Summary
Orf disease is caused by a double-stranded DNA virus of the Parapox family. Human infection is mostly due to occupational hazard and handling infected animals. Our patient was an 18-year-old woman who suffered burns in 2015. Total Burn Surface Area (TBSA) was 22% and cause of burn was flame. One week after hospital admission, she underwent skin grafts of her upper extremities. However, vegetative granulomatous ulcerations developed on the wound, resulting in the grafts failing to take. After careful investigation into the patient’s history, we discovered that the water used to douse the flames was from a drinking trough for sheep. Suspecting Orf disease, we disinfected the wounds and dressing tools with Dakin’s solution. We waited about 12 days to perform a new skin graft, and most of the grafted skin took. PCR test for Parapox virus was positive. Orf disease should be considered a distinct possibility in burn patients with a history of probable contamination. Manipulation of the disease in the early stages of burn wound could potentially spread it and change the degree of the wound, therefore being aware of this possibility can save the patient unnecessary pain and time. To prevent a nosocomial outbreak of Orf, wound care and wound disinfection should be scrupulously carried out. Isolation and disinfection of the entire dressing tool should be considered. Educating wound care providers in burn hospitals and scrupulous wound disinfection would protect the patient from cross contamination and allow skin grafts to take with ease, without the formation of ulcerations associated with Orf
Keywords: Orf, infection, burn wound
Abstract
La maladie de Orf est causée par un virus à ADN bicaténaire du genre Parapox. L’infection humaine est principalement contractée au travail, lors de la manipulation d’animaux infectés. La patiente est une femme de 18 ans, brûlée en 2015 par flamme, sur 22 % SCT, ayant nécessité une greffe des membres supérieurs à semaine. Le développement de lésions granulomateuses ulcérées a entraîné la lyse des greffes. L’enquête étiologique a découvert que l’eau utilisée pour l’extinction des flammes provenait d’un abreuvoir pour moutons, ce qui nous a amenés à suspecter une maladie de Orf et badigeonner les lésions au Dakin. Nous avons effectuer une nouvelle greffe, en grande partie intégrée, 12 j plus tard. La PCR Parapox est revenue positive. Le syndrome d’Orf doit être évoqué chez un patient brûlé chez lequel une contamination est probable. Les interventions sur une zone brûlée infectée sont susceptibles d’acutiser l’infection en faisant évoluer défavorablement la brûlure. Ainsi, son diagnostic et son traitement préalables permettent d’éviter au patient des douleurs et un retard de cicatrisation. La désinfection optimale de la zone infectée et l’isolement du patient permettent d’éviter une dissémination nosocomiale. La formation des soignants aux mesures de prévention et d’hygiène, générales et spécifiques permet d’optimiser la prise en charge de ces patients.
