Abstract
Background:
New highly effective medications are available to treat the hepatitis C virus (HCV). However, little is known about HCV treatment knowledge and readiness among young people who inject drugs (PWID), or factors that may contribute to treatment uptake and adherence in this treatment era.
Purpose:
Using a framework for understanding healthcare utilization, we examined perspectives and experiences of young PWID tied to the HCV care continuum in Boston, Massachusetts, to inform future strategies.
Methods:
We conducted 24 in-depth interviews with active and recent PWID aged 22–30 years living with HCV in Boston February-August 2016. At the time of the interviews, no participants had been prescribed or had taken the new direct acting antivirals. We developed a codebook deductively from the interview guide and coded and analyzed the data into themes using a consensus-based process.
Results:
The following five themes emerged, which captured PWID’s knowledge of and experiences with HCV along the care continuum through social determinants of engagement in care, as well as illness level: (1) deservingness of HCV treatment and stigma, (2) dissatisfaction with provider interactions, (3) perceived lack of referral to treatment and care continuity, (4) disincentives around HCV treatment for PWID; and (5) perceived need for treatment. Young PWID living with HCV face unique barriers to HCV testing, counseling, and treatment.
Conclusion:
Breakdowns in the HCV care continuum may have adverse effects on HCV-treatment readiness and willingness. Improved public health and practice approaches are needed to address these barriers to effectively engage young PWID in care.
Keywords: Hepatitis C, young people who inject drugs, HCV treatment readiness, qualitative research
1. Introduction
HCV infections in the United States have increased significantly among young people during the past decade (Suryaprasad et al., 2014). Between 2002 and 2013, HCV infections in Massachusetts (MA) increased by 137% among those aged 15–29, with more than 2,000 newly reported infections each year (MDPH, 2014). This increase has largely been attributable to injection drug use (MDPH, 2014). In recent years, new treatments have been developed for the hepatitis C virus (HCV) that could potentially be life saving for people who inject drugs (PWID); however, uptake has been less than optimal.
Beginning in 2013, new oral direct acting antivirals (DAAs) were introduced for treatment of HCV. Unlike their interferon predecessors, these new medications have demonstrated high rates of treatment success in clinical trials (>95%) with short treatment durations (≤12 weeks) and minimal side effects (Jacobson et al., 2013). While access to DAAs is still limited in many areas, some restrictions to health insurance coverage for HCV treatment (e.g., sobriety, fibrosis criteria) were recently removed in Massachusetts, paving the way for better access to HCV treatment (NVHR and The Center for Health Law and Policy Innovation, 2016). Further, the state has worked with pharmaceutical companies to significantly lower the cost of DAAs for consumers (Bartlett, 2016). Despite these policy changes, few young PWID who are living with HCV receive DAA treatment. Some barriers impeding widespread treatment of HCV among young PWID include: the high price of the DAAs, psychosocial factors that can contribute to missed doses of medication, emergence of viral resistance, and risk for re-infection (Harris and Rhodes, 2013).
Treatment as prevention is a major component of strategies to combat HIV (identification, linkage, and treatment), but has yet to be widely implemented or evaluated for HCV (Kay et al., 2016). Increasing HCV treatment among young PWID can decrease virus circulation in the community, translating to a decreased infection rate (Cousien et al., 2016). Previous qualitative studies have examined how social factors have influenced HCV prevention and treatment among people who inject drugs, and found that risk perception, uncertain knowledge, and stigma are important determinants (Davis and Rhodes, 2004a; Davis and Rhodes, 2004b, Davis et al., 2004; Clark and Gifford, 2015). These studies have been critical for understanding prevention and treatment in the lives of PWID during the interferon era, prior to the advent of DAAs. Many of the determinants remain highly relevant; however, little is known about HCV treatment knowledge and treatment readiness among young PWID in the current era of DAAs, or factors that may contribute to treatment uptake and adherence, as few studies have examined the willingness to pursue new DAA treatments for HCV among young PWID, and how their experiences with the health care system contribute to – or impede – their preferences for care. The shorter treatment time, higher success rates, and reduced side effects associated with DAA treatment may impact many of the previously documented attitudes and behaviors among young PWID, as prior research on this issue may have a strong tie to side effects of interferon (Batchelder et al., 2015), thus limiting applications to the new treatments.
Qualitative research can help to elucidate issues that are relevant for practice decisions around the HCV care continuum, particularly with respect to testing, treatment availability and access, and aspects of willingness and readiness to initiate treatment. Understanding the barriers and facilitators to care among PWID in Boston, an urban area with gold-standard health care, insurance coverage, and access to HCV treatment experts, can shed light on important barriers impeding access or hindering uptake among this population in the current treatment environment. Using qualitative in-depth interviews, we aimed to provide a greater understanding of the perspectives and experiences of young PWID navigating the HCV care continuum, and in turn, to inform future treatment as prevention strategies.
2. Material and methods
2.1. Conceptual framework and interview guide development
Our study was informed by the Andersen Behavioral Model (1968), along with a more recent framing by Andersen and Newman (2005), which provides a framework for understanding health care utilization among PWID. This framework holds that social determinants influence individual-level determinants, which guide decisions around individuals’ healthcare needs and ultimately their ability to engage in related services (Anderson and Newman, 2005). The framework is rooted in three domains: predisposing determinants, enabling determinants, and illness level. Predisposing determinants are factors such as sociodemographic characteristics (e.g., age, gender), social status and related stigma or health beliefs that increase the likelihood that someone would engage in care (e.g., believing that the new HCV treatment regimens will cure HCV); enabling determinants are factors that facilitate or hinder access to services (e.g., insurance status, sobriety requirements, access to a primary care physician); and illness level involves the individual perception of how ill one is and how engaging with healthcare services will affect this perceived level of illness (Anderson and Newman, 2005). This framework is reflected in our semi-structured interview guide, which captures predisposing determinants (drug use history, family history of drug use, knowledge and beliefs about HCV transmission, stigma, homelessness), enabling determinants (experiences with testing, linkage to care, and medical providers) and perceived illness level (HCV treatment awareness and readiness) as a way to better ascertain the barriers and facilitators to seeking care for HCV among young PWID.
