Abstract
Purpose:
Iniparib is a purported prodrug causing cell death through intracellular conversion to nitro radical ions. We assessed the efficacy and safety of iniparib with standard radiation therapy (RT) and temozolomide (TMZ) in patients with newly diagnosed glioblastoma (GBM).
Experimental Design:
Adults meeting eligibility criteria were enrolled in this prospective, single arm, open-label multi-institution phase II trial with median overall survival (mOS) compared to a historical control as the primary objective. A safety run-in component of RT+TMZ+iniparib (n=5) was followed by an efficacy study (n=76) with the recommended phase II doses of iniparib (8.0 mg/kg IV twice/week with RT + daily TMZ followed by 8.6 mg/kg IV twice/week with 5/28 day TMZ).
Results:
The median age of the 81 evaluable participants was 58 years (63% male). Baseline KPS was ≥80% in 87% of participants. The mOS was 22 months (95%CI: 17–24) and the hazard rate was 0.44 (95%CI: 0.35–0.55) per-person year of follow-up. The 2 and 3 year survival rates were 38% and 25%, respectively. Treatment-related grade 3 adverse events (AE) occurred in 27% of patients; nine patients had AEs requiring drug discontinuation including: infusion-related reaction, rash, gastritis, increased liver enzymes, and thrombocytopenia.
Conclusions:
Iniparib is well tolerated with RT and TMZ in patients with newly diagnosed GBM at up to 17.2mg/kg weekly. The primary objective of improved mOS compared with historical a control was met, indicating potential antitumor activity of iniparib in this setting. Dosing optimization (frequency and sequence) is needed prior to additional efficacy studies.
Keywords: malignant glioma, newly diagnosed, dose finding, cytotoxic, combination therapy
Introduction
The addition of temozolomide (TMZ) to radiation therapy (RT) changed the therapeutic landscape for patients with newly diagnosed glioblastoma (GBM) by demonstrating the activity of TMZ and the predictive importance of the O6-methylguanine–DNA methyltransferase (MGMT) repair enzyme.(1–3) Unfortunately, no drugs have reliably improved median overall survival (mOS) for people with GBM since then. Given that more than 75% of GBM patients who are well enough to enroll on clinical trials die within 2 years of diagnosis, there are multiple efforts underway to improve therapies for patients with GBM.(3–5)
An attractive approach is to identify therapies that enhance the activity of TMZ without increasing its toxicity. Iniparib (4-iodo-3-nitrobenzamide) is a prodrug with demonstrated clinical activity in cancers mediated by mismatch repair defects such as triple negative breast cancer and BRCA2-mutated pancreatic cancer.(6,7) Development of iniparib was initially focused on these tumors as it was thought to be a specific inhibitor of poly (ADP-ribose) polymerase (PARP). PARP inhibitors are hypothesized to enhance the efficacy of alkylating therapies such as RT and TMZ by impairing DNA repair.(6,7) Ultimately, iniparib was shown to have anti-cancer activity through a pro-drug mechanism in which an active nitro radical ion is released through one and two-electron cytosolic activation, rather than direct PARP inhibition.(8–10) The activated nitro radical ion binds to cysteine residues on enzymes critical for reduction–oxidation reactions, including selenoproteins such as thioredoxin reductase (TrxR). This mechanism has the potential to be selectively cytotoxic to cells high in enzymes required for prodrug metabolism or high in targets of the generated metabolites. The proposed mechanism of action paired with extensive clinical data showing iniparib has non-overlapping toxicities with alkylating therapies as well as good access to brain tissue (likely due to the fact that it is lipophilic and MW=292), make it a compelling choice for addition to GBM standard therapy. (7,11,12)
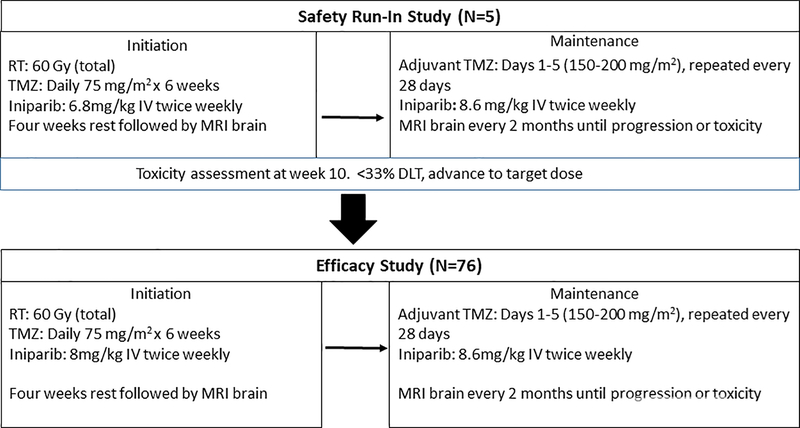
Iniparib was assessed in a dose escalation study in people with newly diagnosed malignant gliomas after completion of chemoradiation. In the setting of adjuvant TMZ, iniparib was well tolerated at doses up to 17.2mg/kg/week.(13) The most common toxicities were fatigue and low blood counts. In addition to encouraging tolerability, the phase I study showed signs of clinical activity with an estimated mOS of 18.9 months (95 % CI 16.2–23.4 months) across all enrolled patients.(13) The current study evaluated the tolerability and efficacy (via a primary endpoint of mOS) of iniparib when given concurrently with RT and TMZ followed by adjuvant TMZ in people with newly diagnosed GBM (Figure 1).
