Abstract
Objective:
To examine the effect of age of first exposure to tackle football on CTE pathological severity and age of neurobehavioral symptom onset in tackle football players with neuropathologically-confirmed CTE.
Methods:
The sample included 246 tackle football players who donated their brains for neuropathological examination. 211 were diagnosed with CTE (126/211 were without comorbid neurodegenerative diseases) and 35 were without CTE. Informant interviews ascertained age of first exposure and age of cognitive and behavioral/mood symptom onset.
Results:
Analyses accounted for decade and duration of play. Age of exposure was not associated with CTE pathological severity, or Alzheimer’s disease or Lewy body pathology. In the 211 participants with CTE, every one year younger participants began to play tackle football predicted earlier reported cognitive symptom onset by 2.44 years (p<0.0001) and behavioral/mood symptoms by 2.50 years (p<0.0001). Age of exposure before 12 predicted earlier cognitive (p<0.0001) and behavioral/mood (p<0.0001) symptom onset by 13.39 and 13.28 years, respectively. In participants with dementia, younger age of exposure corresponded to earlier functional impairment onset. Similar effects were observed in the 126 CTE only participants. Effect sizes were comparable in participants without CTE.
Interpretation:
In this sample of deceased tackle football players, younger age of exposure to tackle football was not associated with CTE pathological severity, but predicted earlier neurobehavioral symptom onset. Youth exposure to tackle football may reduce resiliency to late life neuropathology. These findings may not generalize to the broader tackle football population and informant-report may have affected the accuracy of the estimated effects.
INTRODUCTION
Chronic traumatic encephalopathy (CTE) is a neurodegenerative disease associated with exposure to repetitive head impacts (RHI), such as those from tackle football.1–4 In a convenience sample of 202 deceased tackle football players, CTE was neuropathologically diagnosed in 177 participants.5 CTE is pathologically characterized by an abnormal perivascular deposition of hyperphosphorylated tau (p-tau) in neurons and astrocytes at the depths of the cerebral sulci.2,3 CTE can only be diagnosed through neuropathological examination using defined criteria.2 Provisional research criteria for diagnosing CTE during life have been proposed (e.g., Traumatic Encephalopathy Syndrome; TES).6 CTE presents clinically with early- to mid-life behavioral/mood disturbances (e.g., depression), and/or later-life cognitive impairment (e.g., executive and episodic memory dysfunction).3,6
There is heterogeneity in the clinical expression of CTE and there is inter-individual variability in the age of symptom onset, even in cases with comparable neuropathological load.3,7 A subset of individuals with CTE have been asymptomatic.3 This clinical variability may be related to individual differences in genetic, clinical, or athletic history factors that could influence the expression of the disease. For example, a lower cognitive reserve has been linked with earlier cognitive and behavioral/mood symptom onset in former professional football players with severe CTE.8 CTE pathological severity may be influenced by factors such as duration of tackle football play, age at death,3 and beta-amyloid pathology.7 Notably, the type of contact sport played may modify the presence and severity of CTE pathology and related clinical symptoms. For example, in vivo research has found minimal clinical impairments in former ice hockey and rugby players.9,10 Overall, modifiers of the clinical and pathological expression of CTE continue to be a target of ongoing research.
Living former National Football League (NFL) players who began playing tackle football before age 12 have been shown to exhibit worse cognition11 and fiber path integrity of the anterior corpus callosum12 in middle age compared to those who began playing at age 12 or older. Younger age of first exposure (AFE) to tackle football has also been linked with smaller thalamic volume in former NFL players.13 In a recent study, younger AFE to tackle football (before age 12, in particular) predicted increased odds for self-reported neuropsychiatric and executive impairment in 214 former amateur and professional tackle football players.14 A single season of youth tackle football has corresponded with white matter changes in 8 to 13 year old youth tackle football players.15 The ages of youth tackle football coincide with a timeframe of peak neurodevelopment, including macrostructural, microstructural, and neural network development.16–31 It is possible that youth exposure to RHI may disrupt neurodevelopment to lower the threshold for later life clinical dysfunction. Nevertheless, the evidence on youth tackle football and long-term neurological dysfunction remains unclear, as AFE to tackle football was not associated with clinical outcomes in a sample of 45 former NFL players.32 Cognitive and mood difficulties were also not observed in individuals who played high school tackle football in Wisconsin during the 1950’s.33
The influence of AFE to tackle football on the clinical and pathological expression of CTE has not been examined. This study investigated the relationship between AFE to tackle football and stage of CTE pathology,3 (and other neurodegenerative disease neuropathology) as well as its effect on age of neurobehavioral symptom onset in 211 amateur and professional football players with autopsy-confirmed CTE. Exploratory analyses examined the relationship between AFE and age of neurobehavioral symptom onset in a small subset of deceased football players without CTE.
MATERIALS AND METHODS
Participants
The sample included amateur and professional tackle football players whose brains were donated to the Veteran’s Affairs-Boston University-Concussion Legacy Foundation (VA-BU-CLF) Brain Bank. This study is part of the National Institutes of Health-funded U01 project, entitled, “Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE).” Study details for UNITE have been reported elsewhere.34 For a majority of brain donations, next of kin contacted the brain bank to donate near the time of death or following death. Other brain donors were referred by medical examiners, recruited by the Concussion Legacy Foundation, or agreed to donation during life through the Brain Donation Registry. Inclusion criteria are broad to optimize generalizability and include a history of RHI exposure. Symptomatic status is not part of eligibility criteria. Brain donors were excluded for poor brain and spinal cord specimen quality. Next of kin provided written informed consent for participation and brain donation at Boston University’s CTE Center. The institutional review board at Boston University Medical Center approved brain donation procedures. Study data were collected and managed using REDCap electronic data capture tools.35
This sample of brain donors included 211 tackle football players neuropathologically diagnosed with CTE (i.e., CTE+), some of whom had comorbid neurodegenerative diseases, and 35 football players without CTE (i.e., CTE−). Of the 211 CTE+ participants, 126 were without other neurodegenerative diseases (i.e., CTE only). In the remaining participants, comorbid neurodegenerative disease neuropathological diagnoses included AD (n=27), Lewy body disease (LBD; n=39), frontotemporal lobar degeneration (FTLD-tau=11, FTLD-TDP=8), motor neuron disease (n=13), and/or prion disease (n=2). Nine of the 35 CTE− tackle football players had no (non-terminal) neuropathological diagnostic changes. In the remaining participants, neuropathological diagnoses included AD (n=4), LBD (n=3), FTLD (FTLD-tau=3, FTLD-TDP=1), moderate to severe vascular disease (based on arteriolosclerosis and/or atherosclerosis; n=8), unspecified tauopathy not meeting criteria for CTE (n=7), and non-specific changes (e.g., heme-laden macrophages, axonal injury; n= 9).
