Abstract
BACKGROUND
One driving motivation in the development of point-of-care (POC) diagnostics is to conveniently and immediately provide information upon which healthcare decisions can be based, while the patient is on site. Ambient ionization mass spectrometry (MS) allows direct chemical analysis of unmodified and complex biological samples. This suite of ionization techniques was introduced a decade ago and now includes a number of techniques, all seeking to minimize or eliminate sample preparation. Such approaches provide new opportunities for POC diagnostics and rapid measurements of exogenous and endogenous molecules (e.g., drugs, proteins, hormones) in small volumes of biological samples, especially when coupled with miniature mass spectrometers.
CONTENT
Ambient MS-based techniques are applied in diverse fields such as forensics, pharmaceutical development, reaction monitoring, and food analysis. Clinical applications of ambient MS are at an early stage but show promise for POC diagnostics. This review provides a brief overview of various ambient ionization techniques providing background, examples of applications, and the current state of translation to clinical practice. The primary focus is on paper spray (PS) ionization, which allows quantification of analytes in complex biofluids. Current developments in the miniaturization of mass spectrometers are discussed.
SUMMARY
Ambient ionization MS is an emerging technology in analytical and clinical chemistry. With appropriate MS instrumentation and user-friendly interfaces for automated analysis, ambient ionization techniques can provide quantitative POC measurements. Most significantly, the implementation of PS could improve the quality and lower the cost of POC testing in a variety of clinical settings.
According to the American Clinical Laboratory Association, over 7 billion laboratory tests are performed annually in the US. Nevertheless, the current emphasis is shifting toward point-of-care (POC)3 testing in nonlaboratory settings (e.g., clinician’s office, ambulance, in situ), to empower clinicians in making fast decisions, simplify healthcare delivery, and address challenges on health disparities (1).
Laboratory tests are often performed on blood and urine samples and employ immunoassays or colorimetric screening (2) that can also be used for on-site testing. Profiling and quantification of biomolecules and synthetic drugs are best done by mass spectrometry (MS), usually hyphenated with chromatographic separation techniques [e.g., liquid chromatography (LC)]. In spite of the expense and complexity of the instrumentation and the extensive sample pretreatment required before analysis, the enormous diversity of molecules detectable from complex biological samples—ranging from small synthetic drugs to intact proteins and viruses—justifies the key role that MS-based techniques play in clinical laboratory testing (3). Hyphenated MS methods provide high throughput, great versatility, selectivity, accuracy, and precision in analytical measurements as well as multiplexing capabilities. These features often greatly exceed those of immunoassays. However, the expense and complexity of the instrumentation and analytical protocols make the translation of current hyphenated MS techniques into POC testing unlikely. This is because the requirements are very different from those of laboratory testing, especially in (a) the limited time for sample preparation, which precludes extraction, preconcentration, and reconstitution processes, (b) the individualized nature of the measurements, for which the analytical measurements are specific to a particular patient and dispersed instruments are operated at low efficiency even if they are capable of high throughput when used in a batch mode (e.g., LC-MS/MS), (c) the physical size limitations, and (d) the need for analytical simplicity and automation. New developments in ambient ionization techniques and MS miniaturization (4) present an opportunity for translation of MS technology to POC testing (5). The term ambient ionization refers to a group of ionization techniques that produce gas-phase ions in the open air, removing chromatographic separation and minimizing prior sample preparation. Such ionization techniques promote straightforward sample introduction and analysis that emphasize simplicity, low cost, and speed (6).
This review begins with a brief overview of ambient ionization techniques. The clinical implementation and potential of ambient ionization is illustrated by focusing on paper spray (PS) ionization, highlighting the ability to perform quantitative analysis of small molecules in minute volumes of biofluids. We discuss the capability of performing reactive ambient ionization, i.e., chemical derivatization during ionization, to improve chemical specificity and sensitivity. Furthermore, the current state of miniature MS development is described, because it is a fundamental element of an ambient MS-based POC system. Finally, some challenges and future directions in further developing ambient MS for POC applications are discussed.
Ambient Ionization MS
Ambient ionization was introduced over a decade ago with desorption electrospray ionization (DESI) (7). Since then, a number of ambient techniques have been developed that differ in ionization method (e.g., spray-based, plasma-based, laser-based) and in the degree to which desorption and ionization are coupled (8), but they all share the capability of generating gas-phase ions directly from untreated samples, greatly reducing or eliminating analyte extraction and prior separation (9). Simplicity and rapid analysis are emphasized as characteristics of the ambient techniques (scores of which have been reported, as shown in Table 1 in the Data Supplement that accompanies the online version of this article at http://www.clinchem.org/content/vol62/issue1), which make them well suited in POC applications. They all use the sensitivity and specificity of MS, and rely on mass-to-charge ratios and/or fragmentation to acquire information on individual components of mixtures. Extensive literature exists on qualitative and quantitative analysis of endogenous biomolecules and on therapeutic and illicit drugs, performed in both a targeted and untargeted fashion. A selection of techniques with potential for use in POC applications is listed in Table 1, with some details regarding target analytes, biological matrix, and analytical methodology.
Table 1.
Some clinically relevant ambient ionization techniques and applications.
| Ambient ion sources | Year | Compounds | Biological matrix | Sample volume | Study type | LODa | MS data acquisition | Reference |
|---|---|---|---|---|---|---|---|---|
| DART | 2005 | Ranitidine (exo) | Urine | – | Qualitative | – | HR TOF-MS (exact mass measurement) | Takyi-Williams et al. (76) |
| 2013 | Phenylalanine PKU screening (endo) | DBS | – | Quantitative | 3.0 μmol/L | QQQ-MS(MRM) | Wang et al. (16) | |
| 2014 | Dimethylamylamine (exo) | Urine | – | Qualitative | – | HR TOF-MS (exact mass measurement) | Lesiak et al. (70) | |
| TM-DART | 2014 | Cocaine, diazepam (exo) | Urine, plasma | 0.3–1.5 mL | Quantitative | Cocaine <5 pg/mL, diazepam < 0.5 ng/mL | MRM | Gómez-Ríosa et al. (71) |
| DESI | 2006 | Cocaine and amphetamine-like stimulants | DBS | – | Qualitative | – | QqQLIT(MRM) | Wagner et al. (20) |
| 2007 | Androstadienedione, stigmastadienone, androsteronehemisuccinate, 5α-androstan-3β,17β-diol-16-one, androsterone glucuronide, epitestosterone, and 6-dehydrocholestenone | Urine | 10 μL | Qualitative | 0.6–10 ng/mL | LIT (product MS/MS ion scan) | Huang et al. (72) | |
| 2007 | Phenylalanine, leucine, valine, tyrosine, and methionine | DBS | 3.1 μL | Qualitative | – | QQQ-MS (neutral loss) | Corso et al. (12) | |