Figure 1.
Study schema for the safety run-in and efficacy study. Intention to treat analysis included all patients treated at the target dose (total N=81). RT = radiation therapy; Gy = gray; TMZ = temozolomide; IV = intravenous; DLT = dose limiting toxicity; MRI = magnetic resonance imaging.
Patients and methods
The study was sponsored by the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute (NCI) and conducted by the Adult Brain Tumor Consortium (ABTC, http://www.abtconsortium.org). The study was conducted in accordance with the Declaration of Helsinki and was approved by the institutional review board of each participating institution. All patients provided written informed consent as a condition for participating in the study. Patients eligible for enrollment met the following criteria: ≥18 years old, histologically proven newly diagnosed supratentorial GBM; absolute neutrophil count ≥1,500/μL; platelet count ≥100,000/μL; serum creatinine≤1.7-mg/dL; total bilirubin ≤1.5-mg/dL; aspartate and alanine aminotransferase ≤4 times the upper limit of normal; stable dexamethasone dose for ≥5 days prior to start of treatment; Karnofsky Performance Status (KPS) ≥ 60%. Exclusion criteria included: enzyme inducing anti-epileptic medications; other malignancy within 5 years; pregnant or nursing women; serious concurrent medical condition or other condition that would compromise safety or compliance. Agreement to practice adequate birth control methods was required.
Treatment Plan
This was an open-label, single arm, multi-center, study to estimate the mOS when iniparib is given concurrent with chemoradiation and adjuvant TMZ. The maximum tolerated dose (MTD) in the phase I study of iniparib given with standard dosing of TMZ after completion of chemoradiation (5/28 days 150–200mg/m2) was 8.6mg/kg intravenously (IV) twice weekly (days 1 and 2). The MTD of iniparib when given with metronomic TMZ (75mg/mg/m2 continuous daily dosing for 6 weeks) was 8mg/kg IV twice weekly.(13) Since these doses were determined in people who had already completed chemoradiation, a safety run-in with iniparib at one dose below the MTD (6.8mg/kg IV twice weekly) was first completed to ensure tolerability with concurrent RT and TMZ. Safety evaluation was completed at 10 weeks to assess for tolerability before escalating to 8mg/kg IV twice weekly during RT (Figure 1).
Prescribed RT was 6000 cGy in 30 daily fractions of 200 cGy each with TMZ 75mg/m2/daily with iniparib 8mg/kg IV twice weekly for 6 weeks followed by one month with no therapy. This period of treatment was termed initiation. Subsequently, TMZ was given at 150mg/m2 for 5/28 days (cycle one). If well tolerated, TMZ was increased to 200mg/m2 5/28 days for cycles 2–6. Iniparib 8.6mg/kg IV twice weekly was given with TMZ for all cycles independent of TMZ dose. This period of treatment was termed maintenance. All centers participating in the protocol completed accreditation for conformal techniques (3DCRT/IMRT) according to the procedures outlined in the ABTC Radiation Oncology plan (www.abtconsortium.org).
Iniparib was provided by Sanofi Pharmaceuticals, Inc. (Cambridge, MA) and administered IV on two consecutive days weekly beginning with day 1 of TMZ. If a patient had to stop TMZ for any reason, they stopped iniparib as well. P. carinii prophylaxis was mandatory during initiation and continued until resolution of lymphocytopenia to ≤grade 1. The use of antiemetics was at the discretion of the treating physician during chemoradiation but required during maintenance TMZ. Corticosteroids were prescribed at the discretion of the treating physician, but held constant prior to surveillance imaging and doses were recorded with concurrent medications.
Evaluations
Baseline evaluations included brain magnetic resonance imaging (MRI), medical history and examination, Karnofsky performance status (KPS); complete blood count (CBC); serum chemistry profile; and pregnancy test when appropriate. After initiating treatment, CBC and adverse event (AE) reports were obtained weekly; vital signs and serum chemistries were obtained before each cycle. Brain MRI, clinical examination, and KPS were repeated every other cycle.