Informant Retrospective Clinical Evaluation
Clinical data for the current study were obtained through a combination of online questionnaires and retrospective telephone clinical interviews with informants of participants (see Stern et al.3 and Mez et al.34). Behavioral neurologists and/or neuropsychologists with expertise in neurodegenerative disorders conducted clinical interviews to obtain a detailed medical and clinical history, including the presence, nature, and timeline of symptoms related to cognitive and neuropsychiatric function, as well as other neurological and medical histories and symptoms. Clinicians qualitatively summarized the clinical symptoms and course in a narrative and presented it to a multidisciplinary diagnostic consensus team of clinicians in order to adjudicate whether the participant met criteria for ante-mortem dementia.34
Telephone interviews with informants were also used to determine the absence or presence and age of onset of cognitive and behavior/mood symptoms. Methods for symptom assessment have been described elsewhere.34,36 The specific cognitive domains assessed included attention, episodic memory, executive, language, and visuospatial functioning. For behavioral/mood disturbances, symptoms associated with apathy, depression, mania, anxiety and related disorders (e.g., obsessive compulsive disorder), and impulse control and aggression were evaluated. The informant reported the age cognitive and behavioral/mood symptoms began. Age of onset was used for the initial presenting symptom. Consistent with TES criteria6, behavioral and mood symptoms were combined into a single behavioral/mood domain (i.e., earliest age of onset of behavioral or mood symptoms). The symptoms emphasized in this study are consistent with the clinical features part of the provisional clinical research diagnostic criteria for CTE (i.e., TES).3,6 Informants were also administered the Functional Activities Questionnaire (FAQ) to determine the presence of functional impairment and the age functional impairment began (if present). The FAQ was not initially part of the UNITE protocol and thus 157 of the 246 participants had available FAQ data.
Demographics, educational attainment, athletic history (sports played, level, position, AFE to football, and duration), military history, and traumatic brain injury (TBI) history were evaluated through a combination of the above described telephone interview and online questionnaires completed by the informants. Informants were asked to identify the age the participant began to play organized tackle football. Professional position and duration of play were verified through online databases (justsportsstats.com).
Researchers conducting the interviews were blind to neuropathological findings, and informants were unaware of the neuropathological results. On average, 2.22 (SD=1.45) (N=243) informants participated in the interview. 99 of the informant interviews were with multiple first-degree family members (e.g., children, siblings, parents), 65 were with spouses/significant others, 22 were with children, 30 included a combination of first degree and non-immediate family members, 20 were with parents, 6 were with siblings, and 1 interview was with a friend of the participant. The mean length of relationship between the informant (if multiple informants, longest length of relationship reported was used) and participant was 43.98 (SD=16.60) years (N=193). Clinician’s rated informant reliability using a 0 to 2 scale (0=not reliable, 1=somewhat reliable, 2=very reliable). The sample had an average reliability rating of 1.6 (SD=0.53). (Rating of informant reliability was not initially conducted and was available for 151 participants of this sample).
Pathological Diagnoses
Methodological procedures for pathological processing and evaluation have been published elsewhere.37,38 Neuropathologists were blind to clinical data during examination of the brain tissue. Non-CTE neurodegenerative diseases (i.e., AD, LBD, FTLD, motor neuron disease, prion disease) were diagnosed using established neuropathological diagnostic criteria (refer to Mez et al.5). AD and LBD were the most frequent comorbid neurodegenerative diseases and therefore variables of AD neuropathology (i.e., Braak stage, CERAD neuritic plaque score, Alzheimer’s Disease Neuropathologic Change [ADNC]) and Lewy body pathology (absence or presence of brainstem, limbic, neocortical, amygdala, or olfactory Lewy bodies) served as additional outcomes. The neuropathological diagnosis of CTE was made using neuropathological criteria defined by an National Institutes of Neurological Disease and Stroke (NINDS)/National Institute of Brain Imaging and Behavior (NIBIB) consensus panel.2 Neuropathological criteria for CTE require at least one perivascular lesion of p-tau aggregates in neurons as neurofibrillary tangles (NFTs), astrocytes, and cell processes around a small vessel, typically found at the sulcal depths (“CTE lesion”). CTE pathological severity was graded using a provisional four stage classification scheme that is based on the extent and severity of tau pathology3:
Stage I: 1 or 2 focal perivascular CTE lesions in cerebral cortex at the depths of the sulci.
Stage II: ≥3 CTE lesions in multiple cortical regions and superficial NFTs along the sulcal wall and gyral crests.
Stage III: Multiple CTE lesions, widespread cortical NFTs, and NFTs in the medial temporal lobes.
Stage IV: Multiple CTE lesions, widespread cortical NFTs, NFTs in the medial temporal lobes, widespread astrocytic p-tau pathology, neuronal loss, and gliosis.
This classification of CTE pathological severity has been supported through several empirical studies examining the clinical and neuropathological correlates of CTE.1,3,7,8,39
Statistical Analyses
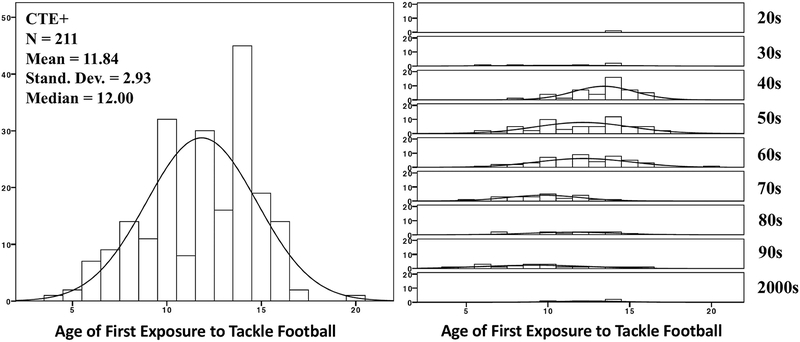
Analyses were first conducted in participants with CTE+ (n = 211) and CTE only (n = 126). To optimize equal group sizes across the CTE stages (stage I as least severe thru stage IV as most severe), stages I and II were combined (n = 61), as were stages III and IV (n = 150). All analyses examined AFE as a continuous variable and dichotomized into before age 12 and 12 or older. Age 12 was examined based on research in living former NFL players.11,12 Participants with AFE to tackle football after 12 were less likely to initiate play after the 1960s (Fig 1). Older participants in the sample may have had less of an opportunity to play organized youth tackle football because youth football did not become nationally promoted until approximately 1960 (http://www.popwarner.com/about_us/history.htm). As such, we used instrumental variable modeling,40,41 with decade at age 12 as the instrument, to examine the effect of AFE to tackle football on CTE pathological severity and age of cognitive and behavioral/mood symptom onset (a separate model for each outcome was performed). Inclusion of decade at age 12 as the instrument assumes that it is associated with the outcome only through its effect on AFE. Because the age at which an individual began to start playing tackle football could determine the decade of tackle football play, the decade at age 12 was used to avoid violation of the assumption that AFE effects the instrumental variable. The instrumental variable model involves a two-stage regression. The first stage estimated the effect of decade at age 12 on AFE. In the second stage, predicted values from the association between decade at age 12 and AFE in the first stage were used to predict stage of CTE pathology and age of cognitive and behavioral/mood symptom onset. Secondary instrumental variable models examined the relationship between AFE to tackle football and variables of AD (Braak stage, CERAD [none/sparse versus moderate/frequent], ADNC) and Lewy body pathology (absence or presence), as well as the effect of AFE to tackle football on age of functional impairment onset in CTE participants who were determined by the consensus panel to have had ante-mortem dementia.
Figure 1. Sample Distribution of Age of First Exposure to Tackle Football and Decade Participants Began to Play Tackle Football.
