Abstract
Aim:
With a perplexing pathogenesis and an incidence rate of approximately 10% among women of reproductive age, endometriosis affects more women in Asia than in any other continent in the world. This paper reviews the available data on the epidemiology and risk factors associated with endometriosis in East Asia.
Methods:
Included studies were published between January 2000 and December 2016. Articles were required to include East Asian patients with a diagnosis of endometriosis and to study epidemiology, such as the prevalence and/or incidence, associated with inherited, environmental, and/or lifestyle factors. A total of 65 candidate articles were retrieved and 22 were included in the final review.
Results:
Only one study provided an estimate of prevalence (6.8%). Short menstrual cycle, family history of endometriosis, and some genetic polymorphisms are associated with the risk of developing endometriosis. Smoking, lower body mass index, and lower parity associated with increased risk of endometriosis were suggested as modifiable factors. Limitations of this study include the poor quality of data identified, and the language barriers behind the study retrieval.
Conclusions:
Data on the epidemiology of endometriosis in the East Asian populations are limited. The available data that examine potential genetic factors do not unveil whether such factors directly contribute to the increased risk of endometriosis. Further extensive studies on endometriosis in Asian women are required to improve the management of this disease.
Keywords: Asia, endometriosis, epidemiology
INTRODUCTION
Endometriosis is a disease characterized by the presence of ectopic endometrial tissue outside the uterine cavity, with clinical presentations of dysmenorrhea, dyspareunia, dyschezia, and sometimes diarrhea.[1,2] Endometriosis is an estrogen-dependent disease strongly influenced by the cyclic change of steroid hormones and it causes inflammatory conditions in the pelvic cavity[3,4] and may present with subfertility, ongoing pelvic pain, and/or pelvic mass despite treatment with oral contraceptives and analgesics.[5,6,7,8] While the incidence of malignant change was rare,[9,10] endometriosis remains an important cause of morbidity impeding quality of life in women of reproductive age.[11] Although the exact etiology and pathogenesis of endometriosis are unclear,[12] environmental and genetic factors which induce complex immunological interactions within the pelvic cavity have been implicated in the disease.[13]
In the Western populations, endometriosis is estimated to occur in 5% to 10% of the population; however, the prevalence of endometriosis is suspected to be higher in Asian women, affecting approximately 15% of women.[5,14,15] Indeed, compared to women in the United States of America, women from Southeast Asia and Japan have a higher prevalence of endometriosis although this may be confounded by socioeconomic status, and the fact that the studies upon which these estimates were based were of poor quality and in some cases were over 40 years old.[11] Despite this, the suggestion that prevalence rates might be higher in Asian populations and the fact that these populations are numerically significantly larger than elsewhere implies that endometriosis is a significantly larger problem for Asian countries than elsewhere in the world. Furthermore, given the plethora of ethnic variations that exist among Asian populations, the epidemiological data and trends from Western countries may not always be relevant to populations in Asia. Combined, this suggested that an updated approach to determining the epidemiology of endometriosis in Asian populations is required.
Understanding the epidemiology and risk factors for endometriosis in the Asian context is important to guide patient care. This includes both the diagnosis and treatment of this debilitating condition. As the clinical impact of the lifestyle and environmental factors on the development of endometriosis is a frequently asked question by the patients and the researchers, the objective of this review was to describe the epidemiology, including the associated inherited, lifestyle, and/or environmental factors with the incidence of endometriosis in the East Asian populations.
METHODS
We undertook a search of English literature from Medline, PubMed, and the Cochrane Library (including the Cochrane Database of Systematic Reviews) for all articles relate to human endometriosis published between January 2000 and December 2016. The MeSH terms included all subheadings, and keywords included "endometriosis risk," "endometriosis association," "endometriosis epidemiology," "endometriosis gene," and "endometriosis environment." All published works were included from the electronic database searches and cross-references pick-up was also performed during the review search if the article was not initially found. Each article was assessed on the abstract for its relevance and only those focusing on epidemiologic information, such as prevalence, incidence, and correlating risk factors, were included. Adenomyosis, which is the endometriotic disorder of the uterus,[16,17,18] was not included in this review as it is a disease different from the peritoneal or ovarian endometriosis.[19] Laboratory studies of disease mechanism were also excluded. In consideration of the ethnic similarity, only studies developed from East Asia (including ethnic groups of Chinese, Taiwanese, Japanese, and Koreans peoples) were included; however, those derived from Northern Asia (such as ethnic groups of Siberia), Central Asia (such as ethnic groups Turkic, Iranian, and Russians peoples), South Asia (including ethnic groups of India, Indochinese Peninsula, and Filipino peoples), and West Asia (such as ethnic groups of Arab peoples and Jews) were not included.
