Abstract
Background
The current study investigated the detection of accessory hepatic veins and their vascular territories in the right hemiliver in rats, guinea pigs, and rabbits, which has become a prerequisite for newly developed clinical procedures. We compared the anatomical continuity of accessory hepatic veins with accessory hepatic veins existing in human livers.
Material/Methods
The analysis of accessory hepatic veins was performed using a corrosion cast method in combination with computer tomography (CT).
Results
In normal livers, accessory hepatic veins were regularly found. The length of these veins was 0.88±0.29 (cm ±SD) in rats, 1.10±0.39 in guinea pigs, and 1.28±0.48 in rabbits. Accessory hepatic veins became a part of the draining vessel draining into segment VI and VII; represented by interpolating and following Chouinard’s segmental concept.
Conclusions
The importance of detecting accessory hepatic veins lies in the identification of structures requiring special attention during surgery, in reduction of surgical complications, and in choosing the best approach to maintain the vitality of a drainage segment. The vascular reconstruction should be done during surgical interventions.
MeSH Keywords: Animals, Laboratory; Hepatic Veins; Imaging, Three-Dimensional; Liver; Tomography
Background
Accessory hepatic veins (AHVs) are one of the vascular variations around the liver that have been discussed in numerous clinical situations in recent years, such as in transplantation, partial resection, hepatectomy, detection of tumour thrombus, and surgical treatment of the liver [1–4]. AHVs are hepatic veins that drain the dorsal part of the liver (mainly segments VI and VII following the Chouinard’s classification system) and have their own independent drainage area with a separated opening into the caudal vena cava. AHVs do not always exist and their drainage territory is usually limited to only 1 segment or a part thereof [5,6]. Considering the embryological origin of the vessel, the embryological basis for the presence of AHV is related to various connections between the ductus venosus and sinusoids of a liver in the course of the intrauterine life. According to a further development of AHV during the vein regression, the closings of cross-communication connections disappear and sinusoids are absorbed. Thus, under those circumstances, several locations in the connections among the sinusoids and the ductus venosus remain stable, which may be the reason for the presence of such veins draining the liver and leading to an independent opening in the vena cava [7].
Clinically, the resection of AHVs can result in worsening the venous drainage and, consequently, cause ischemia, atrophy, or congestion. When removing a hepatic tumour in the right lobe, AHVs require additional surgical steps in order to have them ligated or clamped [8]. Ligation of the hepatic veins prior to the resection of a liver can prevent the growth suppression of cancer cells from spreading into veins, and reduces bleeding [7].
Progress in clinical hepatobiliary surgery has led to an increase in the number of similar procedures performed in experimental surgery. Advances in the field of liver transplantation have been widely supported by animal models [9,10], which are the prerequisite for developing new clinical procedures [11]. The main structure of the major liver lobes in rodent models compared to the human liver segmentation pursuant to Couinaud [12] is similar and the basic structure is comparable [13], which allows use of these models in experimental hepatobiliary surgery [11].
Contrary to the situation in humans, the clinical importance and structural analysis of AHVs in laboratory animals is unclear. The existence of such veins is mentioned in the available literature only in rats [13,14], and in rabbits and guinea pigs, there is no information about AHV in the literature.
The present study is based on performance of anatomical analyses of AHVs in selected laboratory animals, with the aid of computed tomography and corrosion casts. We estimated the number of samples needed to determine the distribution of AHVs within the liver segments and their relation to proper hepatic veins on the right side of the liver. In particular, we considered the potential implications for experimental hepatic surgery. Consequently, the right hemiliver was selected because the majority of AHVs in humans are found in this area.
Material and Methods
Animal models
Twenty healthy adult Wistar rats, 20 healthy adult New Zealand White rabbits, and 20 healthy adult guinea pigs of both sexes (10 males and 10 females in each group) were the subjects of research during ex-vivo imaging of AHVs. The mean weights of animals (mean ±SD) were: rats 495±48 g, rabbits 2980±114 g, and guinea pigs 720±65 g. Animals were examined in the accredited Laboratory of Research Biomodels, the University of P. J. Safarik in Kosice, and in the accredited experimental laboratory at the University of Veterinary Medicine and Pharmacy in Kosice, with the approval of the Institutional Ethical Committee (No. 1827/09-221/3). Animals were sacrificed by intraperitoneal injection of sodium pentobarbital [15] (50 mg/kg, Thiopental Valeant, Valeant Czech Pharma, and Czech Republic). The caudal vena cava was cannulated and the venous system was perfused with 0.9% physiological solution upon the addition of heparin (50 000 IU/kg, Heparin Léčiva, Zentiva, Czech Republic). Continuous perfusion was maintained until casting. Hepatic veins were studied by means of vascular corrosion casting and CT scans. The anatomical nomenclature of hepatic veins was adopted in accordance with Nomina Anatomica Veterinaria [16] and previous reports.