CTE+ = the presence of chronic traumatic encephalopathy and comorbid neurodegenerative disease. The Figure on the right presents the distribution of AFE to tackle football by decade of play in the CTE+ participants. Decade of tackle football play refers to when the subject began to play tackle football and ranges from the 1920s to the 2000s.
An exploratory instrumental variable model (with decade at age 12 as the instrument and duration of football play as a covariate) was conducted to examine the relationship between AFE and age of cognitive and behavioral/mood symptom onset in the 35 CTE− tackle football players. The objective of this analysis was to help clarify whether the effects of AFE to tackle football on later-life clinical status is specific to CTE.
Duration of play served as a covariate in all analyses to account for differences in RHI exposure and capture the unique variance of AFE to tackle football, which we hypothesize is not a marker for duration of play. In addition to duration of play, age at death was included as a covariate in analyses examining stage of CTE pathology (and the other neurodegenerative pathology variables) as the outcome due its association with pathological severity.3Age at death, however, was not included as a covariate in the age of symptom onset models as it is a collider variable with casual pathways from AFE to tackle football and age of symptom onset to age at death. Conditioning of a collider variable (statistical control or stratification) can lead to erroneous estimates due to endogenous selection bias.42 Furthermore, there is a temporal discord between age at death and age of symptom onset, i.e., age at death occurs after age of symptom onset.
RESULTS
AFE and CTE
Tables 1–2 present sample characteristics. Figure 1 shows the sample distribution of AFE to tackle football and decade of tackle football play. Among the 211 participants with CTE+, 183 developed both cognitive and behavioral/mood symptoms prior to death, 8 had only cognitive symptoms, 12 had only behavioral/mood symptoms, 7 did not endorse any symptoms examined in this study, and clinical data were not available for 1 participant. Of the 210 participants with clinical data, three had missing data for age of behavioral/mood symptom onset. Participants with only cognitive or behavioral/mood symptoms were only included in their respective analyses, and asymptomatic participants were not part of analyses examining age of symptom onset. Following exclusion for missing data and consideration of symptomatic status, the final sample size for analyses examining age of cognitive symptom onset was 191, and 192 for age of behavioral/mood symptom onset. 125 of 209 CTE+ participants were determined by the consensus panel to have had ante-mortem dementia (two participants had missing ante-mortem dementia status data). Of the 125 CTE+ participants who were determined to have had ante-mortem dementia, data for age of functional impairment onset was available for 84 participants.
Table 1.
Sample Characteristics of the Deceased Tackle Football Players
| CTE+ (N = 211) |
No CTE (N = 35) |
AFE < 12 (n = 84) |
AFE ≥ 12 (n = 127) |
ap-value | |
|---|---|---|---|---|---|
| DEMOGRAPHIC/ATHLETIC | |||||
| Age of death, mean (SD) years | 62.91 (17.91) | 42.17 (22.40) | 53.45 (16.91) | 69.17 (15.72) | <0.001 |
| Race, n (%) African American | 41 (19.5) | 4 (11.4) | 19 (22.9) | 22 (17.3) | 0.32b |
| Education Level, n (%) | 0.80c | ||||
| Years of football play, mean (SD) | 15.07 (5.56) | 8.07 (4.56) | 16.73 (5.60) | 13.97 (5.27) | <0.001 |
| Decade of football play, mean (SD)d | 5.78 (1.68) | 7.74 (2.19) | 6.42 (1.70) | 5.36 (1.54) | <0.001 |
| Age of first exposure to football, mean (SD) | 11.84 (2.93) | 11.26 (2.76) | 8.79 (1.67) | 13.86 (1.45) | <0.001 |
| Highest level played, n (%) yes | <0.001e | ||||
| Football primary position, n (%) | 0.61f | ||||
| Other contact sport history, n (%) any | 36 (17.1) | 9 (25.7) | 15 (17.9) | 21 (16.5) | 0.80 |
| Military history, n (%) yes | 53 (25.2) | 8 (22.9) | 12 (14.3) | 41 (32.5) | 0.003 |
| MEDICAL/LIFESTYLE, n (%) | |||||
| Heart attack | 38 (21.2) | 5 (16.7) | 11 (15.3) | 27 (25.2) | 0.11 |
| Hypertension | 95 (53.4) | 9 (30.0) | 30 (42.9) | 65 (60.2) | 0.024 |
| Elevated cholesterol | 62 (35.2) | 11 (36.7) | 23 (32.9) | 39 (36.8) | 0.59 |
| Diabetes | 31 (17.2) | 3 (10.0) | 8 (11.4) | 23 (20.9) | 0.10 |
| Stroke | 23 (12.8) | 4 (13.3) | 3 (4.2) | 20 (18.5) | 0.005 |
| Alcohol abuse | 85 (40.7) | 16 (45.7) | 36 (43.4) | 49 (38.9) | 0.52 |
| Illicit drug use | 79 (37.6) | 20 (57.1) | 39 (47.0) | 40 (31.5) | 0.023 |
| Steroid use | 8 (5.8) | 2 (10.0) | 2 (3.6) | 6 (7.1) | 0.39 |
| CLINICAL STATUS | |||||
| Age of cognitive symptom onset, mean (SD) | 53.28 (17.12) | 43.08 (20.48) | 45.65 (16.14) | 58.11 (15.99) | <0.001 |
| Age of behavioral/mood symptom onset, mean (SD) | 46.28 (19.36) | 35.54 (18.22) | 38.97 (17.38) | 50.96 (19.18) | <0.001 |
| Tremor, n (%) yes | 60 (28.7) | 10 (28.6) | 20 (24.1) | 40 (31.7) | 0.23 |
| Falls, n (%) yes | 73 (35.1) | 9 (25.7) | 20 (24.4) | 53 (42.1) | 0.009 |
| Gait disturbance, n (%) yes | 98 (46.9) | 10 (28.6) | 32 (38.6) | 66 (52.4) | 0.050 |
Examined differences between AFE groups in the CTE participants. Independent sample t-tests examined between AFE group differences for age of death, years of tackle football play, age of first exposure, decade of play, and age of cognitive and behavioral/mood symptom onset. Chi-square analysis was used for the remaining variables, with the exception of stage of CTE for which logistic regression was used to control for age of death.
Compared African American vs. other;
compared college degree or greater vs. others;
0 = 1900s, 1 = 1910s, 2 = 1920s, 3 = 1930s, 4 = 1940s, 5 = 1950s, 6 = 1970s, 7 = 1980s, 9 = 1990s, and 10 = 2000s. No participant played before 1920s;
compared professional vs. others;
compared lineman vs. others, excluding other/multiple or unknown.
Missing data: CTE—N = 210 for race, education level, position, military history, and illicit drug use; N = 179 for heart attack and stroke; N = 178 for hypertension; N = 176 for elevated cholesterol; N = 180 for diabetes; N = 209 for alcohol abuse, tremor, and gait disturbance; N = 139 for steroid use; N = 208 for falls. No CTE—N = 34 for education level, years of football, decade of play, and age of first exposure; N = 32 for position; N = 30 for heart attack, hypertension, elevated cholesterol, diabetes, and stroke; N = 20 for steroid use.
Table 2.