| 2010 | Sitamaquine, terfenadine, prazosin (exo) | DBS | 15 μL | Quantitative | – | QQQ-MS (MRM) | Wiseman et al. (10) | |
| DESI coupled with TLME* | 2013 | Methadone, amitriptyline, nortriptyline, andpethidine | Urine | 150 μL | Qualitative | 4–17 ng/mL | LIT (full scan) | Rosting et al. (73) |
| Nanospray DESI | 2008 | DOPA, ephedrine, and ibuprofen | DBS | – | Qualitative, chiral | 5–20 μg | – | Ranc et al. (13) |
| PS | 2011 | Propranolol, atenolol (exo) | DBS | – | Qualitative | – | QQQ-MS (MRM) | Manicke et al. (30) |
| 2011 | Acetaminophen, bezethonium, citalopram, dextrorphan, ibuprofen, paclitaxel, proguanil, simvastatin, sunitinib, telmisartan, verapamil, sitamaquine, amitriptyline (exo) | DBS | 10 μL | Quantitative | ng/mL range | QQQ-MS (MRM) | Manicke et al. (31) | |
| 2012 | Pazopanib, tamoxifen, imatinib, cyclophosphamide, paclitaxel, irinotecan, docetaxel, topotecan (exo) | DBS | 10 μL | Quantitative | 0.5–17 ng/mL | QQQ-MS (MRM) | Espy et al. (29) | |
| 2012 | Acylcarnitines(endo) | Blood, serum | 0.5 μL | Quantitative | 10–100 nmol/L | QQQ-MS (MRM) | Yang et al. (32) | |
| 2013 | Nicotine, cotinine, trans-3′-hydroxycotinine, anabasine (exo) | Blood, oral fluid, urine | 5 μL | Quantitative | <1–5 ng/mL | QQQ-MS (MRM) | Wang et al. (74) | |
| 2013 | Acylcarnitines (C2–C18) | Urine | Quantitative | 6–208 ng/mL | QQQ-MS (MRM) | Naccarato et al. (75) | ||
| 2014 | Amphetamine, methamphetamine, MDA, MDMA, MDEA, morphine, cocaine, Δ9-THC (exo) | Blood | 12 μL | Quantitative | <1–12 ng/mL | QQQ-MS (MRM) | Shi et al. (34) | |
| 2015 | Tacrolimus (exo) | DBS | 10 μL | Quantitative | 0.2 ng/mL | QQQ-MS (MRM) | Edelbroek et al. (33) | |
| 2015 | Sunitinib and benzethonium (exo) | Plasma | 2 μL | Quantitative | 1 ng/mL | QQQ-MS (MRM) | Takyi-Williams et al. (76) | |
| 2015 | Amphetamine, meprobamate, O-desmethyl-cis-tramadol, carisoprodol, tramadol, nordiazepam, EDDP, diazepam, norhydrocodone, hydromorphone, morphine, codeine, temazepam, noroxycodone, alprazolam, methadone, oxycodone, buprenorphine, norbuprenorphine, fentanyl, propoxyphene, 6-monoacetylmorphine (exo) | Oral fluid | 7 μL | Quantitative | ng/mL range | QQQ-MS (MRM) | Unpublished material | |
| SPE-PS | 2015 | Alprazolam, atenolol, caffeine, carbadox, carbamazepine, diazepam, digoxigenin, diltiazem, diphenhydramine, imatinib, propanolol, sulfadiazine, sulfamethazine, sulfathiazole, thiabendazole (exo) | Plasma | 100 μL | Quantitative | ng/mL range | QQQ-MS (MRM) | Zhang et al. (36) |
| Coated blade spray | 2014 | Cocaine, diazepam (exo) | Plasma, urine | 0.3–1.5 mL | Quantitative | pg/mL range | QQQ-MS (MRM) | Gómez-Ríosa et al. (37) |
| Wooden-tip ESI | 2013 | Ketamine, norketamine (exo) | Oral fluid, urine | 2 μL | Quantitative | 20 and 50 ng/mL | QQQ-MS (MRM) | So et al. (23) |
| Touch spray | 2014 | Imatinib (exo) | Blood | – | Qualitative | – | LIT (product MS/MS ion scan) | Kerian et al. (24) |
| 2014 | Lipid profile of pathogenic microorganisms | Oral fluid | 40 μL | Qualitative | – | LIT (full scan) | Jarmusch et al. (26) | |
| Touch spray | 2015 | Cocaine, BZE, ketamine, MDA, MDMA, MDEA, AMP, MAMP, morphine, codeine, 6-MAM, methadone, buprenorphine, Δ9-THC (exo) | Oral fluid | 40 μL | Qualitative | ng/mL range | LIT (product MS/MS/MS ion scan) | Pirro et al. (25) |
LOD, limit of detection; exo, exogenous; HR, high resolution; TD-DART, transmission mode DART; PKU, phenylketonuria; endo, endogenous; QQQ, triple quadrupole; QqQLIT, hybrid triple quadrupole linear ion trap mass spectrometer; TLME, thin liquid membrane extraction; MDA, 3,4-methylenedioxyamphetamine; MDMA, 3,4-methylenedioxymethamphetamine; MDEA, nethylenedioxyethylamphetamine; THC, Δ9-tetrahydrocannabinol; EDDP, 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine; BZE, benzoylecgonine; AMP, amphetamine; MAMP, methamphetamine; 6-MAM, 6-monoacetylmorphine.
Briefly, DESI generates ions via direct desorption and ionization using charged solvent droplets that impact a sample surface. Tissue analysis by DESI-MS, particularly DESI imaging, can provide exogenous drug and/or drug metabolite distributions (10). It also provides diagnostic information on human brain cancers (11) and delineates tumor margins in brain, kidney, and liver via detection of altered lipid profiles that reflect the structural composition of cellular membranes. Moving toward POC diagnostics, DESI has been shown to have a potential application in screening of inborn errors of metabolism (12) and therapeutic drug monitoring (10, 13) by means of direct detection of free amino acids and drugs in dried blood spots (DBS), respectively. In the DBS study, the DESI sprayer was moved laterally to rapidly scan the DBS spots and detect target analytes in the multiple reaction monitoring (MRM) mode. In these early applications, ion suppression due to matrix effects was highlighted as the biggest analytical challenge for direct surface analysis of DBS by ambient MS (10, 13). The selection of the solvent system for the DESI spray allows targeting of the method toward specific analytes, while other parameters like pneumatic pressure (e.g., nitrogen) and geometry of the spray affect the efficiency of the desorption/ionization process and the spatial resolution in the case of DESI imaging experiments (14). More recently, DESI has been used for therapeutic monitoring of salicylic acid using a 3-layer DBS paper card. In this experiment, blood (6μL) was applied on a card yielding DBS with an average diameter of 9 mm that was analyzed by DESI-MS (15). A linear response was achieved over the concentration range 10–2000 mg/L, with relative SDs < 14% and a limit of quantification of 10 mg/L (15). In another experiment, nanospray DESI was reported for chiral analysis (by the kinetic method) of ibuprofen, dihydroxyphenylalanine (DOPA), and ephedrine in DBS (13).
Direct analysis in real time (DART) has been applied to DBS for newborn screening of phenylketonuria (16) and to conduct pharmacokinetic/toxicokinetic studies without additional manipulation of the samples (17). In DART, a discharge occurs far from the sample surface, and a stream of heated gas is used to carry the active species toward the sample. During transit, metastable helium atoms originating in the plasma react with ambient water, oxygen, or other atmospheric components to produce the reactive ions (6).
Laser diode thermal desorption–atmospheric pressure chemical ionization has been used to quantify metformin and sitagliptin in mouse and human DBS (18). An atmospheric pressure thermal desorption chemical ionization (APTDCI) interface has been described for profiling of free carnitine, acylcarnitines, and sterols in dried blood and plasma spots (19). The mechanism of APTDCI involves analyte desorption due to the nitrogen flow, and then analyte gas-phase ionization by APCI from a corona discharge. A liquid microjunction surface sampling probe has also been applied to DBS samples with a chip-based nanoelectrospray infusion system (20). This experiment, also known as nanoDESI (nanospray DESI), provides desorption by means of 2 small glass tubes or capillaries in series, which allows a continuous stream of solvent to be brought in contact with the surface of the sample.
Low temperature plasma (LTP) uses an electric field to generate a plasma that interacts directly with the sample being analyzed to thermally desorb and ionize surface molecules. Biological samples or skin can be analyzed without electrical shock or perceptible heating, including a proof-of-concept of detection of cocaine directly from human skin reported by Harper et al. (21). A handheld LTP probe has also been successfully developed for miniature MS applications and used for in situ testing (e.g., detection of pesticide residues) but not yet applied to clinical diagnostics.