The preparing of the corrosion casts and formation of hepatic vascular trees
The corrosion casts were prepared with Spofacryl® resin (SpofaDental a.s., Jičín, Czech Republic). The casting medium (rats: 15 ml; guinea pigs: 20 ml; rabbits: 30 ml) was injected manually through a plastic cannula (Chiraflex i.v. cannula, inner diameter: 1.3/G18/L45; flow 95 ml/min) using a flow rate of 5 ml/min. The cannula was inserted and fixed in the caudal vena cava. After injection of the resin, animals were left at room temperature for at least 30 min and then submerged in tap water (40–60°C, 0.5–24 h) to fully polymerize the injected resin [17]. Soft tissues were macerated in potassium hydroxide solution (2–4% KOH) for 3 to 6 days at a temperature of 60–70°C. Prior to the drying process, the corroded specimens were submersed in water and dried at room temperature. Corrosion casts provided a 3D view of liver structures with regard to their relationship with other veins. Hepatic veins were evaluated macroscopically and microscopically (Leica M 320, Leica Microsystems Schweiz AG, Heerbrugg, Switzerland).
Acquisition of CT images
The vascular anatomy was reconstructed, analyzed, and used for determining hepatic vascular territories. Corossion casts of hepatic vascular trees extracted by maceration served as a prerequisite for CT imaging. Industrial computed tomography (metrotomography) was used for corrosion casts and to measure AHVs and proper hepatic veins. CT scans were obtained on a Metrotom® (Carl Zeiss, Germany) instrument with the following scanning parameters: 140 kW, 350 uA, integration time 1000 ms, number of scans 1000, and voxel 48.2 um. Consequently, CT images were analyzed with VGStudioMax 2.2 software (Volume Graphics, Germany).
Statistical analysis
Results are expressed as mean ± standard deviation of the mean (SD). Significant differences among groups were analyzed using the Mann-Whitney U test (GraphPad Prism 5.0 for Windows, GraphPad Software, San Diego, CA, USA). Differences among groups of animals were considered as significant at p<0.05.
Results
Evaluation of hepatic and accessory hepatic veins in CT images
Rats
The right hemiliver is located on the right side of the caudal vena cava and consists of 3 obvious parts: the right median lobe, right superior lobe (right lateral lobe), and right inferior lobe (right portion of the caudate lobe). In rats, the main structure of the hepatic venous system was identical to the lobulated liver segmentation. Each lobe has a separate hepatic venous branch that provides the outflow. The right hepatic venous system consists of 3 proper hepatic veins: the right hepatic vein (RHV), superior right hepatic vein (SRHV), and inferior right hepatic vein (IRHV). The outcome was recorded as follows: the RHVs drained the right median lobe, the SRHVs drained the right superior lobe, and the IRHVs drained the right inferior lobe. The length (cm ±SD) of RHVs was 2.43±0.30, while SRHVs had length parameters of 1.18±0.20, and IRHVs had 1.71±0.30 (Table 1).
Table 1.
The values of the length of proper hepatic veins and AHVs in selected laboratory animals (mean ±SD).
| Veins | Lenght (cm) | ||
|---|---|---|---|
| Rat | Guinea pig | Rabbit | |
| RHV | 2.43±0.30 | 2.43±0.67 | 7.56±0.72 |
| SRHV | 1.18±0.20 | 2.18±0.44 | 0 |
| IRHV | 1.71±0.30 | 2.45±0.59 | 5.10±0.65 |
| AHV | 0.88±0.29 | 1.10±0.39 | 1.28±0.48 |
RHV – right hepatic vein; SRHV – superior right hepatic vein; IRHV – inferior right hepatic vein; AHV – accessory hepatic vein.