Demographic, Athletic, and Clinical Characteristics by CTE Severity (N = 211)
| CTE Stage I/II (n = 61) |
CTE Stage III/IV (n = 150) |
p-value* | |
|---|---|---|---|
| DEMOGRAPHIC/ATHLETIC | |||
| Age of death, mean (SD) years | 49.13 (20.30) | 68.51 (13.30) | <0.001 |
| Race, n (%) African American | 9 (15.0) | 32 (21.3) | 0.031a |
| Education Level, n (%) | 0.42b | ||
| Years of football play, mean (SD) | 12.61 (4.48) | 16.07 (5.65) | <0.001 |
| Age of first exposure to football, mean (SD) | 11.07 (3.23) | 12.15 (2.74) | 0.25 |
| Decade of play, mean (SD)c | 7.08 (1.90) | 5.25 (1.25) | 0.24 |
| Highest Level played, n (%) yes: | 0.001d | ||
| Football primary position, n (%): | 0.73e | ||
| Other contact sport history, n (%) any | 16 (26.2) | 20 (13.3) | 0.024 |
| Military history, n (%) yes | 10 (16.4) | 43 (28.9) | 0.09 |
| MEDICAL/LIFESTYLE, n (%) | |||
| Heart attack | 6 (11.8) | 32 (25.0) | 0.47 |
| Hypertension | 22 (42.3) | 73 (57.9) | 0.83 |
| Elevated cholesterol | 15 (30.0) | 47 (37.3) | 0.99 |
| Diabetes | 5 (9.8) | 26 (20.2) | 0.45 |
| Stroke | 6 (11.8) | 17 (13.3) | 0.30 |
| Alcohol abuse | 27 (45.8) | 58 (38.7) | 0.67 |
| Illicit drug use | 26 (42.6) | 53 (35.6) | 0.16 |
| Steroid use | 2 (4.9) | 6 (6.1) | 0.45 |
| CLINICAL STATUS | |||
| Age of cognitive symptom onset, mean (SD) | 42.23 (18.23) | 56.99 (15.07) | 0.35 |
| Age of behavioral/mood symptom onset, mean (SD) | 34.17 (16.08) | 50.77 (18.57) | 0.12 |
| Tremor, n (%) yes | 13 (21.3) | 47 (31.8) | 0.997 |
| Falls, n (%) yes | 13 (21.3) | 60 (40.8) | 0.86 |
| Gait disturbance, n (%) yes | 18 (29.5) | 80 (54.1) | 0.37 |
Logistic regression was used to examine the relationship between the predictor variables and stage of CTE, controlling for age of death (logistic regression for age of death was unadjusted).
compared African American vs. others;
compared college degree or greater vs. others;
Scale included: 0 = 1900s, 1 = 1910s, 2 = 1920s, 3 = 1930s, 4 = 1940s, 5 = 1950s, 6 = 1970s, 7 = 1980s, 9 = 1990s, and 10 = 2000s. No participant played before 1920s;
compared professional vs. others;
compared lineman vs. others, excluding unknown and other/multiple.
Missing data: CTE Stage I/II—N = 60 for race, education level, and position; N = 51 for heart attack, diabetes, and stroke; N = 52 for hypertension; N = 50 for elevated cholesterol; N = 59 for alcohol abuse; N = 41 for steroid use. CTE Stage III/IV—N = 149 for military history; N = 128 for heart attack; N = 126 for hypertension and elevated cholesterol; N = 129 for diabetes; N = 128 for stroke; N = 149 for illicit drug use; N = 98 for steroid use; N = 148 for tremor and gait disturbance; N = 147 for falls
AFE and Neurodegenerative Disease Pathology.
See Table 3 for a description of neuropathology in the sample. The relationship between AFE (continuous or dichotomized based on age 12) and CTE pathological severity was not statistically significant in the CTE+ or CTE only participants (p’s>0.10). In the CTE+ participants, AFE to tackle football (continuous or dichotomized based on age 12) was not associated with Braak stage, CERAD score, ADNC or the presence of Lewy body pathology (p’s>0.05).
Table 3.
Neuropathology Characteristics
| CTE+* | AFE < 12 (n = 84) |
AFE ≥ 12 (n = 127) |
|
|---|---|---|---|
| Stage of CTE pathology, n (%) | |||
| Braak stage, n (%) | |||
| CERAD, n (%) | |||
| ADNC, n (%) | |||
| Lewy body, n (%) present* | 48 (23.1) | 17 (20.5) | 31 (24.8) |
Instrumental variable model showed that AFE to tackle football (continuous and dichotomized into before age 12 and 12 or older) was not associated with stage of CTE pathology, Braak stage, CERAD (none/sparse versus moderate/severe), ADNC, or presence of Lewy body pathology (ps>0.05). Duration of play and age at death were included as a covariates and decade at age 12 served as the instrument variable. Lewy body pathology was determined by absence or presence of brainstem, limbic, neocortical, amygdala, or olfactory Lewy bodies. Abbreviations: CERAD = Consortium to Establish a Registry for Alzheimer’s Disease neuritic plaque score; ADNC = Alzheimer’s Disease Neuropathologic Change. Due to missing data sample sizes include: Braak stage=204, CERAD=208, ADNC=209; Lewy body=208.
CTE+ (N = 211).
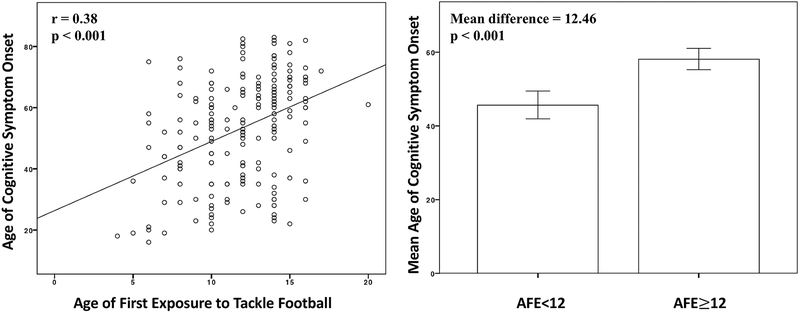
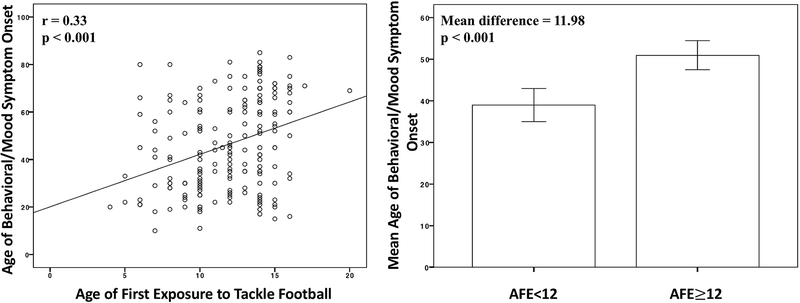
Table 4 shows the unadjusted effects of the predictor variables on age of cognitive and behavioral/mood symptom onset. Figures 2–3 show the unadjusted association between AFE and age of cognitive and behavioral/mood symptom onset. In the 211 tackle football players with CTE, results from the instrumental variable model revealed that every one year younger that participants began to play tackle football resulted in earlier onset of cognitive symptoms by 2.44 years (p<0.0001, 95% CI=1.63, 3.25), and earlier onset for behavioral/mood symptoms by 2.50 years (p<0.0001, 95% CI=1.59, 3.41). AFE before age 12 predicted earlier cognitive (p<0.0001, 95% CI=8.58, 18.20) and behavioral/mood (p<0.0001, 95% CI=7.82, 18.74) symptom onset by 13.39 years and 13.28 years, respectively. See Table 5. Sensitivity analyses showed that statistical significance remained when level of play was added as a covariate (ps<0.01; the 10 semi-professional football players were excluded from these analyses).
