Abstract
Background
This study was conducted to see whether increased values of serum CA125 and BDNF (brain-derived neurotrophic factor) on acute myocardial infarction (AMI) act as predictor for acute heart failure (AHF).
Material/Methods
Seventy-eight patients with clinically diagnosed cardiac function II–IV; and AHF were considered as the study group of this retrospective study and patients who had cardiac function I (without AHF) were considered the control group (n=82). The values of CA125 and BDNF were measured using enzyme-linked immunosorbent assay (ELISA) for developing the correlation with the Killip classification, and the diagnostic value of AHF.
Results
Statistically insignificant difference was noticed between baseline information e.g., blood pressure or smoking status of participants in study group and control group (P>0.05). The higher values of CA125 (5.68±1.8 U/mL or BDNF (19.48±5.3 pg/mL) in the study group had advantage over the control after independent sample t-test (P<0.001). A positive correlation was observed between values of the test substances and Killip classifications (I–IV) of cardiac functioning was observed (r=0.745, P<0.001; Spearman’s rank correlation coefficient). The sensitivity and specificity of area under the curve (AUC) combined with serum CA125 and BDNF levels in the diagnosis of AHF was 91.02% and 81.63%, respectively.
Conclusions
Increased serum level of the test substances indicates severity of AHF-leading AMI. Thus, monitoring is needed to avoid risk of AHF.
MeSH Keywords: Anterior Wall Myocardial Infarction, Diagnostic Test Approval, Prostatitis
Background
Acute myocardial infarction (AMI) is a common critical emergency. AMI refers to the reduction or interruption of coronary blood supply, which causes hypoxia and ischemia in the myocardium of the heart and induces a series of serious complications after myocardial degeneration and necrosis [1,2]. With an increase in population age, changes in eating habits, and a fast-paced lifestyle, the incidence of AMI is increasing every year [3]. Acute heart failure (AHF) is the ultimate fate of cardiovascular diseases, including AMI. Most patients with AMIs also have heart failure with different orders of severity. The incidence of AMI with AHF can reach 32.4%. AHF is the main reason for cessation of cardiovascular events after AMI treatment [4]. Therefore, timely identification and diagnosis of AMI patients with targeted treatments are particularly important for the prognosis of AHF.
CA125 is a heavily glycosylated, high-molecular weight binding mucin that is recognized by the monoclonal antibody 0C125. It is divided into a transmembrane region, an extracellular region, and a cytoplasmic tail region, and its expression is induced by inflammatory mediators and mechanical stress [5]. Previous studies have shown that patient CA125 levels have some connection with the severity of chronic heart failure (CHF), ultrasound parameters, and the degree of hemodynamic disorder. When heart failure worsens, CA125 levels rise significantly [6,7]. Brain-derived neurotrophic factor (BDNF) is a small protein molecule first isolated from the brain of a pig. It is widely distributed in the peripheral and central nervous system, and regulates the growth, differentiation, development, and death of nerve cells [8]. Studies have shown that BDNF is expressed in atherosclerotic vessels, vascular smooth muscle, and endothelial cells, and participates in angiogenesis, inflammation response, and apoptosis of myocardial remodeling after AMI [9,10].
At present, CA125 and BDNF are mostly studied in relation to AMI. There is less research on the correlation between CA125 and BDNF related to the Killip classification system and the combined AMI/AHF diagnostic value. This study explored the role and predictive value of CA125 and BDNF by examining the serum levels of CA125 and BDNF in AMI patients with AHF. The findings will support the practitioners in taking immediate steps against AHF on clinical reports of the test substances.
Material and Methods
Patient sampling
The medical record of 160 patients with AMI admitted to our hospital from March 2016 to April 2018 was analyzed for values of C125 and BDNF (to determine the Killip classification) and status of AHF. According to the Killip classification, 82 patients with cardiac function I without AHF represented the control group, and 78 patients with cardiac function II to IV combined with AHF were considered to be the study group. All study participants had given their written informed consent before participating in the study.
Inclusion and exclusion criteria
Inclusion criteria was based on the AMI Guidelines for the Diagnosis and Treatment of Acute ST-segment Elevation Myocardial Infarction [3] diagnosis criteria; all new-onset AMI cases with complete clinical data were included. Patients with non-coronary atherosclerotic acute myocardial infarction, severe liver and kidney disease, malignant tumors, active infections, chronic respiratory diseases, and hematopoietic dysfunction were excluded from the study. Patients with a previous history of myocardial infarction and patients suffering from mental illness or who had a family history of mental illness were also excluded.
