Abstract
Background:
Disruption of intracellular Ca2+ homeostasis and associated autophagy dysfunction contribute to neuropathology in Alzheimer’s disease (AD).
Objective:
To study the effects of propofol on cell viability via its effects on intracellular Ca2+ homeostasis, and the impact of autophagy, in a neuronal model of presenilin-mutated familial AD (FAD).
Methods:
We treated PC12 cells, stably transfected with either mutated presenilin-1 (L286V) or wild type (WT) controls, with propofol at different doses and durations, in the presence or absence of extracellular Ca2+, antagonists of inositol trisphosphate receptors (InsP3R, xestospongin C) and/or ryanodine receptors (RYR, dantrolene), or an inhibitor of autophagy flux (Bafilomycin). We determined cell viability, cytosolic Ca2+ concentrations ([Ca2+]c), vATPase protein expression, and lysosomal acidification.
Results:
The propofol dose- and time-dependently decreased cell viability significantly more in L286V than WT cells, especially at the pharmacological dose (>50 μΜ), and together with bafilomycin (40 nM). Clinically used concentrations of propofol (<20 μΜ) tended to increase cell viability. Propofol significantly increased [Ca2+]c more in L286V than in WT cells, which was associated with decrease of vATPase expression and localization to the lysosome. Both toxicity and increased Ca2+ levels were ameliorated by inhibiting InsP3R/RYR. However, the combined inhibition of both receptors paradoxically increased [Ca2+]c, by inducing Ca2+ influx from the extracellular space, causing greater cytotoxicity.
Conclusion:
Impairment in autophagy function acts to deteriorate cell death induced by propofol in FAD neuronal cells. Cell death is ameliorated by either RYR or InsP3R antagonists on their own, but not when both are co-administered.
Keywords: Alzheimer’s disease, anesthesia, autophagy, calcium, presenilin
INTRODUCTION
One of the proposed mechanisms for neuropathology in Alzheimer’s disease (AD) is the impairment of autophagy function [1]. This may result in promoting amyloid or tau [2, 3] pathology, increased neurodegeneration [4], and cognitive dysfunction [5]. H+/Ca2+ homeostasis in the lysosome plays an essential role in the regulation of autophagy via autophagy gene transcription, mediated by the transfection factor EB [6, 7]. Recent studies suggested that the mutation of AD’s presenilin 1 (PS1) has abnormally increased Ca2+ release from the endoplasmic reticulum (ER) via over activation of either inositol trisphosphate receptors (InsP3Rs) or ryanodine receptors (RYRs) and has disrupted lysosomal H+/Ca2+ homeostasis. This disruption has led to impaired lysosome and autophagy function [8–10]. The PS2 mutation has also changed the dynamics of ER and mitochondria tethering and the subsequent Ca2+ transfer from the ER to mitochondria, an important process for both cell physiological function and pathological damage [11].
General anesthetics may worsen cognitive function in aged rodents [12, 13]. Through examining various tissue cultures and animal models [14], it is clear that general anesthetics worsen amyloid and tau pathology, neurodegeneration, and cognitive dysfunction in AD although the mechanisms remain unclear. Our previous studies suggested that isoflurane activated InsP3Rs and dysregulated the calcium homeostasis in the ER and the mitochondria and cytosolic space [15, 16], causing cell death. Conversely, over activation of RYRs also contributed to general anesthetics mediated neurotoxicity [17].
General anesthetics also affect the lysosome pH and autophagy function with an unclear molecular mechanism [18]. Herein, we discovered that at pharmacological as opposed to clinically used doses, the commonly used general anesthetic propofol impairs autophagic flux through over activation of InsP3Rs and/or RYRs, disrupting the synthesis or localization of vATPase, and decreasing lysosome acidity in a cell model of FAD.
MATERIALS AND METHODS
Cell cultures
Rat pheochromocytoma cells (PC12) either transfected with wild-type (WT) PS1 or point mutated (L286V) PS1 were utilized to model AD, as L286V cells have been shown to be more vulnerable than WT to various neurotoxin including inhalational anesthetics [19–22]. The cells were maintained in DMEM medium (Life Technologies, Carlsbad, CA, USA) containing 5% fetal calf serum, 10% heat-inactivated horse serum, 200 μg/ml G418 and 1% penicillin/streptomycin [23, 24]. Initial cells were suspended with density of 0.5 × 105 cells/cm2 in culture medium and incubated in T75 flasks in a 95% air, 5% CO2 humidified atmosphere at 37°C. We changed the medium every 48 h.
Cell treatment and viability measurement
Cell were treated with various concentrations of propofol (1, 5, 20, 50, 100, 200, 400 μΜ) for various durations (3, 6, 12 h) as dose- and time-response studies. These concentration and duration are chosen primarily based on the typical clinical range (1–20 μΜ for less than 6 h) [25] and the high pharmacological range for prolonged use (50–400 μΜ and for 12 h). Cell viability was measured by using Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan). One day prior to the experiment, the cells were seeded in a 96-well plate (5000 cells/well) under normal culture condition. Each experiment was repeated for three times to ensure reproducibility. At the end of the various treatments, CCK-8 solution was added to each well at a dilution of 1:10 and the plates were incubated at 37°C for 1 h. Then absorbance measurements at 450 nm were performed by using a microplate reader (Synergy HTX, BioTek, Winooski, VT, USA).
