Abstract
Tracking of tissue oxygenation around chronic foot wounds may help direct therapy decisions in patients with peripheral artery disease (PAD). Novel sensing technology to enable such monitoring was tested over 9 months in a Sinclair mini-pig model. No adverse events were observed over the entire study period. Systemic and acute hypoxia challenges were detected during each measurement period by the microsensors. The median time to locate the sensor signal was 13 s. Lumee Oxygen microsensors appear safe for long-term repeated oxygen measurements over 9 months.
1. Introduction
Approximately 8–12 million people suffer from peripheral artery disease (PAD) in the US alone [1], resulting in a $74B economic burden each year [2]. In PAD, plaque builds up in the vasculature of the lower extremities, reducing blood flow and thus oxygen to the affected limb. Disease progression can be halted or reversed by restoring blood flow to the affected limb, typically done by endovascular balloon angioplasty, stent placement, or bypass surgery. Rates of restenosis after revascularization procedures are high (>30% within 9 months) and amputation will ultimately result if initial or additional interventions are conducted too late in the disease progression [3–6].
While techniques exist to assess perfusion and capillary hemoglobin oxygenation [7], a more direct measurement of tissue (interstitial) oxygen availability may provide a better indicator of tissue health and enable earlier intervention. Transcutaneous oxygen tension (TCOM or TcpO2) and ankle-brachial index (ABI) are two techniques commonly used to monitor PAD patients. TcpO2 is sensitive to temperature and motion artifacts, and takes up to 45 min to warm up. TcpO2 is bulky, requires a skilled operator, and its predictive value is modest [8]. ABI measures the ratio of systolic blood pressure in the leg to the arm. Although ABI measurements are short (<5 min), it has not shown to be a reliable PAD indicator and may be falsely elevated by diabetes, which is prevalent in the PAD population [9].
New injectable, luminescent, hydrogel microsensors (Lumee Oxygen from Profusa), provide minimally invasive continuous tissue oxygen measurements. These measurements may help monitor a PAD patient’s progress and direct therapy decisions [10]. Microsensors consist of a soft, tissue-integrating phosphorescent hydrogel, the optical properties of which fluctuate based on the local dissolved oxygen. The sensor signal is collected non-invasively with near infrared (NIR) light after the initial injection. Microsensors have been used in a variety of applications in which continuous monitoring of tissue oxygen is of interest [11].
The microsensors have regulatory approval (CE Mark) for clinical use over 3 months. The objective of this work was to study functionality of the microsensors beyond 3 months in a pig model.
2. Methods
2.1. Lumee Oxygen Microsensors and Optical Measurement System
Lumee Oxygen microsensors (Profusa, Inc. South San Francisco, CA), consist of a 0.5 × 0.5 × 5 mm injectable hydrogel matrix whose mechanism of sensing is based on oxygen-dependent quenching of palladium porphyrin phosphorescence. Microsensors were injected subcutaneously, and signal was non-invasively measured with a custom NIR reader [10–12]. Data are expressed as a Lumee Oxygen Index (LOI), which is the micromolar concentration of oxygen as calculated from the phosphorescent lifetime (τ) and temperature, using standard phosphorescent quenching calibrations [13]. LOI is the measure of the CE-mark approved Lumee Oxygen microsensors.
2.2. Systemic and Local Oxygen Challenges and Data Analysis
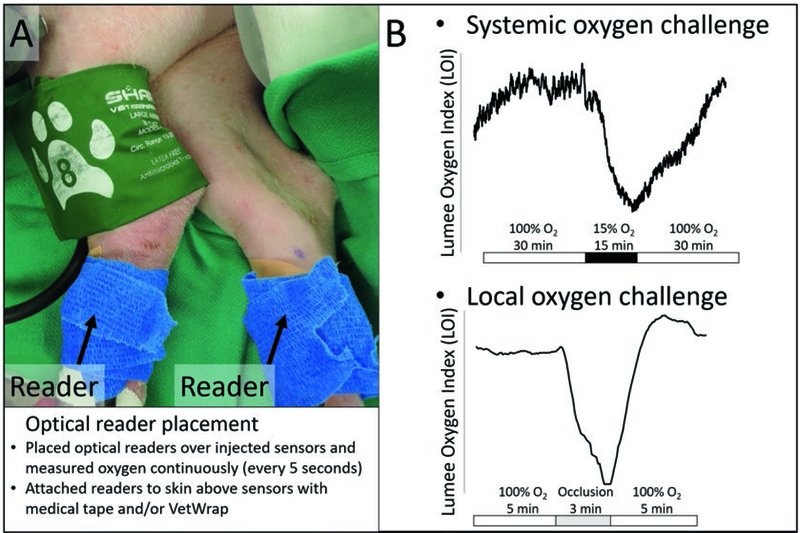
Continuous non-invasive measurements were obtained by attaching optical readers to the skin above microsensors (Fig. 1a). Systemic oxygen challenges were administered by changing the fraction of inspired oxygen (fiO2) to approximately 15% for 15 min (Fig. 1b). Local oxygen challenges were administered by applying a tourniquet or pressure cuff to a limb for 3 min (Fig. 1a, b). Baseline LOI was measured as a 2-min average immediately before each challenge. The absolute change in LOI from baseline during a challenge was measured by taking a 2-min average immediately before ending a systemic or local challenge and subtracting from the baseline LOI. The modulation of tissue oxygen (% ΔLOI) was calculated by dividing the absolute change in LOI by the baseline LOI and multiplying by 100%. SpO2 was measured every second using a CMS60D pulse oximeter (Contec, Qinhuangdao, China) attached to the ear to validate systemic effects during oxygen challenges. The % ΔSpO2 was calculated in the same fashion as % ΔLOI.
