Abstract
Purpose of review
To provide an update on recent studies of human adenoviral (HAdV) infections and to explore the mechanisms of viral persistence and the role of persistent infection in disseminated disease in immunocompromised patients.
Recent findings
Human adenoviruses continue to be a problem in ophthalmology clinics and to cause periodic, limited, global outbreaks of respiratory disease. Ad14p1 remains in worldwide circulation and continues to result in miniepidemics of severe respiratory infections. New variants of Ad4 and Ad7 have emerged in both the United States and Asia. The severity of Ad4 infections in outbreaks appears to depend more on preexisting conditions in patients than on genetically determined, viral virulence factors, in contrast to limited evidence of Ad7 mutations that may convey increased viral pathogenesis. Reactivation of persistent adenovirus infection appears to be the primary source of disseminated infections in immunocompromised patients. New studies suggest that establishment of persistent infection and reactivation are related to variations in interferon-mediated control of viral replication.
Summary
Innate immune responses can create a state of adenoviral persistence, and repression of these host defenses can result in reactivation and dissemination of infection. A better definition of the molecular mechanisms of immune-mediated control of viral replication might lead to new strategies for treatment of HAdV reactivation and dissemination.
Keywords: adenovirus, disseminated infection, immunosuppression, interferons, persistence, reactivation
INTRODUCTION
The discovery of what was eventually termed human adenovirus (HAdV) was reported in 1953 by Rowe et al. [1] who described an ‘adenoid degeneration agent’. While culturing cells from human adenoids surgically harvested from children, they noticed spontaneous cellular degeneration at time points from 1 to 3 weeks after establishment in vitro. Their observations on degeneration of the adenoid tissues and transmissible infection of other cell types provided the classical observation of how HAdVs infect and kill tissue culture cells. In addition, their isolation of HAdV from human lymphoid tissues provided the first evidence that the virus can cause persistent infections in humans.
HAdVs are members of the genus Mastadenovirus in the Adenoviridae family. HAdVs are nonenveloped and contain a linear double-stranded DNA genome. They are grouped into seven species (HAdV-A to G) based on phylogenetic analysis, genomic organization, growth characteristics and oncogenicity. Within those species are over 70 types that were originally classified based on serology (by viral neutralization) but are now classified based on genomic sequence analysis. Tissue tropism of HAdV species determines the clinical manifestations of infection [2]. HAdV can infect conjunctiva (species B, D and E), the upper and lower respiratory tracts (species B, C and E) and the gastrointestinal tract (species F and G). Species A–F circulate globally and can cause limited, periodic outbreaks of infection.
HAdV infections usually present as keratoconjunctivitis or upper or lower respiratory tract infections that occasionally progress to pneumonia, acute respiratory distress syndrome (ARDS) or disseminated infections, depending upon the immunological status of the host. HAdV infections in healthy individuals are usually mild and self-limiting. Most respiratory HAdV outbreaks are seen in closed population clusters, as in military installations, long-term care facilities, schools or hospitals. Disseminated infections usually occur in immuno-suppressed patients. In this review, we will briefly review the recent literature on clinical HAdV infections and then focus on the role of persistent HAdV infection in disseminated disease.
