Abstract
Multiple sclerosis (MS) is a chronic immune-mediated disease of the spinal cord and brain. Many studies have shown that smoking and passive smoking are key environmental risk factors for MS.
Here, we provide an overview of the human leukocyte antigen (HLA) gene studies on smoking and MS risk, and we discuss recent studies on between epigenetics and smoking-induced MS. In addition, in this review we also summarize current research advances in biological pathways and smoking-induced MS.
This review provides an overview of studies on the association between smoking, passive smoking, and MS susceptibility, and the underlying molecular mechanism.
MeSH Keywords: DNA Methylation, HLA Antigens, Multiple Sclerosis, Smoking, Tobacco Smoke Pollution
Background
Multiple sclerosis (MS) is a chronic inflammatory autoimmune disease of the central nervous system that disrupts communication between the brain and other parts of the body. MS is one of the most common causes of neurological disability in young adults. Its incidence in women is higher than that in men. Most MS patients, after the initial deterioration of function (recurrence), enter a recovery period (remission period). With the passage of time, the symptoms cannot be completely reversed, which leads to a gradual decline in function and an increase in the degree of disability. Most people have symptoms of MS for the first time between the ages of 20 and 40 [1–3].
Previous studies showed that many environmental factors, like sunlight, ultraviolet radiation, vitamin D, Epstein-Barr virus (EBV), smoking, and expose to passive smoking, are related to the occurrence of MS [1,4]. Smoking is one of the key environmental risk factors for MS. The purpose of this review is to give an overview of the studies conducted on the association between smoking, passive smoking, and MS susceptibility, and the underlying molecular mechanism.
Smoking and MS
Many studies have shown that smoking increases the risk of MS. In 1965, a study from Israel first suggested that smoking may be related to MS. The study investigated 241 patients with MS and normal individuals matched by age, sex, and location of birth were, showing that many former smokers were found in the patient group (44% vs. 36%, P=0.02) [5]. However, the study did not modify multiple comparisons. Later, in the 1990s, when investigating the relationship between oral contraceptives and MS, researchers found that women who had ever smoked had a higher relative risk (RR) of MS than those who had never smoked (RR for ex-smoker=1.5, RR for 1–14 cigarettes/day=1.6, and RR for at least 15 cigarettes/day=1.8), and the association was almost statistically significant (P=0.054) [6]. Soon thereafter, another similar study from the Royal College of General Practitioners also found a similar phenomenon. Their study demonstrated that women who smoked ≥15 cigarettes per day had a higher incidence of MS (95% CI 0.9–2.2) than those who had never smoked [7]. However, it should be pointed out that the scope of these 2 studies was limited to women, they had a small number of MS cases, and the main research focus was not the relationship between smoking and MS.
The aforementioned prospective cohort studies found that the incidence of MS in smokers was increased, but the number of cases were relatively small and the differences found were not statistically significant. Subsequently, 2 cohort studies of American nurses evaluated the relationship between MS incidence and smoking. After adjusting for age, geography, and ancestry, compared with non-smoking women, the relative prevalence of MS among currently smoking women was 1.6 (95% CI, 1.2–2.1) and that of formerly smoking women was 1.2 (95% CI, 0.9–1.6). With increased cumulative smoking, the relative incidence of MS increased significantly (P<0.05), and similar results were obtained after adjustment for other potential confounding factors [8,9]. Although these results did not confirm the causal relationship between smoking and MS, the researchers believe that smoking increases the risk of MS. A study of male smokers and chronic inflammatory diseases investigated 277 777 men within a cohort of Swedish construction workers who provided information about tobacco use found that ever-smoking was associated with an increased risk MS (95% CI, 1.4–2.6) [10].
Several studies have shown that smoking is related to MS. O’Gorman et al. investigated 646 patients (531 females, 115 males) with MS in Australia. Sex, age, age of onset, exposure to disease-modifying therapy, and smoking status were used as covariates in a Cox proportional hazards analysis. Their study demonstrated that MS occurred approximately 4 years earlier in ever smokers and smoking did not affect age of onset of MS [11]. Another study investigated 1465 patients with MS. The average age of the respondents was 42 years and the average duration of illness was 9.4 years. Of these, 257 of them are still smoking, 428 had smoked but later quit smoking, and 780 never smoked. At the beginning of the survey, smoking patients had more severe MS than the other 2 groups. Subsequently, the researchers conducted an average return visit every 3 years to determine the development of MS among the respondents. As a result, it was found that smoking patients had the highest probability of worsening MS compared with the other 2 groups. The researchers thus concluded that smoking exacerbates MS [12].
The latest study shows that smoking at time of clinically isolated syndrome (CIS) increases the risk of clinically definite MS. The prospective CIS cohort of the researchers included 250 patients aged between 18 and 50 years old, and their smoking status was recorded when the patient first experienced neurological symptoms. They used Cox regression analysis to calculate univariate and multivariate hazard ratios for MS diagnosis in smoking and non-smoking CIS patients; 46% of CIS patients were diagnosed with MS during a mean follow-up of 58 months. In total, 32% of patients smoked at time of CIS, 67% of the smoking CIS patients were diagnosed with MS during follow-up compared to 36% of the non-smoking CIS patients (P<0.001), and smoking at time of CIS was an independent predictor for MS diagnosis. Non-smoking CIS patients who had a history of smoking did not have a higher risk for MS than those who had never smoked [13].
Passive Smoking and MS
It was recently demonstrated that exposure to environmental tobacco smoke is associated with an increased risk of MS. Mikaeloff et al. investigated the relationship between parental smoking at home and MS in children. They conducted a population-based, case-control study with 129 cases of MS and 1038 matched controls. Of these, 62% of children with MS were exposed to smoking by their parents compared with 45.1% in the control group. The RR of a first episode of MS was significantly associated with parental smoking at home (95% CI, 1.43–3.15) and the risk of MS increased significantly with longer duration of exposure [14]. Another study estimated the influence of passive smoking on the risk for MS. The study was limited to patients with MS who had never smoked (695 patients, 1635 matched controls). The risk for MS was increased among never-smokers who had been exposed to passive smoking (OR 1.3, 95% CI 1.1–1.6) compared to never-smokers who had never been exposed. The risk increased with increasing duration of exposure (P=0.003). The above study shows passive smoking is associated with an increased risk for MS [15]. To estimate the effect of exposure to smoking on the risk for MS, Sundström et al. analyzed nicotine metabolite (cotinine) levels in biobank samples from 109 MS cases and 218 matched referents. Their results suggest that the risk of MS increases with the increasing cotinine levels. A similar phenomenon was also found in a small number of individuals collected before the onset of MS, but the change was not statistically significant. Further research shows that the risk of MS in females is related to increased levels of cotinine, but this association was not found in males. Modestly elevated cotinine levels suggestive of passive smoking are associated with an increased risk for MS [16]. Passive smoking may explain the higher incidence of MS in women and children.