Other ambient techniques, such as atmospheric pressure solids analysis probe (22), wooden-tip electrospray (23), and touch spray (24) introduced the concept of adopting the substrate itself as the means for specimen sampling and ionization, allowing for straightforward handling and analysis of intact biofluids, which is ideal for POC testing. Recently, touch spray analysis directly from medical swabs was reported for noninvasive oral fluid analysis for qualitative detection of illicit drugs (25), as well as the detection of bacteria causing strep throat via lipid profiling of pathogenic microorganisms (Fig. 1) (26).
Fig. 1. Strep throat diagnosis.
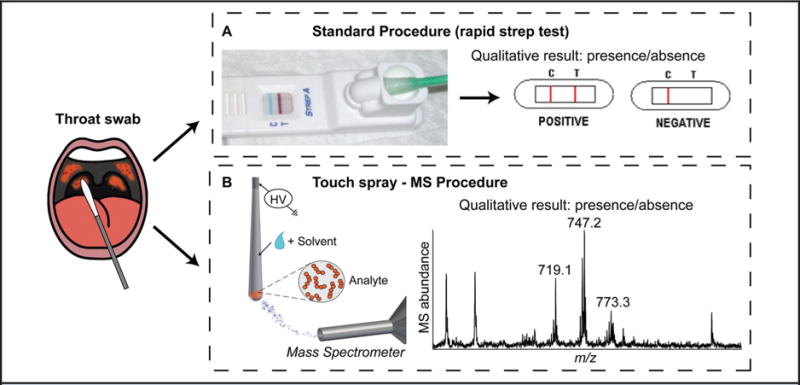
(A), Schematic of the standard on-site procedure for rapid immunoassay testing. (B), Procedure based on touch spray MS with medical swabs described in Jarmusch et al. (26). Fig. was adapted from (26) and reproduced with permission from The Royal Society of Chemistry.
PS for POC Testing
Ambient ionization MS can provide qualitative and quantitative results for clinically relevant analytes in biological matrices. Endogenous and exogenous analytes may be investigated quickly to create quality POC assays that answer the necessary questions for diagnosis. Qualitative analysis by ambient ionization MS seeks to establish whether or not one or more substances are present. These assays may have legal or clinically relevant thresholds that must be met analytically, representing the cutoffs for discrimination between undetected and positive results (i.e., concentrations below or above particular values). Simple, straightforward, and rapid techniques, such as spraying directly from medical swabs (25, 26), are ideal for on-site emergency toxicological screens or roadside drug testing. Providing the patient with the best care justifies the ranking of speed over the most precise analytical result, which is often unnecessary, costly, and slow. Additionally, the ease of sample collection may translate into better patient compliance.
Certainly, there are important questions that require quantitative answers, and PS (27) is currently one of the ambient techniques most developed and investigated for such tasks. Quantification of small analytes in complex matrices is performed by ambient MS using work flows similar to those for LC-MS/MS (Fig. 2). In ambient ionization MS, quantification involves minimal sample handling, usually just internal standard addition, followed by mass spectral analysis in which chemical specificity is usually achieved by MRM, i.e., PS-MS/MS of particular transitions using triple quadrupole mass analyzers. The lack of multistep offline sample preparation and chromatography confers simplicity and speed. Specific examples of analytes quantified in biological matrices by PS-MS/MS are listed in Table 1.
Fig. 2. Comparison of sample treatment, MS analysis, and data output between LC-MS/MS and ambient MS/MS for quantification of small molecules.
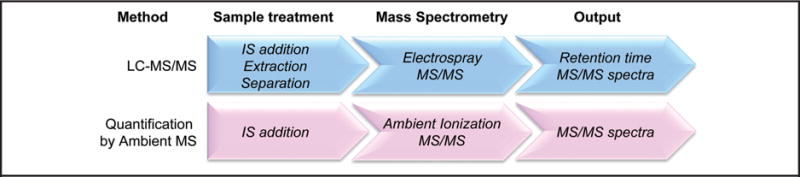
These are characteristic features but exceptions occur. IS, internal standard.
Certainly, the absence of chromatographic separation requires alternative solutions to achieve chemical specificity, as the recognition of isobaric, isomeric, and chiral species is critical in many clinical applications (e.g., identification of dextromethorphan vs levomethorphan). Ambient ionization relies only on the acquisition of high mass resolution measurements and MSn data for specificity, which is based on characteristic fragmentation patterns. However, scans that are multidimensional in mass (MSn) are available to efficiently screen drugs in an untargeted fashion or search for designated classes of compounds in mixtures. Such multidimensional scans (e.g., precursor ion scan, product ion scan, neutral loss scan) are useful in screening known illicit compounds, but they are also capable of screening for classes of compounds, as in detection of minor modifications to molecules due to metabolic processes or intentional chemical modification (e.g., designer synthetic drugs). These are otherwise difficult tasks in clinical–toxicological investigations (see online Supplemental Fig. 1). Reactive ionization experiments described later represent an alternative way of increasing chemical specificity.
PS ionization generates ions directly from paper surfaces cut into a triangular shape, usually cellulose filter paper (e.g., Whatman paper) of various thicknesses. The properties of the paper influence the performance of PS. Recently, Zheng et al. reported detailed methodology to prepare different types of paper substrates without complex surface coating procedures with the aim of enhancing PS performance and facilitating its use (28). A few microliters of biofluid (usually <10μL) is spotted onto the paper substrate and then solvent (typically methanol, acetonitrile, or a combination of these with deionized water and/or doped with formic acid or other modifiers) and high voltage (about 3.5 kV) is applied to the paper (13, 27). High voltage creates a strong electric field at the tip of the paper triangle causing field emission of solution droplets from which gas phase ions are generated (Fig. 3A). The biological matrix (e.g., proteins and salts) interacts with and is partially retained in the cellulose-based paper, thereby minimizing the need for multistep sample pretreatment, especially when dried sample spots are analyzed or when coagulant agents that are not soluble in the solvent used are added to quickly clot blood and allow for analysis of nondried samples (29).
Fig. 3. (A) Schematic of PS analysis.
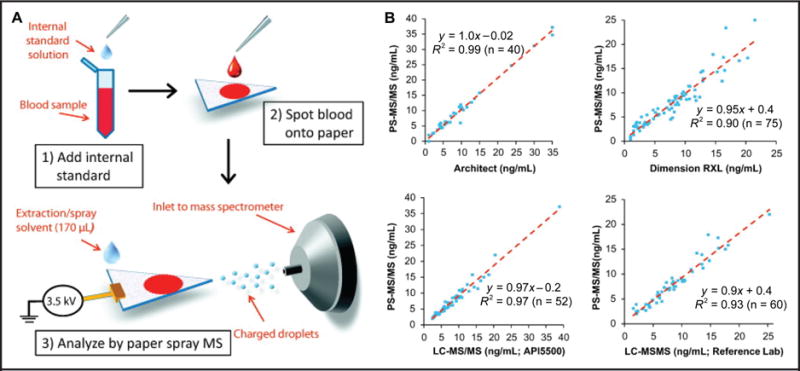
(B) Performance of PS-MS/MS method for quantification of tracrolimus compared with 2 immunoassay tests, and an LC-MS/MS method developed in-house and in a reference laboratory. Fig. adapted and reproduced with permission from Shi et al. (34).