Introduction
The cause of Orf disease is a double-stranded DNA virus belonging to the Parapox family. It is the cause of skin infection in sheep, goats and cows. Human Orf disease is usually due to occupational hazard and mostly occurs in abattoir workers, veterinarians and farm workers.1,2 Orf disease may also occur in some parts of the world like Iran, during the annual feast of sacrifice, where every family slaughters a sheep during a Muslim celebration. On that day, the probability of contamination is greater due a higher level of contact with infected animals.3
In goats and sheep, Orf disease manifests with sore mouth, scabby mouth or contagious postural dermatitis. Human infection is mostly isolated to lesions on the arms and hands, but there have been reports of them on the face or perianal area.4 They are benign neoplastic lesions and are mostly self-limited.5 The patient may also have fever and lymphadenopathy.5
Orf lesions manifest in a different pattern in the human body. The disease may begin as an erythematous maculopapular lesion and it can change to a target-shaped lesion with a red centre. In the regeneration stage, it can present as a dry lesion with black dots on it, and in the next stage it may become papillomatous and have a dry crust.6,7
Diagnosis is based on history of contact with infected animals, the presentation of clinical lesions and viral cultures.3 The disease is usually self-limited. The treatment is supportive care with topical antibiotics, and, when the wound is infected by bacteria, systemic antibiotics may also be applied. Patients usually recover within 2-3 weeks. Larger lesions sometimes need surgery.8
Treatment with Imiquimode and ribavirin has also been suggested.9
Case study
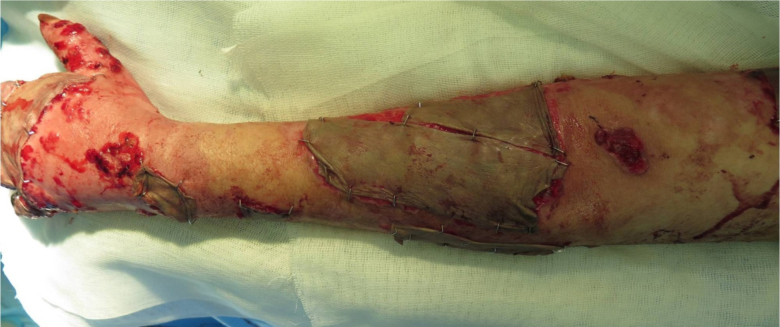
The patient was an 18-year-old female shepherd who was burned on July 10th, 2015 while starting a campfire, and was subsequently admitted to the burn unit in the local hospital. TBSA was 22%, including both upper limbs, trunk and her face. Dressings were changed daily and early surgical debridement was performed. A skin graft was applied to the upper extremity, and the graft took. However, vegetative granulomatous ulceration subsequently appeared on the improved as well as on the grafted areas, 5 days after admission (Fig. 1). At this point, the patient was referred and admitted to the central (Motahari) hospital in Tehran, on August 9th 2015 (30 days after the burn accident). History was taken at the time of admission, and the patient mentioned that after the accident, water from the sheep water trough was used to extinguish the flames.
Fig. 1. Time of admission to referral hospital.
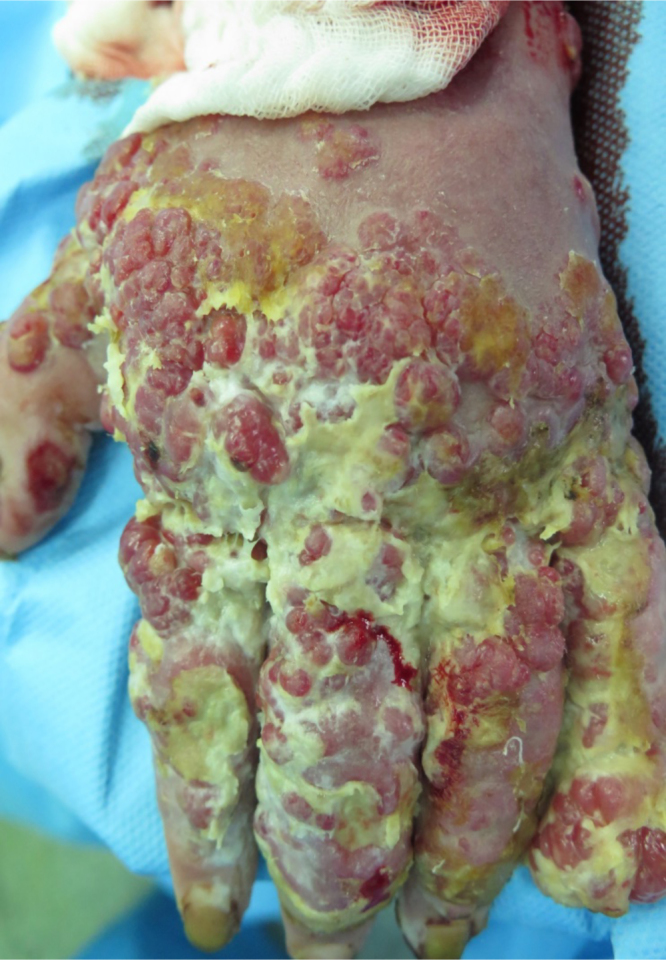
It should be noted that on the day of admission, the patient had a fever, but according to the clinical examination there was no lymphadenopathy.
Lab test results
White blood cell count was normal but hemoglobin levels were a little low. Blood culture was negative.