Potential Immunology Mechanisms and Smoking-Induced MS
The pathology of MS involves autoreactive T cells targeting myelin and being transferred into the central nervous system (CNS) through the blood–brain barrier (BBB) and other barriers, inducing demyelination and loss of axonal function. There are multiple mechanisms involved in the relationship between smoking and progression of MS. MS pathogenesis has been attributed to genetic effects, environmental contributions, and environmental-genetic interactions [17]. Heredity has been reported to account for around 30% of the risk of developing disease [18]. As one of the best-confirmed environmental factors contributing to MS, tobacco smoking may influence MS development, mainly through autoimmune progression and CNS damage.
Immunology regulation pathway
Firstly, smoking exerts regulatory effects on T cells, B cells, and other immune cells, and nicotine has been reported to suppress T cell response [19] and influences the differentiation, phenotype, and dysfunction of antigen-presenting cells (APC) [20–22]. The tobacco glycoprotein in cigarette smoke condensate was reported to stimulate proliferation of T cells and differentiation of B cells [23]. Fas expression level is increased by tobacco smoking, which plays a significant role in immune homeostasis, especially affecting the B and CD4+ lymphocytes [24]. In addition, smoking has an anti-estrogen effect, and estrogen is related to Th1/Th2 balance in the body [25]. Tobacco smoking is associated with pro-inflammatory and anti-inflammatory mediators, increasing the number of inflammatory cytokines, including TNF-induced, and the inflammation process in the CNS [26]. The abnormal ratio of CD4+ and CD8+ is partially attributed to tobacco smoking, which also affects immune response in the CNS [27].
Secondly, the components in cigarette smoke affect the immune system barrier function. For example, nicotine modulates the tight junction proteins and causes higher BBB permeability, which is related with more permeable solute entering the CNS [28]. INOS, an isoform of nitric oxide (NO) synthases (NOS), has also been discovered to contribute to vasodilation and BBB dysfunction, and NO is associated gadolinium-enhanced lesions shown on MRI in blood-cerebrospinal fluid (CSF), an immune-related epithelial barrier, whose dysfunction is related to MS exacerbation [29,30]. Tobacco smoking was also reported to be associated with increased levels of serum metalloproteinase 9, which can degrade extracellular-matrix macromolecules to promote the migration of autoreactive immune cells into the CNS through the BBB [31,32].
Thirdly, tobacco smoking affects antigen presence. It can regulate the release of intracellular antigens via tissue hypoxia or cellular necrosis of toxin-mediation [33]. Moreover, exposure to smoking promotes the induction of autoimmunity to self-antigen [34]. Cigarette smoke suppresses the maturation of dendritic cells and the release of cytokine, which are involved in antigen-presenting activity [35]. The cytoplasm of macrophages is impaired after tobacco use, and can indicate antigen function [36]. TLRs have antigen-presenting functions in many cells, mediating the host defense. Pregnant women who smoke have an impaired response to TLRs, and they have a lower levels of antigen-presenting cell (APC) cytokines than non-smokers [37].
The pathway of CNS damage
Incidental cyanide and NO intoxication in smokers cause demyelination, axonal degeneration, selective loss of oligodendroglia, and neural conduction block [38,39], directly affecting CNS function, which can result in MS. Tobacco smoking also indirectly modulates oligodendrocyte differentiation. Smoking-induced nonspecific lung irritation can trigger the neuroinflammation in the CNS, activating immune response to a number of organ-specific inflammatory diseases [40]. Breathing in tobacco smoke and its derived lung inflammation produces reactive oxygen species (ROS) and reactive nitrogen species (RNS), and hypoxia damages mtDNA and inhibits mitochondrial function [41]. The subsequent energy deficiency cannot maintain the requirement of normal neuroaxonal conduction, promoting an ionic homeostasis imbalance, which may result in excessive accumulation of excitatory neurotransmitters in the CNS and eventually leading to neuronal apoptosis or necrosis.
Genetics and Smoking-Induced MS
MS is a polygenic hereditary disease in which environmental and genetic effects cause allergic immune disorders. The human leukocyte antigen (HLA) gene is the genetic factor that is most definitely associated with MS, accounting for 10% to 40% of the genetic risk factors [42]. In 2005, the International MS Genetics Consortium (IMSGC) reaffirmed the linkage between HLA and MS susceptibility through the results of a chain scan of 4500 SNPs in affected families of more than 730 members. However, other non-HLA loci do not reach the statistical significance in the genomics category [43]. HLA genes are genetic factors that are currently believed to be most closely related to MS. HLA is located in the human chromosome 6p21.31 region, which is a highly polymorphic, tightly linked genome with a total length of 3.60 Mb, also known as the major histocompatibility complex (MHC) [44]. Studies have shown that HLA plays an important role in almost all immune-related diseases, including MS. HLA is divided into HLA-I, -II, and -III regions, of which I and II are the most polymorphic regions in the human genome, which can lead to different combinations of genotypes or haplotypes. Class I contains HLA-expressing A, B, C, and other major sites, and class II mainly includes 3 sub-zones: DP, DQ, and DR [45–47]. Since the 1970s, HLA loci have been reported to be associated with MS [48], and the relationship between HLA genes and MS susceptibility has been increasingly studied.
Based on the Swedish Epidemiological Investigation of MS (EIMS), an interaction between smoking and HLA complex genes regarding risk of MS was reported in 2011. The study involved a total of 843 MS patients and 1209 normal individuals, classified according to their smoking status and HLA DRB1 and HLA-A genotype. The incidence of MS was then compared for subjects with different genotypes and smoking habits, and potential interactions between different genotypes and between genotype and smoking were assessed by calculating the proportion attributed to interactions. The results showed that 2 genetic risk factors were observed in smokers, a significant interaction between the carriage of the HLA-DRB1*15 and the absence of HLA-A*02, but not between non-smokers. The odds ratios vary greatly between groups. Compared with non-smokers with no genetic risk factors, the odds ratio for smokers with 2 genetic risk factors was 13.5 (8.1–22.6). The odds ratio for smokers without genetic risk was 1.4 (0.9–2.1), while the odds ratio for non-smokers with genetic risk factors was 4.9 (3.6–6.6). In those with genetic risk factors, the risk of smoking increased 2.8-fold compared with 1.4-fold for people without genetic risk factors [49]. Hedström et al. replicated and refined the above-mentioned study. They used 6 independent case-control studies from 5 different countries (Sweden, Denmark, Norway, Serbia, United States) and interactions were observed between HLA-DRB*15 and absence of HLA-A*02 and between smoking and each of the genetic risk factors [50]. A similar interaction has been replicated in studies of passive smoke exposure. In this study, an interaction was observed between passive smoking and carriage of HLA-DRB1*15, as well as between passive smoking and absence of HLA-A*02 [51]. In addition, Briggs et al. studied 1588 white MS patients and controls and found that NAT1, which encodes an enzyme involved in metabolism of smoke products, emerged as a genetic effect modifier of passive smoking in MS susceptibility [52]. These findings suggest that the effect of smoking on MS risk depends on the individual’s HLA genotype and other aspects of hereditary composition.