PS is simple and robust and a suitable ionization method for a variety of clinical analytes measured in biological fluids in less than 1 minute (27–32). In contrast to traditional chromatographic MS techniques, which are well suited for cases for which time is less of a concern and clinicians do not need immediate test results, PS can provide rapid optimal results in the opposite situations even without reaching the extreme analytical performance of more exhaustive extraction and purification techniques.
PS has been used for direct analysis of dried sample spots, including whole blood, plasma, urine, and oral fluid, making it particularly amenable to POC testing (33), which would benefit synergistically from the advantages of dried spot analysis (20) and ambient MS. Similarly to dried spot analysis, the position of the spot on the paper surface, type of paper substrate and its thickness, sample volume, solvent used for analysis, solvent application, and internal standard addition are all features that need to be optimized.
Quantitative performance using PS-MS/MS has been demonstrated for a range of analytes (Table 1). The immunosuppressant drug tacrolimus has been quantified by PS-MS/MS in the therapeutic range of 1.5–30 ng/mL, an assay typically performed by either immunoassay or LC-MS/MS (Fig. 3B). Accuracy and precision were comparable to LC-MS/MS techniques (34). Currently, efforts are being made to enhance the multiplexing capabilities of PS-MS/MS because clinical testing often relies on the identification of a pattern of molecules (e.g., toxicological screening needs to cover a wide range of compounds). Recently a PS-MS/MS method was developed to detect 8 traditional illicit drugs in whole blood (35). As PS-MS/MS develops, novel means of improving analytical performance (e.g., improved accuracy and imprecision, reduced matrix effects, lower limits of detection) have been reported, while retaining the rapid and straightforward characteristics of ambient techniques. In a recent study, a solid phase extraction (SPE) column was integrated with the paper spray cartridge to perform extraction and preconcentration before PS and quantify alprazolam, atenolol, carbamazepine, diazepam, and sulfamethazine from bovine plasma. Using isotope-labeled internal standards, limits of detection < 3.0 ng/mL were obtained (36). Another sample preparation method, coated blade spray ionization, which uses a solid-phase micro extraction coated metal substrate shaped to a point, allows sample extraction followed by direct ionization. Using this technique, Gómez-Ríos et al. were able to detect 1.5 ng/mL of cocaine and diazepam in plasma and urine samples (37). In these techniques a larger amount of sample (>100μL) is used than in traditional PS (typically 2–10 μL). An alternative to preconcentration techniques is reactive ambient ionization, in which chemical derivatization to generate a more favorable form of the analyte ions is performed simultaneously with ionization, as described below.
Reactive Ambient Ionization MS
Ambient ionization allows on-line derivatization to be performed concurrently with ionization. Reaction products can be generated on the millisecond timescale of ionization and transferred directly to the mass spectrometer. The speed of the reactions is the result of reaction rate acceleration in microdroplets (38, 39). On-line derivatization has several advantages that are beneficial for ambient ionization and avoid the need for any extraction, clean-up, and desalting process before analysis: (a) increased ionization efficiency and minimized ion suppression in complex biological matrices (40), (b) enhanced chemical specificity to distinguish structural isomers or recover detailed structural information, e.g., double bond positions in lipids (41, 42) or peptide and protein characterization (43), and (c) enhanced structural information via MS/MS fragmentation. Reactive ambient MS has been reported for compounds with a range of functional groups including aldehydes, ketones, alcohols, amines, thiols, and alkenes (38). Table 2 lists examples of online derivatization reactions used in PS, DESI, and LTP and their target analytes. Other types of reactions can also be used in different ambient techniques, like photoinitiated reactions in plasmas and discharges.
Table 2.
Representative derivatization reactions for biomolecules by ambient MS
| Functional Groups | Reagents | Target compounds | Ambient MS method |
|---|---|---|---|
| Aldehydes and ketones | Girard’s reagent T, hydroxylamine, dinitrophenylhydrazine | Cortisone in oral fluid, steroid hormones [Huang et al. (72)], malondialdehyde in tissue [Girod et al. (77)] | PS, DESI |
| Alcohols | Betaine aldehyde | Cholesterol in tissue [Wu et al. (45)] | DESI |
| Phenylboronic acid | Saccharides in urine [Gómez-Ríosa et al. (37)] | DESI | |
| Amines | Bis(sulfosuccinimidyl) suberate, acetone | Cross-linking of peptides containing asparagine, glutamine, arginine, or lysine, amino acids [Gómez-Ríosa et al. (37)] | DESI |
| Disulfides and thiols | Dithiothreitol | Oxidized glutathione and insulin [Peng et al. (43)] | ELDI |
| Alkenes | Ozone, silver nitrate | Unsaturated lipids in bacteria, algae and animal preimplantation embryos [González-Serrano et al. (40), Zhang et al. (41), Jackson et al. (42)] | LTP, PS, DESI |
The detection of oxidized or reduced polycyclic aromatic quinones, compounds implicated in carcinogenesis and in the pathogenesis of respiratory diseases, was performed by reactive PS using minute volumes (2 μL) of urine, serum, and cultured cells. A cysteamine reagent was used to derivatize the quinones. The products were quantified by MS/MS using just a single internal standard. In urine, the limits of quantification for 1,4-naphthoquinone and 1,4-anthraquinone deposited on paper were 1.35 and 2.64 ng (absolute quantity) (44).
Compounds that have permanent charges give the best responses in MS, so charge labeling is an important class of derivatization reactions. Online charge labeling of cholesterol (Chol) can be achieved using betaine aldehyde (BA) as the chloride salt to generate the precharged ester. This reaction was first demonstrated in a DESI tissue imaging experiment (45), and it allowed cholesterol, which is poorly ionized in both positive and negative ion modes, to be readily detected via its product in the positive ion mode. Derivatization of cholesterol in human serum was achieved using a solution of BA directly by PS. Without the derivatization reagent, cholesterol-related peaks, such as the protonated molecule [Chol + H]+ (m/z 387), its dehydration product [Chol + H – H2O]+ of m/z 369, and the sodium adduct [Chol + Na]+ of m/z 409, were not detected. But after online derivatization, the reaction product [Chol + BA]+ was observed at m/z 488 and its identity confirmed by collision-induced dissociation tandem mass spectrometry (45).
The analysis of cortisol in human oral fluid by quantitative reactive PS-MS/MS is yet another example of the applicability of ambient ionization in improving patient care. Cortisol is important in the diagnosis of many diseases and may have prognostic value in critically ill patients. The timescales for testing are wideranging, from medical emergency to long-term hormone concentration monitoring (46). Cortisol concentrations, which plummet as a result of an adrenal crisis, represent a medical emergency and clearly need to be determined urgently. By contrast, the long-term monitoring of a patient with Addison disease merely requires periodic confirmation that the concentration falls within an expected range (47). In both of these cases, a semiquantitative POC determination that allows rapid and confident assessment is all that is required. As shown schematically in online Supplemental Fig. 2, reactive PS-MS/MS can be performed by adding Girard’s Reagent T onto Whatman paper to which 5 μL of oral fluid was previously spotted, to derivatize the ketone functional group of cortisol. The precharged hydrazone product of the reaction needs only to be desorbed for mass spectral analysis. Detection of cortisol is achieved via MRM (m/z 476.2 > 417.2) of the cortisol-Girard’s Reagent T product using a triple-quadrupole mass spectrometer. Quantification in the nanogram per milliliter range is achieved by means of the standard addition method because cortisol is naturally present in oral fluid. The reactive PS-MS/MS method results in improved performance compared to PS-MS/MS with no derivatization, and is thus relevant to POC diagnostic applications.