RESULTS
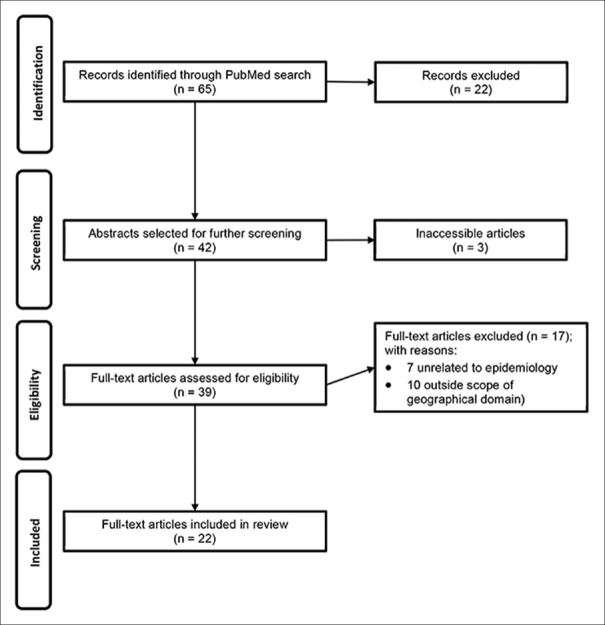
A total of 65 candidate articles were identified fitting the scope of the current review, and finally, 22 eligible articles included for review [Figure 1]. We found five studies of demographic and/or environmental parameters related to endometriosis [Table 1], 12 studies showed positive findings for an association of a genetic polymorphism with endometriosis [Table 2], and another 5 studies found negative results for a correlation of genotypes with endometriosis [Table 3].
Figure 1.
Flow diagram of article selection process
Table 1.
Studies of demographic or environmental parameters
| Reference | Sample Size | Involved factors | Population | Summary |
|---|---|---|---|---|
| Yasui et al., 2015[20] | Cohort: 330 | Menstrual cycle | Japanese | Surgically confirmed endometriosis (Group A, n=210), imaging diagnosed endometriosis without a surgical procedure (Group B, n=120). A short menstrual cycle at 18-22 years of age and cigarette smoking at 30 years of age were associated with significantly increased risk of endometriosis (Group A + Group B) |
| Infertility | ||||
| Cigarette smoking | ||||
| In women with a history of infertility, a short menstrual cycle was associated with a significantly increased risk of endometriosis in both Group A and Group B, but risk profiles of endometriosis were different between Group A and Group B in women without a history of infertility | ||||
| Yi et al., 2009[21] | Cohort: 481 | BMI | Korean | Women in stages III or IV endometriosis had a significantly lower BMI than those in stages I or II disease |
| Itoh et al., 2008[22] | Case: 54 | Cadmium | Japanese | No association between higher urinary cadmium concentration and the risk of endometriosis |
| Control: 74 | ||||
| Tsukino et al., 2005[23] | Case: 58 | Organochlorine | Japanese | No association between higher serum levels of these organochlorine compounds and an increased risk of endometriosis among infertile Japanese women |
| Control: 81 | ||||
| Kashima et al., 2004[24] | Case: 339 | Family history | Japanese | Heritable genetic factors contribute to the development of endometriosis |
| Control: 284 |
BMI: Body mass index
Table 2.
Summary of Ggenetic polymorphism studies with significant differences
| Reference | Sample size | Involved genes | Population | Summary |
|---|---|---|---|---|
| Lee, et al., 2014[25] | Case: 673 | CDKN2B-AS gene | Korean | The CC genotype of the rs10965235 SNP in the CDKN2B-AS gene and the GG genotype of the rs16826658 SNP near the WNT4 gene were significantly associated with endometriosis in Korean population |
| Control: 500 | ||||
| WNT4 gene | ||||
| Cho et al., 2013[26] | Case: 201 | MMP-2 gene | Korean | In MMP-2, G/A haplotype of 9082A>G and 9152A>G in intron 2 was associated with a reduced risk of endometriosis (OR 0.7, 95% CI 0.5-1.0, P=0.04) |
| Control: 183 | TIMP-2 gene | |||
| In TIMP-2, the CC genotype of 42196430T>C and C/C haplotype of 42196041G>C/42196430T>C in the promoter region showed an increased risk of endometriosis (OR 3.0, 95% CI 1.2-8.0, P=0.02; OR 1.6, 95% CI 1.1-2.4, P=0.02) | ||||
| In TIMP-2, the CC genotype of 42183387T>C and the C/G/C haplotype of 42175617C>T/42181597G>A/42183387T>C in intron 1 were associated with a reduced risk (OR 0.5, 95% CI 0.3-0.97, P=0.04; OR 0.6, 95% CI 0.4-0.95, P=0.03) | ||||
| Wang et al., 2012[27] | Case: 300 | HSD17B3 | Taiwanese | These 4 nsSNPs reside ((rs6165, rs6166, rs2066479, rs700519)) in 3 genes related to estrogen synthesis (HSD17B3, FSHR, and CYP19) increases endogenous production of more estrogens may be more strongly associated with the risk of endometriosis |
| Control: 337 | FSHR | |||
| CYP19 | ||||
| Kim et al., 2012[28] | Case: 268 | COX-2 gene | Korean | The C allele for -765G/C of the COX-2 gene was associated with significantly lower risk of advanced-stage endometriosis (OR, 0.14; 95% CI, 0.06-0.30) |
| Control: 242 | ||||
| The -765C allele may be protective against the development of the disease in Korean women | ||||
| Wang et al. 2011[29] | Case: 300 | FSH receptor gene | Taiwanese | The mutant alleles of FSH receptor gene at the position 680 of amino acid (Asn680Ser) (GG genotype, 680Ser/Ser and GA genotype, 680Ser/Asn) may have a protective effect on the development of endometriosis in Taiwanese Chinese women |
| Control: 337 | ||||
| Kang et al., 2010[30] | Case: 421 | FGF2 gene | North Chinese | FGF2 754C/G polymorphism may be associated with a risk of developing endometriosis. The G allele in the FGF2 gene may potentially protect against the disease. |
| Control: 421 | ||||
| Lee et al., 2008[31] | Case: 246 | TNF-α a gene | Korean | This difference at the TNF: g.[-1031T >C] tends to increase in Stage IV endometriosis (P=0.01). The genotype frequencies at the TNF: g.[-1031T>C] and the TNF: g.[-863C >A] sites may be associated with advanced stage endometriosis |
| Control: 248 | ||||
| Kang et al., 2008[32] | Case: 298 | TIMP-2 gene | North Chinese | The frequency of the TIMP-2 -−418C/C homozygote was significantly low in patients with endometriosis (0.7%), the C/C genotype may significantly decrease the risk of endometriosis development, with an odds ratioOR of 0.18 (95% CIconfidence interval, 0.04-0.79). TIMP-2-418C/C homozygote may be a protective factor against the development of endometriosis |
| Control: 324 | MMP-2 gene | |||
| No significant difference in genotype and allelotype distributions of the MMP-2−1306C→T was found between patients and control | ||||