Epigenetics and Smoking-Induced MS
Epigenetic mechanisms mainly include DNA methylation, histone modification, and miRNA regulation. Although research on epigenetics in MS only began about a decade ago, more and more published data suggest that epigenetic changes are associated with development of MS, possibly by regulation of the interaction between environmental risk factors and molecular status, such as epigenetics modification induced by smoking, vitamin D deficiency, and EBV infection.
DNA Methylation and Smoking-Induced MS
The onset of MS and the manifestations of clinical symptoms and signs are associated with chronic autoimmune impairment caused by autoimmune dysfunction. However, the initiating factors and pathogenesis of this autoimmune dysfunction have not been elucidated. Although the current mainstream view categorizes the environmental, infection, and genetic factors as “the external environment affects susceptible individuals,” it is not known how each of these factors influence each other [53]. Specific environmental factors and individual susceptibility factors are still unclear. For individual susceptibility, previous large-scale family studies have found that the risk of family members with MS is significantly higher than that of normal family members, and this risk is gradually reduced with reduced kinship [54]. Thus, the susceptibility genes for the onset of multi-pathogenic sclerosis were mapped to HLA alleles on chromosome 6. This also partially explains why the risk of comorbidity in patients with identical twins is 5 times higher than in normal people [55]. However, there are still many phenomena that cannot be explained simply by relying on traditional susceptibility gene mechanisms. For example, although in homozygous twins the risk of changes in their fellow siblings is greatly increased, the comorbidity rate of identical twins in MS is not 100%, and the highest reported rate was only 30% [56]. In addition, with changes in living environment, such as migration, the incidence of the disease changes. These susceptibilities to individuals strongly suggest a new mechanism, called the epigenetic mechanism, which is different from the conventional genomic inheritance, and may play an important role in the pathogenesis of MS. Epigenetic mechanisms are more susceptible to environmental changes than traditional genomic mechanisms, and the altered epigenetic traits can be reversed under specific circumstances, while the altered traits can be genetically characterized [57]. Therefore, we hope that this new mechanism will provide a new perspective and direction for the study of MS mechanisms.
DNA methylation is one of the epigenetic mechanisms. Its research began in cancer research and was developed in areas such as aging medicine and certain autoimmune diseases such as systemic lupus erythematosus. DNA methylation is the process of selectively adding a methyl group to cytosine to form 5-cytosine under the action of DNA methyltransferase (Dnmt), which was defined as the fifth base when it was discovered. In fact, it is an important epigenetic marker that plays a major role in the regulation of gene expression, maintenance of chromatin structure, genetic imprinting, X chromosome inactivation, and embryonic development. Cytosine, in which the 5-position carbon atom is introduced into a methyl group, can be dispersed in the DNA strands distributed on the chromosomes alone, or closely combined with guanine to form a so-called CpG island [58,59]. The DNA methylation mentioned here mainly refers to the methylation of cytosine occurring in CpG islands. However, these CpG islands have different functions depending on the distribution of the sites on the chromosome. The CpG islands scattered in some chromosomal repeats, such as the centromere region, are mainly related to maintaining the stability of chromosome structure. Others, especially CpG islands of DNA sequences related to gene expression, such as the promoter region of structural genes, are mainly involved in the regulation of gene expression. When methylation of this part of the promoter sequence occurs, it hinders the effective recognition and binding of the corresponding transcription factor with the promoter sequence or changes the spatial conformation of this part of the chromosome, resulting in transcription failure, thus affecting the expression of this segment of the gene. The significance of DNA methylation located in the structural gene promoter sequence lies in the fact that it causes changes in the expression level or molecular structure of certain molecules that play an important role in the biochemical reaction process, and further influences the subsequent biochemical reactions [60].
In the past decade, due to progress in experimental techniques and limitations of the existing pathogenesis in the interpretation of MS, some studies have gradually introduced DNA methylation mechanisms into the study of MS [61,62]. Since the epigenetic content such as DNA methylation embodies the genetic mechanism of tissue-specific changes and serves as the pathological basis of clinical symptoms and pathogenesis, the brain tissues of MS patients and animal experimental models are the first choice for the study of the mechanism of DNA methylation. The experimental modeling of MS animals is currently mature and can provide enough brain tissue samples for research. However, for the patient’s brain tissue, due to the actual clinical activities, most of the patients with MS who are in the acute phase can be significantly relieved of their clinical symptoms after being treated with the corresponding immunoregulatory or immunosuppressive drugs. And there are few difficulties in clinical diagnosis, so the use of brain biopsy or autopsy is rare, so there is little use of brain tissue in this research area. Fabrizio et al., in a study of MS brain tissues, found that abnormally elevated citrullinated myelin basic protein (MBP) levels were observed in the white matter sheath. Further study found that the enzyme that catalyzes the citrullination of MBP is arginine deiminase (PAD2), and the expression level of this enzyme is also significantly increased in the brain tissue of MS patients. In investigating the cause of the abnormal expression of this enzyme, it was found that the methylation level of CpG islands in the promoter sequence of the gene encoding PAD2 was significantly lower than that in non-MS patients. The high expression of this enzyme is associated with a decrease in the methylation level of the expressed gene locus. Surprisingly, the methylation level of the PAD2 gene promoter sequence in the thymic tissue of this subset of patients with MS was not significantly different from that of the thymic tissue of patients without MS. However, the methylation levels of these sites in the brain tissue were significantly lower compared with their own brain tissues. This organ-specific methylation status abnormality suggests that this abnormal change is closely related to the pathogenesis of MS and validates the characteristic of the epigenetic spatial specificity change [63]. Huynh et al. conducted a study of brain tissue samples from patients with MS by examining the appearance of normal-looking brain tissue within the genome-wide CpG island methylation level. Compared with the control group, the level of methylation at the promoter sites of some genes was increased or decreased. Genes with elevated methylation levels are normally associated with the expression of proteins in the physiology of nerve tissue, which is involved in maintaining the normal structure of the tissue. The genes whose levels of methylation are downregulated are mostly protein molecules involved in the process of immune reactions [64].
Although the clinical manifestations of MS are mainly caused by involvement of the CNS, its main pathological damage is also concentrated in the CNS. MS is still primarily regarded as an autoimmune disease [65]. However, it is plausible that MS originates in individuals who are prone to autoimmune system dysfunction, and then another mechanism activates the autoimmune response to the central nervous system [66]. Therefore, it is equally important to study the function of the autoimmune system, not just brain tissue. Current research on the epigenetic mechanism of the human immune system in the pathogenesis of MS is mainly focused on peripheral blood immune cells. Since MS is mainly caused by immune damage mediated by CD4+ or CD8+ T lymphocytes, most studies use blood CD4+ or CD8+ T lymphocytes and their corresponding antigen-presenting cells [67,68]. The DNA methylation level of HLA-DRB1 and HLA-DRB5 has no significant difference in the MS peripheral blood. According to previous research, the locus of HLADRB1 has no influence on the CpG methylation in CD8+ T cells. This demonstrates that HLA DRB1 does not increase MS risk in CD8+ T cells [69]. However, in CD4+ T cells, a relationship was found between HLADRB1 locus and the level of DNA methylation [70]. Additionally, 9 CpGs show increased methylation in MS patients, which were located in the 20-kb region of T cell receptor α, which has an important role in immune recognition [71]. CpG hypermethylation can downregulate the expression of this gene and plays an important role in MS immune deregulation. Recent studies of genome DNA methylation have shown that CD8+ T cells have obvious DNA methylation characteristics [69]. This evidence indicates that DNA methylation in many genes at CpG sites control immune cell movement by influencing microtubule stability in MS. This result shows that DNA methylation is related to the occurrence and development of MS [72]. According to previous studies, various CpGs methylation are related to MS, but the major CpG effect at the MS risk gene HLA-DRB1 locus in CD8+ T cells has not been found [73]. In addition, no significant difference in DNA methylation was detected at a single CpG site [69]. DNA methylation process remodeling may be accompanied by changes in movement and function of CD8+ T cells. The relationship between DNA methylation and MS warrants further study.