Miniature Mass Spectrometers
Implementation of miniature MS instruments and their interfacing with ambient ion sources (48, 49) represents an ideal combination of technologies for POC testing in nonlaboratory settings (Fig. 4). Development of miniature MS instrumentation began in the early 1990s (50, 51). In the 2000s, the reduction of MS system size continued until a handheld mass spectrometer was created (52, 53). Substantial obstacles to miniaturized MS performance were overcome with the introduction of improved pumping technology, minimization of electronics, and, especially, improved atmospheric sampling. Discontinuous atmospheric pressure introduction was a critical step in this development, allowing externally created ions to periodically be introduced into the mass analyzer. Today, a range of miniature mass spectrometers is being developed and commercialized, including ion traps of various geometries (toroidal, cylindrical, rectilinear) (49, 50, 52, 54). Miniature instrumentation based on other mass analyzers has also been developed, including TOF (55) and single quadrupoles, but these lack MS/MS capabilities and thus are less well suited to complex mixture analysis. Recently, a miniature triple quadrupole with capabilities for selected ion fragmentation and mass analysis was reported (56, 57). The detection limits of current miniature MS systems are about 1 order of magnitude less than an analytical lab-scale instrument; however, this difference is expected to decrease and to match or exceed the requirements for many clinical applications (57). Other desirable aspects are the size, power requirements, random access, easy data analysis, and reliability of the system. Miniaturized MS for POC measurements should be evaluated in terms of size, cost, and performance, but certainly should be highly automated and robust so that staff with little or no knowledge of MS can operate the system repeatedly, rapidly, and reliably (49, 58, 59). Great effort should be invested in designing miniature instruments with integrated ambient ion sources for POC systems. Commercialized and partially automated ambient ionization MS sources exist for DESI (10), liquid extraction surface analysis (60), and DART but have been coupled only to benchtop MS instrumentation, although the feasible coupling with miniature instruments (e.g., for DESI, desorption atmospheric pressure chemical ionization, and PS) has been proved with in-house built instruments for research purposes (49, 61).
Fig. 4. Schematic of PS miniature MS POC system.
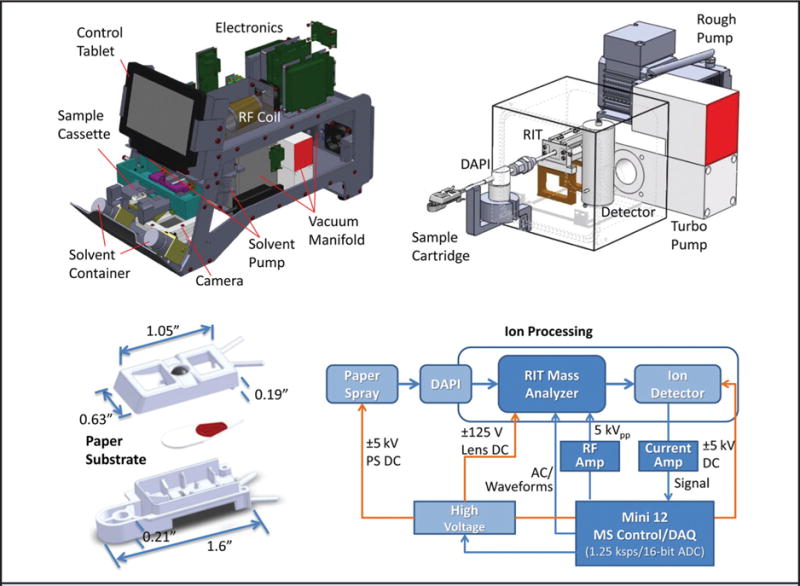
RF, radiofrequency; RIT, rectilinear ion trap; DAPI, discontinuous atmospheric pressure interface; DAQ, data acquisition. Fig. adapted from Li et al. (49) and reproduced with permission from the American Chemical Society.
The field of ambient miniature MS-based POC systems is at an early stage of development, and only a limited number of techniques have been reported. First, the so-called Mini 12, a miniaturized ion trap with a discontinuous atmospheric pressure introduction valve (schematics shown in Fig. 4) equipped with PS as ambient ion source has been used to measure amitriptyline in whole blood at concentrations as low as 7.5 ng/mL (CV 10%), which is below the therapeutic concentration, proving the feasibility of the systems currently available (49). The same apparatus proved suitable for detection of five synthetic cannabinoids in blood and urine (59). Zhai et al. used a continuous atmospheric pressure ionization ion trap system to identify 4 different chemicals (Gly-Pro-Arg-Pro and Met-Arg-Phe-Ala peptides, rhodamine, and reserpine at 100 μg/mL) in a complex matrix by PS (56). The miniature triple quadrupole with a differential pumping interface allows quantification of small molecules, reaching nanogram per milliliter detection for reserpine. Capabilities for precursor and neutral loss scans, which are well suited for searches for designated classes of compounds, were also demonstrated (56, 57).
Final Remarks
Implementation of ambient methods on miniature instruments in clinical diagnostics has great potential for POC testing outside the laboratory environment. This approach not only simplifies the analytical process but also reduces cost and time of analysis. Such a strategy is applicable to toxicological screening, therapeutic drug monitoring, studies of compliance and pharmacokinetics, and metabolic screening. In the latter case, POC testing can be created not only to identify metabolic aberrations indicative of disease (e.g., in newborn screening applications) (3) but also to recover individual molecular fingerprints that can be used to monitor a patient’s state of health longitudinally. It is possible that systematic deviations in repeated measurements of selected biomolecules may reveal the early stages of diseases or physiological changes. Appropriate data handling systems (e.g., multivariate data analysis) can be developed for decision-making strategies, which do not differ much from those used in industrial process monitoring or antidoping controls used to create athletes’ biological passports (62).
Challenges and limitations still need to be faced. First, detection of protein biomarkers has had limited success using ambient MS. Proteins are extremely useful for early detection of disease with quite a number of protein-based POC diagnostic tests (63), because proteins play fundamental roles in life processes. Secondly, ultratrace analysis (> ppb) by ambient MS is challenging. Blending online concentration and extraction techniques with ambient ionization is a strategy that is already in place, but certainly improvements in instrumentation will assist in reaching new limits of detection (now at pg values, absolute, for benchtop instruments). Coupling ambient MS with ion mobility represents an additional strategy to improve analytical performance, providing additional specificity without sample preparation or a substantial increase in time of analysis (64). The application of 3D printing technology to MS has allowed the design of small and cheap ion focusing devices that can be coupled with miniature instruments and so allow ion separation at atmospheric pressure, on top of increasing efficiency of ion transmission in air (65, 66). Modifications to the PS substrate, like carbon nanotube impregnated paper, can advance the development of PS for POC analysis by reducing the magnitude of the external voltage needed to create an electrospray to values as low as 3 V, and so address any safety concerns (67). Lastly, the regulatory environment for MS-based clinical assays is currently uncertain, and this extends to new techniques such as ambient ionization and POC MS. Nowadays in the US, almost all clinical assays performed by MS are developed in individual laboratories that are regulated by Centers for Medicare and Medicaid Services without Food and Drug Administration (FDA) review. The assays described in this review have been developed using research-only instruments because only recently have instrument companies started registering their instruments as class I and class II medical devices. Moreover, the research is still focused on methodology and instrumentation development, and only rarely has internal validation and proficiency testing been conducted. Method validation and the development of shared protocols to follow will be one step needed as the technology spreads. In October 2014, the FDA released a draft framework for regulatory oversight of lab-developed tests, which indicates that the agency has plans to establish a regulatory environment for MS-based assays (2, 68). The use of ambient MS for a number of applications (especially forensics and tissue analysis) is being stimulated by the presence of dedicated companies in this area. Companies involved in POC diagnostics may be challenged by the upcoming changes in the regulatory environment, and ambient MS may be implemented in future with the aid of commercial kits approved by the FDA.