OCULAR INFECTIONS: EPIDEMIC KERATOCONJUNCTIVITIS
Six members of the HAdV D species (HAdV-8, 19, 37, 53, 54 and 56) are the main causes of epidemic keratoconjunctivitis (EKC), a highly contagious infection that can cause severe inflammatory disease of the conjunctiva and cornea. There are an estimated 20–40 million cases annually, with HAdV EKC (A-EKC) being endemic in Japan. HAdV can cause large and prolonged outbreaks in ophthalmology clinics, because of the ability of HAdV to remain viable for many days on instruments and some resistance of these agents to common methods of disinfection. Muller et al. [3] reported an outbreak of A-EKC associated with patients seeing a single physician, where the only shared procedure among those with A-EKC was the use of eye drops, suggesting contaminated multidose vials as the common source of infection. In 2015, there was an epidemic of EKC caused by Ad54 resulting in cases with corneal complications. Suzuki et al. [4] reported a case of a 36-year-old man who acquired Ad54 from his 2-year-old son. Ten years previously, the man had a LASIK procedure. His infected right eye had a severe ocular infection with a conjuctival pseudomembrane and a circular epithelial defect that coincided with the LASIK flap edge, suggesting the possibility that HAdV infection could result in LASIK flap loss. The authors suggested that patients with A-EKC following LASIK should be examined for possible corneal complications. In 2016, the first reported cases of Ad8-associated EKC in mainland China were found in Tibet [5].
RESPIRATORY INFECTIONS: OUTBREAKS, OCCASIONALLY RESULTING IN PNEUMONIA AND ACUTE RESPIRATORY DISTRESS SYNDROME
Respiratory infection with HAdV usually results in mild, self-limited disease in immunocompetent patients. However, there are yearly outbreaks of HAdV causing severe respiratory infections and pneumonias that, in rare cases, progress to ARDS. ARDS appears as bilateral, variable lung opacities that resemble pulmonary edema on chest X-rays or CT scans. Severity of ARDS is scored based on the level of hypoxemia, and treatment of ARDS is mainly supportive care, with mortality rates of 27–45% [6]. Outbreaks of pneumonia and ARDS are usually caused by HAdV from groups B, C and E HAdV that have newly emerged in the population, as a result of viral mutations or recombination events [7▪].
One such emergent strain, Ad14p1 (a variant of HAdV type 14), remains in circulation worldwide and continues to result in outbreaks of influenza-like illness (ILI). For example, in New York State, there was an outbreak among college students of ILI, with clusters of infections with Ad14p1, Ad2 and Ad4 during the 2014–2015 school year [8]. Zhang et al. [9] analyzed the genomes of Ad14p1 from cases in China and found three distinct viral variants, with two of those strains distinct from the US Ad14p1 strain. In an effort to determine the prevalence of Ad14 and Ad55 (an Ad14 – Ad11 recombinant virus) infection in China, Zheng et al. [10] evaluated seropositive responses of over 1000 healthy blood donors. 24.8% of donors were seropositive for neutralizing antibodies against Ad14. 22.4% were seropositive against Ad55. And the rate of seropositivity increased with patient age. In 2016, there was an outbreak of Ad55 in China, involving both patients and staff in a hospital [11]. Ad55 outbreaks in the South Korean military also occurred in the same time frame [12]. The continued appearance of Ad14 (and related variant) outbreaks and the reported seroprevalence rates indicate that Ad14 continues to circulate in the general population, but the reasons for the episodic outbreaks of more severe disease are unknown.
Ad4 and Ad7 strains have re-emerged periodically as causes of local infection outbreaks, linked to both epidemiologic and viral genetic factors. Two epidemiology studies in Japan of HAdV infections in children found similar results. HAdV caused 10–30% of upper respiratory infections. Both studies showed that the infections were predominately from HAdV groups C and B, with few cases resulting from group E (Ad4) infection [13,14▪▪]. Nakamura et al. [14▪▪] speculated that the low rate of Ad4 infection and associated seropositivity in young children might explain the increased risk of Ad4 infections in young adults (e.g. military recruits) because of a lack of acquired ‘herd immunity’ in the older patients. Kajon et al. [15] reviewed Ad4 cases of ILI in New York State from 2011 through 2015. Genomic sequencing of the Ad4 strains identified five different genomic variants in circulation. The majority of mild cases of illness were reported in young otherwise healthy patients. Three of 33 cases progressed to more severe illness. There was no correlation between the viral genotype and illness severity, but there was some association with underlying health problems. Yang et al. [16] did genetic analysis and infection studies of Ad7 isolated from an outbreak among college students in China. The Ad7 strain had a mutation in the VA RNA gene, which they linked to increased expression of L1 52/ 55K protein – a viral packaging protein. This viral genetic change was associated with increased viral yield with time after infection of human cells in vitro, and they concluded that the faster viral growth might be related to its increased clinical virulence [16].