Calcium imaging
Cell were exposed to 200 μΜ propofol to raise [Ca2+]c, which was measured by Fura-2 acetoxymethylester (AM) fluorescence (Invitrogen) on the stage of an inverted microscope (Olympus IX70) and IPLab v3.71 software (Scanalystics, Inc, USA), as previously described [26]. Briefly, the cells were plated onto 35 mm culture dishes for 24 h, washed with Hank’s Balanced Salt Solution (HBSS, Sigma H-4891) for three times, and incubated with 2.5 μΜ Fura-2 AM in the same buffer supplemented with 1% bovine serum albumin (BSA) at 37°C in the dark for 30 min. Then the cells were washed three times with HBSS to remove excess Fura-2 AM. When the cells exposed to various treatment of propofol, in the presence or absence of dantrolene or Xestospongin C, Fura-2 fluorescence intensities were monitored by recording at 340- and 380 nm excitation and at 510 nm emission for up to 10 min. The data are presented as ratios of 340/380 nm of fluorescence intensity normalized to baseline.
Lysosome acidity measurements
Lysosome acidity was measured by staining with LysoTracker Red DND-99 (Molecular Probe, Eugene, OR), a fluorescent dye for labeling and tracking acidic organelles in live cells. The cell suspensions were plated onto glass coverslips placed in 35 mm plastic culture dishes for 24 h. The next day the culture medium were aspirated, the cells were washed three times with HBSS containing 1.8 mM CaCl2 and 0.8 mM MgCl2 (pH 7.4). The cells were loaded with 50 nM LysoTracker working solution and incubated for 1 h at 37°C. After that, the cells were washed three times with PBS and incubated with 4’,6-diamidino-2-phenylindole (DAPI) for 15 min. We measured the fluorescence intensity of the acidic lysosome organelles at excitation maximum of ~577 nm and emission maximum ~590 nm.
Immunofluorescence
The cells were seeded onto glass coverslips placed in a six-well culture dishes at 5000 cells/well and allowed to grow for 24 h. Then the cells were rinsed briefly in PBS and fixed with 4% formaldehyde for 10 min at room temperature. After that, the cells were permeabilized with 10% Triton X-100 for 5 min following three washes with PBS and blocked in 5% normal goat/donkey serum for 1 h. Subsequently, the cells were incubated with primary antibodies diluted in blocking buffer (1:50) overnight at 4°C. After overnight incubation, the cells were washed with PBS three times for 5 min each, and followed by a 1 h (at RT) incubation with a secondary antibody in the dark. Lastly, the coverslips were rinsed once in PBS and stained with DAPI for 5 min and mounted on the slides with ProLong Gold Antifade Mountant Medium (Thermo Fisher Scientific, USA).
Data analysis and statistics
The data is expressed as mean ± SEM and assessed for significance using Student’s unpaired t test, one-way or two-way ANOVA with Tukey post hoc analysis. The significance level for all of our analyses was set at 99% (p < 0.01). We used GraphPad Prism software (GraphPad Software Inc.) for all statistical analyses.
RESULTS
Autophagy impairment in AD worsened propofol induced neurotoxicity
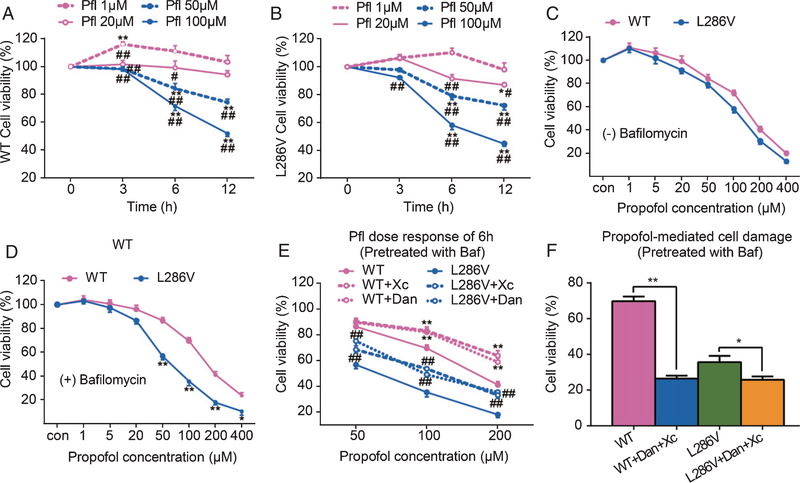
Previous studies demonstrate that propofol is much less potent than inhalational anesthetics in causing cell damage in various types of cells [27] and in developing brains of rodents [16]. The cells featured with AD were more vulnerable to anesthetics toxicity [28]. Similar to in inhalational anesthetics [29], propofol dose- and time-dependently affected the cell viability in both WT and L286V AD cells (Fig. 1A, B). Propofol at the commonly used clinical concentration (1 μM) trended to increase cell viability in both types of cells determined by CCK assay, while its high end concentration within the clinically used range (20 μM) decreased cell viability in L286V AD cells only when used longer than 6 h, but not in WT cells (Fig. 1A, B). Propofol at clinically relevant concentrations (1–20 μM), rarely induced cell damage, but needed very high non-clinical concentrations (>50~100 μM) to cause cell death [30]. Our results here (Fig. 1A) are consistent with previous studies [31] which indicated that in wild type cells clinically relevant concentrations of propofol did not induce obvious cell damage. Although 20 μM induced injury at 6 h of treatment, treatment for 6 h with up to 400 μM of propofol did not cause more cell damage in L286V cells relative to WT cells (Fig. 1C). In contrast, pretreatment of bafilomycin, an agent that inhibits the lysosomal vATPase and the fusion between the autophagosome and the lysosome [32], significantly increased vulnerability of L286V AD cells to propofol toxicity at higher concentrations (>50 μM, Fig. 1D), suggesting that autophagy impairment, as observed in the context of PS1 mutations or with aging, exacerbates propofol induced cell damage. Furthermore, propofol mediated cell damage in the presence of bafilomycin could be inhibited by either Xc or dantrolene (Fig. 1E), suggesting a role for calcium dysregulation via InsP3Rs or RYRs activation in augmenting propofol-induced cell damage. Paradoxically, however, combined use Xc and dantrolene worsened propofol mediated cytotoxicity (Fig. 1F).