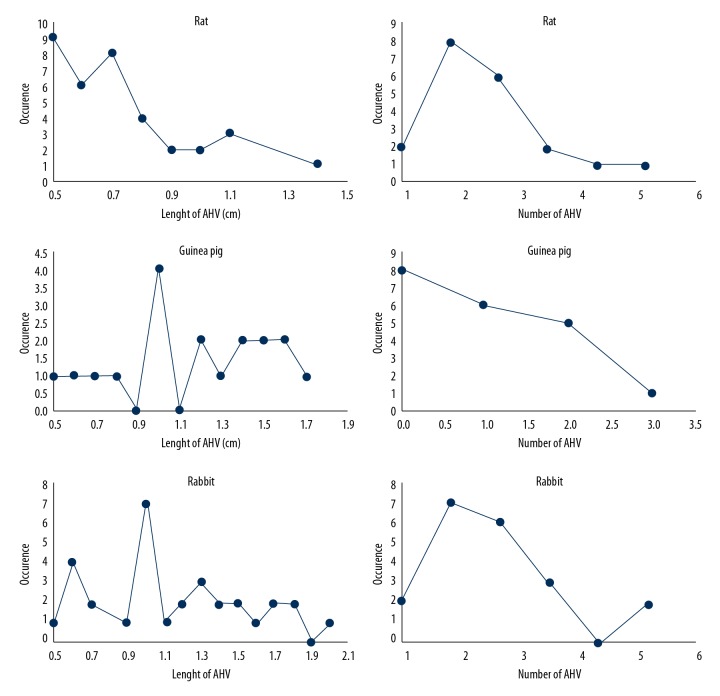
In addition to standard hepatic venous drainage, AHVs participated in formation of the liver venous system (Figures 1, 2). AHVs in normal liver vessels appeared to be regular, and in most cases (in 90% of cases) the presence of 1 or more AHV in the right part of the liver was observed. Only 2 livers did not show any AHVs. In all livers, there were fully identified 35 AHV, ranging from 1 to 5 in number. The frequency of their occurrence is shown in Figure 3. AHVs were found in the whole inferior right lobe and in 10% of cases they were in the caudal periphery of the superior right lobe. The length of these veins ranged from 0.5 to 1.4 cm, with a median length of 0.88±0.29 (cm±SD). Therefore, the distribution area of AHVs was relatively larger than the distribution area of proper hepatic veins. The median length of AHVs was 51% that of the length of SRHVs, and 75% of the length of IRHVs. The distribution area of the AHVs, measured between the inflow of AHVs and inflow of IRHVs down to its junction with the caudal vena cava, had a length of 0.1–0.7 cm cranially, 0.2–0.3 cm caudally, and 0.3–0.5 cm dorsally.
Figure 1.
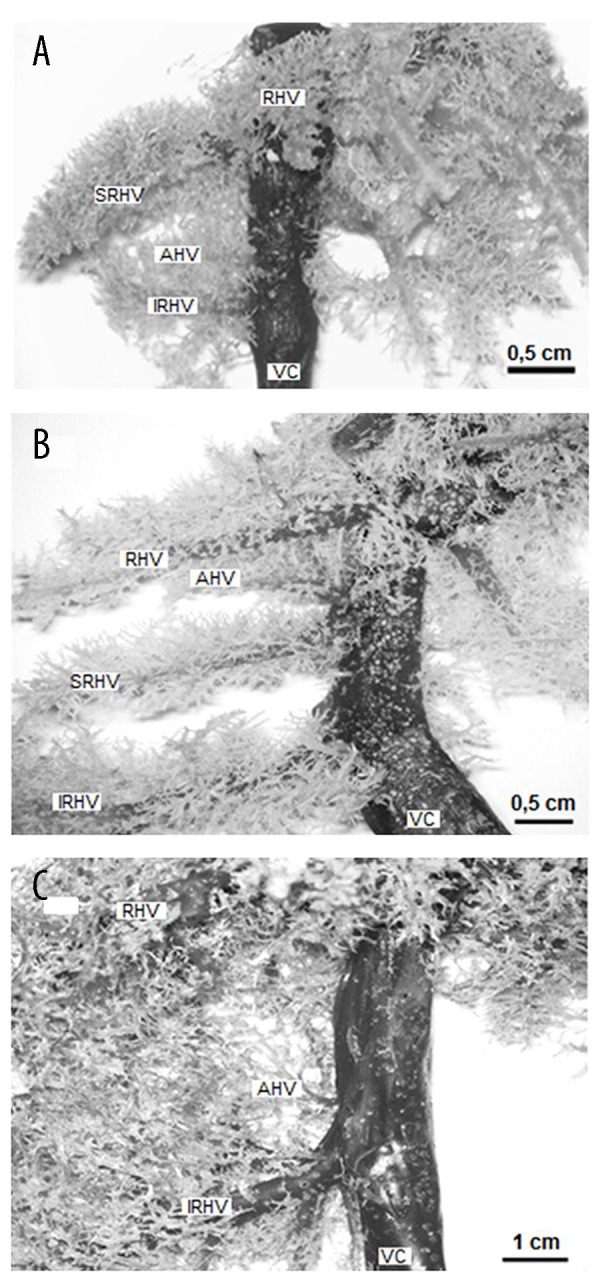
Veins of right hemiliver in a laboratory rat (A), guinea pig (B), and rabbit (C). Corrosion cast, ventral view, RHV – right hepatic vein; SRHV – superior right hepatic vein; IRHV – inferior right hepatic vein; AHV – accessory hepatic veins; VC – caudal vena cava.