Patients who completed the initiation phase of concurrent chemoradiation without tumor progression or dose-limiting toxicity were prescribed 6 cycles of iniparib 8.6mg/kg IV twice weekly with adjuvant TMZ on a 5/28 day schedule (in both the safety run-in and phase two portions of the study). Progression was assessed every two months and defined as: (1) progressive neurologic abnormalities not explained by causes unrelated to tumor progression; (2) greater than 25% increase in the measurement of the contrast enhancing tumor mass by MRI scan on a stable or increasing dose of corticosteroids or the presence of new lesions on MRI outside of the treatment field. Of note, patients for whom therapy was stopped due to radiographic progression that were subsequently histologically determined to be radionecrosis ≤ grade 3 severity were permitted to restart treatment. Patients stopped treatment in the setting of progression, toxicity (a dose limiting toxicity restricting treatment >7 days in initiation or >21 days in maintenance), noncompliance, or if the patient chose to discontinue treatment for any reason. All patients were followed for survival calculated from the date of diagnosis until death from any cause. All enrolled patients were asked to provide tissue for analysis of MGMT promoter methylation status. MGMT status was centrally determined at LabCorp via quantitative methylation-specific polymerase chain reaction.
Statistical Considerations
This was a single-arm, multi-center, open-label phase II study to estimate the safety and efficacy of concurrent iniparib with chemoradiation followed by adjuvant TMZ and iniparib in newly diagnosed adult GBM patients with results compared to the EORTC/NCIC phase III trial.(1) The primary endpoint was mOS and the secondary endpoint was frequency of toxicity associated with the regimen. The trial was designed to have a total of 55 death events to detect a hazard ratio of 0.75, a 25% reduction in hazard of death compared with the EORTC/NCIC trial, with 80% power at an alpha level of 0.1 to be statistically significant.(1) The hazard rate was approximated as 0.45 versus a null of 0.6 and defined as the number of death events per person-year of follow-up based on the hazard rate from the EORTC/NCIC trial.(1)
Eighty-three patients were accrued into the trial from June, 2011 to December, 2012. The trial database was closed on April 2017 for the final analysis with a total of 75 death events. Eighty-one patients were included in the efficacy analysis (intention-to-treat principle); two were ineligible. All 83 treated patients were included in the safety analysis. Survival time was calculated from the date of initial histological diagnosis to the date of death or censored at the time of the analysis if patients were alive. Overall survival was estimated using the Kaplan–Meier method (14) and the confidence interval of median survival was constructed by the method of Brookmeyer-Crowley.(15) The hazard rate was expressed as the hazard of death per person-year of follow-up. Cox regression model was used for survival analysis. Patient baseline characteristics and frequency of observed toxicities attributable to the treatment were summarized using descriptive statistics. Tumor MGMT status was obtained retrospectively. All subgroup analyses were exploratory. The P values reported are two-sided. All analyses were performed using the SAS software (version 9.3; SAS Institute).
Results
For the safety run-in, five patients were enrolled (three female), median age 62 years (range 27–77), all with KPS 80–90, all with prior tumor resection. Two patients stopped treatment for grade 3 and grade 4 thrombocytopenia during the maintenance period and one patient had progressive disease at the end of cycle 5 of the maintenance period. Two patients completed all prescribed treatment without toxicity. For the efficacy portion of the study 78 patients were enrolled across 11 centers. However, two of these patients later had pathology inconsistent with GBM on central pathology review and hence were not eligible for efficacy analysis. All 83 patients were evaluated for safety analyses. The median age was 58 years (range 27–81), 63% male and the median KPS 90 (range 60–100) with 95% of patients undergoing resection (Table 1).
Table 1.
Demographics of patients in the intention-to-treat analysis of the efficacy of iniparib added to radiation and temozolomide.