Fig. 1.
Propofol affects cell viability in autophagy activity-dependent manner via activation of InsP3 (InsP3R) or ryanodine (RYR) receptors. PC12 rat pheochromocytoma cells transfected with knocked in point mutation of presenilin 1 (L286V) or wild type (WT) controls were exposed to different concentrations of propofol (1 to 400 μM) for 3, 6, or 12 h. The Cell Counting Kit-8 (CCK-8) was used to determine cell viability. Propofol dose- and time-dependently affect cell viability in both types of cells equally, with low dose (1 μM) for up to 6 h trended to increase cell viability but high concentrations (over 50 μM) significantly reduced cell viability (A and B). *p < 0.01 and **p < 0.001 compared to the beginning time point at each propofol dose group (N ≥ 3). #p < 0.01 and ##p < 0.001 compared to propofol (1 μM) group at each time point (N ≥ 3). Impairment of autophagy flux by bafilomycin did not worsen the 6 h propofol mediated cell damage in WT cells (C), but significantly increased vulnerability of L286V cells to propofol dose-dependent toxicity (D). *p < 0.01 and **p < 0.001 compared to WT (N ≥ 3). The propofol toxicity in autophagy-disrupted L286V cells was inhibited by antagonists for InsP3Rs (E, xestospongin C, Xc 1 μM) or RYRs (E, dantrolene, 30 μM), but paradoxically worsened by the combined use of Xc and dantrolene (F). *p < 0.05 and **p < 0.01 (N ≥ 3). All data in the figure are expressed as the mean ± SEM from at least three separate experiments with triplicates and analyzed by two-way ANOVA followed by Tukey post multiple comparison tests.
Propofol caused abnormal Ca2+ release from the ER via over activation of InsP3Rs or RYRs in AD cells
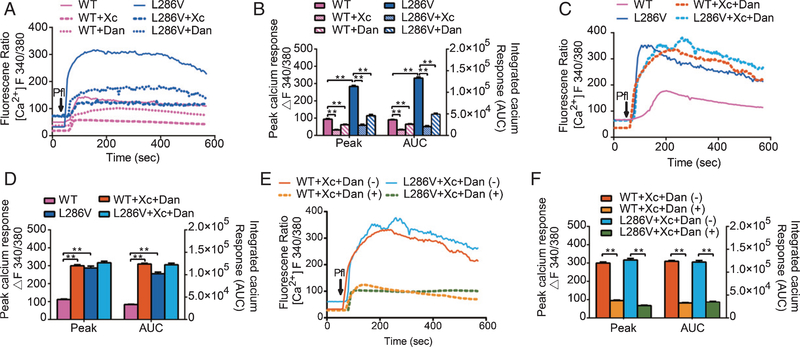
Inhalational anesthetics can affect cytosolic Ca2+ concentrations ([Ca2+]c) via multiple mechanisms. Two such mechanisms include Ca2+ influx from the extracellular space and Ca2+ release from the ER [33]. Inhalational anesthetics at high concentrations for prolonged use could induce cell damage via over activation of InsP3Rs [15] and/or RYRs [34, 35] in a variety of tissue cultures and in animal models [29, 36, 37]. This may be particularly true in cells features with AD [38] or Huntington disease [37]. Similar to inhalational anesthetics, propofol at a high concentration (200 μM) induced Ca2+ release from the ER via activation of InsP3Rs/RYRs. It also elevated both peak and integrated Ca2+ response (area under curve, AUC) [Ca2+]c more significantly in AD than WT PC12 cells, which could be significantly inhibited by either Xc or dantrolene (Fig. 2A, B). Paradoxically, combined use of antagonists for both InsP3R and RYR (Xc+dantrolene) did not further decrease propofol-induced elevation of peak and AUC of [Ca2+]c, as expected, but rather increased it significantly (Fig. 2C, D). This may explain the augmentation of propofol-induced cytotoxicity observed above (Fig. 1F). The paradoxical elevation of [Ca2+]c in the presence of both inhibitors was dependent upon Ca2+ influx from the extracellular space because it was significantly inhibited by ethylene glycol tetraacetic acid (EGTA), a calcium chelator added to the extracellular buffer (Fig. 2E, F).
Fig. 2.
Propofol increased cytosolic Ca2+ concentrations ([Ca2+ ]c) more in L286V than WT cells via activation of InsP3 (InsP3R) or ryanodine (RYR) receptors. A) Average [Ca2+]c response to 200 μM propofol (arrow) in WT or L286V cells in the presence or absence of antagonists for InsP3Rs (Xestospongin C, Xc 1 μM) or RYRs (dantrolene or Dan, 30 μM). B) Propofol increased peak [Ca2+]c and integrated Ca2+ calcium response represented by area under the curve (AUC) significantly more in L286V than in WT cells, which were significantly inhibited by Xc or dantrolene alone. C) Average [Ca2+]c response to 200 μM propofol (arrow) in WT or L286V cells in the presence or absence of combined use of Xestospongin C and dantrolene (Xc+Dan). D) Combined use of Xc and Dan paradoxically elevated both the peak and AUC of [Ca2+]c, only in WT but not L286V cells. E) Average [Ca2+]c response to 200 μM propofol (arrow) with pretreatment of both xestospongin C and dantrolene (Xc+Dan) in WT or L286V cells in the presence or absence of EGTA, a calcium chelator to remove extracellular Ca2+ in the measurement buffer. F) Removal of extracellular Ca2+ from the buffer nearly abolished the increased [Ca2+]c induced by the propofol in the presence of combined inhibition of both InsP3R and RYR. All data (B, D, F) are expressed as the mean ± SEM from two or three separate experiments of at least twenty individual cells (N ≥ 3) and analyzed by one-way ANOVA followed by Tukey multiple comparison tests. *p < 0.01 and **p < 0.001.