Figure 2.
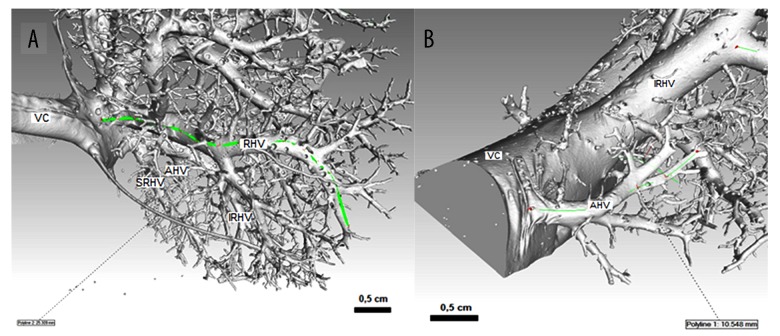
Arrangement and measurement of proper hepatic veins and accessory hepatic veins by using metrotomography in rats (A) and rabbits (B). CT scans; veins are viewed from the lateral side. RHV – right hepatic vein; SRHV – superior right hepatic vein; IRHV – inferior right hepatic vein; AHV – accessory hepatic veins; VC – caudal vena cava.
Figure 3.
The frequency of occurrence and length of accessory hepatic veins in selected laboratory animals. Occurrence was determined by the number of cases within 1 liver. The maximum number of accessory hepatic veins in 1 liver was 5 for rats and rabbits and 3 for guinea pigs. The longest measured length of accessory hepatic veins was 1.4 cm for rats, 1.7 cm for guinea pigs, and 2 cm for rabbits.
Guinea pigs
A similar distribution pattern and arrangement of the 3 proper hepatic veins was seen in guinea pigs (Figure 1). The right hemiliver was drained by RHVs, IRHVs, and SRHVs, which corresponds to the lobe division. The median length of RHVs was 2.43±0.67 (cm ±SD), while the median length of IRHVs was 2.45±0.59, and the median length of SRHVs was 2.18±0.44 (cm ±SD). AHVs were present in only 12 livers, and the total number was 19. Eight livers lacked AHVs. The number of AHVs in 1 liver ranged from 1 to 3 (Figures 3, 4). The length of AHVs varied from 0.5 to 1.7 cm (Figure 3), with a median length of 1.10±0.39 (cm±SD). In comparison with rats, there were fewer AHVs and they were longer, and thus again they may become the large draining vessels within the liver segments. AHVs were distributed in the area of the inferior right lobe and within the whole area of the right superior lobe, with the following percentage compositions: 42% in the superior right lobe, 33% in both segments, and the remaining 25% in the inferior right lobe. The median length of AHVs was 45% that of IRHVs, and 50% that of SRHVs. The position of the outflow of AHVs compared with IRHVs was 0.2–0.3 cm caudally, 0.1–2.1 cm cranially, and 0.2–0.5 cm dorsally.
Figure 4.
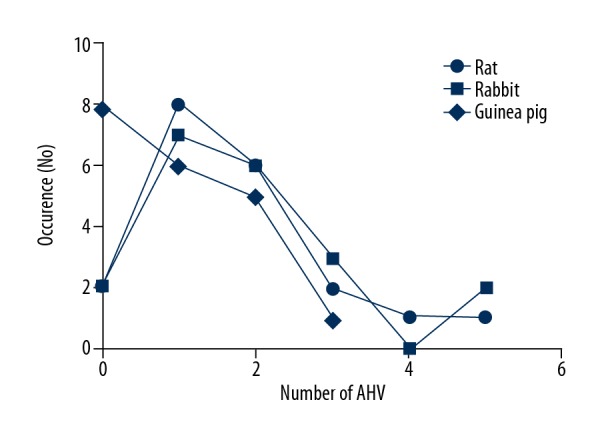
Comparison of incidence of accessory hepatic veins between laboratory rats, guinea pigs, and rabbits. The occurrence is identified with the number of cases within 1 liver. There was a dominant presence of 1 or 2 accessory hepatic veins in all laboratory animals.