Fig. 1.
(a) Image of optical readers attached over sensors secured with wrap (blue) in the hind limbs of a pig and a pressure cuff (green). Optical readers non-invasively measured microsensors continuously during systemic and local oxygen challenges. (b) Example results of tissue oxygen measurements (LOI) versus time and description of protocol for systemic and local oxygen challenges
3. Results
There were no adverse events during the experiment in response to injected Lumee Oxygen microsensors. Additionally, microsensors could be quickly found, as the median time to localize a sensor was 13 s.
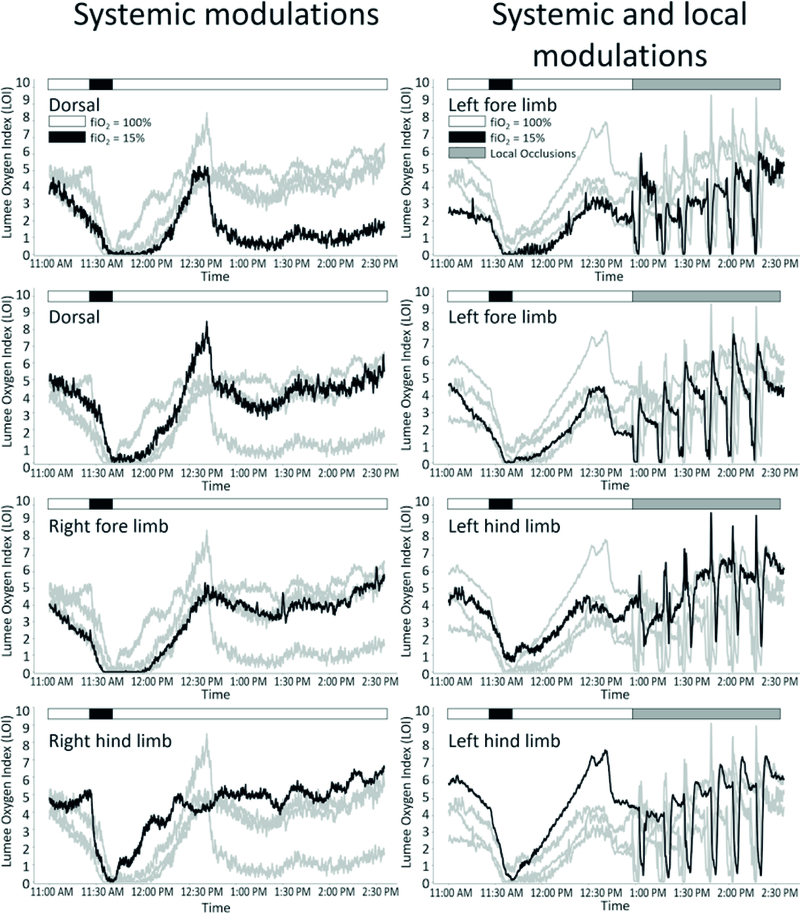
All microsensors responded to oxygen challenges independent of the type of challenge (systemic or local), anatomical location (dorsal or limb) or timepoint (over 9 months). Real-time traces from one representative measurement timepoint (6 months post-injection) show tissue oxygen fluctuations in response to systemic and local challenges (Fig. 2).
Fig. 2.