2.2. Sample recruitment
We recruited participants through a time-location sampling approach, which was done by outreach workers with connections to communities of PWID and in partnership with community-based agencies serving PWID in Boston, MA (e.g., syringe exchange programs, shelters, and social service agencies). Staff members at community-based agencies assisted with recruitment by posting study flyers in community spaces and by identifying qualifying PWID whom they knew were likely to have acquired HCV and would be interested in participating in the study. Research assistants, trained in participant recruitment and qualitative research methods, also conducted targeted street outreach in locations in downtown Boston with high densities of street drug activities. This outreach involved approaching people outside of syringe exchange sites and areas with high concentrations of drug use with a recruitment postcard to provide information about the study and gauge interest in participation. We also reached out via telephone and text messaging to eligible participants who opted to be notified of study opportunities and who had provided contact information during previous studies. Further, we utilized a chain-referral approach, where enrolled participants were asked to refer peers within their networks to the study. Finally, we received referrals from an infectious disease clinician at Tufts Medical Center (TMC) for eligible inpatients (those living with HCV with a history of injection drug use). If recruits were interested, study staff described the study, provided written materials, and set up an appointment for an in-depth interview. Prior to the interview, participants provided written informed consent for both the in-depth interview and for the HCV antibody testing, which was conducted via a commercial rapid testing kit to confirm the presence of HCV antibodies.
Quota sampling was achieved by stratifying based on gender, and experience with drug use (Table 1). Participants were eligible for participation in our study if they: (1) were currently injecting drugs or had injected drugs in the past but were not actively injecting (e.g., they recently entered drug treatment, were on medication-assisted opioid therapy, or stopped using through other modalities); (2) self-disclosed HCV positivity, and (3) were between the ages of 15 and 30. We aimed for a balanced sample with respect to gender, as well as current versus past injection drug use.
Table 1.
Participant Characteristics among Young People Who Inject Drugs (PWID) Living with the Hepatitis C Virus in Boston, Massachusetts, 2016 (n=24)
| Characteristic | Number | Percentage |
|---|---|---|
| Age range (years) | 22–30 | |
| Mean age (years) | 27.2 | |
| Gender | ||
| Female | 12 | 50.0 |
| Male | 12 | 50.0 |
| Race/Ethnicity | ||
| Non-Hispanic white | 21 | 87.5 |
| African American/black | 2 | 8.3 |
| Hispanic/Latino | 1 | 4.2 |
| Mixed race | 1 | 4.2 |
| Use Status (at time of interview) | ||
| Currently injecting | 12 | 50.0 |
| Not currently injecting | 12 | 50.0 |
2.3. Data collection
Each interviewer was trained in qualitative interviewing techniques and followed an interview protocol using a screening script, informed consent form, and semi-structured interview guide. The in-depth, face-to-face interviews were conducted in private rooms at either the Tufts University School of Medicine or in private spaces provided by community-based partners. Interviews were conducted in English, lasted approximately 60 minutes, and were recorded for transcription. Participants were compensated with a $25 gift card or cash at the end of the interview. We transcribed the interviews verbatim using a transcription pedal and WavPedal7 software (WP7SO, Purcellville, VA). Each transcript was crosschecked for clarity and accuracy. All study procedures were approved by the Tufts University Health Sciences Institutional Review Board.
2.4. Data analysis
Our research team used standard qualitative techniques to analyze the data (Miles and Huberman, 1994; Saldaña, 2013). We compiled a preliminary codebook based on the question structure of the interview guide. Using this codebook, two researchers independently coded three transcripts to ensure their completeness and accuracy. The codebook was then shared with the rest of the research team. Through a consensus process with all of the authors, the codebook was edited to include additional codes and refine existing code definitions. We carefully reviewed all areas of disagreement and revised coding definitions and applications until all team members concurred. To determine inter-rater reliability amongst the study authors, the final coding framework was applied to two randomly selected test transcripts using Dedoose qualitative analytic software (V. 7.5.9, Los Angeles, CA), which resulted in an average Cohen’s Kappa score of 0.74. Using the Andersen and Newman framework (2005) as a lens, we interpreted emergent themes with respect to predisposing determinants, enabling determinants, and illness level.
3. Results
Between February and August 2016, we conducted 24 in-depth interviews with participants aged 22–30 who self-identified as having HCV and tested positive for HCV antibodies via the Oraquick ® HCV Rapid Antibody test. The average age of participants was 27 years. At the time of the interviews, no participants had been prescribed or had taken DAAs to treat their HCV. The majority of participants came from street recruitment, chain referral, and local syringe exchange programs (Table 1).
While the interview was structured to address the three areas of predisposing determinants, enabling determinants, and illness level as suggested by Andersen and Newman’s (2005) framework, variability in participants’ sociodemographic characteristics and social structure (e.g., SES) was limited. As a result, the themes that came out from the interviews did not align with these categories as well, specifically as they relate to predisposing and enabling determinants. Therefore, we chose to group ‘predisposing’ and ‘enabling’ determinants into one category of ‘social determinants of engagement in care’ to present the themes. The following themes illustrate the lens through which participants perceived and approached the HCV care continuum, from testing through treatment.