Killip cardiac function rating
AMI cardiac function was assessed according to the Killip classification system [11], starting with Killip I: no heart failure, no lung rales, and no S3 heart sound; Killip II: left heart failure, wet rales in the middle and lower part of the lung (<50% lung field), and no S3 heart sound; Killip III: severe heart failure and pulmonary edema, fine wet rales throughout the lungs, and >50% lung field; and Killip IV: cardiogenic shock with different stages or different degrees of hemodynamic changes.
Sample collection and detection
Samples of 5 mL of venous blood was withdrawn from fasting patients, placed in a vacuum tube without anticoagulants, and centrifuged. The serum was stored in a −20°C low temperature refrigerator. Serum CA125 and BDNF levels were measured using enzyme-linked immunosorbent assay (ELISA) [14], with the testing performed according to the manufacturer’s instructions (Wuhan Merck Biotechnology Co., Ltd.). The sample and the kit were removed from the refrigerator 30 minutes prior to testing to acclimate to room temperature, and to set up a reaction well, a standard well, and a blank well. No samples were added to the blank well. The remaining wells received 50 μL of the sample to be tested or standards with different dilutions and 50 μL of biotin-labeled antibody was then added to all wells. The wells were covered with a membrane and incubated at 37°C for 1 hour. The liquid was removed and washed thrice. Affinity streptavidin (80 uL) was then added to each well. This mixture was mixed and incubated at 37°C for 30 minutes. The liquid was again removed, spun dry, and washed thrice. A working solution of substrate A and B (50 uL) was added to each well. This mixture was mixed and incubated at 37°C for 10 minutes in the dark at room temperature to allow for color development. A stop solution (50 uL) was then added to each well and the O.D. at 450 nm was determined using a fully automated enzyme label analyzer (Meigu Molecular Instruments (Shanghai) Co., Ltd.). This value was used to calculate the levels of CA125 and BDNF.
Statistical method
Statistical analysis was performed using SPSS 20.0 (Beijing Strong Vinda Information Technology Co., Ltd.), and the measurement data were expressed as mean ± standard deviation (x±SD). Comparison of continuous data between groups was carried out using a t-test. Comparison of enumeration data between groups was carried out using the chi-squared test. The correlation between serum CA125 and BDNF levels and the Killip classification was analyzed using Spearman’s rank correlation coefficient. A receiver operating characteristic (ROC) curve was used to evaluate the diagnostic efficacy of serum CA125 and BDNF levels on AHF. The difference was considered statistically significant when P<0.05.
Results
Baseline data of control group and study group
In the control group, there were 51 males and 31 females, aged 51–80 years old, with an average age of 64.15±7.35 years; 32 patients with diabetes; 28 patients with hypertension; and 22 patients with hyperlipidemia. In the study group, there were 54 males and 24 females, aged 52–78 years, with an average age of 65.83±8.29 years; 22 patients with diabetes; 25 patients with hypertension; and 31 patients with hyperlipidemia. There were no statistically significant differences between the control group and the study group in terms of gender, age, body mass index, concomitant disease, smoking status, drinking status, systolic blood pressure, and diastolic blood pressure (all P>0.05) (Table 1).
Table 1.
Baseline data of study group and control group [n(%)]/(x±sd).