PS1 mutation induced defective lysosomal acidification by altered localization and function of the vATPase
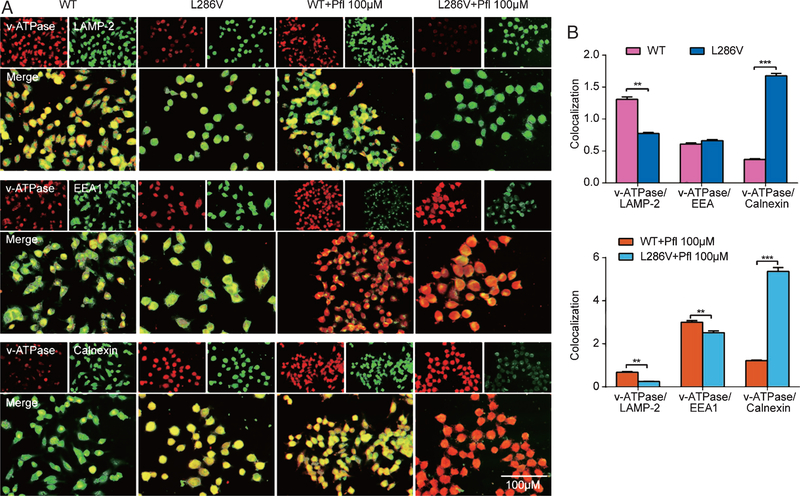
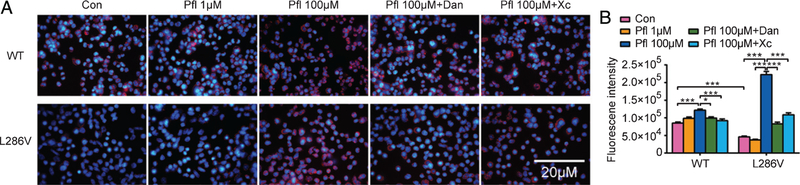
We further explored the vATPase V0a1 subunit as a marker of the proton pump in lysosomes in PS1 mutation cells. Double immunofluorescence labeling analysis demonstrated much stronger colocalization of vATPase with LAMP-2 (lysosome-associated membrane protein-2) in WT cells than that in L286V cells (Fig. 3). Similarly, compared to WT control, acidity in lysosomes determined by LysoTracker in L286V cells also significantly decreased (Fig. 4). Our study is consistent with previous report that the PS1 mutation, like its knock out from cell, significantly decreased acidity in lysosomes by reduced numbers of vATPase. However, propofol at high pharmacological concentration (100 μM) but not at clinically relevant concentration (1 μM) increased LysoTracker staining of acidic compartments, much more dramatically in L286V AD cells, which could be significantly inhibited by either Xc or dantrolene (Fig. 4). Interestingly, although colocalization of vATPase with early endosome antigen-1 (EEA1) was very weak in early endosomes of both WT and L286V cells, it was significantly increased in both types of cells after the treatment of high concentration of propofol (100 μM), suggesting a propofol-mediated vATPase detainment in early endosome and associated decrease in lysosome. Despite an increase of the vATPase V0a1 subunit in ER, decreased Ca2+ concentration in ER may result in a possible block of vATPase assembly and eliminated vATPase-dependent acidification activity, which may responsible for a weaker LysoTracker staining in L286V AD cells in contrast to WT cells.
Fig. 3.
Propofol decreased lysosome vATPase in L286V Alzheimer’s disease cells by over activation of InsP3 (InsP3R) or ryanodine (RYR) receptors. A) Double-immunofluorescence labeling shows the colocalization of vATPase (V0a1 subunit) and lysosome (LAMP-2), or early endosome (EEA1), or ER (calnexin) in WT or L286V AD cells. Scale bar = 50 μm. B) Compared to WT controls, vATPase V0a1 subunit and lysosomal marker, LAMP-2 show little colocalization in L286V AD cells. Compared to WT controls, propofol at high concentration (Pfl 100 μΜ) significantly minimally colocalized vATPase VOal and lysosome marker, LAMP-2, but increased vATPase V0a1 in ER and early endosome. All data are expressed as the mean ± SEM from at least three separate experiments (N ≥ 3) of at least twenty individual cells and analyzed by unpaired t test followed by Tukey multiple comparison tests. *p < 0.01 and **p < 0.001.
Fig. 4.
Propofol significantly increased acidity of lysosome in L286V cells by over activation of InsP3 (InsP3R) or ryanodine (RYR) receptors. A) Representative pictures of lysosome acidity (red staining) determined by Lysotracker. With or without propofol treatment (1 versus 100 μM) and in the presence or absence of antagonists for InsP3Rs (xestospongin C, Xc) or RYRs (dantrolene, Dan). Scale bar = 20 μm. B) Propofol only at non-clinical high concentrations (100 μM) increased acidity, more significantly in L286V cells, which were abolished by Xc or Dan. All data are expressed as the mean ± SEM from at least three separate experiments (N ≥ 3) of at least twenty individual cells and analyzed by two-way ANOVA followed by Tukey multiple comparison tests. *p < 0.01 and **p < 0.001.
DISCUSSION
The primary finding of the current study is that propofol, when administered above commonly used clinical concentrations (>50 μM), sensitized AD neurons to impaired autophagy flux through decreased transport of the vATPase from the ER into the lysosome, after excessive Ca2+ release from the ER due to over activation of InsP3R and/or RYR. Antagonists of InsP3R (Xc) or RYR (dantrolene) alone amelio-rated, while the combined use of Xc and dantrolene paradoxically worsened propofol toxicity in AD cells. Further impairment of autophagy flux by Bafilomycin in AD cells renders these neurons more vulnerable to propofol toxicity than WT cells, consistent to previous study in human stem cells [39]. This study illustrated the molecular mechanisms of propofol mediated neurotoxicity, and developed strategies to mitigate such harmful effects of general anesthetics in neurons featured with AD.