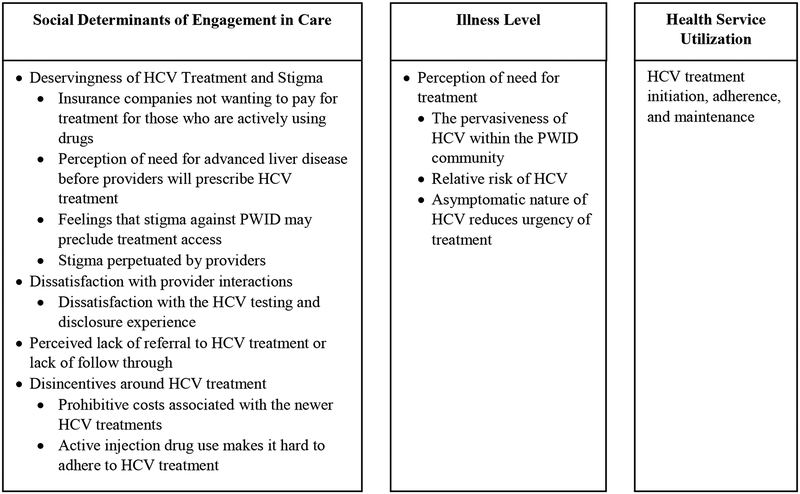
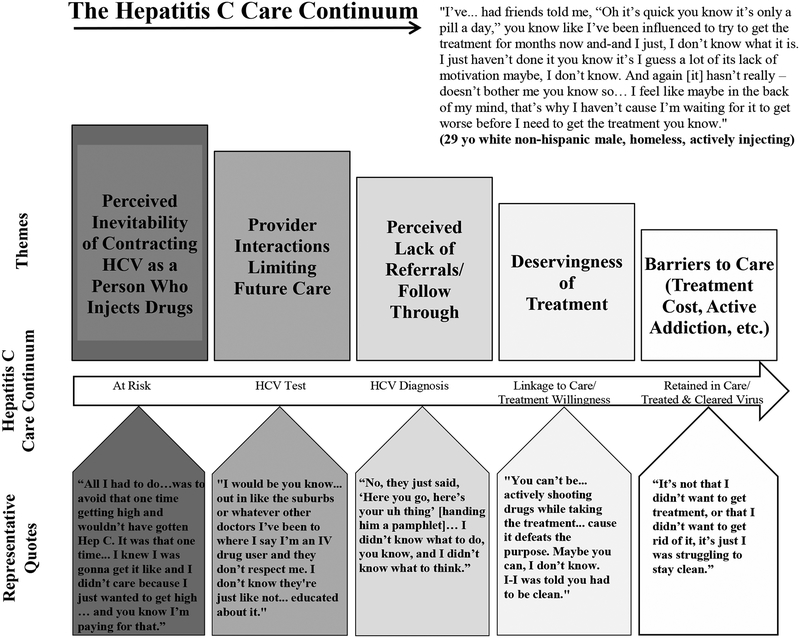
Four major barriers relating to the continuum of care were identified within the context of Social Determinants of Engagement in Care: (1) deservingness of HCV treatment and stigma, (2) dissatisfaction with provider interactions, (3) perceived lack of referral to treatment and care continuity, and (4) disincentives around HCV treatment for PWID; one major barrier was identified for Illness Level: perceived need for treatment. Figure 1 displays a model for understanding determinants of HCV treatment utilization among PWID in Boston, MA and Figure 2 displays the HCV care continuum with corresponding qualitative themes and representative quotes.
Figure 1.
Model for understanding social determinants of hepatitis C virus (HCV) treatment utilization among people who inject drugs (PWID) in Boston, Massachusetts. Adapted from (Anderson and Newman, 2005). Social Determinants of Engagement in Care are factors that influence the likelihood that someone would engage in care or that facilitate or hinder access to services. Illness level refers to the perception of how ill one is and how engaging with healthcare services will affect the perceived level of illness. Themes that emerged from the qualitative data are grouped accordingly.
Figure 2.
The Hepatitis C Virus (HCV) Care Continuum: Qualitative Themes and Representative Quotes among Young People Who Use Drugs Who Were Living with HCV in Boston, Massachusetts, 2016 (n=24).
3.1. Social determinants of engagement in care
Overall levels of HCV knowledge varied among participants. Participants reported that awareness of HCV is growing within the PWID community, although the information shared within this community about the short and long-term health effects of HCV is limited. They were largely uninformed about HCV prior to diagnosis and reported learning more about the virus after they were diagnosed.
Most participants knew about the new DAAs used to treat HCV. Those who were not actively using drugs knew the brand name of Harvoni, whereas those who were actively injecting referred to a “new pill,” but were unable to name specific medications. Participants learned about HCV and HCV treatment options through various avenues (friends, doctors, syringe exchange programs, and on the street) and also through television commercials for treatment. Respondents reported hearing that the new treatments were very expensive, but effective, and had few side effects in comparison to other past treatment options, with which they were familiar.
3.1.1. Deservingness of HCV treatment and stigma.
Participants talked about both whether they thought they “deserved” treatment, as well as how they felt they were being stigmatized and judged by insurance companies and clinicians, therefore reducing their interest or ability to engage in the care continuum. External factors, such as limited insurance coverage for those who were actively injecting drugs and those with less advanced liver disease, reaffirmed the beliefs among participants that they did not deserve HCV treatment.
3.1.1.1. Insurance companies do not want to pay for treatment for those who are actively using drugs.
At the time of the interviews, there were various “sobriety” restrictions among insurance companies, dictating the length of time patients needed to be off drugs before being able to qualify for HCV treatment. In this context, participants recognized that the risk of reinfection with HCV was real, and that insurance companies, if willing to support treatment, would only do so once. For some participants, this translated into the need to be “sober” prior to starting HCV treatment, seeing value in waiting until one is not actively using drugs prior to obtaining HCV treatment, while for others it meant that they should be safer in their injection behaviors, reducing or removing the chance for reinfection.
“…this is like a one-shot deal like health insurance is only gonna cover it one time… I just wanna make sure I don’t catch it back cause I don’t wanna give up my one—financial opportunity.”
[26-year-old, white, non-Hispanic female; homeless; actively injecting cocaine].
“Yeah because what is the point of getting it and then you share a needle again with someone, you’re gonna give it right back to yourself or a different strand back to yourself…So it just makes more sense if…the person’s sober.”
[30-year-old, white, non-Hispanic female; not currently actively injecting].