| Category | Study group (n=78) | Control group (n=82) | t/χ2 | P |
|---|---|---|---|---|
| Gender | 0.877 | 0.406 | ||
| Male | 54 (69.23) | 51 (62.20) | ||
| Female | 24 (30.77) | 31 (37.80) | ||
| Age | 65.83±8.29 | 64.15±7.35 | 1.358 | 0.176 |
| Body mass index (kg/m2) | 25.01±3.47 | 25.49±3.14 | 0.918 | 0.359 |
| Accompanying disease | 3.452 | 0.177 | ||
| Diabetes | 22 (28.21) | 32 (39.02) | ||
| Hypertension | 25 (32.05) | 28 (34.15) | ||
| Hyperlipidemia | 31 (39.74) | 22 (26.83) | ||
| Smoking status | 0.381 | 0.635 | ||
| Have | 39 (50.00) | 37 (45.12) | ||
| No | 39 (50.00) | 45 (54.88) | ||
| Drinking status | 0.229 | 0.639 | ||
| Have | 41 (52.56) | 40 (48.78) | ||
| No | 37 (47.44) | 42 (51.22) | ||
| Systolic blood pressure (mmHg) | 141.52±10.63 | 138.87±11.25 | 1.530 | 0.128 |
| Diastolic blood pressure (mmHg) | 94.25±6.75 | 93.57±5.84 | 0.682 | 0.496 |
Serum CA125 and BDNF levels in the control group and the study group
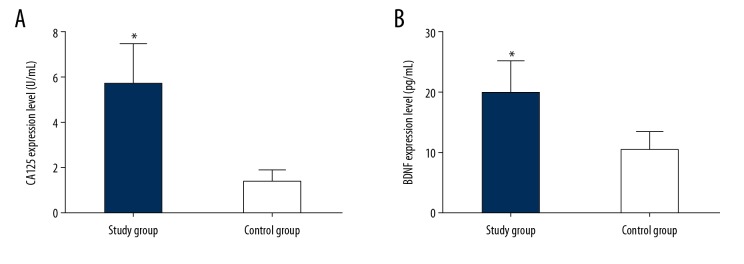
The serum levels of CA125 and BDNF in the study group were significantly higher than those in the control group (t=20.920, P<0.001; t=13.540, P<0.001) (Figure 1A, 1B, Table 2).
Figure 1.
(A, B) Comparison of serum CA125 and BDNF levels between the study group and the control group. (A) Comparison of serum CA125 levels between the control group and the study group; (B) Results comparing serum BDNF levels in the control group and the study group. (* P<0.001 compared with the control group).
Table 2.
Diagnostic value of serum CA125 and BDNF levels in AHF.
| Diagnostic indicator | AUC | 95% CI | Standard error | Cutoff | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| CA125 | 0.910 | 0.865–0.954 | 0.022 | 71.20 | 84.61 | 86.59 |
| BDNF | 0.880 | 0.826–0.934 | 0.027 | 63.94 | 85.59 | 78.05 |
| CA125+BDNF | 0.965 | 0.941–0.989 | 0.012 | 84.92 | 91.02 | 81.63 |
AUC – area under the curve; BDNF – brain-derived neurotrophic factor, AHF – acute heart failure; CI – confidence interval.
Serum CA125 and BDNF levels in patients with different Killip classifications
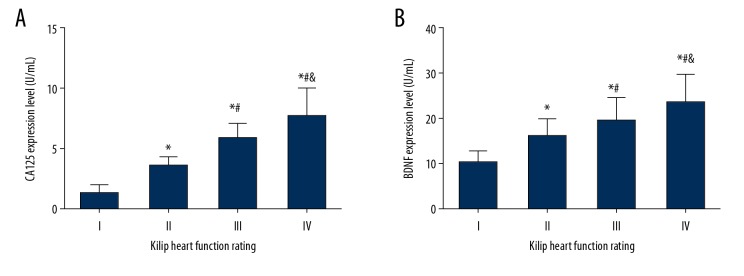
Compared with Killip class I patients, serum CA125 levels were significantly increased in Killip class II, III, and IV patients (all P<0.001). Serum BDNF levels were also significantly increased in Killip class II, III, and IV patients (all P<0.001). Compared with Killip class II patients, serum CA125 levels were significantly increased in Killip class III and IV patients (all P<0.001), with BDNF levels also significantly increased in these 2 groups (P=0.011; P<0.001). Compared with Killip class III patients, serum CA125 levels were significantly increased in Killip class IV patients (P=0.002), and serum BDNF levels were also significantly increased in this group (P=0.011) (Figure 2A, 2B).
Figure 2.
(A, B) Comparison of serum CA125 and BDNF levels in patients with different Killip classifications. (A) Comparison of serum CA125 levels in patients with different Killip classifications. (B) Comparison of serum BDNF levels in patients with different Killip classifications. (* Compared with grade I, P<0.001; # compared with grade II, P<0.05; and compared with grade III, P<0.05).
