Abstract
Objective:
Vascular endothelial growth factor (VEGF) is elevated in joint fluids from patients diagnosed with osteoarthritis (OA). VEGF is known to contribute to vascular tidemark invasion and osteophyte formation, which are classic features of advanced OA. Among the factors that may drive VEGF accumulation in diseased joints, stromal cell-derived factor-1α (SDF-1α) is a likely culprit, as it is enriched in synovial fluids from osteoarthritic joints and is a potent inducer of VEGF expression. Chondrogenic progenitor cells (CPCs) that overexpress SDF-1α are abundant in osteoarthritic cartilage, implicating them in elevating synovial SDF-1α levels. Here we conducted a series of experiments to determine the potential for CPCs to stimulate VEGF expression via autocrine and paracrine mechanisms.
Design:
Immunohistochemistry, immunoblotting, and PCR were used to evaluate the effects of SDF-1α on VEGF expression in CPCs and chondrocytes, and the effects of CPC-conditioned medium on chondrocytes. An SDF-1α receptor antagonist and inhibitors of mitogen-activated protein kinases (MAPKs) were used to probe the pathway linking SDF-1 with VEGF expression in CPCs.
Results:
SDF-1α and CPC-conditioned medium stimulated VEGF expression in chondrocytes. In both chondrocytes and CPCs, SDF-1α stimulated increased VEGF expression via CXCR4, a cell-surface SDF-1α receptor. This response in CPCs is dependent on p38 MAPK activation, but not on ERK or JNK activation.
Conclusions:
By secreting SDF-1α, CPCs stimulate VEGF expression in nearby cells. The co-expression of SDF-1 and CXCR4 in CPCs indicates they are capable of self-sustained VEGF overexpression. Confirmation that this contributes to OA awaits in vivo studies.
Keywords: SDF-1α, CXCR4, vascular endothelial growth factor (VEGF), chondrogenic progenitor cells (CPCs), p38 MAPK
INTRODUCTION:
Osteoarthritis is characterized by slowly progressive degeneration of articular cartilage with joint-space narrowing, subchondral bone alteration, synovitis and osteophyte formation [1, 2]. Recent studies have shed light on the abnormalities of angiogenesis and cartilage metabolism during the development of OA, and confirmed that a variety of cytokines and chemokines are involved in the progression of the disease [3, 4].
Angiogenesis is the growth of new capillaries from pre-existing blood vessels. Abnormal blood vessel formation at the cartilage-subchondral bone interface may contribute to the progression of OA [5]. Osteochondral angiogenesis is accompanied by the generation of sensory and sympathetic nerves, suggesting a role in joint pain [6]. During osteophyte formation, blood vessels also penetrate newly formed cartilage at the joint margins [6, 7]. Vascular endothelial growth factor (VEGF) is a major regulator of vasculogenesis and angiogenesis plays a vital role in tissue regeneration [8]. VEGF can dramatically affect chondrocytic proliferation, apoptosis and metabolism, and induces the release of metalloproteinases (MMPs) and other mediators that contribute to cartilage matrix degradation [9–11]. Hashimoto et al. found that hypertrophic chondrocytes in osteophytes also expressed VEGF, which indicated that VEGF plays a role in angiogenesis during osteophyte formation [12]. More recently, it was reported that VEGF levels in the knee synovial fluid of OA patients was ten-fold higher than that in paired plasma, and VEGF levels in plasma and synovial fluid were both positively correlated with radiographic severity of knee OA [13].
Stromal cell-derived factor 1α (SDF-1α) is an 8-kDa chemokine isolated from bone marrow stromal cells. SDF-1α acts by binding to its cell-surface receptor, CXCR4, a 7 transmembrane G-protein coupled receptor. The interaction of SDF-1α and CXCR4 not only plays an important role in catabolic processes in cartilage through releasing MMPs and interleukin-6 (IL-6), but also regulates the proliferative activity of chondrocytes [14–18]. In addition, in normal human CD34+ cells, megakaryoblasts and acute leukemia cells, SDF-1α can stimulate production of VEGF through binding to CXCR4 [19, 20]. SDF-1α was shown to be dramatically increased in the synovial fluids from OA patients, suggesting that SDF-1α may be involved in the progression of OA [21].