DISSEMINATED INFECTIONS
Disseminated infections with HAdV are almost exclusively seen in immunocompromised patients, and such infections can be life threatening, especially in pediatric patients [17–19] These patients have usually been immunosuppressed prior to and following hematopoietic stem cell transplant (HSCT) or solid organ transplantation. HAdV infection is usually limited by cellular components of the innate and adaptive immune responses to infection and to a lesser extent by virus-specific humoral immunity. Disseminated HAdV infections can arise both from new, exogenous infection and from activation of previously, clinically silent, persistent infection of lymphoid tissues. Both sources of infection may be more common in pediatric transplant patients who are more susceptible to primary infections with viral strains to which they lack prior exposure and immunity and who have a higher burden of persistent virus in tonsilar lymphoid tissue [20]. Last year, Lee et al. [21] wrote a comprehensive review in this journal on HAdV infections in transplant patients and treatment options for patients suffering from disseminated HAdV infection. The remainder of the present review will focus on the biology of persistent HAdV infection and possible mechanisms of reactivation during immunosuppression.
EVIDENCE FOR PERSISTENT HUMAN ADENOVIRUS INFECTION AS THE SOURCE OF DISSEMINATED INFECTION AFTER IMMUNOSUPPRESSION
In retrospect, the first reported evidence for persistent or latent HAdV infection was presented by Rowe et al. [1] in their article that described HAdV isolated from adenoidal lymphoid tissue from pediatric patients. In that era (early 1950s), it was common to remove adenoids and tonsils from children because those lymphoid tissues were enlarged and presumed to be abnormal. It has been appreciated subsequently that such lymphoid tissue enlargement is normal in toddlers; thus, indications for surgery have become much more conservative. In hindsight, it is likely that most of the adenoids studied by Rowe et al. were normal (albeit enlarged) lymphoid tissues with persistent, subclinical HAdV infections. These authors detected what turned out to be HAdV in 33 out of 53 adenoid samples cultured. HAdV has also been detected in the stools of asymptomatic patients for prolonged periods after nasopharyngeal washings have cleared of virus [22,23]. This gut-related isolation of HAdV is probably explained by viral persistence in gut-associated lymphoid tissue in a similar manner to the viral persistence in adenoidal and tonsilar lymphocytes [24,25].
As noted, pediatric recipients of transplants have a several-fold greater risk of disseminated adenovirus infections than adult recipients. Among the risk factors for adenovirus infection are the extent of T-cell depletion and overall intensity of immunosuppression, allogeneic transplantation and exposure to total body irradiation [26]. Viral dissemination can involve multiple organ systems, including the lungs, the hepatobiliary system and the genitourinary tract. Gut involvement with diarrhea is the most common manifestation in pediatric patients [27], suggesting the importance of infection reactivation from gut-associated lymphoid tissue. A study of children receiving allogeneic stem cell transplants showed that HAdV infection risk was correlated with high neutralizing antibody titers prior to transplant and that this antibody response did not protect them from infection with the same viral serotype, suggesting that the infection represented reactivation after transplant-related suppression of cellular immunity, rather than de-novo infection [28]. A 2017 report from Germany described two pediatric patients who developed HAdV infection following allogeneic HSCT, as a result of reactivation of persistent HAdV [29▪▪]. In both cases, there was a drop in donor chimerism percentage (a common measure of transplant engraftment), as the result of expansion of residual, recipient-derived HAdV-specific T cells that survived the myeloablative conditioning process and contributed to control of disseminated HAdV infection. The authors concluded that, in addition to concerns about graft rejection, a fall in donor chimerism during disseminated HAdV infection might also be explained by expansion of autologous host T cells responding to viral infection.