Table 4.
Effects of Decade of Play, Decade at Age 12, and Years of Tackle Football Play on Age of Symptom Onset in 211 Tackle Football Players with CTE
| Predictors | Age of Cognitive Symptom Onset |
Age of Behavioral/Mood Symptom Onset |
||||
|---|---|---|---|---|---|---|
| Unstandardized beta (S.E.) | 95% CI | t-test statistic (p-value) |
Unstandardized beta (S.E.) | 95% CI | t-test statistic (p-value) |
|
| Decade of Tackle Football Play (per increase in decade) | −8.59 (0.45) | −9.47, −7.71 |
−19.31 (<0.001) | −7.80 (0.66) | −9.09, −6.51 |
−11.92 (<0.001) |
| Decade at Age 12 (per increase in decade) |
−8.15 (0.40) | −8.94, −7.35 |
−20.20 (<0.001) | −7.36 (0.59) | −8.53, −6.19 |
−12.39 (<0.001) |
| Years of Tackle Football Play | 0.08 (0.23) | −0.37, 0.52 |
0.33 (0.74) | 0.28 (0.25) | −0.22, 0.78 |
1.11 (0.27) |
Linear regression was used to examine the unadjusted associations between the predictor variables and age of cognitive and behavioral/mood symptom onset. CI = confidence intervals
Figure 2. Relationship between Age of First Exposure to Tackle Football and Age of Cognitive Symptom Onset in 211 Tackle Football Players with CTE.
Left Figure is a scatter plot of the relationship between age of first exposure (AFE) to tackle football and age of cognitive symptom onset. Bivariate correlations tested this relationship and it was statistically significant (r=0.38, p<0.001). The right Figure presents a bar graph comparing the mean age of cognitive symptom onset in participants who began playing tackle football before age 12 and those who began playing at 12 or older. Independent samples t-tests showed this to be a significant difference (p<0.001). Error bars are 95% confidence intervals.
Figure 3. Relationship between Age of First Exposure to Tackle Football and Age of Behavioral/Mood Symptom Onset in 211 Tackle Football Players with CTE.
Left Figure is a scatter plot of the relationship between age of first exposure (AFE) to tackle football and age of behavioral/mood symptom onset. Bivariate correlation tested this relationship and it was statistically significant (r=0.33, p<0.001). The right Figure presents a bar graph comparing the average age of behavioral/mood symptom onset in participants who began playing tackle football before age 12 and those who began playing at 12 or older. Independent samples t-tests showed this to be a significant difference (p<0.001). Error bars are 95% confidence intervals.
Table 5.
Instrumental Variable Modeling Examining AFE to Tackle Football and Age of Symptom Onset in Tackle Football Players with and without CTE
| Cognitive Symptom Onset | |||
|---|---|---|---|
| Unstandardized beta (S.E.) |
t-test statistic (p-value) |
95% CI | |
| CTE+ | |||
| Per year increase in AFE | 2.44 (0.41) | 6.02 (<0.0001) | 1.63, 3.25 |
| AFE<12 | 13.39 (2.44) | 5.49 (<0.0001) | 8.58, 18.20 |
| CTE Only |
Unstandardized
beta (S.E.) |
t-test
statistic (p-value) |
95% CI |
| Per year increase in AFE | 2.93 (0.51) | 5.70 (<0.0001) | 1.92, 3.94 |
| AFE<12 | 16.38 (3.16) | 5.19 (<0.0001) | 10.11, 22.64 |
| CTE− | |||
| Per year increase in AFE | 1.27 (2.17) | 0.58 (0.57) | −3.24, 5.78 |
| AFE<12 | 20.33 (10.33) | 1.97 (0.063) | −1.15, 41.81 |
| Behavioral/Mood Symptom Onset | |||
| Unstandardized beta (S.E.) |
t-test
statistic (p-value) |
95% CI | |
| CTE+ | |||
| Per year increase in AFE | 2.50 (0.46) | 5.44 (<0.0001) | 1.59, 3.41 |
| AFE<12 | 13.28 (2.77) | 4.79 (<0.0001) | 7.82, 18.74 |
| CTE Only | Unstandardized beta (S.E.) |
t-test
statistic (p-value) |
95% CI |
| Per year increase in AFE | 2.66 (0.55) | 4.87 (<0.0001) | 1.57, 3.75 |
| AFE<12 | 13.16 (3.41) | 3.86 (0.0002) | 6.40, 19.92 |
| CTE− | |||
| Per year increase in AFE | 2.49 (1.48) | 1.68 (0.11) | −0.57, 5.55 |
| AFE<12 | 22.42 (6.94) | 3.23 (0.0037) | 8.06, 36.78 |
AFE = Age of first exposure. Duration of play was included as a covariate and decade at age 12 served as the instrument variable, where the instrument assumes that it is associated with age of cognitive and behavioral mood symptom onset only through its effect on AFE. The instrumental variable model is a two-stage regression. The first stage estimated the effect of decade at age 12 on AFE. In the second stage, predicted values from the association between decade at age 12 and AFE in the first stage were used to predict age of cognitive and behavioral/mood symptom onset. Example of parameter estimate interpretation: For CTE+ participants, every one year younger participants started to play tackle football predicted earlier symptom onset by 2.44 and 2.50 years for cognitive and behavioral/mood symptom onset, respectively. AFE before age 12 was associated with onset of cognitive symptoms by 13.39 years earlier, and behavioral/mood symptoms by 13.28 years earlier.
The mean (SD) age of functional impairment onset was 63.10 (12.13). Every one year younger that participants began to play tackle football resulted in earlier onset of functional impairment by 1.17 years (t=2.54, p=0.0131, 95% CI=0.25, 1.17). AFE before age 12 predicted earlier functional impairment onset by 8.02 years (t=2.88, p=0.005, 95% CI=2.49, 8.02).
CTE only (n = 126).
In the 126 tackle football players with CTE only, every one year younger that participants began to play tackle football resulted in earlier onset of cognitive symptoms by 2.93 years (p<0.0001, 95% CI=1.92, 3.94), and earlier onset for behavioral/mood symptoms by 2.66 years (p<0.0001, 95% CI=1.57, 3.75). AFE before age 12 predicted earlier cognitive (p<0.0001, 95% CI=10.11, 22.64) and behavioral/mood (p=0.0002, 95% CI=6.40, 19.92) symptom onset by 16.38 years and 13.16 years, respectively.
Of the 56 CTE only participants who were determined to have had ante-mortem dementia, data for age of functional impairment onset was available for 41 participants. The mean (SD) age of functional impairment onset was 61.29 (12.89). Although there was no statistically significant association between AFE and age of functional impairment onset, this was a result of the reduced sample size. Effect sizes were similar in magnitude to those in the larger CTE+ sample. Every one year younger that participants began to play tackle football resulted in earlier onset of functional impairment by 1.02 years (t=1.36, p=0.182, 95% CI=−0.50, 1.03). AFE before age 12 predicted earlier functional impairment onset by 5.91 years (t=1.35, p=0.1836, 95% CI=−2.92, 5.91).