“I think that a lot of insurance companies have it right, it’s gotta be somebody that has clean time, that’s not gonna re-infect themselves because then it’s just a waste of resources and people’s time to do the treatment if they’re just gonna re-infect themselves right after.”
[29-year-old, white, non-Hispanic male; homeless; not currently actively injecting].
However, some believed that six months was too long of a sobriety requirement and created too high of a bar for those who were actively injecting. Given the realities associated with active injection drug use, participants reflected that being given one treatment chance with a sobriety requirement reaffirms a lack of deservingness of HCV treatment for PWID.
“You know 30 days is enough man, 30 days is a fucking year for an addict… So 30 days is a big step in the right direction… [the current 6-month sobriety period is] almost impossible and-and-It is too long because that’s – it’s simple dude like a lot of people use because their life sucks… so if you’re telling me that I’m not worth helping because I gotta stay clean for 6 months or whatever you know for those 6 months all I’m thinking in my head is – it’s more stress you’re putting on people trying to stay clean. That’s one less stress you should take away from them,,,? How could you play God…You know you could play God in a good way and say, ‘Hey, I’ll reward you with this,’ and you know just show that you’re changing. Show that you’re actively in recovery.”
[30-year-old, African-American male; homeless; actively injecting heroin].
3.1.1.2. Need for advanced liver disease before receiving HCV treatment.
Participants believed that physicians were waiting for patients to have more advanced liver disease before prescribing HCV treatment, which was dictated by the insurance companies. Participants were frustrated by this because they understood the benefits of treating HCV earlier, before it created more severe problems, including cirrhosis.
“I think [people should receive HCV treatment] at any time – I think not just when they’re sick or when their viral load is high, I think when they’re healthy too—especially ‘cause you don’t wanna wait ‘til the damage is already done.”
[26-year-old, white non-Hispanic female; homeless; actively injecting cocaine].
“I think the people who are bad, should get treated, but kinda feel like right now, my life is healthy and everything is good and I wanna get it fixed. I still think they should be able to treat it. They shouldn’t wait until it gets worse to treat it… but that’s what they wanna do – wait until it gets worse.”
[25-year-old, white, non-Hispanic male, not currently actively injecting].
Further, study participants accurately recognized that, at the time, most insurance plans would not initially pay for their HCV treatment if they did not have higher levels of liver disease severity:
“Well I’m trying to get treatment here. It’s difficult because now that I’m doing well, like my numbers and my – I have no liver damage, I have genotype 3, which is a rarer type so getting the insurance to pay for it is a challenge, but they said they’re trying to help work with that and you know something comes along – some kind of study or some kind of you know… new thing…”
[30-year-old, white, non-Hispanic male; not currently actively injecting].
The following participant went on further to emphasize her dismay with the delay to get treatment based on disease severity and provided some additional context with an analogy about cancer, a much less stigmatized disease than HCV among PWID:
“…because we shouldn’t have to wait ‘til it gets bad- oh, now you can get it, you have cirrhosis of the liver…Like that’s not fair…It’s like saying…for example like… we could cure your cancer right now but we’re gonna wait ‘till you have three months left to live…Or…you know, we have a pill to help with your depression but we’re gonna wait ‘til you try to kill yourself first…Doesn’t make sense to me.”
[30-year-old, white, non-Hispanic female; not currently actively injecting].
3.1.1.3. Feelings that stigma against PWID associated with personal characteristics may preclude treatment access.
Several participants felt that the government should cover the costs of treatment; however, there was a sentiment that stigma and judgment towards PWID might preclude such assistance. This may in part be related to the perception that PWID have limited resources and need assistance in paying for clinical care and/or medication.
“I think the State should [pay]… Honestly because people are dying off…But I don’t know. The State’s a little messed up. They could say good riddance, you know what I mean?… These people are eating up the money that we have and they don’t want to get treatment—so why treat them anyways?”
[24-year-old, white, non-Hispanic female; not currently actively injecting].
A number of participants also believed that, while insurance should cover the costs of HCV treatment, the patient should pay what they could, particularly if it was the patient’s “fault” that he or she contracted HCV.
“If it was brought onto themselves, maybe [they should pay]…, and they have the money… to afford it, but like me personally, I brought it onto myself but… I couldn’t afford it… Unless it’s like a co-payment or something.”
[30-year-old, white non-Hispanic female; not currently actively injecting].
“I think health insurance should definitely help pay for it… I mean, maybe health insurance shouldn’t have to pay for the whole thing. You could pay some out of pocket too. You know if you really want the treatment. I mean, what’s a couple thousand? You know. It would be worth it, if you’re gonna get rid of it.”
[26-year-old, white, non-Hispanic female; actively injecting heroin].
“…I think it really depends on your- your, your income and stuff…I think if you’re making, you know, six figures…A year you can afford to pay for more…Than someone who’s making fifteen thousand a year.”
[22-year-old, white, non-Hispanic female; homeless; in recovery].
“I think I should (pay for treatment)… Because it’s my fault… that I got Hepatitis C.”
[30-year-old, white, non-Hispanic male; homeless; not currently actively injecting].
3.1.1.4. Stigma perpetuated by providers.
Participants perceived that providers wanted to treat people for HCV; however, pervasive stigma against treating PWID limited respect and appropriate care.
“They are very uneducated on addiction. They have a big stigma when it comes to addicts. If they find out you’re an addict, their whole demeanor changes. They rush you, they slam things, they are very impatient with you and it’s very saddening to see.”
[24-year-old, white non-Hispanic female; not currently actively injecting].
3.1.2. Dissatisfaction with provider interactions.
While many participants were tested for HCV at various locations (doctors’ offices, jail or prison, in drug treatment or detox programs), several were tested for HCV through a combined state-mandated HIV-HCV testing program, so they were not specifically seeking out HCV testing, though it was provided to them during other routine testing – sometimes without their knowledge.