| Baseline Characteristics: | All Patients (N = 81) |
|---|---|
|
Age (years): Median (range) >70 50–70 <50 |
58 (27–81) 10 (12%) 54 (67%) 17 (21%) |
|
Gender: no. (%) Male |
51 (63) |
|
Race: no. (%) Black or African American White Unknown |
1 (1) 77 (95) 4 (4) |
|
Ethnic Group: no. (%) Hispanic or Latino Not Hispanic or Latino Unknown |
1 (1) 74 (91) 6 (7) |
|
KPS: no. (%) 100 90 80 70 60 |
9 (11) 40 (49) 22 (27) 8 (10) 2 (2) |
|
Surgical Procedure: no. (%) Craniotomy Biopsy |
77 (95) 4 (5) |
|
MGMT: no. (%) Methylated: Unmethylated: Unknown: |
29 (36) 37 (46) 15 (18) |
| Mini Mental Score: median (Range) | 29 (22 – 30) |
|
Diagnosis: no. (%) Glioblastoma Multiforme |
81 (100) |
|
Anticonvulsant Usage: no. (%) Yes No |
64 (79) 17 (21) |
Overall Survival
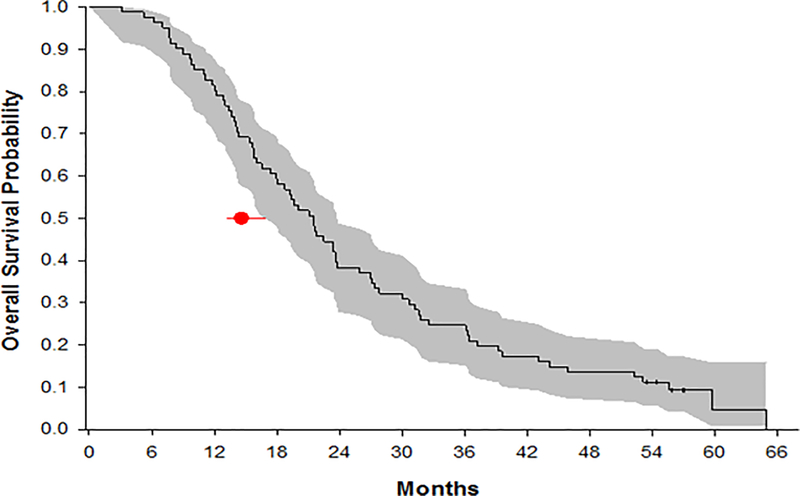
Overall hazard rate was 0.44 (95%CI: 0.35–0.55) per-person year of follow-up with intention-to-treat analysis and the mOS across all patients was 21.6 months (95%CI: 17.4–23.7 months) (Figure 2). An estimated hazard reduction was 27% (hazard ratio 0.73, 0.44 versus 0.6). Known prognostic factors such as age, performance status and MGMT status had significant association with outcome on OS (Table 2). However MGMT promoter methylation status had the greatest impact on overall survival. MGMT was methylated in 29 (36%), unmethylated in 37 (46%) and unknown in 15 (18%) of patients. Overall, 66 patients (81%) had successful centralized MGMT assessment. Of the 15 patients whose tissue could not be assessed, in five instances this was due to technical failures and in 10 instances tissue was not provided for testing. The mOS was 30 months (95% CI: 21.7–37.2), 15.8 months (95% CI: 14.1–19.4), and 25.9 months (95% CI: 11.7–36.7) for patients with MGMT methylated, unmethylated, and unknown, respectively (p=0.0015). Percent survival at two and three years was estimated at 38.3% (95% CI: 27.7–49.7) and 24.7% (95% CI: 15.8–35.5), respectively across all patients. In people whose tumors had MGMT methylation, 2 and 3 year survival rates were 58.6% (95% CI: 38.9–76.5) and 37.9% (95% CI: 20.7–57.7) respectively (Table 3).
Figure 2.
mOS with 95% CI for all patients in the intention to treat analysis. The dot and line correspond to the mOS with 95% CI from EORTC/NCIC (1).
Table 2:
Univariate and multivariate analyses of impact on hazard ratio (N = 81).
| Univariate Analysis | Hazard Ratio (95%CI) | P-value |
|---|---|---|
| Age 50–70 vs. >70 <50 vs. >70 |
0.402 (0.201 – 0.807) 0.222 (0.095 – 0.517) |
0.0104 0.0005 |
| MGMT Methylated vs. Unmethylated Unknown vs. Unmethylated |
0.405 (0.238 – 0.689) 0.467 (0.243 – 0.899) |
0.0008 0.0226 |
| KPS 60–80 vs. 90–100 |
1.756 (1.098 – 2.806) |
0.0187 |
| Multivariate Analysis | ||
| Age 50–70 vs. >70 <50 vs. >70 |
0.458 (0.223 – 0.943) 0.273 (0.107 – 0.7) |
0.0341 0.0069 |
| MGMT Methylated vs. Unmethylated Unknown vs. Unmethylated |
0.308 (0.175 – 0.542) 0.426 (0.213 – 0.852) |
0.0001 0.0158 |
| KPS 60–80 vs. 90–100 |
1.808 (1.051 – 3.108) |
0.0324 |
Table 3:
Percent survival across recent trials for people with newly diagnosed glioblastoma at two and three years for all patients and patients with MGMT promoter methylation.