Adequate Ca2+ release from ER via InsP3Rs and/or RYRs, and the subsequent elevation of [Ca2+]c plays a universal role on many cell functions, including, but not limited to ATP production, cell survival, cell cycles, neurogenesis, autophagy, and cognitive function [40]. However, abnormal and excessive Ca2+ release from the ER via InsP3Rs/RYRs may result in impaired autophagy, mitochondria damage, cell arrest and death [41], which may be the mechanism of some pathology in neurodegenerative diseases [42]. It has been demonstrated that both InsP3Rs and RYRs are excessively activated in AD due to abnormally increased receptor numbers [43], and the elevated receptor hypersensitivity to their agonists [44]. Abnormal and excessive Ca2+ release from the ER not only dangerously increases [Ca2+]c but also detrimentally decreases ER calcium contents. The former can overload mitochondria with Ca2+ and collapse membrane potential, leading to type 1 cell death, apoptosis. The latter may impair protein synthesis and secretion from the ER, causing cell damage. One of the impaired protein synthesis is that of the vATPase, which plays an important role in the uptake of H+ into the lysosome lumen and maintaining its acidity for normal lysosome function. Lack of efficient vATPase on the lysosome membrane in AD cells and resulting low H+, further causes Ca2+ release from lysosome via TRPML1 calcium channel. This leads to increased [Ca2+]c and to a vicious cycle of the disruption of Ca2+ homeostasis in the ER, mitochondria and cytosolic space. These upstream pathologies can result in lysosome dysfunction and in the impairment of autophagy, which causes type 2 autophagy cell death or promotion of apoptosis (Fig. 5). Clearly, the Ca2+ release from the ER via InsP3Rs/RYRs plays a dual role of both promoting cell survival and inducing cell damage, depending on the amount of Ca2+ released from the ER.
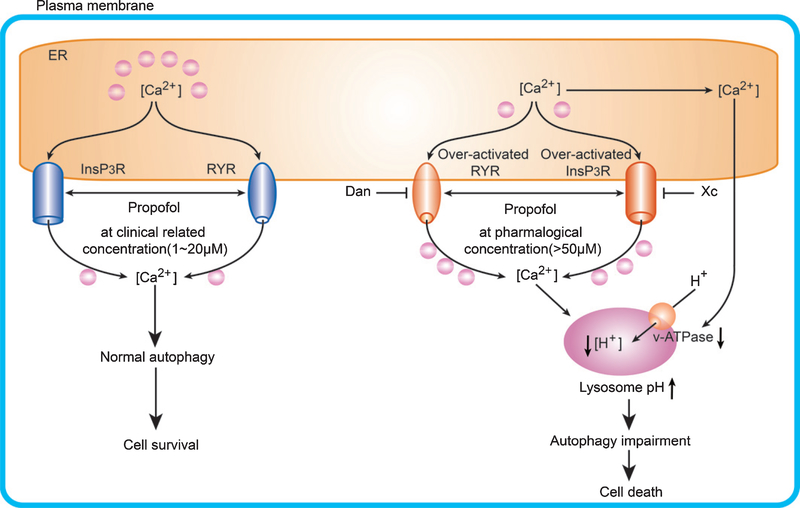
Fig. 5.
Propofol affect cell survival in a dose- and lysosome H+ homeostasis dependent manner via activation of InsP3Rs and/or RYRs. Propofol at clinically relevant concentrations (1–20 μΜ, left side) adequately activate InsP3R and/or RYR, supporting normal lysosome H+/Ca2+ homeostasis and cell survival. However, excessive activation of InsP3Rs and/or RYRs by propofol at pharmacological concentrations (>50 μΜ, right side) and associated depletion of ER Ca2+ together with abnormal elevation of cytosolic Ca2+ concentration ([Ca2+ ]c) resulted in the disruption of lysosome H+/Ca2+ homeostasis, and impairment of endosome-lysosome fusion and autophagic dysfunction, eventually leading to cell death.
We have demonstrated in previous studies [45] that isoflurane at clinically relevant concentrations induced more significantly cell damage in L286V AD cells than in WT. Although previous studies demonstrated that inhalational anesthetics affect autophagy and cell survival via activation InsP3Rs/RYRs [46], the molecular mechanisms that GAs effects on autophagy and cell survival is not clear. Our study here suggests that abnormal Ca2+ release from the ER, induced by excessive propofol, results in ER stress, decreased vATPase, and impaired lysosome and autophagy function. Although propofol significantly decreased lysosome vATPase, it increased the overall intracellular acidity. We presumed that this increased acidity was not originated from lysosome and may be caused by significantly increased vATPase in early endo- some (Fig. 3). It is well known that Ca2+ plays an important role in regulating fusion of multiple types of vesicle including endosome-lysosome fusion. It has been shown that high cytosolic calcium concentration suppressed vesicle fusion, and calcium channel blockers can attenuate saturated fatty acids induced autophagy arrest in mice by promoting autophagosome-lysosome fusion [47]. Therefore, either abnormally high cytosolic [Ca2+] and/or low ER calcium level may contribute to defective lysosomal acidification and autophagosome-lysosome fusion, which may be responsible for lysosomal proteolytic failure and contribute to AD-related accumulation of autophagy and endosomal substrates and neurodegeneration. This defect of lysosomal function contribute to inefficient protein/peptide clearance, amyloid and tau pathology in AD [48].