DNA methylation at multiple CpG sites is affected by various lifestyle factors, like smoking and passive smoking [74]. Previous studies have demonstrated that there is a reproducible association between smoking and DNA methylation at CpG sites [75]. Some DNA methylation sites located in genes associated with pulmonary disease are also associated with smoking [76]. Moreover, there are different related CpGs sites between smokers and non-smokers. A systematic review of recent studies analyzed the results of 14 epigenetic studies with different levels of coverage and different phenotypic definitions of different DNA methylation platforms for smoking exposure. The review only compared statistically significant published results and did not include non-significant differences [77]. A meta-analysis assessed the blood-derived DNA methylation in 15 907 individuals, and found extensive epigenetic effects of smoking. There were 18 760 statistically significant CpGs and about 7000 annotated genes, including approximately one-third of known human genes. Many biological processes are regulated by these genes and affect some smoking-related diseases. Furthermore, studies have found that about 16 000 different methylated CpGs are associated with smoking. The phenotypes of important HLA genotypes in genetic factors are affected by smoking [74]. Additionally, smoking promotes inflammatory lesions in the lung tissue and is associated with neuroinflammation in the CNS. GFI1 is a transcriptional repressor that regulates hematopoietic cell differentiation, and methylation-related downregulation of this gene leads to increasing differentiation of Th17, which is associated with inflammation and demyelination in MS [78]. Moreover, smoking is an important factor involved in the development of mutant genotypes of XRCC1 ARg399GIn, related with the reduced capacity of DNA repair and the antioxidants and repair systems which help in maintaining oxidant-antioxidant balance [79]. High levels of ROS in serum generate oxidative stress products, inducing MS. Additionally, smoking deregulates AHRR/AhR activity through demethylation, and increased its expression level and the number of autologous hematopoietic (CD34+) stem cells, which has a role in the peripheral immune system in MS [80]. Recent studies have found that DNA methylation can modulate the effect of external triggers on cellular functions and the expression network of neuroinflammation, thereby promoting the response to smoking. There is mounting evidence that smoking and passive smoking can affect blood DNA methylation levels, especially the exposure response of smoking load and time. A meta-analysis found that DNA methylation levels of many smoking-related differentially methylated probes returned to normal levels within 5 years of quitting smoking [80]. However, the resilience of methylation levels is closely related to the degree of smoking and has individual differences. To solve this problem, more accurate individual analysis can be performed for larger population samples, or the samples can be traced and analyzed.
Histone Modification and Smoking-Induced MS
Histone is a structural component of nucleosome chromatin, and the modification of histone proteins H2A, H2B, H3, and H4 leads to modulation of gene expression. There are 3 types of modification described: histone variants, ATP-related chromatin remodeling complexes, and modification of histone tails (such as histone acetylation, methylation, citrullination, phosphorylation, and ubiquitylation).
High-level activity of peptidyl arginine deiminase 4 (PAD4) in MS patients and animal models was found to increase the citrullination of nucleosomal histones in white matter, which contributes to change and apoptosis of oligodendrocytes in MS [81]. Histone modification also plays a significant role in regulating the function and differentiation of T cells, especially for CD4+, in autoimmune diseases [82,83]. The function of other immune cells such as macrophages has been linked with histone modification as well. Meanwhile, smoking has been shown to induce the reduction of histone deacetylase (HDAC) in macrophages, consequently inhibiting IL-8 release [84]. The involvement of histone H3 lysine 9 demethylation in the IFN signaling pathway [85] suggests its potential application in MS treatment. More importantly, histone acetylation status was reported to be a response to myelin injury, including oligodendrocyte differentiation and axonal regeneration [86,87], which is related with the CNS function in MS. However, cigarette smoke exposure can induce distinct modification of histone H3 and H4 in lung cells [88], and the acrolein in cigarette smoke can specifically inhibit H3 and H4 acetylation in cytosol [89]. H3 phospho-acetylation [90] and imbalanced histone acetylation status caused enhanced inflammation in smokers [91] and both show an association with cigarette smoking. This evidence suggests a potential mechanism for smoking-induced histone modification in MS, but no direct or clear link has been reported so far.
Micro-RNA Regulators and Smoking-Induced MS
miRNAs are single-stranded non-coding RNA 19–25 nucleotides in length that are associated with post-transcriptional gene silencing by pairing with target mRNAs. Recent research on miRNA regulation showed involvement in MS immune pathogenesis, including the development and differentiation of immune cells, permeability of the BBB, brain development, and neurological disorders.
CD4+ T cells are associated with IFN-γ producing, IL-17, IL-4 producing and secreting of IL-10 and TGF-β, which were known as Th1, Th17, Th2, and Treg. There may be different patterns of expression of miRNA in various types of CD4+, CD8+, B cells, and other immune cells. The regulation of B cell function depending on miRNA regulators was reported and showed the downregulation of 49 miRNAs [92]. The induction of IFN-γ-IL-17A-Foxp3+CD4+ T cells was inhibited through miRNA let-7i regulation by suppressing the expression of IGF1R/TGFBR1 [93], and the changed expression of let-7i-3p was reported to be associated with cigarette smoking [94]. The proliferation of T cells was suppressed by miR-223−/−MO-MDSCs [95], which was downregulated in smokers with lung cancer [96].
Discoveries revealed the critical role for miRNAs in controlling BBB function, and over 65 miRNAs were differently expressed in endothelial cells, which may have potential significance in barrier function of the BBB [97]. The miR-125a-5p was found to directly regulate the migration of monocytes and macrophages [98]. In addition, miR-155 was proposed to be a negative regulator of BBB function in CNS inflammation, and a differential expression level of miR-155 was found in smoking-induced inflammation [99] and in lung cancer [100].
The effect of miRNAs on CNS demyelination and loss of axons is pivotal for MS. A complex role of miR-146a was suggested during the de- and re-myelination, and also a response factor for axonal loss and macrophages infiltration [101], while the alteration of miR-146a expression is particularly responsive to smoke exposure [102], and smoking status is a risk factor for miR-146a polymorphism [103]. Oligodendrocyte (OL) differentiation and myelin maintenance were found to be associated with the regulation of miR-219, miR-138, and other specific miRNAs, which suggests the participation of miRNA in CNS inflammation [104,105]. These factors may indicate a potential epigenetic mechanism for smoking-induced MS.