| 2 year survival All patients (%, 95% CI) |
3 year survival All patients (%, 95% CI) |
2 year survival MGMT methylated (%, 95% CI) |
3 year survival MGMT methylated (%, 95% CI) |
|
|---|---|---|---|---|
| EORTC/NCIC (3) | N = 287 27 (22 – 33) |
N = 287 16 (12 – 20) |
N = 46 49 (34 – 62) |
N = 46 28 (15 – 41) |
| RTOG 0525 (4) | N = 411 34 |
N = 122 estimated 45 |
||
| NABTT RT and TMZ + new agent (5) |
N = 143 37 (29 – 46) |
|||
| CENTRIC EORTC 26071–22072 (16) | N = 272 56 (49–61) |
|||
| ABTC (Iniparib), present study | N = 81 38 (28 – 50) |
N = 81 25 (16 – 36) |
N = 29 59 (39 – 77) |
N = 29 38 (21 – 58) |
Toxicity and tolerability
There were 67 AEs of ≥ grade 3 with attribution to the combination treatment (RT+TMZ+iniparib) (Table 4, Supplemental Table 1). The most common AEs overall were thrombocytopenia (18%), neutropenia (10%), fatigue (5%) and rash (4%) (Table 4). Nine patients stopped treatment due to toxicity and 11 patients refused further treatment or were removed from treatment for non-compliance (7 after completing the initiation phase).
Table 4:
All grades adverse events of all patients (N = 83)
| Adverse Events: N (%) | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total |
|---|---|---|---|---|---|
| Abdominal pain | 2 (2) | 2 (2) | |||
| Acute kidney injury | 1 (1) | 1 (1) | 2 (2) | ||
| Alanine aminotransferase increased | 17 (20) | 2 (2) | 1 (1) | 20 (24) | |
| Albumin | 6 (7) | 2 (2) | 8 (10) | ||
| Alkaline phosphatase increased | 2 (2) | 2 (2) | |||
| Allergic reaction | 2 (2) | 2 (2) | |||
| Alopecia | 35 (42) | 9 (11) | 44 (53) | ||
| Anemia | 41 (49) | 9 (11) | 2 (2) | 52 (63) | |
| Anorexia | 16 (19) | 5 (6) | 21 (25) | ||
| Aspartate aminotransferase increased | 12 (14) | 1 (1) | 13 (16) | ||
| Atrial fibrillation | 1 (1) | 1 (1) | |||
| Blood Bilirubin Increased | 5 (6) | 3 (4) | 8 (10) | ||
| Blurred vision | 2 (2) | 2 (2) | |||
| Bronchial infection | 1 (1) | 1 (1) | 2 (2) | ||
| Cardiac disorders - Other, specify | 1 (1) | 1 (1) | |||
| CD4 lymphocytes decreased | 1 (1) | 1 (1) | |||
| Chest wall pain | 1 (1) | 1 (1) | |||
| Chills | 3 (4) | 3 (4) | |||
| Cognitive disturbance | 1 (1) | 2 (2) | 1 (1) | 4 (5) | |
| Confusion | 1 (1) | 1 (1) | |||
| Conjunctivitis | 1 (1) | 1 (1) | |||
| Constipation | 19 (23) | 7 (8) | 26 (31) | ||
| Creatinine Increased | 6 (7) | 2 (2) | 8 (10) | ||
| Dehydration | 1 (1) | 1 (1) | 2 (2) | ||
| Dermatitis radiation | 20 (24) | 20 (24) | |||
| Diarrhea | 3 (4) | 1 (1) | 4 (5) | ||
| Disseminated intravascular coagulation | 1 (1) | 1 (1) | |||
| Dizziness | 1 (1) | 1 (1) | 1 (1) | 3 (4) | |
| Dry skin | 4 (5) | 4 (5) | |||
| Dysgeusia | 5 (6) | 1 (1) | 6 (7) | ||
| Dyspepsia | 1 (1) | 1 (1) | 2 (2) | ||
| Dysphagia | 1 (1) | 1 (1) | |||
| Dysphasia | 1 (1) | 1 (1) | 1 (1) | 3 (4) | |
| Dyspnea | 1 (1) | 1 (1) | |||
| Ear and labyrinth disorders - Other, specify | 2 (2) | 1 (1) | 3 (4) | ||
| Ear pain | 2 (2) | 2 (2) | |||
| Edema cerebral | 1 (1) | 1 (1) | |||
| Epistaxis | 3 (4) | 3 (4) | |||
| Erythema multiforme | 2 (2) | 2 (2) | |||
| Eye disorders - Other, specify | 2 (2) | 1 (1) | 3 (4) | ||
| Fall | 1 (1) | 1 (1) | |||
| Fatigue | 33 (40) | 27 (33) | 4 (5) | 64 (77) | |
| Fever | 2 (2) | 1 (1) | 3 (4) | ||
| Flatulence | 1 (1) | 1 (1) | |||
| Flu like symptoms | 1 (1) | 1 (1) | 2 (2) | ||
| Flushing | 1 (1) | 1 (1) | 2 (2) | ||
| Gait disturbance | 1 (1) | 1 (1) | |||
| Gastritis | 1 (1) | 1 (1) | |||
| Gastrointestinal disorders - Other, specify | 1 (1) | 1 (1) | |||
| Generalized muscle weakness | 2 (2) | 2 (2) | 4 (5) | ||
| Headache | 13 (16) | 9 (11) | 1 (1) | 23 (28) | |
| Hearing impaired | 1 (1) | 1 (1) | |||
| Hoarseness | 1 (1) | 1 (1) | |||
| Hyperkalemia | 1 (1) | 1 (1) | 2 (2) | ||
| Hypersomnia | 1 (1) | 1 (2) | |||
| Hypertension | 3 (4) | 1 (1) | 4 (5) | ||
| Hypokalemia | 4 (5) | 1 (1) | 1 (1) | 6 (7) | |