Exploratory Analyses: AFE and CTE− (N = 35)
Among the 35 CTE− participants, 25 had both cognitive and behavioral/mood symptoms, 9 had behavioral/mood symptoms only, and 1 did not endorse symptoms examined in this study. Four of the 35 participants had onset of behavioral/mood symptoms before they began to play tackle football and these were excluded from the behavioral/mood analyses; three of these participants had cognitive symptoms that started during or after they began to play tackle football and were included in analyses examining age of cognitive symptom onset. Two participants had missing data for age of behavioral/mood symptom onset, and 1 participant (who had cognitive and behavioral/mood symptoms) had missing data for AFE to tackle football. The final sample size for analyses examining age of cognitive symptom onset was 24, and 27 for age of behavioral/mood onset. Only 12 CTE− participants had ante-mortem dementia and therefore analyses examining the relationship between AFE and age of functional impairment onset in this subgroup were not conducted.
Although not all relationships were statistically significant due to the reduced sample size, there were comparable effect sizes to the CTE+ and CTE only participants. Every one year younger that participants began to play tackle football resulted in earlier onset of cognitive symptoms by 1.27 years (p=0.57, 95% CI=−3.24, 5.78) and behavioral/mood symptoms by 2.49 years (p=0.11, 95% CI=−0.57, 5.55). AFE before age 12 predicted earlier cognitive (p=0.063, 95% CI=−1.15, 41.81) and behavioral/mood (p=0.0037, 95% CI=8.06, 36.78) symptom onset by 20.33 years and 22.42 years, respectively.
Ascertainment Bias Sensitivity Analyses
Ascertainment bias is an important limitation of this study, as individuals or their families may be more likely to donate their brain if the participant experienced symptoms during life. Haneuse et al.43 examined factors that influence brain donation selection in the Adult Changes in Thought (ACT) study, a community-based sample of individuals age 65 and older. Factors that predicted brain donation included dementia status, age, sex, race, education, marital status, and depression. Haneuse et al. used inverse probability weighting (IPW) to statistically adjust for these selection factors in the examination of the relationship between neuropathological measures and risk of dementia and found that the magnitude of the associations strengthened. Factors that influence brain donation in the present autopsy sample of deceased tackle football players from the VA-BU-CLF brain bank are highly distinct from those associated with the older adult sample from the ACT study. However, to explore the potential influence of ascertainment bias in this sample, we repeated the instrumental variable model in the sample of CTE+ participants using IPW with the beta weights (extrapolated form Haneuse et al.) for ante-mortem dementia status, age, race, and depression included in the instrumental variable model regression equation. Due to missing data on the new variables, the sample size was reduced to 208. Sex and marital status were not included because the sample is all male and marital status data were not collected. Education was not included due to restricted variability in this sample. Statistical significance and the magnitude of the associations remained unchanged.
DISCUSSION
In this convenience sample of 211 amateur and professional tackle football players with neuropathologically-confirmed CTE from the UNITE study, there was not a statistically significant association between younger AFE to tackle football and CTE pathological severity, or AD and Lewy body pathology. However, younger AFE to tackle football predicted earlier reported cognitive and behavioral/mood symptom onset. Every one year younger that participants began to play football resulted in earlier reported onset of cognitive and behavioral/mood symptoms by approximately 2.5 years. AFE to football before age 12 corresponded to earlier reported onset of cognitive symptoms by 13.39 years, and behavioral/mood symptoms by 13.28 years. These findings were independent of level (i.e., high school, college, professional) and duration of play. Secondary analyses in a subset of the sample further showed younger AFE to tackle football was associated with earlier onset of functional impairment in participants who were determined by a consensus panel to have had ante-mortem dementia. Nearly identical effects were observed in participants with CTE only. Although many of the relationships between AFE and age of symptom onset were no longer statistically significant in the CTE− participants, this was due to the small sample size given effect sizes were comparable to the CTE participants. These results suggest that the relationship between younger AFE to tackle football and long-term neurobehavioral disturbances may not be specific to CTE.
AFE was not associated with CTE neuropathological severity, or AD and Lewy body pathology. This is comparable to research in AD that shows certain factors (e.g., cognitive reserve) influence clinical status, but are not necessarily related to the neuropathological expression of disease.44,45 RHI intensity and duration and their interaction with variables like age and genetics (e.g., APOE e4)3,7 may be more important determinants of pathology.3,39 In contrast, our findings that show a relationship between AFE and age of symptom onset are consistent with previous research in living former amateur and professional tackle football players that linked younger AFE to tackle football, before age 12 in particular, with worse cognitive, behavior, and mood outcomes.11,14 The clinical presentation of CTE seems to involve early-life behavioral/mood symptoms and/or later-life cognitive impairment. Nearly all reported cases of CTE have exhibited cognitive dysfunction at some point during their life, and those that present with cognitive impairment as an initial symptom tend to develop dementia.3 There is, however, heterogeneity in the presence and severity of symptoms.8,36 The etiologies of the symptoms (particularly in CTE− participants) reported in this sample are likely multifaceted, and could include CTE, axonal injury, idiopathic mental illness, to name a few. Given the exploratory analyses showed that AFE was associated with age of symptom onset in the participants without CTE, the biological mechanism underlying this relationship seems unlikely to be specific to CTE. Of note, nine participants without CTE had no neuropathological diagnostic changes, but still manifested symptoms, potentially related to aging or functional or neurochemical brain changes not captured by neuropathological examination.
The mechanisms for the relationship between AFE to tackle football and earlier symptom onset are unclear. One hypothesis to be tested in the future is that youth exposure to tackle football may reduce one’s ability to clinically compensate for a variety of RHI-related neurological consequences, including CTE or other neuropathology. CTE is characterized by an abnormal perivascular accumulation of p-tau as NFTs and astroglia at the depths of the cortical sulci.2,3 P-tau NFTs accumulate throughout the cortex with disease progression, and extends to involve the medial temporal lobe, diencephalon, and brainstem. Deceased tackle football players can exhibit an array of non-p-tau pathologies related to RHI, including diffuse axonal injury, blood-brain barrier dysfunction, and neuroinflammation. CTE is often comorbid with other neurodegenerative diseases (e.g., AD, LBD), which were present in the CTE+ participants and participants without CTE. Regardless of the neuropathology present, compensatory mechanisms can allow an individual to cope with pathology until a critical threshold is reached,45 a phenomenon known as cognitive reserve. Although this awaits empirical test, RHI from tackle football during critical neurodevelopmental time periods may reduce an individual’s resiliency to late life neuropathology. Brain maturation is at its peak between the ages of 9–12 in males when cortical thickness is at its maximum, particularly within the frontal and temporal lobes,21 as is the subcortical volume of regions such as the hippocampus and amygdala that are undergoing synaptic pruning.17,24 Helmet accelerometer research shows that youth football players can experience a median of 252 head impacts per season that can exceed a force of 80g, but 50% of impacts are between 10g to 20g.46 Exposure to these subconcussive head impacts during youth tackle football has been shown to result in microstructural brain changes following a single season of play.15 RHI, in general, is associated with macro- and microstructural, neurochemical, and functional brain alterations.47 Future work, including experimental animal models, is needed to study mechanisms that underpin the association between youth exposure to RHI and worse clinical function later in life.