Consistent with our previous study [46], the detrimental effects of propofol could be inhibited by antagonists for either InsP3Rs or RYRs in AD cells. Unexpectedly, the combined use of antagonists for InsP3Rs and RYRs not only provided no neuroprotection but also worsened Ca2+ dysregulation and promoted cell death in AD cells. It seems that simultaneous inhibition of both InsP3R/RYR will impair its normal physiologic function and are detrimental to cells. Our results suggest that strategy for treatment of anesthesia toxicity in AD depends on adequate amelioration of InsP3R or RYR. However, excessive inhibition of both receptors disrupts processes that regulate extracellular Ca2+ influx and normal Ca2+ release from the ER, and physiological function can be impaired.
It seems that the existing reduction of vATPase, and impaired lysosome and autophagy function in the AD cells, make them vulnerable to further decrease of vATPase induced by propofol and promotes cell death. Previous studies suggest that inhalational anesthetics, especially isoflurane, at clinically relevant concentrations, can induce significant Ca2+ release from ER via InsP3Rs or RYRs [49] and cell damage. Propofol at clinically relevant concentrations rarely changes cytosolic Ca2+ concentrations and causes cell death, suggesting a less likely possibility of propofol disrupting intracellular Ca2+ homeostasis and a higher chance of cell survival than in inhalational anesthetics. The results from this study seems to support this notion since propofol, only at extremely high concentrations, much higher than clinically relevant concentrations, could impair lysosome acidity and function, damage autophagy function, and induce cell death. More studies are needed to investigate whether this data that a lower potency of propofol causes less cell damage than commonly used inhalational anesthetics is applicable to human beings. Particularly in human cells vulnerable to anesthesia toxicity, such as in AD and developing brains.
The scientific implication of this study is that maintenance of normal intracellular calcium homeostasis is important for cell survival, which was disrupted in AD cells by excessive calcium release from ER via over activation of RyR/InsP3R due to presenlin-1 mutation. Approaches to ameliorate calcium dysregulation by inhibition of RyR/InsP3R should be adequate, with excessive inhibition by simultaneous inhibition of both RyR and InsP3R leading to pathological low cytosolic calcium concentrations unable to maintain physiological need as a second messenger. Notably, the detrimental effects of propofol were observed only at high doses and/or the presence of bafilomycin. This may be relevant in elderly individuals due to declining autophagy function with aging. Nevertheless, taken together with previous studies [21, 50], the clinical implication of this study is that general anesthetics may cause neuronal damage more in AD than WT cells, with inhalational anesthetics more potent than propofol.
The limitations of this study include: 1) We did not measure the direct effects of propofol on autophagy function in the current study, as this has been investigated in human stem cells in previous study [39] and is not our current focus; 2) Lack of direct comparison between propofol and some commonly used inhalational anesthetics (e.g., sevoflurane, desflurane, isoflurane), which warrant further studies in the future; 3) Lack of further studies on molecular mechanisms of Ca2+ influx induced by the combined use of antagonists on InsP3R/RYR. We plan to investigate this mechanism in detail in future studies; 4) The clinical relevance of this study is limited by the use of immortalized cell lines, which are not true neurons from CNS. Future studies may focus on the primary neuronal cultures or neurons directly from AD patients or controls.
In summary, propofol, at pharmacological concentrations caused cell damage in AD cells, sensitized by deficits in autophagy flux, via over activation of InsP3Rs and/or RYRs. These pathological changes can be inhibited by antagonists of either receptors, but not the combination of both.
ACKNOWLEDGMENTS
We appreciate the valuable discussion from Maryellen Eckenhoff, Roderic Eckenhoff and editing assistance from Divakara Goudra at the Department of Anaesthesiology, University of Pennsylvania, Philadelphia, USA.
Supported by grants from the NIH (R01GM084 979, 3R01GM084979-02S1, 2R01GM084979-06A1 to HW and AG026389 to CC).
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/18-0858r1).
Prior Abstract Presentation: 1) Alzheimer Association International Conference, London, Britain, July 2017; 2) Society for Neuroscience in Anesthesia and Critical Care (SNACC), Boston, October 19–20 2017; 3) Society for Neuroscience (SFN) Annual Meeting, Washington DC, November 11–15 2017.