SITES OF PERSISTENT HUMAN ADENOVIRUS INFECTION
It has been observed that the onset of HAdV infection in pediatric transplant patients is usually preceded by the appearance and proliferation of HAdV in the GI tract, as evidenced by increased HAdV copy numbers in stool specimens [30]. Intestinal lymphocytes were shown to be the site of HAdV persistence in the GI tract, predominately in the ileum of 1/3 of the controls [31]. HAdV-positive stem cell transplant patients showed robust HAdV replication in intestinal epithelial cells. A recent paper has reported a quantitative correlation between the amount of HAdV present in stool prior to transplant and increased risk of invasive disease after transplant [32]. Group C HAdV was the predominant species in those studies. Their findings strongly suggest the importance of testing for the presence of persistent HAdV in the stools of patients prior to transplant, to allow for early identification and consideration of therapy of patients at risk for disseminated infection posttransplant.
VIRAL MECHANISMS OF PERSISTENCE
The mechanisms of HAdV persistence in a subclinical state remain to be defined. It has been reported that several HAdV genes expressed prior to viral DNA replication (so-called ‘early’ genes) provide multiple mechanisms through which the virus can evade host immune-mediated elimination [33]. However, those mechanisms do not completely explain viral persistence, without progression to active infection. Zheng et al. [34▪▪] took a novel approach to this question. Type I interferons and interferon-γ can repress HAdV replication, and the virus has evolved mechanisms to block interferon activities [35–40]. In general, interferons fail to prevent HAdV infection in the virally permissive human tumor cell lines that are usually used for viral studies. However, these tumor cells have multiple mutations in cell cycle control pathways that are intimately involved in HAdV replication control. The authors asked whether interferons could block HAdV replication in primary human cells. Interferon treatment efficiently reduced HAdV replication in primary cells by over three orders of magnitude. The interferon-induced repression of HAdV replication was caused by reduced HAdV gene expression – specifically repression of expression of the HAdV E1A gene that encodes the key, viral transcriptional activator and cell cycle control products. The mechanisms of E1A repression by interferons involved blockade of recruitment of the cellular GABP transcription factor to the E1A enhancer and increased enhancer binding and regulation by cellular E2F/Rb family proteins. This interferon-induced repression of E1A activity in virally infected cells was associated with establishment of a persistently infected state, and when interferon was removed from infected cells, viral infection was reactivated and cells progressed to a fully lytic, virus producing state.
POTENTIAL FOR CLINICAL IMMUNOSUPPRESSION AS REACTIVATION TRIGGER
The studies by Zheng et al. [34▪▪] show that reduced interferon levels lead to reactivation of persistent HAdV infection, in an in-vitro infection model. The logical question arising from this observation is whether clinical immunosuppression results in decreased interferon levels, which, in turn, trigger HAdV reactivation and dissemination. Clinical immunosuppression from treatment with chemotherapeutic drugs and corticosteroids causes depletion of T cells and NK cells, both of which are key, interferon-γ producing cells [41–43]. Corticosteroids, used in various types of immunosuppressive chemotherapy including during hematopoietic and solid organ transplantation, also repress type I interferon and interferon-γ signaling pathways and interferon-γ transcription in T cells [44–46]. Toth et al. [47] demonstrated the translational significance of type I interferon signaling in an animal model of HAdV infection, in which there was enhanced viral replication and pathogenicity in hamsters with a knockout of STAT2, a key type I interferon signaling molecule. Overall, these reports support the importance of the host interferon responses in maintaining HAdV infection in its persistent state.