We showed previously that chondrogenic progenitor cells (CPCs), migrate and proliferate in response to cartilage injuries and others have shown that CPCs can be found migrating on damaged cartilage surfaces in osteoarthritic joints [22–25]. CPCs responding to cartilage injuries express SDF-1α and multiple cytokines at higher levels than chondrocytes [25, 26]. Although the role of SDF-1α in VEGF expression has been studied in some cell types, the signaling pathway for SDF-1α in VEGF production in CPCs has not been extensively studied. Based on these findings, we hypothesized that CPCs have the potential to contribute to intra-articular VEGF. We tested this by characterizing the expression of SDF-1α and VEGF by CPCs. To determine if SDF-1α secreted by CPCs was bioactive we measured VEGF expression in chondrocytes cultured in CPC-conditioned medium, with or without AMD3100, an SDF-1α receptor antagonist. Finally, to determine which intracellular kinases were involved in mediating the effects of SDF-1α on VEGF expression, we performed phosphoprotein analysis on CPCs exposed to SDF-1α, and SDF-1α with MAPK inhibitors.
MATERIALS AND METHODS:
Materials
SDF-1α, AMD3100, PD98059 (inhibitor of ERK1/2), SB203580 (inhibitor of p38) and SP600125 (inhibitor of JNK) were all purchased from Sigma Aldrich (St. Louis, MO). Anti-bovine rabbit polyclonal antibody specific for VEGF was purchased from Santa Cruz Biotechnology (Dallas, TX). Rabbit anti-bovine total and phosphorylated P38, ERK1/2, JNK antibodies and HRP-conjugated goat anti-rabbit IgG were purchased from Cell Signaling Technology (Danvers, MA). Biotinylated anti-rabbit IgG (H+L) antibody was purchased from Vector Laboratories (Burlingame, CA).
Cartilage harvest
25mm×25mm Cartilage explants were harvested from tibia plateau of healthy stifle bovine joints (Bud’s Custom Meats, Riverside, IA). The explants were then cultured in culture medium (Dulbecco’s modified Eagle’s medium (DMEM) with nutrient mixture F12 (1:1) containing 10% fetal bovine serum, 50 μg/ml L-ascorbate, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2.5 μg/ml Fungizone) at 37˚C with 5% CO2.
Chondrogenic Progenitor Cells (CPCs) isolation and culture
After two days of equilibration, bovine explants were scratched using by 30 gauge sterile needles to create X-shaped matrix tears of 0.5 mm in depth. After seven days post-injury, explants were treated with 0.25% Trypsin-EDTA (GIBCO, NY) for 10 mins to detach migrating CPCs from the surface. CPCs were collected by centrifuging at 300 G for 5mins, and seeded in T-225 flasks (Corning, NY) (6.3×106 cells per flask). CPCs of the first passage were cultured in 12-well culture plates (0.1×106 cells per well). After two days culture and 24 hrs of serum starvation, cells were treated with SDF-1α (20 ng/ml, 50 ng/ml and 100 ng/ml) with or without pretreated with AMD3100 (200 ng/ml) for 2hrs and then incubated in incubator at 37°C, 5% CO2, 5% O2 for 24 hrs. To examine the downstream signaling pathways involved in SDF-1α treatment, CPCs were pretreated with multiple inhibitors (PD98059, inhibitor of ERK1/2; SB203580, inhibitor of p38; and SP600125, inhibitor of JNK, the concentration of all the three inhibitors is 10μM) for 1 hr before SDF-1α (100 ng/ml) treatment.
Chondrocyte isolation
After isolation of CPCs, the cartilage was shaved off and cut into smaller pieces. Then the cartilage tissue was digested in 0.3% protease (Sigma) and 0.3% collagenase (Sigma) dissolved in serum-free media for 16 hrs. The next day, centrifuged the digestion media at 2000rpm for 10mins and resuspended with culture media and seeded the cells into T-225 flasks (Corning, NY) (6.3×106 cells per flask).