Correlation between serum CA125 and BDNF levels and Killip classifications
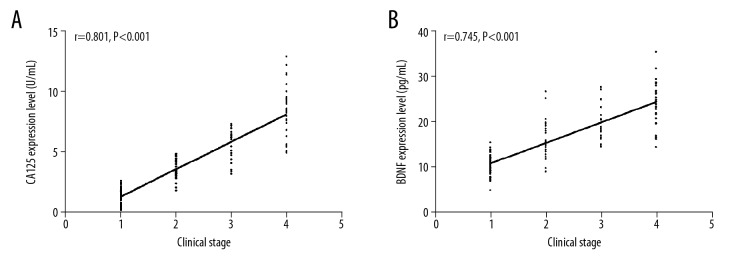
The correlation between serum CA125 and BDNF levels and the Killip classification was determined using Spearman’s rank correlation coefficient after setting Killip class I to 1, class II to 2, class III to 3, and class IV to 4. There was a positive correlation between serum CA125 levels and the Killip classification (r=0.801, P<0.001). There was also a positive correlation between serum BDNF levels and the Killip classification (r=0.745, P<0.001) (Figure 3A, 3B).
Figure 3.
(A, B) Correlation of serum CA125 and BDNF levels with Killip classifications (A) Correlation between serum CA125 and Killip classifications. (B) Correlation between serum BDNF levels and Killip classifications.
Diagnostic value of serum CA125 and BDNF levels in AHF
The area under the curve (AUC) combined with serum CA125 and BDNF levels in the diagnosis of AHF was 0.965 (95% CI: 0.941–0.989), while the diagnostic sensitivity was 91.02% and the specificity was 81.63% (Figure 4).
Figure 4.
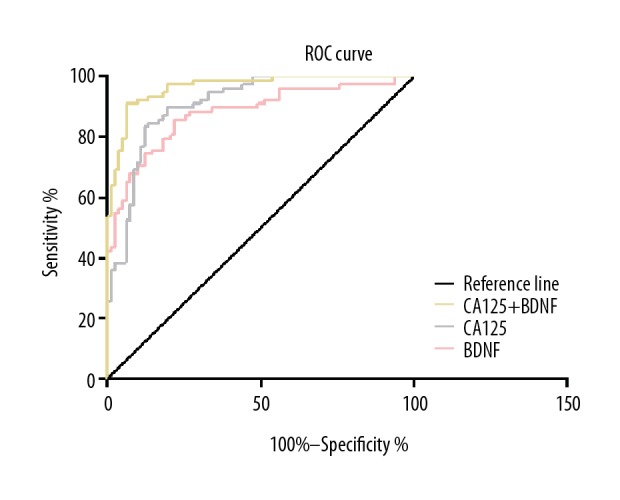
ROC curve for the diagnosis of AHF in combination with serum CA125 and BDNF levels.
Discussion
AMI is a critical emergency in cardiovascular disease and has become a major cause of human disability and death. AMI can induce changes in the extracellular matrix and in cardiomyocyte apoptosis, causing myocardial damage and ventricular remodeling, which is an important cause of AHF [12,13]. AHF is more common in left heart failure, and the prevalence rate increases with age. The elderly population (age >75 years) has a readmission rate of 12% due to AHF, and the clinical mortality rate is even higher. The 5-year survival rate is similar to that of malignant tumors [14]. The early stage of heart failure is often neglected because of non-specific clinical manifestations. Clinicians use certain non-objective factors to judge the degree of heart failure according to patients’ signs and symptoms [15]. Therefore, a timely diagnosis of AMI with targeted treatments are significant for improving the prognosis of and development of AHF.