There are several important limitations to our findings. The present study included a convenience sample of deceased tackle football players and did not include an appropriate control and/or comparison group. The effect of younger AFE to tackle football and earlier neurobehavioral symptom onset is specific to this sample of participants who are from the UNITE study and may not generalize to the broader tackle football population. In this study, informants of individuals who were symptomatic may have been more likely to donate their brain and the present sample may include the most severely affected individuals. Sensitivity analyses that examined the influence of ascertainment bias using results from the ACT study are limited because factors that influence brain donation for tackle football players are unique. In particular, although participation in the UNITE study is for research purposes only and independent of any ongoing class action lawsuits (e.g., those associated with the National Collegiate Athletic Association or NFL), tackle football players may have increased motivation to donate their brain to support their eligibility for compensation. Similarly, because the current study was cross-sectional, associations with age can arise from differential survival or age-related differences in how individuals were selected into the study.
An additional limitation is the use of informant interviews to determine AFE to tackle football and age of symptom onset. With informant-report, there is the possibility of misclassification. Informants of individuals who had severe and early onset symptoms may have been more likely to attribute the participant’s symptoms to tackle football and report an earlier AFE to tackle football and thus the observed associations may have been overestimated. The overestimation of effects may have been further exacerbated by the actual or perceived potential for impending compensation or litigation. However, if informants were similar in their ability to accurately recall events of the past (i.e., non-differential with respect to AFE and age of symptom onset), then the misclassification would be random and the observed associations would be underestimated. The current study is unable to differentiate between informant-reported descriptions of their loved one’s neurobehavioral symptoms from clinically-meaningful impairments determined by evaluation of the decedent during life. Prospective population-based studies of former tackle football players that include objective clinical assessments are needed to address the current limitations associated with sample selection and informant-report and increase our understanding of the effect of AFE to tackle football on the clinical presentation of CTE.
Instrumental variable modeling is an approach used to limit the influence of unmeasured confounders to facilitate inferences in observational studies.40,41 Here, it was used to reduce confound in the relationship between AFE to tackle football and CTE pathological severity and age of symptom onset. We accounted for confound from decade of play by including decade at age 12 as our instrument. Individuals who played tackle football before the 1960s had fewer opportunities to participate in youth tackle football, thereby introducing a relationship between decade of play and AFE. There may also be generational differences in the style that youth tackle football was played (e.g., in terms of equipment, types of exposure, number of games, type of fields played). The instrumental variable model makes several theoretical assumptions about the relationships among variables, including that decade at age 12 only affects symptom onset through AFE to tackle football and that there is not another variable that affects both decade at age 12 and age of symptom onset. Although this is a homogeneous sample of mostly professional tackle football players, potential differences in environmental and lifestyle factors could have violated these assumptions.
In unadjusted analyses, there was not a statistically significant relationship between duration of tackle football play and age of cognitive or behavioral/mood symptom onset (Table 4). This finding was unexpected, given more years of tackle football was associated with worse CTE pathological severity in this sample. Although we examined duration of tackle football play, decade of play, and position(s) and level(s) played, other exposure factors were not included in this study (e.g., cumulative exposure to RHI, location of head trauma, severity of head trauma, practice type). Our group is currently conducting studies using advanced RHI exposure modeling to better understand the relationship between AFE, cumulative RHI exposure, and CTE symptoms and pathology.
CONCLUSIONS
AFE to tackle football was not associated with CTE neuropathological severity (or AD and Lewy body pathology) in this convenience sample of 211 amateur and professional tackle football players with neuropathologically-confirmed CTE from the UNITE study. However, younger AFE to tackle football, before age 12 in particular, predicted earlier reported neurobehavioral symptom onset by more than 13 years in participants with neuropathologically-confirmed CTE. Similar effects on neurobehavioral symptoms were observed in participants without CTE, suggesting that the relationship between younger AFE to tackle football and long-term neurobehavioral disturbances may not be specific to CTE. Selection bias into the brain bank may limit the generalizability of these findings to the broader tackle football population. Informant-report of AFE and age of symptom onset may also have affected the accuracy of the estimated effect of AFE to tackle football on age of symptom onset. Given the growing public health concerns for participation in tackle football, prospective studies of former tackle football players that include objective clinical assessments are needed to better understand the relationship between youth tackle football exposure and long-term neurobehavioral outcomes.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the use of the resources and facilities at VA Boston Healthcare System (Boston, MA) and the Edith Nourse Rogers Memorial Veterans Hospital (Bedford, MA, USA). We also gratefully acknowledge the help of all members of the Boston University CTE Center, the VA-BU-CLF Brain Bank, and the Concussion Legacy Foundation, as well as the individuals and families whose participation and contributions made this work possible.
This work was supported by the National Institute of Neurological Disorders and Stroke (1U01NS086659–01, U01NS093334, R01NS078337, R56NS078337), Department of Defense (W81XWH-13-2-0064), Department of Veterans Affairs, the Veterans Affairs Biorepository (CSP 501), the National Institute of Aging Boston University Alzheimer’s Disease Center (P30AG13846; supplement 0572063345–5), Department of Defense Peer Reviewed Alzheimer’s Research Program (DoD- PRARP #13267017), the National Institute of Aging Boston University Framingham Heart Study (R01AG1649), the National Operating Committee on Standards for Athletic Equipment and the Concussion Legacy Foundation. This work was also supported by unrestricted gifts from the Andlinger Foundation, the WWE and the NFL. Michael L. Alosco and research reported in this publication is supported by the National Institutes of Health under grant number F32NS096803 and a Pilot Grant from the Boston University Alzheimer’s Disease Center (AG013846). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Jesse Mez is supported by the National Institutes of Health under grant number K23 AG046377. Funding sources did not have restrictions on the design and conduct of the study, collection, management, analysis, and interpretation of data, or the preparation, review, or approval of the manuscript, or decision to submit the manuscript for publication.
Footnotes
POTENTIAL CONFLICTS OF INTEREST
The authors have no potential conflicts of interest to report.