| Shan et al., 2007[33] | Case: 152 | E-cadherin gene | North Chinese | There was a significant difference in the frequency of the E-cadherin 3’- UTR C --> T genotypes between endometriosis and controls (P=0.01) |
| Control: 189 | ||||
| When compared with the T/T+T/C genotypes, the C/C genotype had a significantly increased susceptibility to endometriosis, with an adjusted odds ratioOR of 1.79 (95% confidence intervalCI=1.17-2.76) | ||||
| E-cadherin 3’- UTR C --> T polymorphism, the -160 A/-347 GA haplotype of two promoter polymorphisms suggesting a potential role in endometriosis development | ||||
| Kitawaki et al., 2006[34] | Case: 202 | IL-6 gene | Japanese | The IL-6-634C/G and ICAM-1 469K/E polymorphisms synergistically affect the susceptibility for endometriosis. The frequency of ICAM-1 EE homozygotes who concomitantly carried the IL-6-634G allele was significantly higher in patients with endometriosis (P=0.0396, d.f. 2) |
| Control: 236 | ICAM-1 gene | |||
| No differences in the IL-6-634C/G genotypes and allele frequencies or the ICAM-1 469K/E polymorphisms between patients and control | ||||
| Tsuchiya et al., 2005[35] | Case: 79 | HSD17B1 gene CYP19 gene | Japanese | Individuals having at least one A-allele (A/G or A/A genotype) of HSD17B1 showed a significantly increased risk of endometriosis (A/G genotype: adjusted OR, 3.06; 95%CI 1.21-0.74; A/A genotype: adjusted OR, 3.02; 95%CI 1.08-8.43) |
| Control: 59 | ||||
| There was a significant trend associating A/G + A/A genotypes with severity of endometriosis (P for trend <0.01) | ||||
| No statistically significant association was found for the CYP19 polymorphism | ||||
| Kitawaki et al., 2004[13] | Case: 185 | IFNG gene | Japanese | The IFNG CA-repeat polymorphism is associated with susceptibility to endometriosis |
| Control: 176 | IL4 gene | No differences in IL-4-590C/T genotypes and allele frequencies between control women and patients with endometriosis |
SNP: Single -nucleotide polymorphism, TNF-: Tumor necrosis factor- alpha, GST: Glutathione-S-transferases, CI: Confidence interval, OR: Odds ratio
Table 3.
Summary of genetic polymorphism studies with no association or significance
| Reference | Sample size | Involved genes | Population | Summary |
|---|---|---|---|---|
| Matsuzaka et al., 2012[36] | Case: 100 | ESR1 gene | Japanese | No statistically significant differences were noted in the SNP allele frequencies and genotypes between the cases and controls in ESR1 gene. ESR1 gene polymorphisms are not significantly associated with the development of endometriosis |
| Control: 143 | ||||
| Chae et al., 2010[37] | Case: 390 | ICAM-1 gene | Korean | The K469E and G241R polymorphisms in the ICAM-1 gene and the C-634G polymorphism in the IL-6 gene may not be genetic factors related to susceptibility to advanced-stage endometriosis |
| Control: 351 | IL-6 gene | |||
| Lee et al., 2009[38] | Case: 237 | IL-2R β gene | Korean | The C627T polymorphism of the IL-2R β gene is not associated with advanced stage endometriosis in a Korean population |
| Control: 164 | ||||
| Kim et al., 2008[39] | Case: 105 | VEGF genes | Korean | Endostatin G (4349) A and VEGF C (936) T polymorphisms was not associated with endometriosis |
| Control: 101 | ||||
| Serum endostatin levels (but not VEGF levels) were negatively correlated with the development of endometriosis, specifically in early-stage endometriosis patients | ||||
| Hur et al., 2005[40] | Case: 194 | GSTM1 gene GSTT1 gene | Korean | No association was noted between the genetic polymorphisms of GSTM1, GSTT1, and GSTP1 with the development of endometriosis in Korean women |
| Control: 259 | ||||
| GSTP1 gene |
SNP: Single- nucleotide polymorphism, TNF-: Tumor necrosis factor- alpha, GST: Glutathione-S-transferases
Epidemiology
The majority of included studies were either retrospective cohort studies or case–control studies, neither of which is able to produce reliable incidence nor prevalence estimates. Indeed, there was only one study from which an estimate of prevalence was obtainable. This was a large survey of nurses from Japan, in which 1025 of 15,019 (6.8%) self-reported endometriosis.[20] The authors of this study found that 89% of women with self-reported endometriosis had a diagnosis confirmed by laparoscopy.[20]
Body mass index
Only one study reported the effect of body mass index (BMI) on the severity of endometriosis. Korean women with early or mild endometriosis have a significantly higher BMI compared to those with advanced disease even after adjusting for age, parity, and menstrual factors.[21]
Parity
Only one study reported the effect of parity on the development of endometriosis. Korean women with only one child experience a more severe disease than those with more than one child.[21]
Environmental exposure
Environmental exposure includes cigarette smoking and exposure to industrial chemicals. One study reported that cigarette smoking is associated with an increased risk of endometriosis in women with surgically confirmed endometriosis from Japan.[20]
A number of industrial chemicals and environmental pollutants are known to either mimic or antagonize endogenous hormones.[41] Of particular interest are the organochlorides, found in pesticides, and heavy metals. However, in two studies of infertile women from Japan, there was very little difference in the levels of these compounds in women with or without endometriosis.[22,23] Yet, given these women were infertile, the influence of such environmental toxins in an unselected group of women with endometriosis remains unclear.