3.1.2.1. Dissatisfaction with HCV testing and disclosure experience.
Many participants expressed feeling uncared for or dismissed during interactions with healthcare providers, and viewed this mistreatment as owing to their status as a PWID. They expressed that the process of testing and counseling requires more sensitivity, care, time, and attention on the part of those conducting the test and providing the results.
“Like if they were just like, ‘here like you know like yeah you’re positive, this is what you can go do about it, you know like, here’s the numbers you need.’ Like… give people more hope more than just like, ‘Oh yeah, you have Hep C. See you later’ you know, go figure it out.”
[30-year-old, white, non-Hispanic male; homeless; actively injecting heroin].
(On what the participant would change to make the process better for young people who are injecting)
“The sensitivity of the people delivering the message, diagnosis, options in treatment, and counseling.”
[29-year-old, white, non-Hispanic male; actively injecting heroin].
Some respondents discussed wanting the HCV disclosure process to be more professional, whereas others said that they wanted it to be more comfortable, and there was a spectrum of satisfaction with care depending on where participants were tested and were disclosed their HCV status.
“[Doctors] seem like kind of above you. Like people at [the syringe exchange program] are like on your level. They don’t think like any like lower or like higher, like you know what I mean, we’re all in the same – we’re all the same pretty much.”
[29-year-old, mixed race, female; homeless, actively injecting heroin].
Some participants reported finding out about their status through non-clinic-based settings, and wished they had heard about their status from a clinician.
“I kinda wish that I had got tested in a different environment than prison but… that’s where it happened but they didn’t really offer any… help or resources or you know…. information or anything. I didn’t seek help ‘til I got out and went to a specialist later on.”
[26-year-old, white non-Hispanic female; homeless; actively injecting cocaine].
“I shoulda went right to my primary care, yeah I would’ve. You know I shoulda went to my primary care but it shoulda, woulda, coulda you know… [The counselor] should’ve sat me down and really spoke about this and taught me like really look… this is what you should do… You know what I mean? But they didn’t, yes. I mean they should’ve, you know?”
[30-year-old, white non-Hispanic male, homeless, actively injecting heroin].
Ultimately, participants expressed dissatisfaction with the HCV testing and disclosure experience and desired more respect and less judgment under the circumstances.
3.1.3. Lack of referral to HCV treatment and care continuity.
Once their HCV status was disclosed to them, participants who received testing and counseling from physicians’ offices generally reported they were given a referral to a specialist, but there was not much follow-up – either by the physician’s office or the participant. Additionally, those tested for HCV in other contexts, such as prison or through detoxification, often were not provided with referrals.
(On receiving HCV test results in jail):
“They gave me flyers and um pamphlets… So no, they don’t provide me with shit. And when I was leaving, they basically told me go see a doctor – and that’s it man, that’s the end of that.”
[30-year-old, African American male; homeless; actively injecting heroin].
(On receiving HCV test results in a detoxification center):
“So they just sort of said you have Hep C… follow up with your primary care doctor and all that.”
[27-year-old, white, non-Hispanic male; actively injecting Heroin].
3.1.4. Disincentives around HCV Treatment.
Additional disincentives around HCV treatment were raised in the interviews, including barriers related to costs of the new treatment and challenges in adhering to regimens while addicted to drugs.
3.1.4.1. Prohibitive costs associated with the new HCV treatments.
As previously stated, the majority of the young PWID participants believed that insurance should cover the cost of treatment. However, they realized that working with insurance plans could be challenging because of the cost.
“I’ve had my share of sicknesses and illnesses but, you know this is the first one that like it’s a challenge to get the… treatment …because of the cost.”
[30-year-old, white, non-Hispanic male; not currently actively injecting].
“All I’ve heard is that there’s really expensive pill that costs like $1,000 and that it’s like uh you take it twice… once or twice a month and that you have to be approved by it and you have to have the right genotype for it.”
[30-year-old, white, non-Hispanic male; not currently actively injecting].
3.1.4.2. Active injection drug use makes it hard to adhere to HCV treatment.
There was a general sentiment that actively injecting drugs would make it hard for people to adhere to an HCV treatment regimen.
“‘Cause a lot of people who are using… like they’re out on the streets. They’re not really thinking about taking their meds… or even if they’re not on the streets like which you’re trying to get high all day like it’s gonna be hard unless you’re in recovery like and you actually care about your own well-being.”
[29-year-old, mixed-race female; homeless; actively injecting heroin].
“‘Cause they’d still be using and then they’d just nod off and forget about it.”
[27-year-old, white, non-Hispanic female; actively injecting heroin].
The feeling about the need for PWID to be sober when they receive treatment was different for people if they were talking about themselves compared to others. While many participants felt that they themselves could adhere to an HCV medication regimen, even while using drugs, the general consensus was that it would be difficult for others who are actively using to adhere to treatment.
“I would say that – yeah, for others it probably would [be hard], you know. And half these people if you gave it to them, they’d probably lose it. I’ll tell you the truth you know… Get robbed, or you know or something stupid… They get their pills stolen every day or their money or this or that you know and… – the street life, you know.”
[30-year-old, white, non-Hispanic male; homeless; actively injecting heroin].
Ultimately, participants recognized the importance of HCV treatment, but understood real barriers as they relate to lack of HCV symptoms, coverage by insurance companies, and active injection drug use.
3.2. Illness level
3.2.1. Perceived need for treatment.
In the context of the HCV care continuum, perceptions of the pervasiveness and severity of HCV as a chronic disease may influence the desire and readiness for treatment among young PWID.
3.2.1.1. The pervasiveness of HCV within the PWID community.
Participants believed that most people who inject drugs would ultimately contract HCV, but were unaware of the virus before they started injecting. Most participants knew or guessed that they would test positive or knew they were at risk because they had shared syringes and because HCV is prevalent within the PWID community. Respondents reported being nervous about getting tested for HIV and HCV, because even though they thought they might test positive, getting the news would make their suspicion a reality that they would then have to deal with.