CONCLUSION
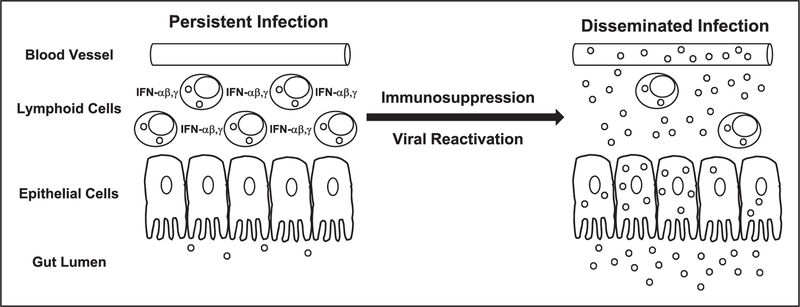
There is increasing evidence that reactivation of persistent HAdV infections may be the most common source of disseminated infections in immuno-compromised patients. On the basis of information and reports reviewed here, we propose the following model for establishment of HAdV persistence and reactivation in the immunosuppressed patient (Fig. 1) [31,34▪▪]. Following an otherwise self-limiting infection, HAdV establishes persistent infection in lymphoid tissues of the upper respiratory and GI tracts, and this infection remains in its subclinical state in the presence of intact immune activation responses – especially those mediated by interferons. In infected lymphoid cells, there is limited HAdV replication that can result in persistent, low-level viral shedding of virus. However, in epithelial cells associated with lymphoid tissue, HAdV replication is blocked by interferon activity, such as described by Zheng et al. [34▪▪]. Immunosuppressive chemotherapy depletes interferon producing innate immune cells (T and NK) and blocks signaling of virus-induced interferon activation in residual immune cells and other host cells. This loss of interferon responses relieves the repression of viral E1A expression in infected epithelial cells associated with persistently infected lymphoid tissues, resulting in increased expression of HAdV genes, viral replication and dissemination of infection, until there is posttransplantation expansion or reconstitution of the HAdV-specific host cellular immune response. The details of each step in this sequence of initial infection; long-term viral persistence, immunosuppression-related activation and dissemination will require continued study to complete the understanding of the control of these processes. This could provide the basis for development of new antiviral strategies that can be used to prevent the morbidity and mortality associated with disseminated disease.
FIGURE 1.
Model of HAdV persistence and reactivation in the gut. During initial adenovirus infection, sufficient interferon (IFN)-α, β and γ are produced to create a state of viral persistence. Adenovirus (o) is found primarily in lymphoid cells in the gut but is largely absent from highly permissive epithelial cells. Small amounts of virus are produced and shed in the feces. Chemotherapy-induced immunosuppression depletes IFN producing lymphoid cells and represses IFN expression in residual lymphoid cells and other host cells. Repression of the host IFN response allows increased expression of the viral E1A gene, which, in turn, results in increased viral replication in lymphoid cells and lymphoid tissue-associated epithelial cells that can produce large amounts of virus. This viral amplification can result in increased fecal viral shedding, increased tissue invasion and blood borne dissemination of infection.
KEY POINTS.
A state of persistent adenoviral infection can be established in human lymphoid cells, not epithelia cells.
Type I interferon and interferon-γ are key factors in adenoviral persistence as a result of their repression of expression of E1A, the viral transactivator and cell cycle regulator.
Immunosuppression reduces the numbers of interferon producing cells and blocks the interferon activation response in residual host cells.
Loss of the interferon activation response results in recovery of E1A expression, reactivation of viral replication and the potential for disseminated viral infection.
Acknowledgements
The views expressed here are those of the authors and do not reflect the opinion or policy of the VA or the Government of the United States.
Financial support and sponsorship
J.R.R. is a junior investigator at the Idaho Infectious Disease COBRE and is supported by NIH Grant No. P20GM109007 (National Institute of General Medical Sciences). J.L.C. is a coinvestigator on the Loyola University Chicago Experimental Immunology Training Grant supported by NIH Grant No. 2T32AI007508–16.
Footnotes
Conflicts of interest
The authors have no conflicts of interest.