Rabbit
The arrangement of veins in rabbit livers differs from the rat and guinea pig livers. There are only 2 proper veins that provide drainage of the right hemiliver entering the caudal vena cava. The right hemiliver was drained by RHVs with the median length of 7.56±0.72 (cm ±SD) and the caudally situated IRHVs (Figures 1–3) had a median length of 5.10±0.65 (cm ±SD). There were 32AHVs and these veins were present in 18 livers. Similar to the situation in rats, there were only 2 livers without AHVs. The length of AHVs varied from 0.5 to 2 cm (Figure 3), with a median length of 1.28±0.48 (cm ±SD, Figure 5), and were 25% that of the length of IRHVs. The distribution territory of AHVs compared with IRHVs was 0.2–0.8 cm caudally, 0.3–1.1 cm cranially, and 0.3–0.8 mm dorsally.
Figure 5.
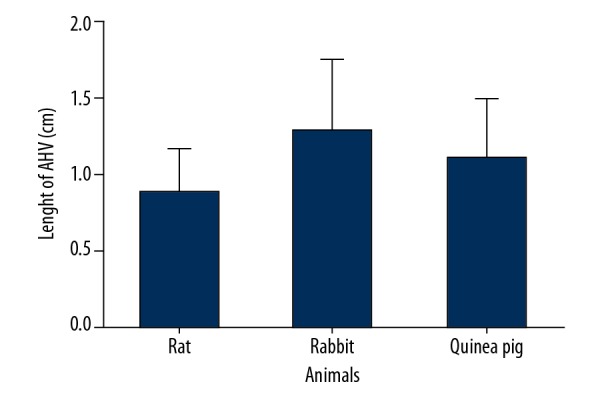
Comparison of the length of accessory hepatic veins among laboratory rats, guinea pigs, and rabbits. The length of accessory hepatic veins (mean ±SD) was 0.88±0.29 in rats, 1.28±0.48 in rabbits, and 1.10±0.39 in guinea pigs.
Analysis of the reconstructed veins
Overall, the main study outlines how the venous drainage of a right hemiliver in selected laboratory animals was provided by the combination of proper hepatic veins and AHVs in all 3 species. Proper hepatic veins were the dominant vessel in all cases. The area covered by the accessory hepatic vein was entirely covered by the accessory hepatic vein as a draining vein, so it drained the isolated the area in the liver lobe and did not show any communication to the proper hepatic veins or to the other AHVs. The RHVs, SRHVs, and IRHV did not enter the area covered by the accessory hepatic vein. Drainage could be extensive and if the accessory hepatic veins were long enough, they could drain a significant amount of blood. However, the presence of accessory hepatic veins in each liver did not affect the length of proper vessels; it had an effect on the width of the distribution area of the proper veins, which became remarkably smaller. We performed the statistical analysis of the length of AHVs among rats, rabbits, and guinea pigs. Differences among groups of animals were non-significant (p>0.05). The diameters of veins for which lengths were measured are shown in Table 2.
Table 2.
The diameters of veins at their endings to which lengths were measured. All values were expressed as mean ±SD.
| Veins | Diameter (cm) | ||
|---|---|---|---|
| Rat | Guinea pig | Rabbit | |
| RHV | 0.21±0.09 | 0.23±0.11 | 0.42±0.13 |
| SRHV | 0.15±0.06 | 0.20±0.06 | 0 |
| IRHV | 0.18±0.04 | 0.26±0.14 | 0.71±0.17 |
| AHV | 0.07±0.02 | 0.16±0.08 | 0.19±0.09 |
RHV – right hepatic vein; SRHV – superior right hepatic vein; IRHV – inferior right hepatic vein; AHV – accessory hepatic vein.
