.1016/0304-3835(89)90101-8. [DOI] [PubMed] [Google Scholar]
- 41.Maman N, Dhami S, Phillips D, et al. Kinetic and equilibrium studies of incorporation of di-sulfonated aluminum phthalocyanine into unilamellar vesicles. Biochim. Biophys. Acta. 1999;1420(1–2):168–178. doi: 10.1016/s0005-2736(99)00093-0. [DOI] [PubMed] [Google Scholar]
- 42.Madsen SJ, Gonzales J, Zamora G, et al. Comparing the effects of light- or sonic-activated drug delivery: photochemical/sonochemical internalization. J. Environ. Pathol. Toxicol. Oncol. 2016;35(1):91–98. doi: 10.1615/JEnvironPatholToxicolOncol.2016015463. [DOI] [PubMed] [Google Scholar]
- 43.Berg K, Nordstrand S, Selbo PK, et al. Disulfonated tetraphenyl chlorin (TPCS2a), a novel photosensitizer developed for clinical utilization of photochemical internalization. Photochem. Photobiol. Sci. 2011;10(10):1637–1651. doi: 10.1039/c1pp05128h. [DOI] [PubMed] [Google Scholar]
- 44.Lilletvedt M, Tonnesen HH, Hogset A, et al. Physicochemical characterization of the photosensi-tizers TPCS2a and TPPS2a 1. Spectroscopic evaluation of drug-solvent interactions. Pharmazie. 2010;65(8):588–595. [PubMed] [Google Scholar]
- 45.Gonzales J, Nair RK, Madsen SJ, et al. Focused ultrasound-mediated sonochemical internalization: an alternative to light-based therapies. J. Biomed. Opt. 2016;21(7):78002. doi: 10.1117/1.JBO.21.7.078002. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Demonstration of synergy SDT and drug; sonochemical internalization.
- 46.Selbo PK, Weyergang A, Høgset A, et al. Photochemical internalization provides time- and space-controlled endolysosomal escape of therapeutic molecules. J. Control Rel. 2010;148(1):2–12. doi: 10.1016/j.jconrel.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 47.Prasmickaite L, Høgset A, Selbo PK, et al. Photochemical disruption of endocytic vesicles before delivery of drugs: a new strategy for cancer therapy. Br. J. Cancer. 2002;86(4):652–657. doi: 10.1038/sj.bjc.6600138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guicciardi ME, Leist M, Gores GJ. Lysosomes in cell death. Oncogene. 2004;23(16):2881–2890. doi: 10.1038/sj.onc.1207512. [DOI] [PubMed] [Google Scholar]
- 49.Kessel D, Vicente MGH, Reiners JJ. Initiation of apoptosis and autophagy by photodynamic therapy. Autophagy. 2006;2(4):289–290. doi: 10.4161/auto.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reiners JJ, Agostinis P, Berg K, et al. Assessing autophagy in the context of photodynamic therapy. Autophagy. 2010;6(1):7–18. doi: 10.4161/auto.6.1.10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inguscio V, Panzarini E, Dini L. Autophagy contributes to the death/survival balance in cancer photodynamic therapy. Cells. 2012;1(3):464–491. doi: 10.3390/cells1030464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pepe J, Rincon M, Wu J, et al. Experimental comparison of sonoporation and electro-poration in cell transfection applications. Acoust. Res. Lett. Onl. 2004;5:62–67. [Google Scholar]
- 53.Wu J, Pepe J, Rinco M. Sonoporation, anti-cancer drug and antibody delivery using ultrasound. Ultrasonics. 2006;44(Suppl. 1):e21–e25. doi: 10.1016/j.ultras.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 54.Iwanaga K, Tominaga K, Yamamoto K, et al. Local delivery system of cytotoxic agents to tumors by focused sonoporation. Cancer Gene Ther. 2007;14(4):354–363. doi: 10.1038/sj.cgt.7701026. [DOI] [PubMed] [Google Scholar]
- 55.Yudina A, Lepetit-coiffé M, Moonen CT. Evaluation of the temporal window following ultrasound-mediated cell membrane permeability enhancement. Mol. Imaging Biol. 2011;13(2):239–249. doi: 10.1007/s11307-010-0346-5. [DOI] [PubMed] [Google Scholar]
- 56.Osaki T, Yokoe I, Uto Y, et al. Bleomycin enhances the efficacy of sonodynamic therapy using aluminum phthalocyanine disulfonate. Ultrason. Sonochem. 2016;28:161–168. doi: 10.1016/j.ultsonch.2015.07.013. [DOI] [PubMed] [Google Scholar]; • In vivo demonstration of efficacy of SDT and drug.