Supplementary Material
Footnotes
Nonstandard abbreviations: POC, point-of-care; MS, mass spectrometry; LC, liquid chromatography; PS, paper spray; DESI, desorption electrospray ionization; DBS, dried blood spots;MRM,multiple reaction monitoring; DOPA, dihydroxyphenylalanine; DART, direct analysis in real time; APTDCI, atmospheric pressure thermal desorption chemical ionization; LTP, low temperature plasma; SPE, solid phase extraction; Chol, cholesterol; BA, betaine aldehyde; FDA, Food and Drug Administration.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership: Z. Ouyang, Purspec Technologies Inc.; R.G. Cooks, guest editor, Clinical Chemistry, AACC.
Consultant or Advisory Role: None declared.
Stock Ownership: Z. Ouyang, Purspec Technologies Inc.
Honoraria: None declared.
Research Funding: Z. Ouyang, institutional funding from NIH.
Expert Testimony: None declared.
Patents: None declared.
References
- 1.NIH. Point-of-care diagnostic testing. http://report.nih.gov/nihfactsheets/ViewFactSheet.aspx?csid=112 (Accessed June 2015)
- 2.Arnaud CH. Mass spec welcome in clinical labs. C&EN. 2015;93:32–4. [Google Scholar]
- 3.La Marca G. Mass spectrometry in clinical chemistry: the case of newborn screening. J Pharm Biomed Anal. 2014;101:174–82. doi: 10.1016/j.jpba.2014.03.047. [DOI] [PubMed] [Google Scholar]
- 4.Cooks RG, Manicke NE, Dill AL, Ifa DR, Eberlin LS, Costa AB, et al. New ionization methods and miniature mass spectrometers for biomedicine: DESI imaging for cancer diagnostics and paper spray ionization for therapeutic drug monitoring. Faraday Discuss. 2011;149:247–67. doi: 10.1039/c005327a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corso G, D’Apolito O, Gelzo M, Paglia G, Dello Russo A. A powerful couple in the future of clinical biochemistry: in situ analysis of dried blood spots by ambient mass spectrometry. Bioanalysis. 2010;2:1883–91. doi: 10.4155/bio.10.149. [DOI] [PubMed] [Google Scholar]
- 6.Venter A, Nefliu M, Cooks RG. Ambient desorption ionization mass spectrometry. Trends Anal Chem. 2008;27:284–90. [Google Scholar]
- 7.Takats Z, Wiseman JM, Gologan B, Cooks RG. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science. 2004;306:471–3. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- 8.Venter AR, Douglass KA, Shelley JT, Hasman G, Jr, Honarvar E. Mechanisms of real-time, proximal sample processing during ambient ionization mass spectrometry. Anal Chem. 2014;86:233–49. doi: 10.1021/ac4038569. [DOI] [PubMed] [Google Scholar]
- 9.Monge ME, Harris GA, Dwivedi P, Fernandez FM. Mass spectrometry: recent advances in direct open air surface sampling/ionization. Chem Rev. 2013;113:2269–308. doi: 10.1021/cr300309q. [DOI] [PubMed] [Google Scholar]
- 10.Wiseman J, Evans CA, Bowen CL, Kennedy JH. Direct analysis of dried blood spots utilizing desorption electrospray ionization (DESI) mass spectrometry. Analyst. 2010;135:720–5. doi: 10.1039/b922329k. [DOI] [PubMed] [Google Scholar]
- 11.Eberlin LS, Norton I, Orringer D, Dunn IF, Liu X, Ide JL, et al. Ambient mass spectrometry for the intraoperative molecular diagnosis of human brain tumors. Proc Natl Acad Sci USA. 2013;110:1611–6. doi: 10.1073/pnas.1215687110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corso G, Paglia G, Garofalo D, D’Apolito O. Neutral loss analysis of amino acids by desorption electrospray ionization using an unmodified tandem quadrupole mass spectrometer. Rapid Commun Mass Spectrom. 2007;21:3777–84. doi: 10.1002/rcm.3280. [DOI] [PubMed] [Google Scholar]
- 13.Ranc V, Havlicek V, Bednar P, Lemr K. Nano-desorption electrospray and kinetic method in chiral analysis of drugs in whole human blood samples. Eur J Mass Spectrom. 2008;14:411–7. doi: 10.1255/ejms.978. [DOI] [PubMed] [Google Scholar]
- 14.Eberlin LS. DESI-MS imaging of lipids and metabolites from biological samples. Methods MolBiol. 2014;1198:299–311. doi: 10.1007/978-1-4939-1258-2_20. [DOI] [PubMed] [Google Scholar]
- 15.Siebenhaar M, Küllmer K, de Barros Fernandes NM, Hüllen V, Hopf C. Personalized monitoring of the rapeutic salicylic acid in dried blood spots using a three-layer setup and desorption electrospray ionization mass spectrometry. Anal Bioanal Chem. 2015;407:7229–38. doi: 10.1007/s00216-015-8887-8. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, Zhu H, Cai Z, Song F, Liu Z, Liu S. Newborn screening of phenylketonuria using direct analysis in real time (DART) mass spectrometry. Anal Bioanal Chem. 2013;405:3159–64. doi: 10.1007/s00216-013-6713-8. [DOI] [PubMed] [Google Scholar]
- 17.Crawford E, Gordon J, Wu JT, Musselman B, Liu R, Yu S. Direct analysis in real time coupled with dried spot sampling for bioanalysis in a drug-discovery setting. Bio-analysis. 2011;3:1217–26. doi: 10.4155/bio.11.99. [DOI] [PubMed] [Google Scholar]
- 18.Swales JG, Gallagher RT, Denn M, Peter RM. Simultaneous quantitation of metformin and sitagliptin from mouse and human dried blood spots using laser diode thermal desorption tandem mass spectrometry. J Pharm Biomed Anal. 2011;55:544–51. doi: 10.1016/j.jpba.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 19.Corso G, D’Apolito O, Garofalo D, Paglia G, Dello Russo A. Profiling of acylcarnitines and sterols from dried blood or plasma spot by atmospheric pressure thermal desorption chemical ionization (APTDCI) tandem mass spectrometry. Biochim Biophys Acta. 2011;1811:669–79. doi: 10.1016/j.bbalip.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Wagner M, Tonoli D, Varesio E, Hopfgartner G. The use of mass spectrometry to analyze dried blood spots. Mass Spectrom Rev. doi: 10.1002/mas.21441. [Epub ahead of print 2014 Sep 22] [DOI] [PubMed] [Google Scholar]
- 21.Harper JD, Charipar NA, Mulligan CC, Zhang X, Cooks RG, Ouyang Z. Low-temperature plasma probe for ambient desorption ionization. Anal Chem. 2008;80:9097–104. doi: 10.1021/ac801641a. [DOI] [PubMed] [Google Scholar]
- 22.Doué M, Dervilly-Pinel G, Gicquiau A, Pouponneau K, Monteau F, Le Bizec B. High throughput identification and quantification of anabolic steroid esters by atmospheric solids analysis probe mass spectrometry for efficient screening of drug preparations. Anal Chem. 2014;86:5649–55. doi: 10.1021/ac501072g. [DOI] [PubMed] [Google Scholar]
- 23.So PK, Ng TT, Wang H, Hu B, Yao ZP. Rapid detection and quantitation of ketamine and norketamine in urine and oral fluid by wooden-tip electrospray ionization mass spectrometry. Analyst. 2013;138:2239–43. doi: 10.1039/c3an36641c. [DOI] [PubMed] [Google Scholar]