REFERENCES
- 1.Bieniek KF, Ross OA, Cormier KA, et al. Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol 2015;130:877–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKee AC, Cairns NJ, Dickson DW, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 2016;131:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013;136:43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tagge CA, Fisher AM, Minaeva OV, Gaudreau-Balderrama A, Moncaster JA, Zhang XL, Wojnarowicz MW, Casey N, Lu H, Kokiko-Cochran ON, Saman S, Ericsson M, Onos KD, Veksler R, Senatorov VV, Kondo A, Zhou XZ, Miry O, Vose LR, Gopaul KR, Upreti C, Nowinski CJ, Cantu RC, Alvarez VE, Knorad J, Hamilton JA, Hua N, Tripodis Y, Anderson AT, Howell GR, Kaufer D, Hall GF, Lu KP, Ransohoff RM, Cleveland RO, Kowall NW, Huber BR, Stein TD, Lamb BT, Moss WC, Friedman A, Stanton PK, McKee AC, Goldstein LE Concussion, microvascular injury, and early tauopathy in young athletes after impact head injury and an impact concussion mouse model. Brain 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mez J, Daneshvar DH, Kiernan PT, et al. Clinicopathological Evaluation of Chronic Traumatic Encephalopathy in Players of American Football. JAMA 2017;318:360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montenigro PH, Baugh CM, Daneshvar DH, et al. Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res Ther 2014;6:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein TD, Montenigro PH, Alvarez VE, et al. Beta-amyloid deposition in chronic traumatic encephalopathy. Acta Neuropathol 2015;130:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alosco ML, Mez J, Kowall NW, et al. Cognitive Reserve as a Modifier of Clinical Expression in Chronic Traumatic Encephalopathy: A Preliminary Examination. J Neuropsychiatry Clin Neurosci 2017;29:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esopenko C, Chow TW, Tartaglia MC, et al. Cognitive and psychosocial function in retired professional hockey players. J Neurol Neurosurg Psychiatry 2017;88:512–9. [DOI] [PubMed] [Google Scholar]
- 10.McMillan TM, McSkimming P, Wainman-Lefley J, et al. Long-term health outcomes after exposure to repeated concussion in elite level: rugby union players. J Neurol Neurosurg Psychiatry 2017;88:505–11. [DOI] [PubMed] [Google Scholar]
- 11.Stamm JM, Bourlas AP, Baugh CM, et al. Age of first exposure to football and later-life cognitive impairment in former NFL players. Neurology 2015;84:1114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stamm JM, Koerte IK, Muehlmann M, et al. Age at First Exposure to Football Is Associated with Altered Corpus Callosum White Matter Microstructure in Former Professional Football Players. J Neurotrauma 2015;32:1768–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schultz V, Stern RA, Tripodis Y, et al. Age at First Exposure to Repetitive Head Impacts Is Associated with Smaller Thalamic Volumes in Former Professional American Football Players. J Neurotrauma 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alosco ML, Kasimis AB, Stamm JM, Chua AS, Baugh CM, Daneshvar DH, Robbins CA, Mariani M, Hayden J, Conneely S, Au R, Torres A, McClean MD, McKee AC, Cantu RC, Mez J, Nowinski CJ, Martin BM, Chaisson CE, Tripodis Y, Stern RA Age of First Exposure to American Football and Long-Term Neuropsychiatric and Cognitive Outcomes. Translational Psychiatry in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bahrami N, Sharma D, Rosenthal S, et al. Subconcussive Head Impact Exposure and White Matter Tract Changes over a Single Season of Youth Football. Radiology 2016;281:919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epstein HT. Stages of increased cerebral blood flow accompany stages of rapid brain growth. Brain Dev 1999;21:535–9. [DOI] [PubMed] [Google Scholar]
- 17.Caviness VS Jr., Kennedy DN, Richelme C, et al. The human brain age 7–11 years: a volumetric analysis based on magnetic resonance images. Cereb Cortex 1996;6:726–36. [DOI] [PubMed] [Google Scholar]
- 18.Giedd JN. The teen brain: insights from neuroimaging. J Adolesc Health 2008;42:335–43. [DOI] [PubMed] [Google Scholar]
- 19.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 1999;2:861–3. [DOI] [PubMed] [Google Scholar]
- 20.Shaw P, Greenstein D, Lerch J, et al. Intellectual ability and cortical development in children and adolescents. Nature 2006;440:676–9. [DOI] [PubMed] [Google Scholar]
- 21.Shaw P, Kabani NJ, Lerch JP, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci 2008;28:3586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Courchesne E, Chisum HJ, Townsend J, et al. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology 2000;216:672–82. [DOI] [PubMed] [Google Scholar]
- 23.Lebel C, Walker L, Leemans A, et al. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 2008;40:1044–55. [DOI] [PubMed] [Google Scholar]
- 24.Uematsu A, Matsui M, Tanaka C, et al. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS One 2012;7:e46970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snook L, Paulson LA, Roy D, et al. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage 2005;26:1164–73. [DOI] [PubMed] [Google Scholar]
- 26.Thatcher RW. Maturation of the human frontal lobes. Physiological evidence for staging. Developmental Neuropsychology 1991;7:397–419. [Google Scholar]
- 27.Klingberg T, Vaidya CJ, Gabrieli JD, et al. Myelination and organization of the frontal white matter in children: a diffusion tensor MRI study. Neuroreport 1999;10:2817–21. [DOI] [PubMed] [Google Scholar]
- 28.Gogtay N, Thompson PM. Mapping gray matter development: implications for typical development and vulnerability to psychopathology. Brain Cogn 2010;72:6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marek S, Hwang K, Foran W, et al. The Contribution of Network Organization and Integration to the Development of Cognitive Control. PLoS Biol 2015;13:e1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherman LE, Rudie JD, Pfeifer JH, et al. Development of the default mode and central executive networks across early adolescence: a longitudinal study. Dev Cogn Neurosci 2014;10:148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol 1987;22:487–97. [DOI] [PubMed] [Google Scholar]
- 32.Solomon GS, Kuhn AW, Zuckerman SL, et al. Participation in Pre-High School Football and Neurological, Neuroradiological, and Neuropsychological Findings in Later Life: A Study of 45 Retired National Football League Players. Am J Sports Med 2016;44:1106–15. [DOI] [PubMed] [Google Scholar]
- 33.Deshpande SK, Hasegawa RB, Rabinowitz AR, et al. Association of Playing High School Football With Cognition and Mental Health Later in Life. JAMA Neurol 2017;74:909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mez J, Solomon TM, Daneshvar DH, et al. Assessing clinicopathological correlation in chronic traumatic encephalopathy: rationale and methods for the UNITE study. Alzheimers Res Ther 2015;7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stern RA, Daneshvar DH, Baugh CM, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology 2013;81:1122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vonsattel JP, Amaya Mdel P, Cortes EP, et al. Twenty-first century brain banking: practical prerequisites and lessons from the past: the experience of New York Brain Bank, Taub Institute, Columbia University. Cell Tissue Bank; 2008;9:247–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vonsattel JP, Del Amaya MP, Keller CE. Twenty-first century brain banking. Processing brains for research: the Columbia University methods. Acta Neuropathol 2008;115:509–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cherry JD, Tripodis Y, Alvarez VE, et al. Microglial neuroinflammation contributes to tau accumulation in chronic traumatic encephalopathy. Acta Neuropathol Commun 2016;4:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Angrist JD, Imbens, G.W., Rubin, D.B. Identification of causal effects using instrumental variables. J Am Stat Assoc 1996;81:444–55. [Google Scholar]
- 41.Winship C, Morgan SL The estimation of causal effects from observational data. Ann Rev Sociol 1999;25. [Google Scholar]
- 42.Cole SR, Platt RW, Schisterman EF, et al. Illustrating bias due to conditioning on a collider. Int J Epidemiol 2010;39:417–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haneuse S, Schildcrout J, Crane P, et al. Adjustment for selection bias in observational studies with application to the analysis of autopsy data. Neuroepidemiology 2009;32:229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borenstein AR, Copenhaver CI, Mortimer JA. Early-life risk factors for Alzheimer disease. Alzheimer Dis Assoc Disord 2006;20:63–72. [DOI] [PubMed] [Google Scholar]
- 45.Stern Y Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 2012;11:1006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munce TA, Dorman JC, Thompson PA, et al. Head Impact Exposure and Neurologic Function of Youth Football Players. Med Sci Sports Exerc 2015;47:1567–76. [DOI] [PubMed] [Google Scholar]
- 47.Ng TS, Lin AP, Koerte IK, et al. Neuroimaging in repetitive brain trauma. Alzheimers Res Ther 2014;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]