REFERENCES
- [1].Nixon RA, Yang DS (2011) Autophagy failure in Alzheimer’s disease-locating the primary defect. Neurobiol Dis 43, 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yang DS, Stavrides P, Mohan PS, Kaushik S, Kumar A, Ohno M, Schmidt SD, Wesson D, Bandyopadhyay U, Jiang Y, Pawlik M, Peterhoff CM, Yang AJ, Wilson DA, St George-Hyslop P, Westaway D, Mathews PM, Levy E, Cuervo AM, Nixon RA (2011) Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer’s disease ameliorates amyloid pathologies and memory deficits. Brain 134, 258–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hamano T, Gendron TF, Causevic E, Yen SH, Lin WL, Isidoro C, Deture M, Ko LW (2008) Autophagic-lysosomal perturbation enhances tau aggregation in transfectants with induced wild-type tau expression. Eur J Neurosci 27, 1119–1130. [DOI] [PubMed] [Google Scholar]
- [4].Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K (2006) Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441, 880–884. [DOI] [PubMed] [Google Scholar]
- [5].Roberson ED, Halabisky B, Yoo JW, Yao J, Chin J, Yan F, Wu T, Hamto P, Devidze N, Yu GQ, Palop JJ, Noebels JL, Mucke L (2011) Amyloid-beta/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer’s disease. J Neurosci 31, 700–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R, Montefusco S, Scotto-Rosato A, Prezioso C, Forrester A, Settembre C, Wang W, Gao Q, Xu H, Sandri M, Rizzuto R, De Matteis MA, Ballabio A (2015) Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol 17, 288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tong Y, Song F (2015) Intracellular calcium signaling regulates autophagy via calcineurin-mediated TFEB dephosphorylation. Autophagy 11, 1192–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lee JH, McBrayer MK, Wolfe DM, Haslett LJ, Kumar A, Sato Y, Lie PP, Mohan P, Coffey EE, Kompella U, Mitchell CH, Lloyd-Evans E, Nixon RA (2015) Presenilin 1 maintains lysosomal Ca(2+) homeostasis via TRPML1 by regulating vATPase-mediated lysosome acidification. Cell Rep 12, 1430–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].D’Adamio L, Castillo PE (2013) Presenilin-ryanodine receptor connection. Proc Natl Acad Sci USA 110, 14825–14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cheung K-H, Mei L, Mak D-OD, Hayashi I, Iwatsubo T, Kang DE, Foskett JK (2010) Gain-of-function enhancement of IP3 receptor modal gating by familial Alzheimer’s disease-linked presenilin mutants in human cells and mouse neurons. Sci Signaling 3, ra22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zampese E, Fasolato C, Kipanyula MJ, Bortolozzi M, Pozzan T, Pizzo P (2011) Presenilin 2 modulates endoplasmic reticulum (ER)-mitochondria interactions and Ca2+ crosstalk. Proc Natl Acad Sci USA 108, 2777–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bianchi SL, Tran T, Liu C, Lin S,Li Y, Keller JM, Eckenhoff RG, Eckenhoff MF (2008) Brain and behavior changes in 12-month-old Tg2576 and nontransgenic mice exposed to anesthetics. Neurobiol Aging 29, 1002–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cao L, Li L, Lin D, Zuo Z (2012) Isoflurane induces learning impairment that is mediated by interleukin 1beta in rodents. PLoS.One 7, e51431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Seitz DP, Shah PS, Herrmann N, Beyene J, Siddiqui N (2011) Exposure to general anesthesia and risk of alzheimer’s disease: A systematic review and meta-analysis. BMC Geriatrics 11, 83–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhao Y, Liang G, Chen Q, Joseph DJ, Meng Q, Eckenhoff RG, Eckenhoff MF, Wei H (2010) Anesthetic-induced neurodegeneration mediated via inositol 1, 4, 5-trisphosphate receptors. J Pharmacol Exp Ther 333, 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yang B, Liang G, Khojasteh S, Wu Z, Yang W, Joseph D, Wei H (2014) Comparison of neurodegeneration and cognitive impairment in neonatal mice exposed to propofol or isoflurane. PLoS One 9, e99171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Peng J, Liang G, Inan S, Wu Z, Joseph DJ, Meng Q, Peng Y, Eckenhoff MF, Wei H (2012) Dantrolene ameliorates cognitive decline and neuropathology in Alzheimer triple transgenic mice. Neurosci Lett 516, 274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yang M, Wei H (2017) Anesthetic neurotoxicity: Apoptosis and autophagic cell death mediated by calcium dysregulation. Neurotoxicol Teratol 60, 59–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Guo Q, Sopher BL, Fumkawa K, Pham DG, Robinson N, Martin GM, Mattson MP (1997) Alzheimer’s presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: Involvement of calcium and oxyradicals. J Neurosci 17, 4212–4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Keller JN, Guo Q, Holtsberg FW, Bruce-Keller AJ, Mattson MP (1998) Increased sensitivity to mitochondrial toxin-induced apoptosis in neural cells expressing mutant presenilin-1 is linked to perturbed calcium homeostasis and enhanced oxyradical production. J Neurosci 18, 4439–4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liang G, Wang QJ, Li Y, Kang B, Eckenhoff MF, Eckenhoff RG, Wei HF (2008) A presenilin-1 mutation renders neurons vulnerable to isoflurane toxicity. Anesth Analg 106, 492–500. [DOI] [PubMed] [Google Scholar]
- [22].Wei HF, Liang G, Yang H, Wang QJ, Hawkins B, Madesh M, Wang SP, Eckenhoff RG (2008) The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1, 4, 5-trisphosphate receptors. Anesthesiology 108, 251–260. [DOI] [PubMed] [Google Scholar]
- [23].Chan SL, Mayne M, Holden CP, Geiger JD, Mattson MP (2000) Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J Biol Chem 275, 18195–18200. [DOI] [PubMed] [Google Scholar]
- [24].Wei H, Kang B, Wei W, Liang G, Meng QC, Li Y, Eckenhoff RG (2005) Isoflurane and sevoflurane affect cell survival and BCL-2/BAX ratio differently. Brain Res 1037, 139–147. [DOI] [PubMed] [Google Scholar]
- [25].Bhattacharya AA, Curry S, Franks NP (2000) Binding of the general anesthetics propofol and halothane to human serum albumin. High resolution crystal structures. J Biol Chem 275, 38731–38738. [DOI] [PubMed] [Google Scholar]