Individual microsensor traces from eight separate sensors during systemic and local oxygen challenges 6 months post-injection. Graphs in the left column were tested with systemic oxygen challenges. Graphs in the right column were tested with systemic and local oxygen challenges. Anatomical location of black sensor trace is indicated on each graph. Grey traces are the other sensors with the same modulation types (systemic only or systemic and local oxygen challenges). Displayed are the LOI measurements versus time. Microsensors detected tissue oxygen reductions in response to systemic and local hypoxic events
Systemic Measurements
Both LOI (tissue oxygen) and SpO2 (pulsatile blood hemoglobin saturation) showed simultaneous modulation in response to systemic oxygen challenges (Table 1); however, tissue oxygen showed much more dramatic fluctuations than blood oxygen (e.g. 49–96% in tissue compared to 6–14% modulation in blood). The % ΔSpO2 was not predictive of the % ΔLOI, indicating that tissue-level sensors provide different specific information about the state of the tissue, which may be relevant to PAD and other chronic healing states of tissue. The tissue oxygen is a balance between the supply from capillaries and metabolic demand of the cells, while SpO2 is an arterial blood saturation dependent on supply from the lungs.
Table 1.
Tissue and blood oxygenation responses to systemic and local oxygen challenges over time
| Timepoint (days) | Systemic modulations (average ± SEM) | Local modulations (average ± SEM) | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample size | Baseline (LOI) | Modulation (% LOI) | Δ SpO2 (%) | Sample size | Baseline (LOI) | Modulation (% LOI) | Δ Sp02 (%) | |
| 15 | 8 | 20.3 ± 2.7 | 62.9 ± 19.6% | 14.4 ± 0.6% | 0 | – | – | – |
| 29 | 8 | 15.1 ± 2.2 | 65.2 ± 5.1% | 7 ± 2.1% | 24 | 23.0 ± 2.3 | 98.0 ± 0.3% | 0.0 ± 0.0% |
| 57 | 8 | 9.3 ± 1.2 | 62.4 ± 4.6% | 6.7 ± 1.3% | 24 | 12.4 ± 0.8 | 92.9 ± 1.3% | 0.0 ± 0.1% |
| 71 | 8 | 9.2 ± 1.4 | 49.1 ± 7.6% | 6.5 ± 1.9% | 24 | 12.1 ± 1.0 | 95.3 ± 1.2% | 0.1 ± 0.3% |
| 97 | 7 | 7.5 ± 2.1 | 65.1 ± 10.7% | 7.7 ± 0.9% | 16 | 8.9 ± 1.3 | 89.7 ± 1.8% | – |
| 169 | 8 | 3.0 ± 0.4 | 96.0 ± 7.7% | 9.3 ± 0.5% | 24 | 4.0 ± 0.3 | 48.0 ± 7.9% | 0.0 ± 0.4% |
| 275 | 6 | 9.3 ± 3.2 | 78.9 ± 38.8% | 10.8 ± 0.9% | 18 | 6.7 ± 1.0 | 40.3 ± 8.5% | 0.1 ± 0.7% |
Tissue oxygen data (LOI) are presented as average ± standard error of the mean (SEM) over the sample sizes described. SpO2 data are presented as average ± standard deviation of the SpO2 measurements from one device. SpO2 data confirmed systemic modulations in blood oxygen to decreased fiO2 as well as confirmed that the local modulations did not have systemic effects. Sample sizes for systemic modulations are for individual sensors (six to eight sensors). Sample sizes for local modulations are for up to four sensors with up to six modulations each
On average, baseline tissue oxygen around the microsensors varied between 3 and 23 LOI over time (Table 1) with a declining trend over the first three measurement timepoints. It is hypothesized that initial oxygen concentrations are elevated above normal due to the stimulation of insertion. There may be greater utility in characterizing the relative tissue oxygen trends, rather than baseline measurements, for providing a metric on PAD status. One systemic and two local challenge baseline LOIs were calculated to be 0 LOI on one measurement timepoint (275 days), but this is likely due to poor temperature measurements used in the calibration as the raw sensor signal responded to the systemic and local oxygen challenges (data not shown). The inclusion of these data on timepoint 275 days also resulted in a lower precision (increased SEM) for that timepoint (Table 1).
Acute Measurements
Local pressure applied in the left limbs induced modulations ranging from 40% to 98% LOI over 9 months (Table 1). As expected, tissue oxygen as measured by the microsensors in the right limbs and dorsum was not affected by local occlusion applied to the left limbs, and no changes in SpO2 were detected with the ear clip. Reduced modulation was observed after 97 days (~90% decreasing to ~40% modulation). However, systemic modulations at the same timepoints show detection capability for quite large modulations (79–96%); therefore, the decreased local modulations are believed to be due to insufficient cuff occlusion due to pig growth, not reduced sensor function.