| Hypomagnesemia | 1 (1) | 1 (1) | |||
| Hyponatremia | 3 (4) | 3 (4) | |||
| Hypotension | 1 (1) | 1 (1) | |||
| Hypoxia | 1 (1) | 1 (1) | |||
| Infections and infestations - Other, specify | 1 (1) | 1 (1) | |||
| Infusion related reaction | 4 (5) | 4 (5) | |||
| Infusion site extravasation | 1 (1) | 1 (1) | |||
| Injection site reaction | 3 (4) | 3 (4) | |||
| Insomnia | 1 (1) | 1 (1) | |||
| Lethargy | 3 (4) | 3 (4) | |||
| Localized edema | 1 (1) | 1 (1) | |||
| Lymphocyte count decreased | 4 (5) | 4 (5) | |||
| Memory impairment | 3 (4) | 2 (2) | 5 (6) | ||
| Mucosal infection | 2 (2) | 2 (2) | |||
| Mucositis oral | 1 (1) | 1 (1) | |||
| Muscle weakness left-sided | 1 (1) | 1 (1) | |||
| Myalgia | 1 (1) | 1 (1) | |||
| Nasal congestion | 2 (2) | 2 (2) | |||
| Nausea | 26 (31) | 7 (8) | 2 (2) | 35 (42) | |
| Nervous system disorders - Other, specify | 1 (1) | 1 (1) | |||
| Neutrophil Count Decreased | 4 (5) | 9 (11) | 3 (4) | 5 (6) | 21 (25) |
| Olfactory nerve disorder | 1 (1) | 1 (1) | |||
| Otitis media | 1 (1) | 1 (1) | |||
| Pain | 2 (2) | 2 (2) | |||
| Paresthesia | 2 (2) | 2 (2) | |||
| Peripheral motor neuropathy | 1 (1) | 1 (1) | |||
| Platelet Count Decreased | 40 (48) | 6 (7) | 4 (5) | 11 (13) | 61 (73) |
| Pruritus | 3 (4) | 3 (4) | 6 (7) | ||
| Radiation recall reaction (dermatologic) | 1 (1) | 1 (1) | |||
| Rash acneiform | 4 (5) | 1 (1) | 5 (6) | ||
| Rash maculo-papular | 1 (1) | 1 (1) | 3 (4) | 5 (6) | |
| Rash pustular | 1 (1) | 1 (1) | |||
| Respiratory, thoracic and mediastinal disorders - Other, specify | 1 (1) | 1 (1) | |||
| Seizure | 4 (5) | 4 (5) | |||
| Sinus bradycardia | 1 (1) | 1 (1) | |||
| Skin and subcutaneous tissue disorders - Other, specify | 5 (6) | 1 (1) | 6 (7) | ||
| Skin hyperpigmentation | 1 (1) | 1 (1) | |||
| Somnolence | 1 (1) | 1 (1) | |||
| Stroke | 1 (1) | 1 (1) | |||
| Thromboembolic event | 2 (2) | 2 (2) | |||
| Tremor | 1 (1) | 1 (1) | |||
| Urinary frequency | 1 (1) | 1 (1) | |||
| Urinary incontinence | 1 (1) | 1 (1) | |||
| Urticaria | 1 (1) | 2 (2) | 3 (4) | ||
| Vertigo | 1 (1) | 1 (1) | |||
| Vomiting | 8 (10) | 2 (2) | 1 (1) | 11 (13) | |
| Watering eyes | 1 (1) | 1 (1) | 2 (2) | ||
| Weight loss | 1 (1) | 1 (1) | |||
| White Blood Cell Decreased | 22 (27) | 14 (17) | 5 (6) | 3 (4) | 44 (53) |
Discussion
This study reached its primary endpoint with a hazard rate across all patients of 0.44 (95%CI: 0.35–0.55) per person year of follow-up compared to 0.6 from the landmark EORTC/NCIC data.(1) This translates into an approximately 27% reduction in hazard of death with the addition of iniparib to standard RT and TMZ. As in the EORTC/NCIC trial and subsequent studies, MGMT methylation status was an important prognostic factor. Patients with MGMT methylation had substantially better survival than those with unmethylated MGMT in this study (mOS 30 months methylated versus 15.8 months unmethylated, p=0.0015). In addition, iniparib showed a favorable tolerability and safety profile when added to RT and TMZ. Specifically, the rates of fatigue and leukopenia in this study were similar to those seen in studies assessing RT and TMZ alone.(1,4)
These encouraging results, however, must be considered in the context of recent similar efforts to define optimal therapies for people with newly diagnosed GBM. Over the last 10 years, multiple studies have shown improved survival with new agents and schedules added to the standard treatment established by EORTC/NCIC.(4,5,16) Such improvement may be attributable to improved overall supportive care and experience rather than to any one active agent.