- [26].Zhao X, Yang Z, Liang G, Wu Z, Peng Y, Joseph DJ, Inan S, Wei H (2013) Dual effects of isoflurane on proliferation, differentiation, and survival in human neuroprogenitor cells. Anesthesiology 118, 537–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhang Y, Zhen Y, Dong Y, Xu Z, Yue Y, Golde TE, Tanzi RE, Moir RD, Xie Z (2011) Anesthetic propofol attenuates the isoflurane-induced caspase-3 activation and Abeta oligomerization. PLoS One 6, e27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Xie Z, Dong Y, Maeda U, Moir RD, Xia W, Culley DJ, Crosby G, Tanzi RE (2007) The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid β-protein accumulation. J Neurosci 27, 1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zou X, Liu F, Zhang X, Patterson TA, Callicott R, Liu S, Hanig JP, Paule MG, Slikker W, Wang C Jr (2011) Inhalation anesthetic-induced neuronal damage in the developing rhesus monkey. Neurotoxicol Teratol 33, 592–597. [DOI] [PubMed] [Google Scholar]
- [30].Long B, Li S, Xue H, Sun L, Kim DH, Liu Y (2017) Effects of propofol treatment in neural progenitors derived from human-induced pluripotent stem cells. Neural plasticity 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Qiao H, Li Y, Xu Z, Li W, Fu Z, Wang Y, King A, Wei H (2017) Propofol affects neurodegeneration and neurogenesis by regulation of autophagy via effects on intracellular calcium homeostasis. Anesthesiology 127, 490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mauvezin C, Neufeld TP (2015) Bafilomycin A1 disrupts autophagic flux by inhibiting both V-ATPase-dependent acidification and Ca-P60A/SERCA-dependent autophagosome-lysosome fusion. Autophagy 11, 1437–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wei H, Inan S (2013) Dual effects of neuroprotection and neurotoxicity by general anesthetics: Role of intracellular calcium homeostasis. Prog Neuropsychopharmacol Biol Psychiatry 47, 156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang H, Dong Y, Zhang J, Xu Z, Wang G, Swain CA, Zhang Y, Xie Z (2014) Isoflurane induces endoplasmic reticulum stress and caspase activation through ryanodine receptors. Br J Anaesth 113, 695–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Stutzmann GE, Smith I, Caccamo A, Oddo S, Parker I, Laferla F (2007) Enhanced ryanodine-mediated calcium release in mutant PS1-expressing Alzheimer’s mouse models. Ann N YAcad Sci 1097, 265–277. [DOI] [PubMed] [Google Scholar]
- [36].Zhu C, Gao J, Karlsson N, Li Q, Zhang Y, Huang Z, Li H, Kuhn HG, Blomgren K (2010) Isoflurane anesthesia induced persistent, progressive memory impairment, caused a loss of neural stem cells, and reduced neurogenesis in young, but not adult, rodents. J Cereb Blood Flow Metab 30, 1017–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang Q, Liang G, Yang H, Wang S, Eckenhoff MF, Wei H (2011) The common inhaled anesthetic isoflurane increases aggregation of huntingtin and alters calcium homeostasis in a cell model of Huntington’s disease. Toxicol Appl Pharmacol 250, 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Shilling D, Muller M, Takano H, Mak DO, Abel T, Coulter DA, Foskett JK (2014) Suppression of InsP3 receptor- mediated Ca2+ signaling alleviates mutant presenilin-linked familial Alzheimer’s disease pathogenesis. J Neurosci 34, 6910–6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Qiao H, Li Y, Xu Z, Li W, Fu Z, Wang Y, King A, Wei H (2017) Propofol affects neurodegeneration and neurogenesis by regulation of autophagy via effects on intracellular calcium homeostasis. Anesthesiology 127, 490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].La Rovere RML, Roest G, Bultynck G, Parys JB (2016) Intracellular Ca2+ signaling and Ca2+ microdomains in the control of cell survival, apoptosis and autophagy. Cell Calcium 60, 74–87. [DOI] [PubMed] [Google Scholar]
- [41].Celsi F, Pizzo P, Brini M, Leo S, Fotino C, Pinton P, Rizzuto R (2009) Mitochondria, calcium and cell death: A deadly triad in neurodegeneration. Biochim Biophys Acta 1787, 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zündorf G, Reiser G (2011) Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxid Redox Signal 14, 1275–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang Y, Shi Y, Wei H (2017) Calcium dysregulation in Alzheimer’s disease: A target for new drug development. J Alzheimers Dis Parkinsonism 7, 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pitake S, Ochs RS (2016) Membrane depolarization increases ryanodine sensitivity to Ca(2+) release to the cytosol in L6 skeletal muscle cells: Implications for excitation-contraction coupling. Exp Biol Med 241, 854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wei H, Liang G, Yang H, Wang Q, Hawkins B, Madesh M, Wang S, Eckenhoff RG (2008) The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1, 4, 5-trisphosphate receptors. Anesthesiology 108, 251–260. [DOI] [PubMed] [Google Scholar]
- [46].Ren G, Zhou Y, Liang G, Yang B, Yang M, King A, Wei H (2017) General anesthetics regulate autophagy via modulating the inositol 1, 4, 5-trisphosphate receptor: Implications for dual effects of cytoprotection and cytotoxicity. Sci Rep 7, 12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Park H-W, Park H, Semple IA, Jang I, Ro S-H, Kim M, Cazares VA, Stuenkel EL, Kim J-J, Kim JS, Lee JH (2014) Pharmacological correction of obesity-induced autophagy arrest using calcium channel blockers. Nat Commun 5, 4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cataldo AM, Petanceska S, Terio NB, Peterhoff CM, Durham R, Mercken M, Mehta PD, Buxbaum J, Haroutunian V, Nixon RA (2004) Abeta localization in abnormal endosomes: Association with earliest Abeta elevations in AD and Down syndrome. Neurobiol Aging 25, 1263–1272. [DOI] [PubMed] [Google Scholar]
- [49].Joseph JD, Peng Y, Mak DO, Cheung KH, Vais H, Foskett JK, Wei H (2014) General anesthetic isoflurane modulates inositol 1,4,5-trisphosphatereceptor calcium channel opening. Anesthesiology 121, 528–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zhang Y, Zhen Y, Dong Y, Xu Z, Yue Y, Golde TE, Tanzi RE, Moir RD, Xie Z (2011) Anesthetic propofol attenuates the isoflurane-induced caspase-3 activation and Abeta oligomerization. PLoS.One 6, e27019. [DOI] [PMC free article] [PubMed] [Google Scholar]