Conclusions
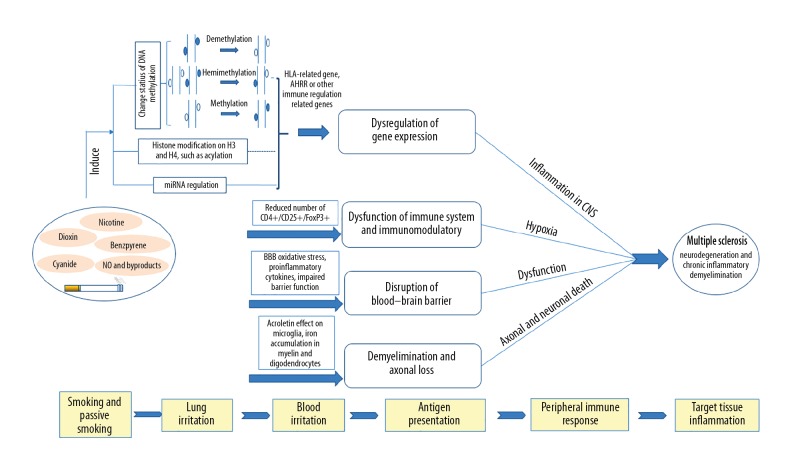
Increasing evidence shows that smoking and passive smoking can increase the risk of MS. Comprehensive environmental and genetics research, especially on molecular mechanisms, can increase awareness of MS (Figure 1). Smoking is one of the key environmental risk factors for MS. So far, we only know that the effect of smoking on MS risk is based on individual differences in HLA genotypes. However, this difference has stimulated further study of specific molecular mechanism of MS. The pathogenesis of MS is also accompanied by a series of epigenetic changes. Recent research results show that smoking can lead to methylation of DNA in peripheral blood cells of MS patients and affects the expression of downstream genes, but its downstream molecular mechanism remains unclear. At present, the study of the pathogenesis of smoking-induced MS is still in its infancy. With the development of epigenetics and molecular biology, in-depth study of the pathogenesis of MS will help find new therapeutic targets.
Figure 1.
Smoking affects the immune system to trigger MS.
Footnotes
Conflict of interests
None.
Source of support: This study was funded by the Zhejiang Basic Public Welfare Research Project (LGF18H090018) and the Young Research Foundation of Shaoxing People’s Hospital (2017A10)
References
- 1.Milo R, Kahana EW. MS: Geoepidemiology, genetics and the environment. Autoimmun Rev. 2010;9(5):A387–94. doi: 10.1016/j.autrev.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Ramagopalan SV, Byrnes JK, Orton SM, et al. Sex ratio of MS and clinical phenotype. Eur J Neurol. 2010;17(4):634–37. doi: 10.1111/j.1468-1331.2009.02850.x. [DOI] [PubMed] [Google Scholar]
- 3.Kamm CP, Uitdehaag BM, Polman CH. MS: Current knowledge and future outlook. Eur Neurol. 2014;72(3–4):132–41. doi: 10.1159/000360528. [DOI] [PubMed] [Google Scholar]
- 4.Hedstrom AK, Olsson T, Alfredsson L. Smoking is a major preventable risk factor for MS. Mult Scler. 2016;22(8):1021–26. doi: 10.1177/1352458515609794. [DOI] [PubMed] [Google Scholar]
- 5.Antonovsky A, Leibowitz U, Smith HA, et al. Epidemiologic study of MS in Israel. I. An overall review of methods and findings. Arch Neurol. 1965;13:183–93. doi: 10.1001/archneur.1965.00470020073010. [DOI] [PubMed] [Google Scholar]
- 6.Villard-Mackintosh L, Vessey MP. Oral contraceptives and reproductive factors in MS incidence. Contraception. 1993;47(2):161–68. doi: 10.1016/0010-7824(93)90088-o. [DOI] [PubMed] [Google Scholar]
- 7.Thorogood M, Hannaford PC. The influence of oral contraceptives on the risk of MS. Br J Obstet Gynaecol. 1998;105(12):1296–99. doi: 10.1111/j.1471-0528.1998.tb10008.x. [DOI] [PubMed] [Google Scholar]
- 8.Hernan MA, Olek MJ, Ascherio A. Cigarette smoking and incidence of MS. Am J Epidemiol. 2001;154(1):69–74. doi: 10.1093/aje/154.1.69. [DOI] [PubMed] [Google Scholar]
- 9.Hernan MA, Jick SS, Logroscino G, et al. Cigarette smoking and the progression of MS. Brain. 2005;128(Pt 6):1461–65. doi: 10.1093/brain/awh471. [DOI] [PubMed] [Google Scholar]
- 10.Carlens C, Hergens MP, Grunewald J, et al. Smoking, use of moist snuff, and risk of chronic inflammatory diseases. Am J Respir Crit Care Med. 2010;181(11):1217–22. doi: 10.1164/rccm.200909-1338OC. [DOI] [PubMed] [Google Scholar]
- 11.O’Gorman C, Bukhari W, Todd A, et al. Smoking increases the risk of MS in Queensland, Australia. J Clin Neurosci. 2014;21(10):1730–33. doi: 10.1016/j.jocn.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Healy BC, Ali EN, Guttmann CR, et al. Smoking and disease progression in MS. Arch Neurol. 2009;66(7):858–64. doi: 10.1001/archneurol.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Vuurst de Vries RM, Mescheriakova JY, Runia TF, et al. Smoking at time of CIS increases the risk of clinically definite MS. J Neurol. 2018;265(5):1010–15. doi: 10.1007/s00415-018-8780-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mikaeloff Y, Caridade S, Suissa M, et al. KIDSEP Study Group. Clinically observed chickenpox and the risk of childhood-onset MS. Am J Epidemiol. 2009;169(10):1260–66. doi: 10.1093/aje/kwp039. [DOI] [PubMed] [Google Scholar]
- 15.Hedstrom AK, Baarnhielm M, Olsson T, Alfredsson L. Exposure to environmental tobacco smoke is associated with increased risk for MS. Mult Scler. 2011;17(7):788–93. doi: 10.1177/1352458511399610. [DOI] [PubMed] [Google Scholar]
- 16.Sundstrom P, Nystrom L, Hallmans G. Smoke exposure increases the risk for MS. Eur J Neurol. 2008;15(6):579–83. doi: 10.1111/j.1468-1331.2008.02122.x. [DOI] [PubMed] [Google Scholar]
- 17.Bar-Or A. Immunology of MS. Neurol Clin. 2005;23(1):149–75. doi: 10.1016/j.ncl.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Beecham AH, Patsopoulos NA, Xifara DK, et al. Analysis of immune-related loci identifies 48 new susceptibility variants for MS. Nat Genet. 2013;45(11):1353–60. doi: 10.1038/ng.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sopori ML, Kozak W. Immunomodulatory effects of cigarette smoke. J Neuroimmunol. 1998;83(1–2):148–56. doi: 10.1016/s0165-5728(97)00231-2. [DOI] [PubMed] [Google Scholar]
- 20.Floto RA, Smith KG. The vagus nerve, macrophages, and nicotine. Lancet. 361(9363):1069–70. doi: 10.1016/S0140-6736(03)12902-9. 200. [DOI] [PubMed] [Google Scholar]
- 21.Guinet E, Yoshida K, Nouri-Shirazi M. Nicotinic environment affects the differentiation and functional maturation of monocytes derived dendritic cells (DCs) Immunol Lett. 2004;95(1):45–55. doi: 10.1016/j.imlet.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Nourishirazi M, Tinajero R, Guinet E. Nicotine alters the biological activities of developing mouse bone marrow-derived dendritic cells (DCs) Immunol Lett. 2007;109(2):155–64. doi: 10.1016/j.imlet.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34(3):J258–65. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Hoser G, Wasilewska D, Domagała-Kulawik J. Expression of Fas receptor on peripheral blood lymphocytes from patients with non-small cell lung cancer. Folia Histochem Cytobiol. 2005;42(4):249–52. [PubMed] [Google Scholar]