Restenosis rates in patients after revascularization procedures are >30% at 9 months and the end result is frequently debilitating amputations [3–6]. Long-term monitoring of local tissue oxygen may enable early detection of restenosis. While further study in a PAD population is needed, the current study demonstrates that the Lumee Oxygen microsensors continue to detect oxygen fluctuations for over 9 months. Previously, microsensor function has been measured up to 2.5 years post-injection in individual human cases, indicating potential function well past the 9 months presented here [11]. Human studies are underway to evaluate the application of Lumee Oxygen microsensors in PAD.
4. Conclusions
Injectable Lumee Oxygen microsensors demonstrated function for over 9 months to repeated systemic and local oxygen challenges in a pig model. Further study is needed to refine application to PAD. Long-term oxygen monitoring may be beneficial in detecting restenosis in PAD patients and improving patient outcomes.
Acknowledgments
This work was funded in part by R01EB016414, R42HL127933, R44HL134532, and R44HL131366 from the National Institutes of Health, W911NF-16–1-0341, W911NF-11–1-0119 and W31P4Q-12-C-0205 from the Defense Advanced Research Projects Agency and the U.S. Army Research Office. The authors would like to thank Lauren A. Russell and Sierra J. Guidry for pre-clinical support.
Contributor Information
Scott P. Nichols, Profusa, Inc., College Station, TX, USA
Mary K. Balaconis, Profusa, Inc., College Station, TX, USA
Rebecca M. Gant, Profusa, Inc., College Station, TX, USA
Kit Y. Au-Yeung, Profusa, Inc., South San Francisco, CA, USA
Natalie A. Wisniewski, Profusa, Inc., South San Francisco, CA, USA
References
- 1.Hirsch A, Criqui M, Treat-Jacobson D et al. (2001) Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 286:1317–1324 [DOI] [PubMed] [Google Scholar]
- 2.Nowygrod R, Egorova N, Greco G et al. (2006) Trends, complications, and mortality in peripheral vascular surgery. J Vasc Surg 43:417–418 [DOI] [PubMed] [Google Scholar]
- 3.Stanley J, Veith F, Wakefield T (2014) Current therapy in vascular and endovascular surgery Elsevier Health Sciences, Philadelphia [Google Scholar]
- 4.Wolfram R, Budinsky A, Pokrajac B et al. (2006) Endovascular brachytherapy for prophylaxis of restenosis after femoropopliteal angioplasty: five-year follow-up: prospective randomized study. Radiology 240:878–884 [DOI] [PubMed] [Google Scholar]
- 5.Laird J, Jaff M, Biamino G et al. (2005) Cryoplasty for the treatment of femoropopliteal arterial disease: results of a prospective, multicenter registry. J Vasc Inerv Radiol 16:1067–1073 [DOI] [PubMed] [Google Scholar]
- 6.Kasapis C, Henke PK, Chetcuti SJ et al. (2009) Routine stent implantation vs. percutaneous transluminal angioplasty in femoropopliteal artery disease: a meta-analysis of randomized controlled trials. Eur Heart J 30:44–55. 10.1093/eurheartj/ehn514 [DOI] [PubMed] [Google Scholar]
- 7.Harrison DK (2002) Optical measurement of tissue oxygen saturation. Int J Lower Extremity Wounds 1:191–201 [DOI] [PubMed] [Google Scholar]
- 8.Thomsen A (2012) The tcpO2 handbook. Radiometer Medical ApS, Brønshøj, Denmark [Google Scholar]
- 9.Anderson C (2010) Noninvasive assessment of lower extremity hemodynamics in individuals with diabetes mellitus. J Vasc Surg 52:76S–80S [DOI] [PubMed] [Google Scholar]
- 10.Montero-Baker MF, Au-Yeung KY, Wisniewski NA et al. (2015) The first-in-man “Si Se Puede” study for the use of micro-oxygen sensors (MOXYs) to determine dynamic relative oxygen indices in the feet of patients with limb-threatening ischemia during endovascular therapy. J Vasc Surg 61:1501–1509. 10.1016/j.jvs.2014.12.060 [DOI] [PubMed] [Google Scholar]
- 11.Wisniewski N, Nichols S, Gamsey S et al. (2017) Tissue-integrating oxygen sensors: continuous tracking of tissue hypoxia. Adv Exp Med Biol 977:377–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chien JS, Mohammed M, Eldik H et al. (2017) Injectable phosphorescence-based oxygen biosensors identify post ischemic reactive hyperoxia. Sci Rep 7:8255 10.1038/s41598-017-08490-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo LW, Koch CJ, Wilson DF (1996) Calibration of oxygen-dependent quenching of the phosphorescence of Pd-meso-tetra (4-carboxyphenyl) porphine: a phosphor with general application for measuring oxygen concentration in biological systems. Anal Biochem 236:153–160. 10.1006/abio.1996.0144 [DOI] [PubMed] [Google Scholar]