Immunohistochemical staining for VEGF
6 mm diameter osteochondral plugs without subchondral bone were harvested from bovine tibia plateaus of healthy stifle joints using biopsy punches, and then incubated in culture medium. Following two days in culture, osteochondral plugs were treated with 100 ng/ml SDF-1α with or without pretreatment of AMD3100 (200 ng/ml for 2 hrs). After 24 hrs incubation, osteochondral plugs were coated with TFM Tissue Freezing Medium (Electron Microscopy Sciences, Hatfield, PA) and stored at −20°C for future usage. Five micron sections were cut with a Reichert-Jung microtome for immunohistochemical staining. Sections were fixed in 10% neutral buffered formalin for 30 seconds, and treated with 0.3% hydrogen peroxide for 15 mins to block endogenous peroxidase. They were also treated with 10% normal goat serum 1% BSA in TBST for 40 mins to reduce non-specific binding. Sections were then treated with rabbit anti-bovine VEGF (1:50) overnight at 4°C. On the following day, they were incubated with biotinylated secondary antibody for 30 mins. Sections were treated with Elite ABC reagent (Vector Laboratories), followed by emersion for 5 mins in diaminobenzidine (DAB) substrate for development.
Gene expression analysis
CPCs were treated with SDF-1 for 24 hrs with or without 2 hr-pretreatment of 200 ng/ml AMD3100. To determine the effect of MAPK inhibitor on SDF-1 stimulated VEGF expression, CPCs were also treated with 50 ng/ml SDF-1 for 24 hrs with or without 1 hr-pretreatment of 10 μM MAPK inhibitor (SB203580, PD98059, SP600125). Total RNA was extracted with the combination for Trizol Reagent (Life Technologies) and RNeasy Mini Kit (QIAGEN, Valencia, CA), according to the manufacturer’s instructions. Real time PCR analysis was carried out using TaqMan reverse transcription reagents and SYBR green PCR Master Mix (Applied Biosystems). Custom specific primers were purchased from Integrated DNA Technologies (Coralville, IA) (Table 1). β-actin was used as an internal reference gene. Relative quantitative method (2-ΔΔCT) was used to calculate the relative expression levels of VEGF among different groups.
Table 1.
Primers Sequences for RT-PCR of VEGF
| Gene | Forward primer | Reverse primer |
|---|---|---|
| VEGF | 5’GCTCAGAGCGGAGAAAGCAT3’ | 5’ GCAACGCGAGTCTGTGTTTT3’ |
| β-actin | 5’ TCGACACCGCAACCAGTTCGC3’ | 5’ CATGCCGGAGCCGTTGTCGA3’ |
Western blot analysis
For VEGF protein expression and kinase activation studies, CPCs were plated on 6-well plates and treated with SDF-1α for different concentration and time intervals (0, 5 mins, 15 mins, 30 mins, 1 hr, 2 hrs). In addition, groups of chondrocytes were stimulated with CPC conditioned media (0, 20%, 40%, 60% and 80%) for 24 hrs with or without AMD3100 (200 ng/ml for 2 hrs) were also prepared. All the cultured cells were lysed for 20 mins with cold lysis buffer: 10 mM Tris-base, 150 mM NaCl, 0.1% sodium dodecyl sulfate (SDS), 1% Triton X-1α00, 0.1% sodium deoxycholate, 50 mM NaF, 1 mM ethyleneglycol bis tetraacetic acid (EGTA), 1 mM glycerol phosphate, 1 mM Na3VO4, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM ethylenediaminetetraacetic acid (EDTA), 2.5 mM sodium pyrophosphate decahydrate with 1:100 fold dilution of protease inhibitor cocktail III. Equal volume and weight of the protein was applied per lane, and electrophoresis was then performed under denaturing conditions on a 10% SDS gel, and transferred to a nitrocellulose membrane at 4°C overnight. The blots were blocked with 5% BSA in TBST for 1 hr at room temperature and the probed with rabbit anti-bovine antibodies against VEGF (1:200), total phosphorylated P38 (1:1000), ERK1/2 (1:1000), JNK (1:1000) and β-actin (1:2000) antibodies at 4°C overnight. After three washes, the blots were subsequently incubated with a HRP-conjugated goat anti-rabbit IgG secondary antibody (1:10000) for 1 hr at room temperature. The blots were visualized by Super Signal Chemiluminescent Substrate kit. The blot images were analyzed using Quantity One software.