- 25.Zivadinov R, Weinstockguttman B, Hashmi K, et al. Smoking is associated with increased lesion volumes and brain atrophy in MS. Neurology. 2009;73(7):504–10. doi: 10.1212/WNL.0b013e3181b2a706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J, Taneja V, Vassallo R. Cigarette smoking and inflammation: cellular and molecular mechanisms. J Dent Res. 2012;91(2):142–49. doi: 10.1177/0022034511421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fusby JS, Kassmeier MD, Palmer VL, et al. Cigarette smoke-induced effects on bone marrow B-cell subsets and CD4+: CD8+ T-cell ratios are reversed by smoking cessation: influence of bone mass on immune cell response to and recovery from smoke exposure. Inhalat Toxicol. 2010;22(9):785–96. doi: 10.3109/08958378.2010.483258. [DOI] [PubMed] [Google Scholar]
- 28.Petecchia L, Sabatini F, Varesio L, et al. Bronchial airway epithelial cell damage following exposure to cigarette smoke includes disassembly of tight junction components mediated by the extracellular signal-regulated kinase 1/2 pathway. Chest. 2009;135(6):1502–12. doi: 10.1378/chest.08-1780. [DOI] [PubMed] [Google Scholar]
- 29.Liu JS, Zhao ML, Brosnan CF, Lee SC. Expression of inducible nitric oxide synthase and nitrotyrosine in MS lesions. Am J Pathol. 2001;158(6):2057–66. doi: 10.1016/S0002-9440(10)64677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rejdak K, Eikelenboom MJ, Petzold A, et al. CSF nitric oxide metabolites are associated with activity and progression of MS. Neurology. 2004;63(8):1439–45. doi: 10.1212/01.wnl.0000142043.32578.5d. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura T, Ebihara I, Shimada N, Koide H. Effect of cigarette smoking on plasma metalloproteinase-9 concentration. Clinica Chimica Acta. 1998;276(2):173–77. doi: 10.1016/s0009-8981(98)00104-1. [DOI] [PubMed] [Google Scholar]
- 32.Rosenberg GA. Matrix metalloproteinases biomarkers in MS. Lancet. 2005;365(9467):1291–93. doi: 10.1016/S0140-6736(05)61008-2. [DOI] [PubMed] [Google Scholar]
- 33.Francus T, Klein RF, Staiano-Coico L, et al. Effects of tobacco glycoprotein (TGP) on the immune system. II. TGP stimulates the proliferation of human T cells and the differentiation of human B cells into Ig secreting cells. J Immunol. 1988;140(6):1823–29. [PubMed] [Google Scholar]
- 34.Herberth G, Bauer M, Gasch M, et al. Maternal and cord blood miR-223 expression associates with prenatal tobacco smoke exposure and low regulatory T-cell numbers. J Allergy Clin Immunol. 2014;133(2):543–50.e4. doi: 10.1016/j.jaci.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 35.Robbins CS, Franco F, Mouded M, et al. Cigarette smoke exposure impairs dendritic cell maturation and T cell proliferation in thoracic lymph nodes of mice. J Immunol. 2008;180(10):6623–28. doi: 10.4049/jimmunol.180.10.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sköld CM, Lundahl J, Halldén G, et al. Chronic smoke exposure alters the phenotype pattern and the metabolic response in human alveolar macrophages. Clin Exp Immunol. 2010;106(1):108–13. doi: 10.1046/j.1365-2249.1996.d01-805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nizri E, Ironytursinai M, Lory O, et al. Activation of the cholinergic anti-inflammatory system by nicotine attenuates neuroinflammation via suppression of Th1 and Th17 responses. J Immunol. 2009;183(10):6681–88. doi: 10.4049/jimmunol.0902212. [DOI] [PubMed] [Google Scholar]
- 38.Coleman JW. Nitric oxide in immunity and inflammation. Int Immunopharmacol. 2001;1(8):1397–406. doi: 10.1016/s1567-5769(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 39.Hernán MA, Oleky MJ, Ascherio A. Cigarette smoking and incidence of MS. Am J Epidemiol. 2001;154(1):69–74. doi: 10.1093/aje/154.1.69. [DOI] [PubMed] [Google Scholar]
- 40.Olsson T, Barcellos LF, Alfredsson L. Interactions between genetic, lifestyle and environmental risk factors for MS. Nat Rev Neurol. 2017;13(1):25–36. doi: 10.1038/nrneurol.2016.187. [DOI] [PubMed] [Google Scholar]
- 41.Dendrou CA, Fugger L, Friese MA. Immunopathology of MS. Nat Rev Immunol. 2015;15(9):545–58. doi: 10.1038/nri3871. [DOI] [PubMed] [Google Scholar]
- 42.Wang M, Claesson MH. Classification of human leukocyte antigen (HLA) supertypes. Methods Mol Biol. 2014;1184:309–17. doi: 10.1007/978-1-4939-1115-8_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parmeggiani U, Pisano G, Canonico S, Piegari V. [Surgical treatment of hyperthyroidism in the elderly]. Ann Ital Chir. 1989;60(5):387–90. [in Italian] [PubMed] [Google Scholar]
- 44.Cree BA. MS genetics. Handb Clin Neurol. 2014;122:193–209. doi: 10.1016/B978-0-444-52001-2.00009-1. [DOI] [PubMed] [Google Scholar]
- 45.Olsson T, Barcellos LF, Alfredsson L. Interactions between genetic, lifestyle and environmental risk factors for MS. Nat Rev Neurol. 2017;13(1):25–36. doi: 10.1038/nrneurol.2016.187. [DOI] [PubMed] [Google Scholar]
- 46.Yates RL, Esiri MM, Palace J, et al. The influence of HLA-DRB1*15 on motor cortical pathology in MS. Neuropathol Appl Neurobiol. 2015;41(3):371–84. doi: 10.1111/nan.12165. [DOI] [PubMed] [Google Scholar]
- 47.Mack SJ, Udell J, Cohen F, et al. High resolution HLA analysis reveals independent class I haplotypes and amino-acid motifs protective for MS. Genes Immun. 2018 doi: 10.1038/s41435-017-0006-8. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shepherd DI, Downie AW. Prevalence of MS in north-east Scotland. Br Med J. 1978;2(6133):314–16. doi: 10.1136/bmj.2.6133.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hedstrom AK, Sundqvist E, Baarnhielm M, et al. Smoking and two human leukocyte antigen genes interact to increase the risk for MS. Brain. 2011;134(Pt 3):653–64. doi: 10.1093/brain/awq371. [DOI] [PubMed] [Google Scholar]
- 50.Hedstrom AK, Katsoulis M, Hossjer O, et al. The interaction between smoking and HLA genes in MS: replication and refinement. Eur J Epidemiol. 2017;32(10):909–19. doi: 10.1007/s10654-017-0250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hedstrom AK, Bomfim IL, Barcellos LF, et al. Interaction between passive smoking and two HLA genes with regard to MS risk. Int J Epidemiol. 2014;43(6):1791–98. doi: 10.1093/ije/dyu195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Briggs FB, Acuna B, Shen L, et al. Smoking and risk of MS: Evidence of modification by NAT1 variants. Epidemiology. 2014;25(4):605–14. doi: 10.1097/EDE.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 53.Ascherio A. Environmental factors in MS. Expert Rev Neurother. 2013;13(12 Suppl):3–9. doi: 10.1586/14737175.2013.865866. [DOI] [PubMed] [Google Scholar]