Papers of particular interest, published within the annual period of review, have been highlighted as:
of special interest
of outstanding interest
REFERENCES AND RECOMMENDED READING
- 1.Rowe WP, Huebner RJ, Gilmore LK, et al. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc Soc Exp Biol Med 1953; 84:570–573. [DOI] [PubMed] [Google Scholar]
- 2.Ghebremedhin B Human adenovirus: viral pathogen with increasing importance. Eur J Microbiol Immunol (Bp) 2014; 4:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller MP, Siddiqui N, Ivancic R, Wong D. Adenovirus-related epidemic keratoconjunctivitis outbreak at a hospital-affiliated ophthalmology clinic. Am J Infect Control 2018; 10.1016/j.ajic.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki S, Kawamura T, Saeki Y, et al. A case of type 54 human mastadenovirus keratoconjunctivitis causing severe broad epithelial defect ten years after LASIK surgery. Jpn J Infect Dis 2017; 70:597–598. [DOI] [PubMed] [Google Scholar]
- 5.Lei Z, Zhu Z, Wang BMC, et al. Outbreaks of epidemic keratoconjunctivitis caused by human adenovirus type 8 in the Tibet Autonomous Region of China in 2016. PLoS One 2017; 12:e0185048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med 2017; 377:562–572. [DOI] [PubMed] [Google Scholar]
- 7.▪.Cook J, Radke JR. Mechanisms of pathogenesis of emerging adenoviruses. F1000Res 2017; 6:90. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review article highlights the varied genetic mechanisms through which human adenoviruses have evolved into more pathogenic strains. It presents the hypothesis that the emergence of pathogenic adenoviral strains might be related to subwild-type levels of viral gene expression that can result in a more pathogenic phenotype.
- 8.Lamson DM, Kajon A, Shudt M, et al. Detection and genetic characterization of adenovirus type 14 strain in students with influenza-like illness, New York, USA, 2014–2015. Emerg Infect Dis 2017; 23:1194–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Q, Jing S, Cheng Z, et al. Comparative genomic analysis of two emergent human adenovirus type 14 respiratory pathogen isolates in China reveals similar yet divergent genomes. Emerg Microbes Infect 2017; 6:e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng X, Rong X, Feng Y, et al. Seroprevalence of neutralizing antibodies against adenovirus type 14 and 55 in healthy adults in Southern China. Emerg Microbes Infect 2017; 6:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yi L, Zou L, Lu J, et al. A cluster of adenovirus type B55 infection in a neurosurgical inpatient department of a general hospital in Guangdong, China. Influenza Other Respir Viruses 2017; 11:328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoo H, Gu SH, Jung J, et al. Febrile respiratory illness associated with human adenovirus type 55 in South Korea Military, 2014–2016. Emerg Infect Dis 2017; 23:1016–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiroi S, Morikawa S, Nakata K, Kase T. Surveillance of adenovirus respiratory infections in children from Osaka, Japan. Jpn J Infect Dis 2017; 70:666–668. [DOI] [PubMed] [Google Scholar]
- 14.▪▪.Nakamura H, Fujisawa T, Suga S, et al. Species differences in circulation and inflammatory responses in children with common respiratory adenovirus infections. J Med Virol 2018; 90:873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]; These authors suggest that one reason for the higher incidence of severe HAdV-4 infections in otherwise healthy young adults is the lack of herd immunity, resulting from the low incidence of HAdV-4 infections in childhood.