- 57.Osaki T, Ono M, Uto Y, et al. Sonodynamic therapy using 5-aminolevulinic acid enhances the efficacy of bleomycin. Ultrasonics. 2016;67:76–84. doi: 10.1016/j.ultras.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 58.Selbo PK, Kaalhus O, Sivam G, et al. 5-amino-levulinic acid-based photochemical internalization of the immunotoxin MOC31-gelonin generates synergistic cytotoxic effects in vitro . Photochem. Photobiol. 2001;74(2):303–310. doi: 10.1562/0031-8655(2001)074<0303:aabpio>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 59.Liang L, Xie S, Jiang L, et al. The combined effects of hematoporphyrin monomethyl ether-SDT and doxorubicin on the proliferation of qbc939 cell lines. Ultrasound Med. Biol. 2013;39(1):146–160. doi: 10.1016/j.ultrasmedbio.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 60.Wang X, Jia Y, Su X, et al. Combination of protoporphyrin IX-mediated sonodynamic treatment with doxorubicin synergistically induced apoptotic cell death of a multidrug-resistant leukemia k562/dox cell line. Ultrasound Med. Biol. 2015;41(19):2731–2739. doi: 10.1016/j.ultrasmedbio.2015.06.001. [DOI] [PubMed] [Google Scholar]; • Overcoming drug resistance by combined SDT + drug treatment.
- 61.Shen S, Wu L, Liu J, et al. Core-shell structured Fe3O4@TiO2-doxorubicin nanoparticles for targeted chemo-sonodynamic therapy of cancer. Int. J. Pharm. 2015;486(1–2):380–388. doi: 10.1016/j.ijpharm.2015.03.070. [DOI] [PubMed] [Google Scholar]
- 62.McEwan C, Kamila S, Owen J, et al. Combined sonodynamic and antimetabolite therapy for the improved treatment of pancreatic cancer using oxygen loaded microbubbles as a delivery vehicle. Biomaterials. 2016;80:20–32. doi: 10.1016/j.biomaterials.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 63.Prasmickaite L, Høgset A, Murberg V, et al. Photochemically enhanced gene transfection increases the cytotoxicity of the herpes simplex virus thymidine kinase gene combined with ganciclovir. Cancer Gene Ther. 2004;11(7):514–523. doi: 10.1038/sj.cgt.7700720. [DOI] [PubMed] [Google Scholar]
- 64.Berg K, Berstad M, Prasmickaite L, et al. Photochemical internalization (PCI). A new tool for gene and oligonucleotide delivery. Top. Curr. Chem. 2010;296:251–281. doi: 10.1007/128_2010_63. [DOI] [PubMed] [Google Scholar]
- 65.Zamora G, Wang F, Sun CH, et al. Photochemical internalization-mediated nonviral gene transfection: polyamine core-shell nanoparticles as gene carrier. J. Biomed. Opt. 2014;19(10):105009. doi: 10.1117/1.JBO.19.10.105009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weyergang A, Selbo PK, Berstad ME, et al. Photochemical internalization of tumor-targeted protein toxins. Lasers Surg. Med. 2011;43(7):721–733. doi: 10.1002/lsm.21084. [DOI] [PubMed] [Google Scholar]
- 67.Mathews MS, Shih EC, Zamora G, et al. Glioma cell growth inhibition following photochemical internalization enhanced non-viral PTEN gene transfection. Lasers Surg. Med. 2012;44(9):746–754. doi: 10.1002/lsm.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Norum OJ, Giercksky KE, Berg K. Photochemical internalizationas an adjunct to marginal surgery in a human sarcoma model. Photochem. Photobiol. Sci. 2009;8(6):758–762. doi: 10.1039/b821129a. [DOI] [PubMed] [Google Scholar]
- 69.Selbo PK, Bostad M, Olsen CE, et al. Photochemical internalisation, a minimally invasive strategy for light-controlled endosomal escape of cancer stem cell-targeting therapeutics. Photochem. Photobiol. Sci. 2015;14(8):1433–1450. doi: 10.1039/c5pp00027k. [DOI] [PubMed] [Google Scholar]
- 70.Weyergang A, Berstad ME, Bull-Hansen B, et al. Photochemical activation of drugs for the treatment of therapy-resistant cancers. Photochem. Photobiol. Sci. 2015;14(8):1465–1475. doi: 10.1039/c5pp00029g. [DOI] [PubMed] [Google Scholar]