- 54.Sadovnick AD, Baird PA, Ward RH. MS: Updated risks for relatives. Am J Med Genet. 1988;29(3):533–41. doi: 10.1002/ajmg.1320290310. [DOI] [PubMed] [Google Scholar]
- 55.Hawkes CH, Macgregor AJ. Twin studies and the heritability of MS: A conclusion. Mult Scler. 2009;15(6):661–67. doi: 10.1177/1352458509104592. [DOI] [PubMed] [Google Scholar]
- 56.Fagnani C, Neale MC, Nistico L, et al. Twin studies in MS: A meta-estimation of heritability and environmentality. Mult Scler. 2015;21(11):1404–13. doi: 10.1177/1352458514564492. [DOI] [PubMed] [Google Scholar]
- 57.Kucukali CI, Kurtuncu M, Coban A, et al. Epigenetics of MS: An updated review. Neuromolecular Med. 2015;17(2):83–96. doi: 10.1007/s12017-014-8298-6. [DOI] [PubMed] [Google Scholar]
- 58.Jeltsch A, Jurkowska RZ. New concepts in DNA methylation. Trends Biochem Sci. 2014;39(7):310–18. doi: 10.1016/j.tibs.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 59.Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38(1):23–38. doi: 10.1038/npp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meng H, Cao Y, Qin J, et al. DNA methylation, its mediators and genome integrity. Int J Biol Sci. 2015;11(5):604–17. doi: 10.7150/ijbs.11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koch MW, Metz LM, Kovalchuk O. Epigenetic changes in patients with MS. Nat Rev Neurol. 2013;9(1):35–43. doi: 10.1038/nrneurol.2012.226. [DOI] [PubMed] [Google Scholar]
- 62.Li X, Xiao B, Chen XS. DNA Methylation: A new player in MS. Mol Neurobiol. 2017;54(6):4049–59. doi: 10.1007/s12035-016-9966-3. [DOI] [PubMed] [Google Scholar]
- 63.Mastronardi FG, Noor A, Wood DD, et al. Peptidyl argininedeiminase 2 CpG island in MS white matter is hypomethylated. J Neurosci Res. 2007;85(9):2006–16. doi: 10.1002/jnr.21329. [DOI] [PubMed] [Google Scholar]
- 64.Huynh JL, Garg P, Thin TH, et al. Epigenome-wide differences in pathology-free regions of MS-affected brains. Nat Neurosci. 2014;17(1):121–30. doi: 10.1038/nn.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kipp M, van der Valk P, Amor S. Pathology of MS. CNS Neurol Disord Drug Targets. 2012;11(5):506–17. doi: 10.2174/187152712801661248. [DOI] [PubMed] [Google Scholar]
- 66.Weissert R. The immune pathogenesis of MS. J Neuroimmune Pharmacol. 2013;8(4):857–66. doi: 10.1007/s11481-013-9467-3. [DOI] [PubMed] [Google Scholar]
- 67.Hoppmann N, Graetz C, Paterka M, et al. New candidates for CD4 T cell pathogenicity in experimental neuroinflammation and MS. Brain. 2015;138(Pt 4):902–17. doi: 10.1093/brain/awu408. [DOI] [PubMed] [Google Scholar]
- 68.Solti I, Kvell K, Talaber G, et al. Thymic atrophy and apoptosis of CD4+CD8+ thymocytes in the cuprizone model of MS. PLoS One. 2015;10(6):e0129217. doi: 10.1371/journal.pone.0129217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Handel AE, De Luca GC, Morahan J, et al. No evidence for an effect of DNA methylation on MS severity at HLA-DRB1*15 or HLA-DRB5. J Neuroimmunol. 2010;223(1–2):120–23. doi: 10.1016/j.jneuroim.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 70.Maltby VE, Graves MC, Lea RA, et al. Genome-wide DNA methylation profiling of CD8+ T cells shows a distinct epigenetic signature to CD4+ T cells in MS patients. Clin Epigenetics. 2015;7:118. doi: 10.1186/s13148-015-0152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Graves MC, Benton M, Lea RA, et al. Methylation differences at the HLA-DRB1 locus in CD4+ T-Cells are associated with MS. Mult Scler. 2014;20(8):1033–41. doi: 10.1177/1352458513516529. [DOI] [PubMed] [Google Scholar]
- 72.Shintani T, Ihara M, Tani S, et al. APC2 plays an essential role in axonal projections through the regulation of microtubule stability. J Neurosci. 2009;29(37):11628–40. doi: 10.1523/JNEUROSCI.2394-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shin J, Bourdon C, Bernard M, et al. Layered genetic control of DNA methylation and gene expression: A locus of MS in healthy individuals. Hum Mol Genet. 2015;24(20):5733–45. doi: 10.1093/hmg/ddv294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Joehanes R, Just AC, Marioni RE, et al. Epigenetic signatures of cigarette smoking. Circ Cardiovasc Genet. 2016;9(5):436–47. doi: 10.1161/CIRCGENETICS.116.001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zeilinger S, Kuhnel B, Klopp N, et al. Tobacco smoking leads to extensive genome-wide changes in DNA methylation. PLoS One. 2013;8(5):e63812. doi: 10.1371/journal.pone.0063812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guida F, Sandanger TM, Castagne R, et al. Dynamics of smoking-induced genome-wide methylation changes with time since smoking cessation. Hum Mol Genet. 2015;24(8):2349–59. doi: 10.1093/hmg/ddu751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gao X, Jia M, Zhang Y, et al. DNA methylation changes of whole blood cells in response to active smoking exposure in adults: A systematic review of DNA methylation studies. Clin Epigenetics. 2015;7:113. doi: 10.1186/s13148-015-0148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kurebayashi Y, Nagai A, Ikejiri A, et al. PI3K-Akt-mTORC1-S6K1/2 axis controls Th17 differentiation by regulating Gfi1 expression and nuclear translocation of RORγ. Cell Rep. 2012;1(4):360–73. doi: 10.1016/j.celrep.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 79.Karahalil B, Orhan G, Ak F. The impact of detoxifying and repair gene polymorphisms and the levels of serum ROS in the susceptibility to MS. Clin Neurol Neurosurg. 2015;139:288–94. doi: 10.1016/j.clineuro.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 80.Marabita F, Almgren M, Sjöholm LK, et al. Smoking induces DNA methylation changes in MS patients with exposure-response relationship. Sci Rep. 2017;7(1):14589. doi: 10.1038/s41598-017-14788-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mastronardi FG, Wood DD, Mei J, et al. Increased citrullination of histone H3 in MS brain and animal models of demyelination: A role for TNF-induced PAD4 translocation. J Neurosci. 2006;26(26):11387–96. doi: 10.1523/JNEUROSCI.3349-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Z, Yin H, Lau CS, Lu Q. Histone posttranslational modifications of CD4+T Cell in autoimmune diseases. Int J Mol Sci. 2016;17(10):1547. doi: 10.3390/ijms17101547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Castro K, Casaccia P. Epigenetic modifications in brain and immune cells of MS patients. Mult Scler. 2018;24(1):69–74. doi: 10.1177/1352458517737389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang SR, Chida AS, Bauter MR, et al. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol. 2006;291(1):46–57. doi: 10.1152/ajplung.00241.2005. [DOI] [PubMed] [Google Scholar]