Discussion
Although the AHVs in humans have been frequently discussed [5,7,18,19], structural analysis of AHV in laboratory animals has not been performed. The classical description of the human liver segmentation determined by Couinaud [20] divides the human liver into 9 segments [21]. Interestingly, a study by Kogure et al. [13] that compared human and rat livers observed that lobes of the rat liver are equivalent to the liver of a human. The caudal lobe of the rat (Spiegel lobe and paracaval portion) corresponds to the human segment I–XI, the left lobe corresponds to segment II, the middle lobe corresponds to segments III, IV, V, and VIII, and the rat right lobe corresponds with segments VI and VII. According to Kogure et al. [13], the right lobe of the rat liver is divided into 2 sub-lobes; R-1 (which corresponds to the superior right lobe) and R-2 (which corresponds to the inferior right lobe). R-1 and R-2 corresponds to the segment VI and VII in accordance with the Couinaud classification [13]. This implies that the superior and inferior right lobe corresponds to segments VI and VII. We have extended and applied this segmentation scheme of the rat and human liver to 2 other species: rabbits and guinea pigs.
In our study, we found that the venous drainage of the right hemiliver was provided by the ensemble flow of the drainage system of proper hepatic veins and AHVs in all 3 selected laboratory animal species. The incidence of AHV was 90% in rats and rabbits and 60% in guinea pigs. Only 10% of rats and rabbits and 40% of guinea pigs did not show any AHVs, only the proper hepatic vessel. The arrangement of the AHVs was very regular. The numbers and sizes of AHVs varied in each case within a single species. AHVs in rats and rabbits were distributed in all areas of the inferior right lobe and the caudal part of the superior right lobe. In other words, the existence of AHV was bound to segment VI and fused with the caudal part of segment VII, following Chouinard’s liver segment classification and utilizing terminology derived from this classification. In contrast to the rat and rabbit liver, the AHV distribution in guinea pigs ranged in the area of the inferior right lobe and in the whole area of the superior right lobe, thus completely intervening with segment VII. Our results indicate that the number of AHVs varied from 1 to 5 veins in rats and rabbits, and from 1 to 3 veins in guinea pigs. Overall, we found 35 AHVs in rats, 19 in guinea pigs, and 32 in rabbits. The drainage area was principally supplied by the proper hepatic vein in each case and partly by the AHVs in humans, which agrees with the conclusions of Kogure et al. [22], who showed that the inferior right lobe had a systematized hepatic venous system that consisted of 1 or 2 proper hepatic veins and a number of accessory hepatic veins. Livers with 1 or 2 AHVs were most common [22] and this assessment correlates with our findings. Kogure et al. [13] in their other study reported that the AHV in the human liver could be compared to the vessels occurring in rats. They pointed out that each lobe in the rat liver had its own draining vein and the right lobe had veins, such as AHV, entering the vena cava. Myitaky et al. [14] in their study also reported the venous system of the liver in rats. Likewise, they found that there was a caudal lobe with short hepatic veins, which bear a resemblance to the AHV; however, they did not perform further structural analysis of these veins.
The occurrence of human AHV was described by several authors. Orguc et al. [23] found that out of 100 cases, 47 cases were of AHV, whereas in 13 cases more than 1 accessory hepatic vein was observed. Buhe et al. [5] observed the presence of AHVs in 60 human livers: 15 out of 60 livers (15/60) had 1 AHV, 26/60 had 2 AHVs, 13/60 had 3 AHVs, 4/60 had 4 AHVs, and 2/60 had 5 AHVs. Five AHVs was the highest number found in a single human liver, which agrees with our observation in laboratory animals. Buhe et al. [5] reported that AHVs were distributed in the dorsal region of the right liver lobe, and all veins were distributed in the area within segments VI and VII and never stretched outside these segments. Veins did not show any communications to the standard hepatic veins. In many cases, the range of the vein distribution was not extensive [5]. According to Van Leeuwen et al. [24], the conventional pattern of hepatic veins was seen in 2/10 instances. In the other 8 instances, 9 additional veins were found: 5 AHV on the right side, 1 in the middle, and 3 in the left hemiliver. Catalano et al. [8] reported that AHVs usually drain segments VI and VII, but rarely segment V. AHVs in laboratory animals are therefore more common than in humans.
In pre-surgical planning, it is essential to focus not only on the presence of AHVs but also their location, size, and the distance from the main hepatic venous drainage. For instance, if the distance is larger, it may be technically difficult to implant a vein or more of the veins into the recipient’s vena cava, and surgical techniques must be modified as well [8]. The present study found that the distance between the inflow of the standard vein of the inferior right lobe and the inflow of the AHV into the caudal vena cava ranged from 0.1 to 0.7 in rats, 0.1 to 1.1 in rabbits, and from 0.2 to 2.1 cm in guinea pigs.