- 15.Kajon AE, Lamson DM, Bair CR, et al. Adenovirus type 4 respiratory infections among civilian adults, Northeastern United States, 2011–2015. Emerg Infect Dis 2018; 24:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, Wang Q, Liang B, et al. An outbreak of acute respiratory disease caused by a virus associated RNA II gene mutation strain of human adenovirus 7 in China, 2015. PLoS One 2017; 12:e0172519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feghoul L, Chevret S, Cuinet A, et al. Adenovirus infection and disease in paediatric haematopoietic stem cell transplant patients: clues for antiviral preemptive treatment. Clin Microbiol Infect 2015; 21:701–709. [DOI] [PubMed] [Google Scholar]
- 18.van Tol MJ, Kroes AC, Schinkel J, et al. Adenovirus infection in paediatric stem cell transplant recipients: increased risk in young children with a delayed immune recovery. Bone Marrow Transplant 2005; 36:39–50. [DOI] [PubMed] [Google Scholar]
- 19.Lion T Adenovirus infections in immunocompetent and immunocompromised patients. Clin Microbiol Rev 2014; 27:441–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garnett CT, Talekar G, Mahr JA, et al. Latent species C adenoviruses in human tonsil tissues. J Virol 2009; 83:2417–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee YJ, Prockop SE, Papanicolaou GA. Approach to adenovirus infections in the setting of hematopoietic cell transplantation. Curr Opin Infect Dis 2017; 30:377–387. [DOI] [PubMed] [Google Scholar]
- 22.Fox JP, Brandt CD, Wassermann FE, et al. The virus watch program: a continuing surveillance of viral infections in metropolitan New York families. VI. Observations of adenovirus infections: virus excretion patterns, antibody response, efficiency of surveillance, patterns of infections, and relation to illness. Am J Epidemiol 1969; 89:25–50. [DOI] [PubMed] [Google Scholar]
- 23.Fox JP, Hall CE, Cooney MK. The Seattle Virus Watch. VII. Observations of adenovirus infections. Am J Epidemiol 1977; 105:362–386. [DOI] [PubMed] [Google Scholar]
- 24.Garnett CT, Erdman D, Xu W, Gooding LR. Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes. J Virol 2002; 76:10608–10616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy S, Calcedo R, Medina-Jaszek A, et al. Adenoviruses in lymphocytes of the human gastro-intestinal tract. PLoS One 2011; 6:e24859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Tol MJD, Claas ECJ, Heemskerk B, et al. Adenovirus infection in children after allogeneic stem cell transplantation: diagnosis, treatment and immunity. Bone Marrow Transplant 2005; 35(Suppl 1S1):S73–S76. [DOI] [PubMed] [Google Scholar]
- 27.de Mezerville MH, Tellier R, Richardson S, et al. Adenoviral infections in pediatric transplant recipients: a hospital-based study. Pediatr Infect Dis J 2006; 25:815–818. [DOI] [PubMed] [Google Scholar]
- 28.Veltrop-Duits LA, van Vreeswijk T, Heemskerk B, et al. High titers of preexisting adenovirus serotype-specific neutralizing antibodies in the host predict viral reactivation after allogeneic stem cell transplantation in children. Clin Infect Dis 2011; 52:1405–1413. [DOI] [PubMed] [Google Scholar]
- 29.▪▪.Schultze-Florey RE, Tischer S, Ku€hnau W, et al. Persistent recipient-derived human adenovirus (HAdV)-specific T cells promote HAdV control after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2017; 52:609–611. [DOI] [PubMed] [Google Scholar]; This paper shows that a fall in donor chimerism in HSCT patients can be the result of the host response to reactivation of persistent HAdV infection after transplantation. The fall in chimerism percentage resulted from amplification of residual, recipient HAdV-specific T cells in response to viral reactivation. The results suggest caution for clinicians interpreting a fall in chimerism as sign of graft rejection, without ruling out HAdV infection.