CA125 is a high-molecular weight glycoprotein with a relative molecular mass of 200 kD, which can be produced under the action of inflammatory and mechanical stress, and can enter the blood through the surface of mesothelial cells under enzymatic splitting action [16]. CA125 levels are widely used in clinical screening, diagnosis, disease assessment, and the treatment prognosis of ovarian cancer, and are found in gastric and endometrial cancer [17,18]. Previous studies have shown that CA125 was elevated in CHF patients and was expressed in cardiovascular diseases such as reduced left ventricular output, ejection fraction retention, and chronic stable heart failure, even showing a potential advantage for the treatment prognosis assessment of these diseases [19]. It has been reported that an increase of CA125 levels was associated with an increase of inflammatory factors and fluid load in the body. In the development of heart failure, CHF can promote the secretion of CA125 by mesothelial cells under the action of mechanical stress caused by a large release of inflammatory factors and fluid retention, which is closely related to the presence of serous liquids [20]. BDNF, a member of the neurotrophic factor family, can mediate the survival of endothelial cells, promote the migration and proliferation of ischemic local endothelial cells, and play an important role in ischemic tissue angiogenesis [21]. In recent years, BDNF has been reported to be expressed in vascular smooth muscle cells, endothelial cells, and atherosclerotic vessels. BDNF is a key cytokine that regulates vascular development [22]. BDNF is expressed in both infarcted and non-infarcted areas of AMI, and is highly expressed in cardiomyocytes in the infarct border areas [23]. The results of this study showed that serum CA125 and BDNF levels in the study group were significantly higher than those in the control group. Serum CA125 and BDNF levels were positively correlated with the Killip classifications, suggesting that CA125 and BDNF were involved in the occurrence and development of AMI with AHF. Serum CA125 and BDNF levels increased significantly, and were positively correlated with, the severity of AHF after an AMI. It has been reported that AMI patients who had a high degree of cardiac dysfunction at admission, also had a relatively high hospital mortality rate. The Killip classification is an independent risk factor for AMI prognosis [24]. The sensitivity of serum CA125 for AHF diagnosis was 84.61% and the specificity was 86.59%. The sensitivity of serum BDNF for AHF diagnosis was 85.59% and the specificity was 78.05%. The sensitivity of serum CA125 and BDNF levels for the AHF combined diagnosis was 91.02%, and the specificity was 81.63%, suggesting that both CA125 and BDNF have a good diagnostic predictive value for AHF, and that combined detection can improve the sensitivity of an AHF diagnosis.
Conclusions
This study was conducted in strict accordance with inclusion and exclusion criteria. The differences between the control group and the study group were not statistically significant with respect to the clinical baseline data of gender, age, body mass index, concomitant disease, smoking status, drinking status, and systolic and diastolic blood pressure, ensuring the rigor and reliability of this study. This study did not explore the role of CA125 and BDNF in the prognosis of AMI patients with AHF, and therefore it has certain limitations. In future studies, the study time should be extended to investigate the role of CA125 and BDNF in the prognosis of AMI patients with AHF.
In summary, CA125 and BDNF play a role in the occurrence and development of AMI with AHF. As the disease progresses, serum CA125 and BDNF levels increase significantly, and are positively correlated with the severity of AMI combined with AHF. Both CA125 and BDNF have a good diagnostic value for AHF, and their combined detection can improve the sensitivity of an AHF diagnosis.
Footnotes
Conflict of interests
None.
Source of support: This work was supported by Medical Science Research Key Project of Hebei Province (grant number: 20180163)
References
- 1.Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. Lancet. 2017;389:197–210. doi: 10.1016/S0140-6736(16)30677-8. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JL, Morrow DA. Acute myocardial infarction. N Engl J Med. 2017;376:2053–64. doi: 10.1056/NEJMra1606915. [DOI] [PubMed] [Google Scholar]
- 3.Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119–77. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 4.Zeymer U, Werdan K, Schuler G, et al. Editor’s Choice – Impact of immediate multivessel percutaneous coronary intervention versus culprit lesion intervention on 1-year outcome in patients with acute myocardial infarction complicated by cardiogenic shock: Results of the randomised IABP-SHOCK II trial. Eur Heart J Acute Cardiovasc Care. 2017;6:601–9. doi: 10.1177/2048872616668977. [DOI] [PubMed] [Google Scholar]