Infertility
Infertility is significantly associated with increased risk of endometriosis in women with surgically confirmed endometriosis from Japan although whether endometriosis is the causal factor for infertility remains a possibility.[20] Unsurprisingly then, there appears to be an association of a diagnosis of infertility in women who are subsequently diagnosed with endometriosis.
Menstrual cycle length
A short menstrual cycle length, at age 18–22 years of age, was significantly associated with an increased risk of endometriosis in women surgically confirmed endometriosis from Japan.[20] However, in Korean women, no association between menstrual cycle length and disease severity was found.[21]
Family history and genetics
In a Japanese study, the prevalence of endometriosis in siblings of women diagnosed with endometriosis was 8.8% compared to 1.5% of controls, suggesting that there is a familial tendency for endometriosis.[24] There may therefore be a genetic link between specific polymorphisms and the development of endometriosis.
Several genes have been studied [Tables 2 and 3]. An increased risk of endometriosis has been reported in women with CDKN2B-AS/rs10965253 and WNT4/rs16826658 single-nucleotide polymorphisms (SNPs);[25] TIMP-2 promoter region;[26] estrogen synthesis and metabolism genes (nonsynonymous SNPs rs6165, rs6166, rs2066479, and rs700519);[27] FGF2 754C/C polymorphism;[30]-C805 or -1031T/C TNFA gene polymorphism;[31] A264C HSD17B1 polymorphisms in cytochrome P450 CYP19;[34] a13 allele in interferon gamma (IFN-γ); and IFN-γ CA repeat polymorphisms;[34] A264C HSD17B1 polymorphisms in cytochrome P450 CYP19;[35] and a13 allele in IFN-γ and IFN-γ CA repeat polymorphisms.[13]
In contrast, alterations in the TIMP-2 intron 1 region and MMP-2,[26]-765C allele of COX-2 gene,[29] SNPs in the follicle-stimulating hormone receptor FSHR gene,[29] FGF2 754C/G or G/G,[30]-863C/A TNFA gene polymorphism,[31]418C/C TIMP-2 polymorphism,[32] and appear to be protective. Increased serum endostatin levels (but not VEGF levels) are negatively correlated with development of endometriosis.[39]
Other genetic alterations have been reported to have no association with the development of endometriosis. These include ESR1 gene polymorphisms (although the authors speculated this may be due to low sample size),[36] K469E and G241R polymorphisms in ICAM-1;[37] C627T in IL-2R β gene;[38] G (4349) A, C (936) T, 405G,-406C>T VEGF polymorphisms;[39] and GSTM1, GSTT1, and GSTP1.[40]
The role of the E-cadherin gene is controversial, with several studies reporting a significant association with endometriosis risk, particularly with 160C/-347 GA and 30-UTR C/T polymorphisms,[33] while other polymorphisms are not associated with increased risk (-160C/A and -347 G/GA).[33]
Risk factors
One study[20] proposed an idea that some inherited or constitutional factors which increased the risk of endometriosis could hardly be changed, while some others which correlated to lifestyle or environmental factors could be modifiable. Collectively, for the current review, nonmodifiable risk factors include infertility, menstrual cycle length, early menarche, family history, and genetics; however, factors include diet, BMI, length of breastfeeding, physical activity, and exposure to heavy metals and pesticides could be modifiable to the development of endometriosis [Table 4].
Table 4.
Factors associated with increased risk of endometriosis
| Modifiable risk factors | Non-modifiable risk factors |
|---|---|
| Parity | Genetics |
| BMI | Family history |
| Early menarche | |
| Infertility | |
| Factors of unknown significance | |
| Smoking | Menstrual cycle length |
| Heavy metals | |
| Organochlorines |
BMI: Body mass index
DISCUSSION
In contrast to the abundant articles focused on the molecular biologic studies,[4,12,42] there was limited data on the epidemiology of endometriosis in the East Asian populations despite earlier reports suggesting that endometriosis is more common in women from Asia than from Western countries.[5,11,14,15] In the present review, it appears that the best characterized risk factors in the East Asian populations are the various genetic polymorphisms that have been studied. In contrast to the genetic or familial factors which are nonmodifiable, factors such as parity, smoking, and probably the BMI could be the modifiable factors for the development of endometriosis. Regretfully, almost all these found articles were limited by their low strength of evidence and lack of the strong support of causal relationship, so these factors finally could only conclude an association or correlation with endometriosis.