- 30.Lion T, Kosulin K, Landlinger C, et al. Monitoring of adenovirus load in stool by real-time PCR permits early detection of impending invasive infection in patients after allogeneic stem cell transplantation. Leukemia 2010; 24:706–714. [DOI] [PubMed] [Google Scholar]
- 31.Kosulin K, Geiger E, Ve´csei A, et al. Persistence and reactivation of human adenoviruses in the gastrointestinal tract. Clin Microbiol Infect 2016; 22:381; e1–381.e8. [DOI] [PubMed] [Google Scholar]
- 32.Kosulin K, Berkowitsch B, Matthes S, et al. Intestinal adenovirus shedding before allogeneic stem cell transplantation is a risk factor for invasive infection posttransplant. Ebiomedicine 2018; 28:114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hendrickx R, Stichling N, Koelen J, et al. Innate immunity to adenovirus. Hum Gene Ther 2014; 25:265–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.▪▪.Zheng Y, Stamminger T, Hearing P. E2F/Rb family proteins mediate interferon induced repression of adenovirus immediate early transcription to promote persistent viral infection. PLoS Pathog 2016; 12:e1005415. [DOI] [PMC free article] [PubMed] [Google Scholar]; This seminal report describes the cellular mechanisms through which type I interferon and interferon-g block adenoviral replication in normal human cells and how loss of the interferon response can result in reactivation of HAdV infection from a state of viral persistence to one of fully active viral production.
- 35.Chahal JS, Gallagher C, DeHart CJ, Flint SJ. The repression domain of the E1B 55-kilodalton protein participates in countering interferon-induced inhibition of adenovirus replication. J Virol 2013; 87:4432–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chahal JS, Qi J, Flint SJ. The human adenovirus type 5 E1B 55 kDa protein obstructs inhibition of viral replication by type I interferon in normal human cells. PLoS Pathog 2012; 8:e1002853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ullman AJ, Reich NC, Hearing P. Adenovirus E4 ORF3 protein inhibits the interferon-mediated antiviral response. J Virol 2007; 81:4744–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schreiner S, Wimmer P, Sirma H, et al. Proteasome-dependent degradation of Daxx by the viral E1B-55K protein in human adenovirus-infected cells. J Virol 2010; 84:7029–7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitajewski J, Schneider RJ, Safer B, et al. Adenovirus VAI RNA antagonizes the antiviral action of interferon by preventing activation of the interferon-induced eIF-2alpha kinase. Cell 1986; 45:195–200. [DOI] [PubMed] [Google Scholar]
- 40.Ullman AJ, Hearing P. Cellular proteins PML and Daxx mediate an innate antiviral defense antagonized by the adenovirus E4 ORF3 protein. J Virol 2008; 82:7325–7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verma R, Foster RE, Horgan K, et al. Lymphocyte depletion and repopulation after chemotherapy for primary breast cancer. Breast Cancer Res 2016; 18:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Öhrmalm L, Smedman C, Wong M, et al. Decreased functional T lymphocyte-mediated cytokine responses in patients with chemotherapy-induced neutropenia. J Intern Med 2013; 274:363–370. [DOI] [PubMed] [Google Scholar]
- 43.Mackall CL. T-cell immunodeficiency following cytotoxic antineoplastic therapy: a review. Stem Cells 2000; 18:10–18. [DOI] [PubMed] [Google Scholar]
- 44.Arya SK, Wong-Staal F, Gallo RC. Dexamethasone-mediated inhibition of human T cell growth factor and gamma-interferon messenger RNA. J Immunol 1984; 133:273–276. [PubMed] [Google Scholar]
- 45.Flammer JR, Dobrovolna J, Kennedy MA, et al. The type I interferon signaling pathway is a target for glucocorticoid inhibition. Mol Cell Biol 2010; 30:4564–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu X, Li W-P, Meng C, Ivashkiv LB. Inhibition of IFN-gamma signaling by glucocorticoids. J Immunol 2003; 170:4833–4839. [DOI] [PubMed] [Google Scholar]
- 47.Toth K, Lee SR, Ying B, et al. STAT2 knockout syrian hamsters support enhanced replication and pathogenicity of human adenovirus, revealing an important role of Type I interferon response in viral control. PLoS Pathog 2015; 11:e1005084. [DOI] [PMC free article] [PubMed] [Google Scholar]