- 85.Ahmed CM, Noon-Song EN, Kemppainen K, et al. Type I IFN receptor controls activated TYK2 in the nucleus: Implications for EAE therapy. J Neuroimmunol. 2013;254(1–2):101–9. doi: 10.1016/j.jneuroim.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ntranos A, Casaccia P. Bromodomains: Translating the words of lysine acetylation into myelin injury and repair. Neurosci Lett. 2016;625:4–10. doi: 10.1016/j.neulet.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koreman E, Sun X, Lu QR. Chromatin remodeling and epigenetic regulation of oligodendrocyte myelination and myelin repair. Mol Cell Neurosci. 2018;87:18. doi: 10.1016/j.mcn.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sundar IK, Nevid MZ, Friedman AE, Rahman I. Cigarette smoke induces distinct histone modifications in lung cells: Implications for the pathogenesis of COPD and lung cancer. J Proteome Res. 2014;13(2):982–96. doi: 10.1021/pr400998n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen D, Fang L, Li H, et al. Cigarette smoke component acrolein modulates chromatin assembly by inhibiting histone acetylation. J Biol Chem. 2013;288(30):21678–87. doi: 10.1074/jbc.M113.476630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang SR, Valvo S, Yao H, et al. IKKα causes chromatin modification on pro-inflammatory genes by cigarette smoke in mouse lung. Am J Respir Cell Mol Biol. 2008;38(6):689–98. doi: 10.1165/rcmb.2007-0379OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Szulakowski P, Crowther AJL, Jiménez LA, et al. The effect of smoking on the transcriptional regulation of lung inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174(1):41–50. doi: 10.1164/rccm.200505-725OC. [DOI] [PubMed] [Google Scholar]
- 92.Sievers C, Meira M, Hoffmann F, et al. Altered microRNA expression in B lymphocytes in MS: Towards a better understanding of treatment effects. Clin Immunol. 2012;144(1):70–79. doi: 10.1016/j.clim.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 93.Kimura K, Hohjoh H, Fukuoka M, et al. Circulating exosomes suppress the induction of regulatory T cells via let-7i in MS. Nat Commun. 2018;9(1):17. doi: 10.1038/s41467-017-02406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang J, Wu J, Li Y, et al. Deregulation of serum microRNA expression is associated with cigarette smoking and lung cancer. Biomed Res Int. 2014;2014 doi: 10.1155/2014/364316. 364316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cantoni C, Cignarella F, Ghezzi L, et al. Mir-223 regulates the number and function of myeloid-derived suppressor cells in MS and experimental autoimmune encephalomyelitis. Acta Neuropathologica. 2017;133(1):61–77. doi: 10.1007/s00401-016-1621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pavel AB, Campbell JD, Liu G, et al. Alterations in bronchial airway microRNA expression for lung cancer detection. Cancer Prev Res. 2017;10(11):651–59. doi: 10.1158/1940-6207.CAPR-17-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kamphuis WW, Derada TC, Reijerkerk A, et al. The blood–brain barrier in MS: microRNAs as key regulators. CNS Neurol Disord Drug Targets. 2015;14(2):157–67. doi: 10.2174/1871527314666150116125246. [DOI] [PubMed] [Google Scholar]
- 98.Reijerkerk A, Lopez-Ramirez MA, van Het Hof B, et al. MicroRNAs regulate human brain endothelial cell-barrier function in inflammation: Implications for MS. J Neurosci. 2013;33(16):6857–63. doi: 10.1523/JNEUROSCI.3965-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Conickx G, Avila Cobos F, van den Berge M, et al. microRNA profiling in lung tissue and bronchoalveolar lavage of cigarette smoke-exposed mice and in COPD patients: a translational approach. Sci Rep. 2017;7(1):12871. doi: 10.1038/s41598-017-13265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mohamed MA, Mohamed EI, Saa EK, et al. Underexpression of miR-486-5p but not overexpression of miR-155 is associated with lung cancer stages. Microrna. 2018;7(2):120–27. doi: 10.2174/2211536607666180212124532. [DOI] [PubMed] [Google Scholar]
- 101.Martin NA, Molnar V, Szilagyi GT, et al. Experimental demyelination and axonal loss are reduced in microRNA-146a deficient mice. Front Immunol. 2018;9:490. doi: 10.3389/fimmu.2018.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maccani MA, Avissarwhiting M, Banister CE, et al. Maternal cigarette smoking during pregnancy is associated with downregulation of miR-16, miR-21, and miR-146a in the placenta. Epigenetics. 2010;5(7):583–89. doi: 10.4161/epi.5.7.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang AX, Xu B, Tong N, et al. Meta-analysis confirms that a common G/C variant in the pre-miR-146a gene contributes to cancer susceptibility and that ethnicity, gender and smoking status are risk factors. Genet Mol Res. 2012;11(3):3051–62. doi: 10.4238/2012.August.31.2. [DOI] [PubMed] [Google Scholar]
- 104.Li JS, Yao ZX. MicroRNAs: novel regulators of oligodendrocyte differentiation and potential therapeutic targets in demyelination-related diseases. Mol Neurobiol. 2012;45(1):200–12. doi: 10.1007/s12035-011-8231-z. [DOI] [PubMed] [Google Scholar]
- 105.Galloway DA, Moore CS. miRNAs as emerging regulators of oligodendrocyte development and differentiation. Front Cell Dev Biol. 2016;4:59. doi: 10.3389/fcell.2016.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]