(5) This possibility may confound the interpretation of modern single arm studies. For example, although it is notable that the mOS with iniparib added to RT and TMZ exceeds the mOS for RT and TMZ alone either in the original EORTC/NCIC or the more modern RTOG 0525 study (mOS 21.6 months with iniparib and TMZ versus 16.6 months with TMZ alone), the two year survival rates are relatively overlapping across these studies (Table 3). This is particularly notable when considering the two year survival results of the CENTRIC EORTC study that showed no difference in mOS between patients with newly diagnosed MGMT methylated GBM randomized to RT and TMZ alone versus cilengitide added to RT and TMZ in a large, randomized and blinded study.(16) In addition, the report of mOS is influenced by the time point from which survival is calculated. This study and the single RT and TMZ studies reported by NABTT (or ABTC) calculate survival from the date of diagnosis (5). In contrast, randomized studies calculate OS from the time of randomization (1,4,16). This may result in up to a month difference in calculated mOS and is an additional caveat that must be considered when interpreting the mOS from this study. Hence, although the results of this single arm phase 2 study suggest important biologic activity and safety for iniparib in adults with GBM based on reduction in hazard of death and percent survival at two years, more work is required before advancing to confirmatory efficacy studies.
In addition, there remains lack of clarity about the mechanism of action of iniparib in conjunction with RT and TMZ. Iniparib was initially selected for combination with RT and TMZ as it was thought to be a PARP inhibitor that would enhance the efficacy of these therapies by preventing DNA repair.(6,7,13) However, subsequent investigations indicate that iniparib is not a dedicated PARP inhibitor, but rather a prodrug that when activated, modulates enzymatic activity critical for maintaining redox homeostasis in cells.(8,9,17,18) Cancer cells are particularly susceptible to shifts in reactive oxygen species (ROS) and it is possible that iniparib is making both dividing and quiescent malignant glioma cells more vulnerable to RT and TMZ by increasing ROS intracellularly.(8,17) Further, there is evidence suggesting that iniparib cytotoxicty is potentiated in states of low glutathione.(8,18) This is compelling as there is new data indicating that mutations in isocitrate dehydrogenase 1 (IDH1) may mediate chemosensitivity via reduced ROS response and decreased glutathione.(19) Further studies are needed to evaluate if iniparib enhances RT and TMZ effects in a similar manner, allowing IDH1 wild type tumors to behave more like IDH1 mutant tumors or if the effect is limited to IDH1 mutant tumors.
The initial enthusiasm for iniparib as an active anti-cancer agent dwindled after disappointing late stage studies and evidence that its activity is not as a specific PARP inhibitor.(7,9,10) The emerging data that iniparib may act to enhance RT and TMZ by increasing intracellular ROS needs to be evaluated in GBM model systems. Future studies should evaluate markers of oxidative stress such as 4-HNE-His adducts and immunohistochemistry for protein nitration in pre- and post-treatment GBM tissue to assess the impact iniparib has on oxidative stress in these cells alone and in combination with RT and TMZ.(20) Finally, pre-clinical studies are needed to determine if iniparib has increased activity in settings of low glutathione. If confirmed, this would be highly relevant to both low- and high-grade gliomas as it would present an opportunity to sequence iniparib with RT and TMZ in order to modulate mutant IDH1 effects in malignant gliomas.
Supplementary Material
Acknowledgments
Acknowledgements: The authors would like to thank Joy Fisher for assistance with data collection oversight on behalf of the Adult Brain Tumor Consortium, the team at Cancer Therapy Evaluation Program, National Cancer Institute for their oversight and collaboration through the Adult Brain Tumor Consortium and Sanofi for the provision of the drug.