- 5.Falcao F, de Oliveira FRA, da Silva M, Sobral Filho DC. Carbohydrate antigen 125: A promising tool for risk stratification in heart diseases. Biomark Med. 2018;12:367–81. doi: 10.2217/bmm-2017-0452. [DOI] [PubMed] [Google Scholar]
- 6.Cavusoglu YY, Altinbas ME, F Mutlu F, et al. Chronic kidney disease in heart failure is associated with higher CA 125 and hsCRP levels. Poster session. Eur J Heart Fail. 2017:P2209. [Google Scholar]
- 7.Nunez J, Nunez E, Bayes-Genis A, et al. Long-term serial kinetics of N-terminal pro B-type natriuretic peptide and carbohydrate antigen 125 for mortality risk prediction following acute heart failure. Eur Heart J Acute Cardiovasc Care. 2017;6:685–96. doi: 10.1177/2048872616649757. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen HL, Yarzebski J, Lessard D, et al. Ten-year (2001–2011) trends in the incidence rates and short-term outcomes of early versus late onset cardiogenic shock after hospitalization for acute myocardial infarction. J Am Heart Assoc. 2017;6(6) doi: 10.1161/JAHA.117.005566. pii: e005566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim B, Park SJ, Kwon JS, et al. Fimasartan protects cardiac myocyte damage in ischemic heart diseases. Poster seeesion. Eur J Heart Fail. 2016:P1784. [Google Scholar]
- 10.Hang P, Sun C, Guo J, et al. BDNF-mediates down-regulation of microRNA-195 inhibits ischemic cardiac apoptosis in rats. Int J Biol Sci. 2016;12:979–89. doi: 10.7150/ijbs.15071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garg P, Broadbent DA, Swoboda PP, et al. Extra-cellular expansion in the normal, non-infarcted myocardium is associated with worsening of regional myocardial function after acute myocardial infarction. J Cardiovasc Magn Reson. 2017;19:73. doi: 10.1186/s12968-017-0384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishikita A, Matoba T, Ikeda G, et al. Nanoparticle-mediated delivery of mitochondrial division inhibitor 1 to the myocardium protects the heart from ischemia-reperfusion injury through inhibition of mitochondria outer membrane permeabilization: A new therapeutic modality for acute myocardial infarction. J Am Heart Assoc. 2016;5(7) doi: 10.1161/JAHA.116.003872. pii: e003872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heusch G, Gersh BJ. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: A continual challenge. Eur Heart J. 2017;38:774–84. doi: 10.1093/eurheartj/ehw224. [DOI] [PubMed] [Google Scholar]
- 14.Nkhoma E, Ptaszynska A, Gomez A. Characterizing the incidence of heart failure following hospitalizations for acute myocardial infarction. Circulation. 134(Suppl 1) Abstract 15164. [Google Scholar]
- 15.Santhanakrishnan R, Wang N, Larson MG, et al. Atrial fibrillation begets heart failure and vice versa: Temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133:484–92. doi: 10.1161/CIRCULATIONAHA.115.018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becerra-Munoz VM, Sobrino-Marquez JM, Rangel-Sousa D, et al. Long-term prognostic role of CA-125 in noncongestive patients undergoing a cardiac transplantation. Biomark Med. 2017;11:239–43. doi: 10.2217/bmm-2016-0247. [DOI] [PubMed] [Google Scholar]
- 17.LaVigne K, Dao F, Abu-Rustum N, et al. HE4 is a biomarker for newly diagnosed and recurrent high-grade serous ovarian cancers with normal CA-125 values. Clinical Cancer Resarch. 2017;23(11) Abstract AP01. [Google Scholar]
- 18.Mehri JS, Farnaz S, Ali DT, et al. Diagnostic value of tumor biomarkers CA125 and CA72-4 in differentiation of epithelial ovarian cancer and endometrioma. Biomed Res. 2018;29(8) [Google Scholar]
- 19.Isilak Z, Kozan H, Yalcin M, et al. PP-166 The relationship between carbohydrate antigen 125 and left ventricular echocardiographic parameters in stable chronic systolic heart failure patients: a comparative study with NT-pro BNP. Am J Cardiol. 2016;117:S100. [Google Scholar]
- 20.Kosar F, Aksoy Y, Ozguntekin G, et al. Relationship between cytokines and tumour markers in patients with chronic heart failure. Eur J Heart Fail. 2006;8:270–74. doi: 10.1016/j.ejheart.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Pius-Sadowska E, Machalinski B. BDNF – A key player in cardiovascular system. J Mol Cell Cardiol. 2017;110:54–60. doi: 10.1016/j.yjmcc.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Ejiri J, Inoue N, Kobayashi S, et al. Possible role of brain-derived neurotrophic factor in the pathogenesis of coronary artery disease. Circulation. 2005;112:2114–20. doi: 10.1161/CIRCULATIONAHA.104.476903. [DOI] [PubMed] [Google Scholar]
- 23.Simkhovich BZ, Marjoram P, Poizat C, et al. Brief episode of ischemia activates protective genetic program in rat heart: A gene chip study. Cardiovasc Res. 2003;59:450–59. doi: 10.1016/s0008-6363(03)00399-7. [DOI] [PubMed] [Google Scholar]
- 24.Forrester JS, Diamond GA, Swan HJ. Correlative classification of clinical and hemodynamic function after acute myocardial infarction. Am J Cardiol. 1977;39:137–45. doi: 10.1016/s0002-9149(77)80182-3. [DOI] [PubMed] [Google Scholar]