Endometriosis is a dynamic and complex disorder, involving the interplay of genetic and environmental factors. The current review revealed a number of risk factors for endometriosis, including early menarche,[43] increased BMI,[44,45,46] environmental factors (including cigarette smoking),[47] and genetic factors.[48,49] It seems some of our findings were consistent with other studies that early menarche, shorter menstrual length, duration of infertility, and family history of endometriosis are strongly associated with endometriosis,[8,50,51] while higher parity and higher BMI are associated with decreased risk.[8] However, some associations have not yet been proven or disproven, such as smoking and the influence of diet.[8,50,52,53] A Japanese study in the current review found cigarette smoking significantly increased the risk of endometriosis, while another study from Boston, USA, found smoking was associated with decreased risk,[8] and a study from Sweden found no significant association between smoking and endometriosis.[50] The same study from Sweden also found no significant associations with level of education, BMI, oral contraceptive use, coffee consumption, or alcohol intake.
BMI is significantly associated with disease severity in one Korean study,[21] those with lower BMI being more likely to have more severe disease compared to those with higher BMI. Other studies have also suggested that women with a low BMI are at greater risk of endometriosis.[8,44,45,46] This might explain the difference in the prevalence in Asian women compared to women from the United States,[11] given that average BMIs are much higher in the US population. However, a critical question is whether the low BMI is the cause or the consequence of developing endometriosis even though these studies suggested a correlation. Besides, if BMI is a potential predisposing factor of endometriosis, it is still unknown if there are any BMI-related genetic or environmental factors which could be involved.
Factors contributing toward or against endometriosis development are far from clearly understood, and the interaction between genetic susceptibility and environmental factors is inadequately studied, despite over 50 years of hypothesis-driven research.[3] The epidemiological data reviewed in the current study suggested that the development of endometriosis in the East Asian populations is strongly driven by genetic factors; however, almost all these studies were focused on the genetic SNPs. It is still unclear the exact biological significance of these SNPs or the mechanisms of subsequent signaling.
There are several limitations to the current review. First, we only included articles that were published in English, which may lead to bias considering this review is specifically focused on the East Asian populations. Second, as with all reviews, there is possible publication bias and we have not formally assessed this. Third, the retrieved publications were from only several countries within East Asia (Japan, Taiwan, Korea, and China). Although we also found some available data from India[54,55,56,57,58,59] (South Asia), epidemiology-related researches from other Asian countries remains sparse. Fourth, probably because of the difficulties of epidemiologic studies, the level of evidence for lifestyle research may not be strong enough. Although these data are still worthy as a reference of an East Asian population, surely, there was no strong evidence to support if those proposed "modifiable" risk factors could really change the development of endometriosis. Fifth, links between the genetic and mechanistic studies of the development of endometriosis were lacking. From a cohort viewpoint, the clinical impact of the genetic polymorphism in a woman's life is unclear, and it is not clear whether those revelations would be altered after conception or not, as the incidence of endometriosis significantly declines after successful pregnancy. Obviously, there are many questions yet to be answered.
Data on the epidemiologic factors of endometriosis in the Asian populations are limited. The available data that examine potential genetic factors, however, do not reveal whether such factors directly contribute to increased risk of endometriosis. Smoking, low parity, and probably BMI could be modifiable risk factors associated with endometriosis among East Asian women. Further extensive studies on endometriosis in Asian women are required to identify other risk factors for the management of this condition.[60]
Financial support and sponsorship
This study was partially supported by the research grants of National Science Council, Ministry of Science and Technology (grant no. NMRPG3G0521 (MOST 106-2314-B-182A-150-)) to Dr. C.F. Yen.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Editorial support for the development of this article was provided by MediTech Media Asia Pacific; this support was funded by Takeda Pharmaceutical (Asia Pacific) Pte. Ltd. Medical writing services provided by Dr. Belinda Butcher CMPP from Write Source Medical were funded by Takeda in accordance with Good Publication Practice (GPP3) guidelines (http://ismpp.org/gpp3). This study was partially supported by the research grants of the National Science Council, Ministry of Science and Technology (grant no. NMRPG3G0521 (MOST 106-2314-B-182A-150-)) to Dr. C. F. Yen.