- 24.Kerian KS, Jarmusch AK, Cooks RG. Touch spray mass spectrometry for in situ analysis of complex samples. Analyst. 2014;139:2714–20. doi: 10.1039/c4an00548a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pirro V, Jarmusch AK, Vincenti M, Cooks RG. Direct drug analysis from oral fluid using medical swab touch spray mass spectrometry. Anal Chim Acta. 2015;861:47–54. doi: 10.1016/j.aca.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarmusch AK, Pirro V, Kerian KS, Cooks RG. Detection of strep throat causing bacterium directly from medical swabs by touch spray-mass spectrometry. Analyst. 2014;139:4785–9. doi: 10.1039/c4an00959b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Wang H, Manicke NE, Lin JM, Cooks RG, Ouyang Z. Development, characterization, and application of paper spray ionization. Anal Chem. 2010;82:2463–71. doi: 10.1021/ac902854g. [DOI] [PubMed] [Google Scholar]
- 28.Zheng Y, Zhang Z, Yang H, Liu X, Zhang X, Zhang Z. Facile preparation of paper substrates coated with different materials and their applications in paper spray mass spectrometry. Anal Meth. 2015;7:5381–6. [Google Scholar]
- 29.Espy RD, Manicke NE, Ouyang Z, Cooks RG. Rapid analysis of whole blood by paper spray mass spectrometry for point-of-care therapeutic drug monitoring. Analyst. 2012;137:2344–9. doi: 10.1039/c2an35082c. [DOI] [PubMed] [Google Scholar]
- 30.Manicke NE, Qian Y, He W, Sheran O, Zheng O, Cooks RG. Assessment of paper spray ionization for quantification of pharmaceuticals in blood spots. Int J Mass Spectrom. 2011;300:123–9. [Google Scholar]
- 31.Manicke NE, Abu-Rabie P, Spooner N, Ouyang Z, Cooks RG. Quantitative analysis of therapeutic drugs in dried blood spot samples by paper spray mass spectrometry: an avenue to therapeutic drug monitoring. J Am Soc Mass Spectrom. 2011;22:1501–7. doi: 10.1007/s13361-011-0177-x. [DOI] [PubMed] [Google Scholar]
- 32.Yang Q, Manicke NE, Wang H, Petucci C, Cooks RG, Ouyang Z. Direct and quantitative analysis of underivatizedacylcarnitines in serum and whole blood using paper spray mass spectrometry. Anal Bioanal Chem. 2012;404:1389–97. doi: 10.1007/s00216-012-6211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edelbroek PM, van der Heijden J, Stolk LM. Dried blood spot techniques in therapeutic drug monitoring: techniques, assays, and pitfalls. Ther Drug Monit. 2009;31:327–36. doi: 10.1097/FTD.0b013e31819e91ce. [DOI] [PubMed] [Google Scholar]
- 34.Shi RZ, El Gierari el TM, Manicke NE, Faix JD. Rapid measurement of tacrolimus in whole blood by paper spray-tandem mass spectrometry (PS-MS/MS) Clin Chim Acta. 2015;441:99–110. doi: 10.1016/j.cca.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 35.Espy RD, Teunissen SF, Manicke NE, Ren Y, Ouyang Z, van Asten A, Cooks RG. Paper spray and extraction spray mass spectrometry for the direct and simultaneous quantification of eight drugs of abuse in whole blood. Anal Chem. 2014;86:7712–8. doi: 10.1021/ac5016408. [DOI] [PubMed] [Google Scholar]
- 36.Zhang C, Manicke NE. Development of a paper spray mass spectrometry cartridge with integrated solid phase extraction for bioanalysis. Anal Chem. 2015;87:6212–9. doi: 10.1021/acs.analchem.5b00884. [DOI] [PubMed] [Google Scholar]
- 37.Gómez-Ríos GA, Pawliszyn J. Development of coated blade spray ionization mass spectrometry for the quantification of target analytes present in complex matrices. Angew Chem Int Ed Engl. 2014;126:14731–5. doi: 10.1002/anie.201407057. [DOI] [PubMed] [Google Scholar]
- 38.Espy RD, Wleklinski M, Yan X, Cooks RG. Beyond the flask: reactions on the fly in ambient mass spectrometry. Trend Anal Chem. 2014;57:135–46. [Google Scholar]
- 39.Badu-Tawiah AK, Li A, Jjunju FP, Cooks RG. Peptide cross-linking at ambient surfaces by reactions of nanosprayed molecular cations. Angew Chem Int Ed Engl. 2012;51:9417–21. doi: 10.1002/anie.201205044. [DOI] [PubMed] [Google Scholar]
- 40.González-Serrano AF, Pirro V, Ferreira CR, Oliveri P, Eberlin LS, Heinzmann J, et al. Desorption electrospray ionization mass spectrometry reveals lipid metabolism of individual oocytes and embryos. PLoS One. 2013;8:e74981. doi: 10.1371/journal.pone.0074981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang JI, Tao WA, Cooks RG. Facile determination of double bond position in unsaturated fatty acids and esters by low temperature plasma ionization mass spectrometry. Anal Chem. 2011;83:4738–44. doi: 10.1021/ac1030946. [DOI] [PubMed] [Google Scholar]
- 42.Jackson AU, Shum T, Sokol E, Dill A, Cooks RG. Enhanced detection of olefins using ambient ionization mass spectrometry: Ag+ adducts of biologically relevant alkenes. Anal Bioanal Chem. 2011;399:367–76. doi: 10.1007/s00216-010-4349-5. [DOI] [PubMed] [Google Scholar]
- 43.Peng IX, Ogorzalek Loo RR, Shiea J, Loo JA. Reactive-electrospray-assisted laser desorption/ionization for characterization of peptides and proteins. Anal Chem. 2008;80:6995–7003. doi: 10.1021/ac800870c. [DOI] [PubMed] [Google Scholar]
- 44.Zhou X, Pei J, Huang G. Reactive paper spray mass spectrometry for in situ identification of quinones. Rapid Commun Mass Spectrom. 2015;29:100–6. doi: 10.1002/rcm.7092. [DOI] [PubMed] [Google Scholar]
- 45.Wu C, Ifa DR, Manicke NE, Cooks RG. Rapid, direct analysis of cholesterol by charge labeling in reactive desorption electrospray ionization. Anal Chem. 2009;81:7618–24. doi: 10.1021/ac901003u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tarjányi Z, Montskó G, Kenyeres P, Márton Z, Hágendorn R, Gulyás E, et al. Free and total cortisol levels are useful prognostic markers in critically ill patients: a prospective observational study. Eur J Endocrinol. 2014;171:751–9. doi: 10.1530/EJE-14-0576. [DOI] [PubMed] [Google Scholar]
- 47.Swords FM. Uncertainties in endocrine substitution therapy for central hypocortisolism. Handb Clin Neurol. 2014;124:387–96. doi: 10.1016/B978-0-444-59602-4.00026-5. [DOI] [PubMed] [Google Scholar]
- 48.Hendricks PI, Dalgleish JK, Shelley JT, Kirleis MA, Mc-Nicholas MT, Li L, et al. Autonomous in situ analysis and real-time chemical detection using a backpack miniature mass spectrometer: concept, instrumentation development, and performance. Anal Chem. 2014;86:2900–8. doi: 10.1021/ac403765x. [DOI] [PubMed] [Google Scholar]
- 49.Li L, Chen TC, Ren Y, Hendricks PI, Cooks RG, Ouyang Z. Mini 12, miniature mass spectrometer for clinical and other applications–introduction and characterization. Anal Chem. 2014;86:2909–16. doi: 10.1021/ac403766c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaiser RE, Jr, Cooks RG, Stafford GC, Jr, Syka JEP, Hemberger PH. Operation of a quadrupole ion trap mass spectrometer to achieve high mass/charge ratios. Int J Mass Spectrom Ion Proc. 1991;106:79–115. [Google Scholar]