Wound at the time of admission
There were some vegetated granular lesions in the wound. Some parts of the wound were disinfected with Dakin’s solutions, and were excised after 4 days of dressing changes. Sulfamylon (Mafenide) cream was used on excised areas; the wound was covered with allograft 4 days later. After 5 days, most of the allograft had taken, and there was no infection in the wound (Fig. 2).
Fig. 2. Wound covered with Allograft.
The allograft was replaced with an autograft after 12 days, and all the grafts took. The patient had been in the referral hospital for 22 days.
The result of the first wound culture performed in the Motahari Hospital was multidrug-resistant Pseudomonas (MDR). Suitable antibiotic therapy with Colestin and Ceftazidim was started.
The result of the histology report from the previous hospital was: hyperkeratosis and pseudoepitheliomatous hyperplasia, dermal edema and congestion with dermal spindle cell proliferation.
The result of the patient’s tissue culture (discussed below) in the Motahari hospital was granulation type hemangioma with ulceration.
With regard to the tissue culture in the virus lab, viral DNA was extracted for a tissue biopsy using High Pure DNA extraction kit (ROCHE, Germany). The sample was tested for camel pox, Capri pox, Sheep pox and Goat pox using PCR by specific printing. The PCR test for Parapox virus (causative agent of Orf) was positive.
Discussion
Orf disease is a disease that occurs in goats, sheep and cows.1
Humans are infected by direct contact with infected animals.5 The disease occurs worldwide, but it has been more frequently reported in Europe and New Zealand due to high numbers of sheep and cattle.10 The virus survives in the skin of the animal for up to one month after improvement.5
In people who work with animals, the disease mostly appears as a solitary lesion on the finger, forearm and/or hand in the form of a small papule. Its appearance will change to a lens-shaped nodule with a red centre and pale circle within 7 days.11
Over the following days, the lesion grows larger and can become exudative. Afterwards, in the regenerative phase, black spots will form on the surface. In the fifth phase, it appears as a small papilloma. Eventually, in the final phase the lesion heals.5
Orf disease is a self-limited disease in humans and does not need any special treatment. However, some references suggest treatment with Imiquimode and Ribavirin.9
Reports of Orf disease peaked in Norway in 197512 and in New Zealand in 1983.13
Shamsedini et al.’s study reports that, among 15,012 patients over 20 years of age seen in a dermatology center in Iran between 1991-1996, the incidence of Orf was about 0.4%.14
Nedim et al. reported 5 cases of children with Orf disease over a decade, among farming families in western Ireland.15 Shirzad et al. report a case of Orf disease in a patient who had a history of cutting meat with an infected knife. The patient was infected following a finger injury. Contact with such a wound will transmit the disease.11
Midilli conducted a study in which 9 patients were reported to have Orf disease. They were infected by scissors, ointments and water tanks.16
Roess et al. published a study on two North American patients who contracted Parapoxvirus infections from dressing white-tailed deer. This demonstrates that the risk of this infection is possible in any country, and as white-tailed deer populations rise, infection will be more and more possible.17
In our case, the patient was an 18-year-old woman with 22% TBSA burns (2nd and 3rd degree) who was affected by vegetative and granulomatous lesions on her hands, forearms, arms and trunk.
The result of our tissue culture PCR test for the DNA virus was Parapox.
Conclusion
Orf disease can infect burn wounds, and even the healing superficial burn can be deepened, possibly requiring skin grafting. The disease should be considered in burn patients with a history of probable contamination.
Manipulation of the disease in the early stages of the burn wound could potentially spread the disease and change the degree of the wound, as was the case in our patient. To prevent a nosocomial outbreak of Orf, wound care and wound disinfection should be scrupulously carried out and all dressing tools should be isolated and disinfected. Suitable training should be given to wound care providers in burn units, and wounds thoroughly disinfected to protect the patient from cross contamination following this phase.
Acknowledgments
Conflict of interest.None of the authors has any financial interest in any of the products or drugs mentioned in this study.
Ethical approval.Approval was granted by the Ethics Committee of the Iran University of Medical Sciences.