“… pretty much everybody that shoots heroin gets Hep C. [I] just told myself like I’m gonna end up getting it anyways because I’m a heroin addict and I had no care about my life whatsoever. So, like I kind of already knew there’s like a 90% chance I have it. So, it wasn’t like a {gasp} ‘Oh my God moment’. It was like I kind of knew.”
[24 year-old, white, non-Hispanic female; not currently actively injecting]
“I’m sure there’s some people who aren’t honest but everybody that I can think of that I know that injects, they have Hep C… that’s how scary – it’s so common.”
[26-year-old, white, non-Hispanic female; homeless; actively injecting cocaine].
“But it’s kinda like, everybody kind of knows about it. Everybody’s aware about it and like how like it seems like there’s more awareness about it now than there was maybe when I first started using ‘cause I didn’t really hear anything about Hep C until I went into programs and stuff like that… But now it’s more on the streets and people know about it.”
[29-year-old, mixed race female; homeless; actively injecting heroin].
3.2.1.2. Asymptomatic nature of HCV reduces urgency of treatment.
There was a sentiment among participants that because people can live with HCV for a long time asymptomatically, there is no rush to get treatment.
3.2.1.3. Relative risk of HCV.
Some participants expressed either not thinking of HCV as a major health issue or hearing that sentiment among friends or other PWID. The following participant reflected on a comment from her partner, with whom she used to inject:
“He would always say, ‘Well, Hep C’s no big deal, Hep C’s like the common cold for the junkie,’ … it might take five years away from your, you know, your life but, you know, we’re not even gonna live that long anyways so who cares about it anyway.”
[28-year-old, Hispanic/Latina female; not currently injecting].
4. Discussion
As the HCV epidemic surges among young PWID in the United States, our findings from Boston provide a first glimpse into young PWID perspectives and experiences surrounding the HCV care continuum in the new era of DAAs. Young PWID represent a growing fraction of the opioid epidemic in the United States and are likely to live with HCV for long periods without significant symptoms, increasing their likelihood of spreading the virus. Building upon findings from prior qualitative research with this population in the interferon treatment era (Davis and Rhodes, 2004a; Davis and Rhodes, 2004b, Davis et al., 2004; Clark and Gifford, 2015), our study revealed that major barriers to care related to social determinants of engagement in care, as well as illness level include a lack of deservingness of treatment, dissatisfaction with provider interactions, perceived lack of referral to treatment and care continuity, stigma among healthcare providers, and policies that disincentivize HCV treatment. These persistently limit care, even when insurance and access to addiction medicine experts with training in HCV treatment are prevalent, as is the case in Boston. We found that negative interactions with healthcare providers during the HCV testing process and perceived inadequate access to HCV treatment served to reinforce the perception that young PWID are undeserving of treatment, resulting in a reduced willingness to ultimately pursue newly available treatment options.
At the core of many of the interviews were misconceptions about the availability of DAAs for PWID. We found that participants knew about DAAs, but that this determinant was not enough to facilitate their engagement with treatment. High prevalence of HCV within the PWID community and inaccurate knowledge about the consequences of HCV contributed to the belief that infection was inevitable and that seeking treatment was not urgent. Experiences with healthcare professionals who responded casually to positive tests only reinforced this perception, as when people believe that a chronic disease is not severe, they are less likely to want to engage in treatment, particularly if barriers are high (Scharloo and Kaptein, 2013). In this case, the perceived illness level was low, resulting in a lack of engagement with services. Furthermore, participants reflected misconceptions regarding the consequences of HCV, availability of treatment, and referral to care. This is highly relevant as research has highlighted the importance and prevalence of poor HCV knowledge, its contribution to missed treatment opportunities, continued transmission, and poorer health outcomes (Treloar et al., 2012).
Despite increasing need for HCV treatment and newly expanded access to DAAs for many young PWID with HCV, linkage to treatment has been infrequent, and barriers to treatment persist. Our study revealed barriers throughout the care continuum that discourage utilization within this population. Echoing prior research (Ditah et al., 2015), high costs were noted as obstacles to pursuing and adhering to treatment, which reinforced deeply entrenched barriers in this community, namely that patients were undeserving of care and were unlikely to receive it. Interactions with providers, including perceived doubt associated with PWID’s ability and willingness to adhere to treatment also reinforced these beliefs.
As of August 2016, Massachusetts lifted the restrictions on sobriety and disease severity requirements and the decision to treat HCV among PWID has been left to the discretion of providers (NVHR, 2016). This was a critical move because these restrictions disproportionately affect young people living with HCV and PWID, who are more likely to transmit HCV to others. Although health and insurance policies tied to HCV treatment have become less restrictive in Massachusetts, which has helped to expand treatment availability to populations previously excluded from care, participants still experienced significant stigma in healthcare encounters reinforcing their beliefs that PWID were not deserving of care and that care was not available for them. Even if participants did not support long sobriety periods before HCV treatment, they generally understood medical providers’ apprehension related to reinfection or “wasting” treatment on someone who is not “ready,” which could affect the likelihood of insurance companies providing any treatments for this community in the future. Participants expressed that a perceived high risk of HCV reinfection among PWID led physicians to steer people who are actively injecting drugs away from treatment; however, there is little evidence to support that clinically significant resistant mutations arise due to incomplete adherence (Svarovskaia et al., 2014). Additionally, research has shown that active PWID living with HIV can adhere to HIV medications, leading to successful viral suppression (Sherer, 1998). Even when HCV treatment regimens were more complex, studies have demonstrated successful adherence in PWID using a multi-pronged approach (Jeffrey et al., 2007; Reimer et al., 2013).