REFERENCES
- 1.Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389–98. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan KN, Kitajima M, Hiraki K, Fujishita A, Nakashima M, Masuzaki H. Visible and occult microscopic lesions of endometriosis. Gynecol Minim Invasive Ther. 2014;3:109–14. [Google Scholar]
- 3.Wu MH, Hsiao KY, Tsai SJ. Endometriosis and possible inflammation markers. Gynecol Minim Invasive Ther. 2015;4:61–7. [Google Scholar]
- 4.Bulun SE, Monsivais D, Kakinuma T, Furukawa Y, Bernardi L, Pavone ME, et al. Molecular biology of endometriosis: From aromatase to genomic abnormalities. Semin Reprod Med. 2015;33:220–4. doi: 10.1055/s-0035-1554053. [DOI] [PubMed] [Google Scholar]
- 5.Ozkan S, Murk W, Arici A. Endometriosis and infertility: Epidemiology and evidence-based treatments. Ann N Y Acad Sci. 2008;1127:92–100. doi: 10.1196/annals.1434.007. [DOI] [PubMed] [Google Scholar]
- 6.Khine YM, Taniguchi F, Harada T. Clinical management of endometriosis-associated infertility. Reprod Med Biol. 2016;15:217–25. doi: 10.1007/s12522-016-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takebayashi A, Shimizu Y, Takahashi A, Yamanaka A, Takashima A, Kimura F, et al. Comparison of the outcome of in vitro fertilization after laparoscopic laser ablation surgery versus laparoscopic cystectomy for endometrioma. Gynecology and Minimally Invasive Therapy. 2013;2:27–9. [Google Scholar]
- 8.Parasar P, Ozcan P, Terry KL. Endometriosis: Epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. 2017;6:34–41. doi: 10.1007/s13669-017-0187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuo HH, Huang CY, Ueng SH, Huang KG, Lee CL, Yen CF, et al. Unexpected epithelial ovarian cancers arising from presumed endometrioma: A 10-year retrospective analysis. Taiwan J Obstet Gynecol. 2017;56:55–61. doi: 10.1016/j.tjog.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Nomura H, Okuda K, Saito N, Fujiyama F, Nakamura Y, Yamashita Y, et al. Mini-laparoscopic surgery versus conventional laparoscopic surgery for patients with endometriosis. Gynecology and Minimally Invasive Therapy. 2013;2:85–8. [Google Scholar]
- 11.Mangtani P, Booth M. Epidemiology of endometriosis. J Epidemiol Community Health. 1993;47:84–8. doi: 10.1136/jech.47.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo SW. Keep the pressure on for more transparency of clinical trials on endometriosis. Gynecol Minim Invasive Ther. 2013;2:73–4. [Google Scholar]
- 13.Kitawaki J, Koshiba H, Kitaoka Y, Teramoto M, Hasegawa G, Nakamura N, et al. Interferon-gamma gene dinucleotide (CA) repeat and interleukin-4 promoter region (-590C/T) polymorphisms in Japanese patients with endometriosis. Hum Reprod. 2004;19:1765–9. doi: 10.1093/humrep/deh337. [DOI] [PubMed] [Google Scholar]
- 14.Kvaskoff M, Mu F, Terry KL, Harris HR, Poole EM, Farland L, et al. Endometriosis: A high-risk population for major chronic diseases? Hum Reprod Update. 2015;21:500–16. doi: 10.1093/humupd/dmv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto A, Johnstone EB, Bloom MS, Huddleston HG, Fujimoto VY. A higher prevalence of endometriosis among Asian women does not contribute to poorer IVF outcomes. J Assist Reprod Genet. 2017;34:765–74. doi: 10.1007/s10815-017-0919-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrarelli P, Yen CF, Arcuri F, Funghi L, Tosti C, Wang TH, et al. Myostatin, follistatin and activin type II receptors are highly expressed in adenomyosis. Fertil Steril. 2015;104:744–520. doi: 10.1016/j.fertnstert.2015.05.032. [DOI] [PubMed] [Google Scholar]
- 17.Carrarelli P, Yen CF, Funghi L, Arcuri F, Tosti C, Bifulco G, et al. Expression of inflammatory and neurogenic mediators in adenomyosis. Reprod Sci. 2017;24:369–75. doi: 10.1177/1933719116657192. [DOI] [PubMed] [Google Scholar]
- 18.Yen CF, Liao SK, Huang SJ, Tabak S, Arcuri F, Lee CL, et al. Decreased endometrial expression of leukemia inhibitory factor receptor disrupts the STAT3 signaling in adenomyosis during the implantation window. Reprod Sci. 2017;24:1176–86. doi: 10.1177/1933719116681515. [DOI] [PubMed] [Google Scholar]
- 19.Yen CF, Huang SJ, Lee CL, Wang HS, Liao SK. Molecular characteristics of the endometrium in uterine adenomyosis and its biochemical microenvironment. Reprod Sci. 2017;24:1346–61. doi: 10.1177/1933719117691141. [DOI] [PubMed] [Google Scholar]
- 20.Yasui T, Hayashi K, Nagai K, Mizunuma H, Kubota T, Lee JS, et al. Risk profiles for endometriosis in Japanese women: Results from a repeated survey of self-reports. J Epidemiol. 2015;25:194–203. doi: 10.2188/jea.JE20140124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi KW, Shin JH, Park MS, Kim T, Kim SH, Hur JY, et al. Association of body mass index with severity of endometriosis in Korean women. Int J Gynaecol Obstet. 2009;105:39–42. doi: 10.1016/j.ijgo.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Itoh H, Iwasaki M, Nakajima Y, Endo Y, Hanaoka T, Sasaki H, et al. A case-control study of the association between urinary cadmium concentration and endometriosis in infertile Japanese women. Sci Total Environ. 2008;402:171–5. doi: 10.1016/j.scitotenv.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Tsukino H, Hanaoka T, Sasaki H, Motoyama H, Hiroshima M, Tanaka T, et al. Associations between serum levels of selected organochlorine compounds and endometriosis in infertile Japanese women. Environ Res. 2005;99:118–25. doi: 10.1016/j.envres.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Kashima K, Ishimaru T, Okamura H, Suginami H, Ikuma K, Murakami T, et al. Familial risk among Japanese patients with endometriosis. Int J Gynaecol Obstet. 2004;84:61–4. doi: 10.1016/s0020-7292(03)00340-0. [DOI] [PubMed] [Google Scholar]