In general, the hepatic arteries are arteries of resistance and the portal and hepatic veins are vessels of capacitance. Because more than 40% of hepatic blood is held in large capacitance vessels [25], accessory hepatic veins contain some blood volume, but if they reach 25% to 75% (according to laboratory animal species) the length of standard hepatic veins, they can account for a significant portion of the blood.
A comprehensive understanding and application of surgically important vessels of the liver is essential in improving and maintaining the excellent results of surgical intervention or segmental liver transplantation in research [6]. Pre-surgical evaluation of AHVs should be known prior to transplantation surgery [26]. One of the key points to a successful living donor liver transplantation is to keep the balance between the blood supply and venous drainage of the graft [8]. If AHVs are not found and are not reconstructed during the surgery, graft congestion and liver failure can occur [27,28]. Venous congestion may severely damage the graft, and even small hepatic venous branches that run down the dissection plane need to be left intact or reconstructed [29].
Conclusions
In conclusion, our study determined the area that is drained by the AHV and, in accordance with the Chouinard’s classification, is provided as an isolated zone of the liver in segments VI and VII. This area has a special blood circulation system, provided not only with the proper hepatic veins, but in most cases, also with AHVs. There are more AHVs in laboratory animals than in human liver vasculature. Advancing knowledge of AHV helps to determine the best hepatectomy in order to avoid transection of the major venous branches, allowing precise identification of areas at risk for venous congestion or devascularisation, which is mandatory for surgical planning in order to help reduce post-surgical complications.
Footnotes
Conflicts of interest
None.
Source of support: This study was funded by the grant VEGA No. 1/0046/16 from the Ministry of Education, Science, Research, and Sport of the Slovak Republic, and it was realized within the framework of the project ITMS No. 26220120066 “Centre of excellence for biomedical technologies”, which is supported by the Operational Program “Research and Development” financed through the European Regional Development Fund
References
- 1.Peng Y, Gong J, Yan L, et al. Improved two-cuff technique for orthotopic liver transplantation in rat. Hepatobiliary Pancreat Dis Int. 2004;3:33–37. [PubMed] [Google Scholar]
- 2.Peng SY, Liu YB, Xu B, et al. Role and significance of extra hepatic control of hepatic vein and inferior vena cava in difficult hepatectomies for patients with liver tumors. Chin J Surg. 2004;42:260–64. [PubMed] [Google Scholar]
- 3.Qiao J, Han CH, Zhang J, et al. A new rat model of auxiliary partial heterotopic liver transplantation with liver dual arterial blood supply. Exp Ther Med. 2015;9:367–71. doi: 10.3892/etm.2014.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu S, Xu Y, Yin T, et al. Fabrication and data harvesting of casting sample of rat liver blood vessels. Hepatobiliary Pancreat Dis Int. 2005;4:582–84. [PubMed] [Google Scholar]
- 5.Buhe S, Miyaki T, Saito T, et al. A study of the accessory hepatic vein to segments VI and VII with morphological reconsideration of the human liver. Surg Radiol Anat. 2008;30:201–7. doi: 10.1007/s00276-008-0315-8. [DOI] [PubMed] [Google Scholar]
- 6.Deshpande RR, Heaton ND, Rela M. Surgical anatomy of segmental liver transplantation. Br J Surg. 2002;89:1078–88. doi: 10.1046/j.1365-2168.2002.02153.x. [DOI] [PubMed] [Google Scholar]
- 7.Shilal P, Tuli A. Anatomical variations in the pattern of the right hepatic veins draining the posterior segment of the right lobe of the liver. J Clin Diagn Res. 2015;9:8–12. doi: 10.7860/JCDR/2015/8736.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catalano OA, Singh AH, Uppot RN, et al. Vascular and biliary variants in the liver: implications for liver surgery. Radiographics. 2008;28:359–78. doi: 10.1148/rg.282075099. [DOI] [PubMed] [Google Scholar]