From a clinical perspective, PWID who are currently injecting drugs or who have injected drugs in the past are often treated poorly in clinical environments (Neale et al., 2008), which was confirmed by participants in our study. This was true even in an area such as Boston, which has a strong infrastructure to support this population. Our results highlight the need for patient-oriented care in a safe setting where young PWID feel respected and destigmatized. Participants reflected that providers should become more educated about addiction and approach HCV treatment with this population in a manner that allows them to feel like people with a chronic condition, rather than merely “addicts.” Ultimately, positive clinical interactions may have a subsequent positive impact on their readiness and willingness to seek HCV treatment. Furthermore, recent policy changes loosening restrictions on sobriety and disease severity requirements and on treating PWID offer promising and critical advancements; however, these policies may not be sufficient to increase utilization of HCV medication, especially among young PWID, as determinants of care (negative provider interactions, stigma, perceived lack of deservingness of treatment, perceived lack of referral to treatment, perceived and actual insurance challenges, disincentives to pursue treatment), and illness level (perceived need for treatment) were clear barriers to seeking treatment, above and beyond cost and availability.
4.1. Limitations
The current study has some important limitations. First, while we aimed to recruit participants between the ages of 15 and 30, we were unable to recruit those under 22, which may have implications around barriers and facilitators to treatment that may be unique to younger PWID. Second, while there was diversity with respect to age and gender, participants primarily identified as non-Hispanic white, so the results may not be reflective of the lived experiences of those from different racial/ethnic backgrounds. We conducted interviews only in English, which may have limited our understanding of other issues that are present for speakers of other languages with respect to navigating clinical systems and perceived access to HCV medication. Future studies with Spanish speaking PWID in particular may be merited given the disproportionate infection rates among Latinos (Lelutiu-Weinberger et al., 2009). Finally, due to the recruitment locations, participants may have been somewhat more connected with services than PWID who live and congregate in areas distant from needed services.
5. Conclusions
Through qualitative interviews informed by a model for understanding healthcare utilization, we were able to gain important insights into the perceptions of young PWID with respect to social determinants of engagement in care, as well as illness levels, related to the HCV care continuum. Evidence suggests that PWID can successfully complete DAA treatment and can be cleared of HCV (Grebely et al., 2013; Grebely and Dore, 2014). Our study highlights that young PWID may have the determinants of believing that the new HCV medications are effective and also the desire to pursue treatment, but face many obstacles that hinder linkage to care. Reducing stigma among healthcare professionals, which cuts across the different levels of the HCV care continuum, improving referral patterns and continuity of care, better informing people about their HCV status through patient-oriented testing and disclosure experiences, and reducing perceptions of personal responsibility for disease are crucial next steps to increasing treatment as prevention, in the long-term effort to reduce the HCV burden among PWID.
Highlights.
New highly effective medications are available to treat hepatitis C virus (HCV).
Young HCV-infected people who inject drugs (PWID) face unique barriers to testing, counseling, and treatment.
The HCV care continuum could influence HCV-treatment readiness and willingness.
Acknowledgements
The authors acknowledge our funding sources for this work: The Tufts Institute for Innovation, the National Institutes of Health (Awards UL1TR001064 and KL2TR001063), and the Lifespan/Tufts/Brown Center for AIDS Research (P30 AI042853). The authors also acknowledge the full Tufts REACTs team for their collaboration and support during the data collection and manuscript preparation phases of this project. We also acknowledge Harsha Amaravadi and Margaret Levene for their help with editing the final manuscript draft.
Role of the Funding Source
The study team was supported by a grant from the Tufts Institute for Innovation Pilot Study program (PI: Stopka) through Tufts University for data collection, analysis, and manuscript preparation. Additional support for Dr. Stopka’s time during manuscript preparation came from the National Institutes of Health, Clinical and Translational Science Award (UL1TR001064) (Stopka), and the Lifespan/Tufts/Brown Center for AIDS Research (P30 AI042853). Additional support for Dr. Ladin’s time during manuscript preparation came from the National Institutes of Health, National Center for Advancing Translational Sciences (KL2TR001063). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or other funding sources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
No conflict declared.
References
- Andersen R, 1968. A behavioral model of families’ use of health services University of Chicago, Chicago, Illinois. [Google Scholar]
- Anderson R, and Newman JF, 2005. Societal and individual determinants of medical care utilization in the united states. Milbank Q 83 doi: 10.1111/j.1468-0009.2005.00428.x. [DOI] [PubMed] [Google Scholar]
- Bartlett J, 2016. “State lowers requirements for MassHealth patients to get hepatitis C drugs”. Retrieved from: http://www.bizjournals.com/boston/blog/health-care/2016/07/state-lowers-requirements-for-masshealth-patients.html?ana=yahoo.