- 25.Lee GH, Choi YM, Hong MA, Yoon SH, Kim JJ, Hwang K, et al. Association of CDKN2B-AS and WNT4 genetic polymorphisms in Korean patients with endometriosis. Fertil Steril. 2014;102:1393–7. doi: 10.1016/j.fertnstert.2014.07.1237. [DOI] [PubMed] [Google Scholar]
- 26.Cho YJ, Kim NH, Jeong KA, Lee JY, Moon HS, Kim HL, et al. Association between MMP-2 and TIMP-2 gene polymorphisms and advanced-stage endometriosis in Korean women. Am J Reprod Immunol. 2013;69:73–84. doi: 10.1111/aji.12020. [DOI] [PubMed] [Google Scholar]
- 27.Wang HS, Wu HM, Cheng BH, Yen CF, Chang PY, Chao A, et al. Functional analyses of endometriosis-related polymorphisms in the estrogen synthesis and metabolism-related genes. PLoS One. 2012;7:e47374. doi: 10.1371/journal.pone.0047374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim HY, Cho S, Choi YS, Yang HI, Lee KE, Seo SK, et al. Cyclooxygenase-2 (COX -2) gene-765G/C polymorphism and advanced-stage endometriosis in Korean women. Am J Reprod Immunol. 2012;68:238–43. doi: 10.1111/j.1600-0897.2012.01151.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang HS, Cheng BH, Wu HM, Yen CF, Liu CT, Chao A, et al. A mutant single nucleotide polymorphism of follicle-stimulating hormone receptor is associated with a lower risk of endometriosis. Fertil Steril. 2011;95:455–7. doi: 10.1016/j.fertnstert.2010.07.1092. [DOI] [PubMed] [Google Scholar]
- 30.Kang S, Li SZ, Wang N, Zhou RM, Wang T, Wang DJ, et al. Association between genetic polymorphisms in fibroblast growth factor (FGF) 1 and FGF2 and risk of endometriosis and adenomyosis in Chinese women. Hum Reprod. 2010;25:1806–11. doi: 10.1093/humrep/deq128. [DOI] [PubMed] [Google Scholar]
- 31.Lee GH, Choi YM, Kim SH, Hong MA, Oh ST, Lim YT, et al. Association of tumor necrosis factor-{alpha} gene polymorphisms with advanced stage endometriosis. Hum Reprod. 2008;23:977–81. doi: 10.1093/humrep/den016. [DOI] [PubMed] [Google Scholar]
- 32.Kang S, Zhao XW, Wang N, Chen SC, Zhou RM, Li Y, et al. Association of polymorphisms of the MMP-2 and TIMP-2 genes with the risk of endometriosis in North Chinese women. Fertil Steril. 2008;90:2023–9. doi: 10.1016/j.fertnstert.2007.09.068. [DOI] [PubMed] [Google Scholar]
- 33.Shan K, Xiao-Wei M, Na W, Xiu-Feng Z, Deng-Gui W, Wei G, et al. Association of three single nucleotide polymorphisms of the E-cadherin gene with endometriosis in a Chinese population. Reproduction. 2007;134:373–8. doi: 10.1530/REP-07-0104. [DOI] [PubMed] [Google Scholar]
- 34.Kitawaki J, Kiyomizu M, Obayashi H, Ohta M, Ishihara H, Hasegawa G, et al. Synergistic effect of interleukin-6 promoter (IL6 -634C/G) and intercellular adhesion molecule-1 (ICAM-1 469K/E) gene polymorphisms on the risk of endometriosis in Japanese women. Am J Reprod Immunol. 2006;56:267–74. doi: 10.1111/j.1600-0897.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- 35.Tsuchiya M, Nakao H, Katoh T, Sasaki H, Hiroshima M, Tanaka T, et al. Association between endometriosis and genetic polymorphisms of the estradiol-synthesizing enzyme genes HSD17B1 and CYP19. Hum Reprod. 2005;20:974–8. doi: 10.1093/humrep/deh726. [DOI] [PubMed] [Google Scholar]
- 36.Matsuzaka Y, Kikuti YY, Izumi S, Goya K, Suzuki T, Cai LY, et al. Failure to detect significant association between estrogen receptor-alpha gene polymorphisms and endometriosis in Japanese women. Environ Health Prev Med. 2012;17:423–8. doi: 10.1007/s12199-011-0259-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chae SJ, Lee GH, Choi YM, Hong MA, Kim JM, Lee KS, et al. Intercellular adhesion molecule-1 and interleukin-6 gene polymorphisms in patients with advanced-stage endometriosis. Gynecol Obstet Invest. 2010;70:34–9. doi: 10.1159/000284398. [DOI] [PubMed] [Google Scholar]
- 38.Lee GH, Choi YM, Kim SH, Hong MA, Ku SY, Kim SH, et al. Interleukin-2 receptor beta gene C627T polymorphism in Korean women with endometriosis: A case-control study. Hum Reprod. 2009;24:2596–9. doi: 10.1093/humrep/dep242. [DOI] [PubMed] [Google Scholar]
- 39.Kim JG, Kim JY, Jee BC, Suh CS, Kim SH, Choi YM, et al. Association between endometriosis and polymorphisms in endostatin and vascular endothelial growth factor and their serum levels in Korean women. Fertil Steril. 2008;89:243–5. doi: 10.1016/j.fertnstert.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 40.Hur SE, Lee JY, Moon HS, Chung HW. Polymorphisms of the genes encoding the GSTM1, GSTT1 and GSTP1 in Korean women: No association with endometriosis. Mol Hum Reprod. 2005;11:15–9. doi: 10.1093/molehr/gah127. [DOI] [PubMed] [Google Scholar]