- 51.Sinha MP, Tomassian AD. Development of a miniaturized, lightweight magnetic-sector for a field-portable mass spectrograph. Rev SciInstrum. 1991;62:2618–20. [Google Scholar]
- 52.Gao L, Song Q, Patterson GE, Cooks RG, Ouyang Z. Handheld rectilinear ion trap mass spectrometer. Anal Chem. 2006;78:5994–6002. doi: 10.1021/ac061144k. [DOI] [PubMed] [Google Scholar]
- 53.Gao L, Sugiarto A, Harper JD, Cooks RG, Ouyang Z. Design and characterization of a multisource hand-held tandem mass spectrometer. Anal Chem. 2008;80:7198–205. doi: 10.1021/ac801275x. [DOI] [PubMed] [Google Scholar]
- 54.Lammert SA, Rockwood AA, Wang M, Lee ML, Lee ED, Tolley SE, et al. Miniature toroidal radio frequency ion trap mass analyzer. J Am Soc Mass Spectrom. 2006;17:916–22. doi: 10.1016/j.jasms.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 55.Shimma S, Nagao H, Aoki J, Takahashi K, Miki S, Toyoda M. Miniaturized high-resolution time-of-flight mass spectrometer MULTUM-S II with an infinite flight path. Anal Chem. 2010;82:8456–63. doi: 10.1021/ac1010348. [DOI] [PubMed] [Google Scholar]
- 56.Zhai Y, Feng Y, Wei Y, Wang Y, Xu W. Development of a miniature mass spectrometer with continuous atmospheric pressure interface. Analyst. 2015;140:3406–14. doi: 10.1039/c5an00462d. [DOI] [PubMed] [Google Scholar]
- 57.Wright S, Malcolm A, Wright C, O’Prey S, Crichton E, Dash N, et al. A microelectromechanical systems-enabled, miniature triple quadrupole mass spectrometer. Anal Chem. 2015;87:3115–22. doi: 10.1021/acs.analchem.5b00311. [DOI] [PubMed] [Google Scholar]
- 58.Kirby AE, Lafrenière NM, Seale B, Hendricks PI, Cooks RG, Wheeler AR. Analysis on the go: quantification of drugs of abuse in dried urine with digital microfluidics and miniature mass spectrometry. Anal Chem. 2014;86:6121–9. doi: 10.1021/ac5012969. [DOI] [PubMed] [Google Scholar]
- 59.Ma Q, Bai H, Li W, Wang C, Cooks RG, Ouyang Z. Rapid analysis of synthetic cannabinoids using a miniature mass spectrometer with ambient ionization capability. Talanta. 2015;142:190–6. doi: 10.1016/j.talanta.2015.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin NJ, Griffiths RL, Edwards RL, Cooper HJ. Native liquid extraction surface analysis mass spectrometry: analysis of non-covalent protein complexes directly from dried substrates. J Am Soc Mass Spectrom. 2015;26:1320–7. doi: 10.1007/s13361-015-1152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keil A, Talaty N, Janfelt C, Noll RJ, Gao L, Ouyang Z, Cooks RG. Ambient mass spectrometry with a handheld mass spectrometer at high pressure. Anal Chem. 2007;79:7734–9. doi: 10.1021/ac071114x. [DOI] [PubMed] [Google Scholar]
- 62.Saugy M, Lundby C, Robinson N. Monitoring of biological markers indicative of doping: the athlete biological passport. Br J Sports Med. 2014;48:827–32. doi: 10.1136/bjsports-2014-093512. [DOI] [PubMed] [Google Scholar]
- 63.Warsinke A. Point-of-care testing of proteins. Anal Bioanal Chem. 2009;393:1393–405. doi: 10.1007/s00216-008-2572-0. [DOI] [PubMed] [Google Scholar]
- 64.Manicke NE, Belford M. Separation of opiate isomers using electrospray ionization and paper spray coupled to high-field asymmetric waveform ion mobility spectrometry. J Am Soc Mass Spectrom. 2015;26:701–5. doi: 10.1007/s13361-015-1096-z. [DOI] [PubMed] [Google Scholar]
- 65.Baird Z, Pu W, Cooks RG. Ion creation, ion focusing, ion/molecule reactions, ion separation, and ion detection in the open air in a small plastic device. Analyst. 2015;140:696–700. doi: 10.1039/c4an01929f. [DOI] [PubMed] [Google Scholar]
- 66.Salentijn GI, Permentier HP, Verpoorte E. 3D-printed paper spray ionization cartridge with fast wetting and continuous solvent supply features. Anal Chem. 2014;86:11657–65. doi: 10.1021/ac502785j. [DOI] [PubMed] [Google Scholar]
- 67.Narayanan R, Sarkar D, Cooks RG, Pradeep T. Molecular ionization from carbon nanotube paper. Angew Chem Int Ed Engl. 2014;53:5936–40. doi: 10.1002/anie.201311053. [DOI] [PubMed] [Google Scholar]
- 68.Food and Drug Administration. Framework for regulatory oversight of laboratory developed tests (LDTS) 2014 http://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm416685.pdf (Accessed June 2015)
- 69.Cody RB, Laramée JA, Durst HD. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal Chem. 2005;77:2297–302. doi: 10.1021/ac050162j. [DOI] [PubMed] [Google Scholar]
- 70.Lesiak A, Adams KJ, Domin MA, Henck C, Shepard JR. DART-MS for rapid, preliminary screening of urine for DMAA. Drug Test Anal. 2014;6:788–94. doi: 10.1002/dta.1540. [DOI] [PubMed] [Google Scholar]
- 71.Gómez-Ríosa GA, Pawliszyn J. Solid phase microextraction (SPME)-transmission mode (TM) pushes down detection limits in direct analysis in real time (DART) Chem Commun. 2014;50:12937–40. doi: 10.1039/c4cc05301j. [DOI] [PubMed] [Google Scholar]
- 72.Huang G, Chen H, Zhang X, Cooks RG, Ouyang Z. Rapid screening of anabolic steroids in urine by reactive desorption electrospray ionization. Anal Chem. 2007;79:8327–32. doi: 10.1021/ac0711079. [DOI] [PubMed] [Google Scholar]
- 73.Rosting C, Pedersen-Bjergaard S, Hansen SH, Janfelt C. High-throughput analysis of drugs in biological fluids by desorption electrospray ionization mass spectrometry coupled with thin liquid membrane extraction. Analyst. 2013;138:5965–72. doi: 10.1039/c3an00544e. [DOI] [PubMed] [Google Scholar]
- 74.Wang H, Ren Y, McLuckey MN, Manicke NE, Park J, Zheng L, et al. Direct quantitative analysis of nicotine alkaloids from biofluid samples using paper spray mass spectrometry. Anal Chem. 2013;85:11540–4. doi: 10.1021/ac402798m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Naccarato A, Moretti S, Sindona G, Tagarelli A. Identification and assay of underivatized urinary acylcarnitines by paper spray tandem mass spectrometry. Anal Bioanal Chem. 2013;405:8267–76. doi: 10.1007/s00216-013-7232-3. [DOI] [PubMed] [Google Scholar]
- 76.Takyi-Williams J, Dong X, Gong H, Wang Y, Jian W, Liu CF, Tang K. Application of paper spray-MS in PK studies using sunitinib and benzethonium as model compounds. Bioanalysis. 2015;7:413–23. doi: 10.4155/bio.14.288. [DOI] [PubMed] [Google Scholar]
- 77.Girod M, Moyano E, Campbella DI, Cooks RG. Accelerated bimolecular reactions in microdroplets studied by desorption electrospray ionization mass spectrometry. Chem Sci. 2011;2:501–10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