Funding: National Institutes of Health (U01 CA-62475 to S.G., U01 CA-137443 to S.G.); Sanofi Pharmaceuticals, Inc.
Footnotes
Conflict of Interest: The authors JOB, SAG, AC, TM, MRR, MSA, LBN, AE, SD, XY declare no potential conflicts of interest. IGR was an employee of Sanofi at the time of the design and launch of the study.
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96. [DOI] [PubMed] [Google Scholar]
- 2.Hegi ME, Liu L, Herman JG, Stupp R, Wick W, Weller M, et al. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol 2008;26:4189–99. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459–66. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: A randomized phase III clinical trial. J Clin Oncol 2013;31:4085–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the united states. Clin Cancer Res 2010;16:2443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fogelman DR, Wolff RA, Kopetz S, Javle M, Bradley C, Mok I, et al. Evidence for the efficacy of iniparib, a PARP-1 inhibitor, in BRCA2-associated pancreatic cancer. Anticancer Res 2011;31:1417–20. [PubMed] [Google Scholar]
- 7.O’Shaughnessy J, Osborne C, Pippen JE, Yoffe M, Patt D, Rocha C, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med 2011;364:205–14. [DOI] [PubMed] [Google Scholar]
- 8.Mendeleyev J, Kirsten E, Hakam A, Buki KG, Kun E. Potential chemotherapeutic activity of 4-iodo-3-nitrobenzamide metabolic reduction to the 3-nitroso derivative and induction of cell death in tumor cells in culture. Biochem Pharmacol 1995;50:705–14. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Shi Y, Maag DX, Palma JP, Patterson MJ, Ellis PA, et al. Iniparib nonselectively modifies cysteine-containing proteins in tumor cells and is not a bona fide PARP inhibitor. Clin Cancer Res 2012;18:510–23. [DOI] [PubMed] [Google Scholar]
- 10.Patel AG, De Lorenzo SB, Flatten KS, Poirier GG, Kaufmann SH. Failure of iniparib to inhibit poly(ADP-ribose) polymerase in vitro. Clin Cancer Res 2012;18:1655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novello S, Besse B, Felip E, Barlesi F, Mazieres J, Zalcman G, et al. A phase II randomized study evaluating the addition of iniparib to gemcitabine plus cisplatin as first-line therapy for metastatic non-small-cell lung cancer. Ann Oncol 2014;25:2156–62. [DOI] [PubMed] [Google Scholar]
- 12.Castro M, Li L, Stallings TE. Pharmacokinetics of BSI-201, a poly (ADP-ribose) polymerase-1 (PARP1) inhibitor, in cerebrospinal fluid (CSF) of a patient with breast cancer with carcinomatous meningitis. J Clin Oncol 2010; 28:15_suppl, e13559–e13559. [Google Scholar]
- 13.Blakeley JO, Grossman SA, Mikkelsen T, Rosenfeld MR, Peereboom D, Nabors LB, et al. Phase I study of iniparib concurrent with monthly or continuous temozolomide dosing schedules in patients with newly diagnosed malignant gliomas. J Neurooncol 2015;125:123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P Nonparametric estimation from incomplete observations. J. Amer. Statist. Assn 1958; 53:457–81. [Google Scholar]
- 15.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics 1982; 38:29–41. [Google Scholar]
- 16.Stupp R, Hegi ME, Gorlia T, Erridge SC, Perry J, Hong YK, et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071–22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1100–8. [DOI] [PubMed] [Google Scholar]
- 17.Kun E, Mendeleyev J, Hakam A, Kirsten E. Enzymatic mechanism of the tumoricidal action of 4-iodo-3-nitrobenzamide. Mol Med Rep 2009;2:739–42. [DOI] [PubMed] [Google Scholar]
- 18.Kun E, Kirsten E, Mendeleyev J. Synergistic anticancer action of reversibly and irreversibly acting ligands of poly (ADP-ribose) polymerase. Int J Mol Med 2003;11:191–3. [PubMed] [Google Scholar]
- 19.Shi J, Sun B, Shi W, Zuo H, Cui D, Ni L, et al. Decreasing GSH and increasing ROS in chemosensitivity gliomas with IDH1 mutation. Tumour Biol 2015;36:655–62. [DOI] [PubMed] [Google Scholar]
- 20.Cipak Gasparovic A, Zarkovic N, Zarkovic K, Semen K, Kaminskyy D, Yelisyeyeva O, Bottari SP. Biomarkers of oxidative and nitro-oxidative stress: conventional and novel approaches. Br J Pharmacol. 2017;174:1771–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.