- 41.Safe SH. Endocrine disruptors and human health – Is there a problem?. An update. Environ Health Perspect. 2000;108:487–93. doi: 10.1289/ehp.00108487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matarese G, De Placido G, Nikas Y, Alviggi C. Pathogenesis of endometriosis: Natural immunity dysfunction or autoimmune disease? Trends Mol Med. 2003;9:223–8. doi: 10.1016/s1471-4914(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 43.Nnoaham KE, Webster P, Kumbang J, Kennedy SH, Zondervan KT. Is early age at menarche a risk factor for endometriosis?. A systematic review and meta-analysis of case-control studies. Fertil Steril. 2012;98:702–12e6. doi: 10.1016/j.fertnstert.2012.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Darrow SL, Vena JE, Batt RE, Zielezny MA, Michalek AM, Selman S, et al. Menstrual cycle characteristics and the risk of endometriosis. Epidemiology. 1993;4:135–42. doi: 10.1097/00001648-199303000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Raimondo D, Mabrouk M, Zannoni L, Arena A, Zanello M, Benfenati A, et al. Severe ureteral endometriosis: Frequency and risk factors. J Obstet Gynaecol. 2018;38:257–60. doi: 10.1080/01443615.2017.1349083. [DOI] [PubMed] [Google Scholar]
- 46.Backonja U, Hediger ML, Chen Z, Lauver DR, Sun L, Peterson CM, et al. Beyond body mass index: Using anthropometric measures and body composition indicators to assess odds of an endometriosis diagnosis. J Womens Health (Larchmt) 2017;26:941–50. doi: 10.1089/jwh.2016.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sadeu JC, Hughes CL, Agarwal S, Foster WG. Alcohol, drugs, caffeine, tobacco, and environmental contaminant exposure: Reproductive health consequences and clinical implications. Crit Rev Toxicol. 2010;40:633–52. doi: 10.3109/10408444.2010.493552. [DOI] [PubMed] [Google Scholar]
- 48.Pabalan N, Jarjanazi H, Christofolini DM, Bianco B, Barbosa CP. Association of the protein tyrosine phosphatase non-receptor 22 polymorphism (PTPN22) with endometriosis: A meta-analysis. Einstein (Sao Paulo) 2017;15:105–11. doi: 10.1590/S1679-45082017RW3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pabalan N, Kunjantarachot A, Ruangpratheep C, Jarjanazi H, Christofolini DM, Barbosa CP, et al. Potential of RASSF1A promoter methylation as biomarker for endometrial cancer: A systematic review and meta-analysis. Gynecol Oncol. 2017;146:603–8. doi: 10.1016/j.ygyno.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 50.Saha R, Kuja-Halkola R, Tornvall P, Marions L. Reproductive and lifestyle factors associated with endometriosis in a large cross-sectional population sample. J Womens Health (Larchmt) 2017;26:152–8. doi: 10.1089/jwh.2016.5795. [DOI] [PubMed] [Google Scholar]
- 51.Moini A, Malekzadeh F, Amirchaghmaghi E, Kashfi F, Akhoond MR, Saei M, et al. Risk factors associated with endometriosis among infertile Iranian women. Arch Med Sci. 2013;9:506–14. doi: 10.5114/aoms.2013.35420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parazzini F, Viganò P, Candiani M, Fedele L. Diet and endometriosis risk: A literature review. Reprod Biomed Online. 2013;26:323–36. doi: 10.1016/j.rbmo.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Nazni P. Association of western diet and lifestyle with decreased fertility. Indian J Med Res. 2014;140(Suppl):S78–81. [PMC free article] [PubMed] [Google Scholar]
- 54.Govatati S, Tangudu NK, Deenadayal M, Chakravarty B, Shivaji S, Bhanoori M, et al. Association of E-cadherin single nucleotide polymorphisms with the increased risk of endometriosis in Indian women. Mol Hum Reprod. 2012;18:280–7. doi: 10.1093/molehr/gar079. [DOI] [PubMed] [Google Scholar]
- 55.Lakshmi KV, Shetty P, Vottam K, Govindhan S, Ahmad SN, Hasan Q, et al. Tumor necrosis factor alpha – C850T polymorphism is significantly associated with endometriosis in Asian Indian women. Fertil Steril. 2010;94:453–6. doi: 10.1016/j.fertnstert.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 56.Rai P, Deenadayal M, Shivaji S. Absence of activating somatic mutations of PI3KCA and AKT1 genes in South Indian women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2010;152:78–82. doi: 10.1016/j.ejogrb.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 57.Bhanoori M, Arvind Babu K, Pavankumar Reddy NG, Lakshmi Rao K, Zondervan K, Deenadayal M, et al. The vascular endothelial growth factor (VEGF) +405G & gt; C 5’-untranslated region polymorphism and increased risk of endometriosis in South Indian women: A case control study. Hum Reprod. 2005;20:1844–9. doi: 10.1093/humrep/deh852. [DOI] [PubMed] [Google Scholar]
- 58.Bhanoori M, Babu KA, Deenadayal M, Kennedy S, Shivaji S. The interleukin-6 -174G/C promoter polymorphism is not associated with endometriosis in South Indian women. J Soc Gynecol Investig. 2005;12:365–9. doi: 10.1016/j.jsgi.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 59.Govatati S, Deenadayal M, Shivaji S, Bhanoori M. Mitochondrial displacement loop alterations are associated with endometriosis. Fertil Steril. 2013;99:1980–6.e9. doi: 10.1016/j.fertnstert.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 60.Guo SW. A call to end the beauty contest in China's science and technology. Gynecol Minim Invasive Ther. 2014;3:103–4. [Google Scholar]