- 9.Madrahimov N, Dirsch O, Broelsch CH, Dahmen U. Marginal hepatectomy in the rat. From anatomy to surgery. Ann Surg. 2006;244:89–98. doi: 10.1097/01.sla.0000218093.12408.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oldani G, Lacotte S, Morel P, et al. Orthotopic liver transplantation in rats. J Vis Exp. 2012;65 doi: 10.3791/4143. pii: 4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sänger C, Schenk A, Schwen LO, et al. Intrahepatic vascular anatomy in rats and mice – variations and surgical implications. PLoS One. 2015;10(11):e0141798. doi: 10.1371/journal.pone.0141798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Couinaud C. Portal segmentation. In: Couinaud C, editor. Controlled hepatectomies and exposure of the intrahepatic bile duct. Paris: Couinaud; 1981. pp. 9–27. [Google Scholar]
- 13.Kogure K, Ishizaki M, Nemoto M, et al. A comparative study of the anatomy of rat and human livers. J Hepatobiliary Pancreat Surg. 1999;6:171–75. doi: 10.1007/s005340050101. [DOI] [PubMed] [Google Scholar]
- 14.Miyaki T, Alimjan S, Saito T, Ito M. [The distribution of the portal and hepatic veins in rats]. Keitai Kagaku (Morphol Sci) 2006;10:27–31. [in Japanese] [Google Scholar]
- 15.Chio CC, Hsu CC, Tian YF, et al. Combined hemorrhagic shock und unilateral common carotid occlusion induces neurological injury in adult male rats. Int J Med Sci. 2017;14:1327–34. doi: 10.7150/ijms.21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danko J, Simon F, Artimova J. Nomina anatomica veterinaria. Kosice: UVLF; 2011. [Google Scholar]
- 17.Lametschwandtner A, Lametschwandtner U, Weiger T. Scanning electron microscopy of vascular corrosion casts-technique and applications: updated review. Scanning Microsc. 1990;4:889–40. [PubMed] [Google Scholar]
- 18.Fang CH, You J, Lau WY, et al. Anatomical variations of hepatic veins: Three-dimensional computed tomography scans of 200 subjects. World J Surg. 2012;36:120–24. doi: 10.1007/s00268-011-1297-y. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Li Z, Liu S, et al. The study on sectional anatomy and imaging of accessory hepatic veins. Surg Radiol Anat. 2009;31:739–43. doi: 10.1007/s00276-009-0513-z. [DOI] [PubMed] [Google Scholar]
- 20.Couinaud C. The paracaval segments of the liver. J Hepatobiliary Pancreat Surg. 1994;1:145–51. [Google Scholar]
- 21.Martins PNA, Neuhaus P. Surgical anatomy of the liver, hepatic vasculature and bile ducts in the rat. Liver Int. 2007;27:384–92. doi: 10.1111/j.1478-3231.2006.01414.x. [DOI] [PubMed] [Google Scholar]
- 22.Kogure K, Kuwano H, Fujimaki N, Makuuchi M. Relation among portal segmentation, proper hepatic vein, and external notch of the caudate lobe in the human liver. Ann Surg. 2000;231:223–28. doi: 10.1097/00000658-200002000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orguc S, Tercan M, Bozoklar A, et al. Variations of hepatic veins: Helical computerized tomography experience in 100 consecutive living liver donors with emphasis on right lobe. Transplant Proc. 2004;36:2727–32. doi: 10.1016/j.transproceed.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Van Leeuwen MS, Fernandez MA, Van Es HW, et al. Variations in venous anatomy of the liver: Two- and three-dimensional MR imaging in healthy volunteers. Am J Roentgenol. 1994;162:1337–45. doi: 10.2214/ajr.162.6.8191995. [DOI] [PubMed] [Google Scholar]
- 25.Greenway CV, Stark RD. Hepatic vascular bed. Physiol Rev. 1971;51:23–65. doi: 10.1152/physrev.1971.51.1.23. [DOI] [PubMed] [Google Scholar]
- 26.Saylisoy S, Atasoy C, Ersöz S, et al. Multislice CT angiography in the evaluation of hepatic vascular anatomy in potential right lobe donors. Diagn Interv Radiol. 2015;11:51–59. [PubMed] [Google Scholar]
- 27.Kamel IR, Kruskal JB, Keogan MT, et al. Multidetector CT of potential right-lobe liver donors. Am J Roentgenol. 2001;177:645–51. doi: 10.2214/ajr.177.3.1770645. [DOI] [PubMed] [Google Scholar]
- 28.Pannu HK, Maley WR, Fishman EK. Liver transplantation: preoperative CT evaluation. Radiographics. 2001;21:133–46. doi: 10.1148/radiographics.21.suppl_1.g01oc03s133. [DOI] [PubMed] [Google Scholar]
- 29.Kamel IR, Lawler LP, Fishman EK. Variations in anatomy of the middle hepatic vein and their impact on formal right hepatectomy. Abdom Imaging. 2003;28:668–74. doi: 10.1007/s00261-002-0088-1. [DOI] [PubMed] [Google Scholar]