- Batchelder AW, Peyser D, Nahvi S, Arnsten JH, and Litwin AH., 2015. “Hepatitis C treatment turned me around:” Psychological and behavioral transformation related to hepatitis C treatment. Drug Alcohol Depend 153, 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JA, and Gifford AL, 2015. Resolute efforts to cure hepatitis C: Understanding patients’ reasons for completing antiviral treatment. Health 19, 473–489. [DOI] [PubMed] [Google Scholar]
- Cousien A, Tran VC, Deuffic-Burban S, Jauffret‐ Roustide M, Dhersin J, and Yazdanpanah Y, 2016. Hepatitis C treatment as prevention of viral transmission and liver‐ related morbidity in persons who inject drugs. Hepatology 63, 1090–1101. doi: 10.1002/hep.28227. [DOI] [PubMed] [Google Scholar]
- Davis M, and Rhodes T, 2004. Beyond prevention? Injecting drug user narratives about hepatitis C. Int. J. Drug Policy 15, 123–131. [Google Scholar]
- Davis M, and Rhodes T, 2004. Managing seen and unseen blood associated with drug injecting: Implications for theorising harm reduction for viral risk. Int. J. Drug Policy 15, 377–384. [Google Scholar]
- Davis M, Rhodes T, and Martin A, 2004. Preventing hepatitis C: ‘Common sense’, ‘the bug’ and other perspectives from the risk narratives of people who inject drugs. Soc. Sci. Med 59, 1807–1818. [DOI] [PubMed] [Google Scholar]
- Ditah I, Al Bawardy B, Gonzalez HC, Saberi B, Ditah C, Kamath PS, Charlton M, 2015. Lack of health insurance limits the benefits of hepatitis C virus screening: Insights from the national health and nutrition examination hepatitis C follow-up study. Am. J. Gastroenterol 110, 1126–1133. doi: 10.1038/ajg.2015.31. [DOI] [PubMed] [Google Scholar]
- Grebely J, and Dore GJ, 2014. Can hepatitis C virus infection be eradicated in people who inject drugs? Antiviral Res 104, 62–72. doi: 10.1016/j.antiviral.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Grebely J, Matthews GV, Lloyd AR, and Dore GJ, 2013. Elimination of hepatitis C virus infection among people who inject drugs through treatment as prevention: Feasibility and future requirements. Clin. Infect. Dis 57, 1014–1020. doi: 10.1093/cid/cit377. [DOI] [PubMed] [Google Scholar]
- Harris M, and Rhodes T, 2013. Hepatitis C treatment access and uptake for people who inject drugs: A review mapping the role of social factors. Harm Reduct. J 10, 7. doi: 10.1186/1477-7517-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson IM, Gordon SC, Kowdley KV, Yoshida ME, Rodriguez-Torres M, Sulkowski MS, Shiffman ML, Lawitz E, Everson G, Bennett M, Schiff E, Al-Assi MT, Subramanian GM, An D, Ling M, McNally J, Brainard D, Symonds WT, McHutchinson JG, Patel K, Feld J, Pianko S, Nelson DR, 2013. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. New Engl. J. Med 368, 1867. [DOI] [PubMed] [Google Scholar]
- Jeffrey GP, MacQuillan G, Chua F, Galhenage S, Bull J, Young E, Hulse G, O’Neil G, 2007. Hepatitis C virus eradication in intravenous drug users maintained with subcutaneous naltrexone implants. Hepatology 45, 111–117. doi: 10.1002/hep.21470. [DOI] [PubMed] [Google Scholar]
- Kay ES, Batey SD, and Mugavero MJ, 2016. The HIV treatment cascade and care continuum: Updates, goals, and recommendations for the future. AIDS Res. and Ther 13, 35. doi: 10.1186/s12981-016-0120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelutiu-Weinberger C, Pouget ER, Des Jarlais DD, Cooper HL, Scheinmann R, Stern R, Strauss SM, Hagan H, 2009. A meta-analysis of the hepatitis C virus distribution in diverse racial/ethnic drug injector groups. Soc. Sci. Med 68, 579–590. doi: 10.1016/j.socscimed.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massachusetts Department of Public Health (MDPH), 2014. Clinical advisory: Routine screening for hepatitis C Executive Office of Health and Human Services Department of Public Health, Boston. [Google Scholar]
- Miles MB, and Huberman MA, 1994. Qualitative data analysis SAGE, London. [Google Scholar]
- Neale J, Tompkins C, and Sheard L, 2008. Barriers to accessing generic health and social care services: A qualitative study of injecting drug users. Health Soc. Care Commun 16, 147–154. doi: 10.1111/j.1365-2524.2007.00739.x. [DOI] [PubMed] [Google Scholar]
- National Viral Hepatitis Roundtable (NVHR) and the Center for Health Law and Policy Innovation., 2016. Hepatitis C: The state of medicaid access preliminary findings: National summary report Retrieved from http://www.chlpi.org/wp-content/uploads/2013/12/HCV-Report-Card-National-Summary_FINAL.pdf.
- Reimer J, Schmidt CS, Schulte B, Gansefort D, Gölz J, Gerken G, Scherbaum N, Verthein U, Backmund M, 2013. Psychoeducation improves hepatitis C virus treatment during opioid substitution therapy: A controlled, prospective multicenter trial. Clin. Infect. Dis 57 Suppl. 2, S104. doi: 10.1093/cid/cit307. [DOI] [PubMed] [Google Scholar]
- Saldaña J, 2013. The coding manual for qualitative researchers (2. ed.). SAGE, Los Angeles. [Google Scholar]
- Scharloo M, and Kaptein A, 2013. “Measurement of illness perceptions in patients with chronic somatic illnesses: A review” In Petrie KJ, and Weinman JA (Eds.), Perceptions of health and illness (pp. 103–154). Routledge, New York. [Google Scholar]
- Sherer R, 1998. Adherence and antiretroviral therapy in injection drug users. JAMA 280, 567–568. doi: 10.1001/jama.280.6.567. [DOI] [PubMed] [Google Scholar]
- Suryaprasad AG, White JZ, Xu F, Eichler BA, Hamilton J, Patel A, Hamdounia SB, Church DR, Barton K, Fisher C, Macomber K, Stanley M, Guilfoyle SM, Sweet K, Liu S, Iqbal K, Tohme R, Sharapov U, Kupronis BA, Ward JW, Holmberg SD, 2014. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006–2012. Clin. Infect. Dis, 59, 1411–1419. doi: 10.1093/cid/ciu643. [DOI] [PubMed] [Google Scholar]
- Svarovskaia ES, Dvory-Sobol H, Parkin N, Hebner C, Gontcharova V, Martin R, Ouyang W, Han B, Xu S, Ku K, Chiu S, Gane E, Jacobson IM, Nelson DR, Lawitz E, Wyles DL, Bekele N, Brainard D, Symonds WT, McHutchinson JG, Miller MD, Mo H, 2014. Infrequent development of resistance in genotype 1–6 hepatitis C virus-infected subjects treated with sofosbuvir in phase 2 and 3 clinical trials. Clin. Infect. Dis 59, 1666–1674. doi: 10.1093/cid/ciu697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar C, Hull P, Dore GJ, and Grebely J, 2012. Knowledge and barriers associated with assessment and treatment for hepatitis C virus infection among people who inject drugs. Drug Alcohol Rev 31, 918–924. doi: 10.1111/j.1465-3362.2012.00468.x. [DOI] [PubMed] [Google Scholar]