Consent.Written consent was obtained from the patients for publication of the case reports and accompanying images.
Availability of data and materials.Please contact the corresponding author for data requests.
Competing interests.The authors declare that they have no competing interests.
Acknowledgements.The authors wish to thank Miss Parisa Pahlevanpoor and Miss Rafat Babajani for their cooperation in conducting the present study.
References
- 1.Afzali H, Momen-Heravi M. Human Orf Disease: a case series in Kashan City, Iran. International Archives of Health Science. 2015;2(3):129–132. [Google Scholar]
- 2.Becher P, Konig M, Muller G, Siebert U, Thiel HJ. Characterization of sealpox virus, a separate member of the parapoxvirus. Arch Virol. 2002;147(6):1133–1140. doi: 10.1007/s00705-002-0804-8. [DOI] [PubMed] [Google Scholar]
- 3.Uzel M, Sasmaz S, Bakaris S, Cetinus E. A viral infection of the hand commonly seen after the feast of sacrifice: Human Orf (orf of the hand). Epidemiol Infec. 2005;133(4):653–657. doi: 10.1017/s0950268805003778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayindir Y, Bayracdar M, Karadag N, Ozcan H. Investigation and analysis of a human orf outbreak among people living on the same farm. New Microbiologica. 2011;34(1):37–43. [PubMed] [Google Scholar]
- 5.Taghipour M, Babamahmoodi F, Arashnia P, Taghipour S. Orf Virus in humans (Echtyma Contagiosum): a report of eight cases in the North of Iran. Int J Med Invest. 2015;4(1):183–186. [Google Scholar]
- 6.Zimmerman JL. Orf. JAMA. 1991;266(4):476. [PubMed] [Google Scholar]
- 7.Mendez B, Burnett JW. Orf. JAMA. 1991;266(4):476. [PubMed] [Google Scholar]
- 8.Buller RML. Poxviruses. Infectious Diseases. 2010:1577–1582. [Google Scholar]
- 9.Mandell D, Bennet JE, Dolin R, Blaster MJ. DNA virus Chapter in “Mandell, Douglas, and Bennett’s Principle and Practice of Infectious Disease”, Churchill Livingstone Elsevier. 2015 [Google Scholar]
- 10.Damon IK. Other poxvirus that infect humans: Parapoxvirus, Molluscomcontagiosum, and Yatapoxviruse. Mandell, Douglas, and Bennett’s Principle and Practice of Infectious Disease. 2010 [Google Scholar]
- 11.Shirazi MR, Pedram N. Orf: report of eleven cases in five Iranian families. Iran J Clinic Infect Dis. 2007;2(2):83–85. [Google Scholar]
- 12.Johannessen JV, Krogh HK, Solberg L, Delan A. Human Orf. Cutan Pathol. 1975;2(6):265–283. doi: 10.1111/j.1600-0560.1975.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 13.Robinson AJ, Petersen GV. Orf virus infection of workers in the meat industry. N Z Med J. 1983;96(725):81–85. [PubMed] [Google Scholar]
- 14.Shamsaldini S, Rezaei A. Incidence of Orf disease in dermatology center clinic in Kerman. Razi J Med Sci. 1999;5(2):30–35. [Google Scholar]
- 15.Nadeem M, Curran P, Ryan CA, Connolly K. Orf: contagious pustular dermatitis. Ir Med J. 2010;103(5):152–153. [PubMed] [Google Scholar]
- 16.Midilli K, Erikilic A, Kuskuko M, Analay H, Benzonana N. Nosocomial outbreak of disseminated Orf infection in a burn unit, Gazinatep, Turkey, October to December 2012. European Journal of Infectious Disease Epidemiology Prevention and Control. 2013;18(11):204–225. doi: 10.2807/ese.18.11.20425-en. [DOI] [PubMed] [Google Scholar]
- 17.Roess AA, Galan A, Kitces E, Li Y, Zhao H, Christopher D. Novel deer-associated parapoxvirus infection in deer hunters. N Engl J Med. 2010;363:2621–2627. doi: 10.1056/NEJMoa1007407. [DOI] [PubMed] [Google Scholar]