Statistical Analysis
For western blots, we calculated the relative optical density by Quantity One (version 4.62). And the quantity graphs of western blot results were obtained through comparing the relative value of the target protein to β-actin. For immunohistochemical quantitative comparisons, data of each group were obtained from more than 6 visual fields. All quantitative data are presented as mean with 95% confidence interval (95% CI). Statistical tests were performed using SPSS software version 21 (SPSS, Sigma Stat). One-way analysis of variance (ANOVA) was used for mean comparisons. P values less than 0.05 were deemed to indicate a statistically significant difference. Each data point represents 3 independent experiments performed with different batches of cells and tissue.
RESULTS
Immunohistochemical staining for VEGF
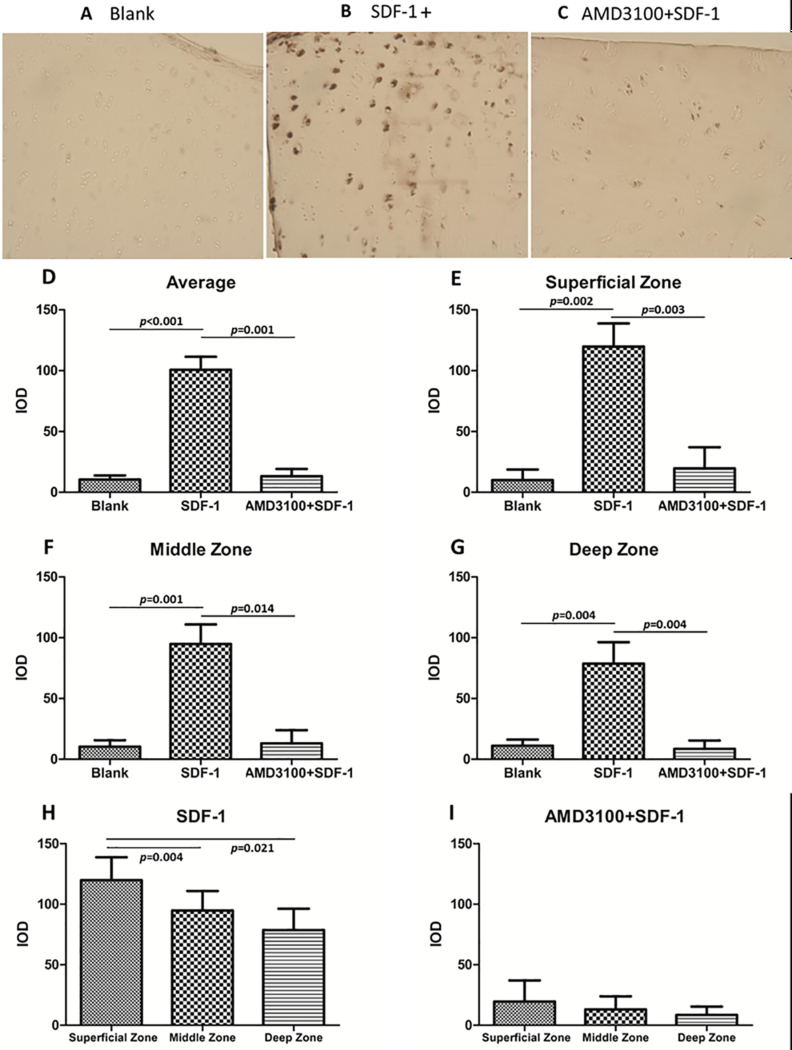
The role of CXCR4 in SDF-1α-induced VEGF expression in articular cartilage was determined by immunohistochemical staining for VEGF in osteochondral explants that were exposed to SDF-1α with or without pre-treatment with AMD3100, an SDF-1α receptor antagonist. Integrated optical density was applied to quantify the results. This analysis showed that SDF-1α significantly increased VEGF expression in cartilage (Fig.1 A, B, D-G), especially in the superficial zone (Fig.1 A, B, H). AMD3100 strongly suppressed VEGF induction by SDF-1α (Fig.1 B, C, D-G), implicating CXCR4 in the response pathway.
Figure 1.
Immunohistochemical staining for VEGF showed significantly increased expression of VEGF in the SDF-1α treated group (B, D-G) especially in the superficial zone of the cartilage (H), while AMD3100 impaired the SDF-1α-induced expression of VEGF (C, D-G) (10×10). P values indicate the statistical differences between groups. (IOD: integrated optical density)
Western blot for VEGF
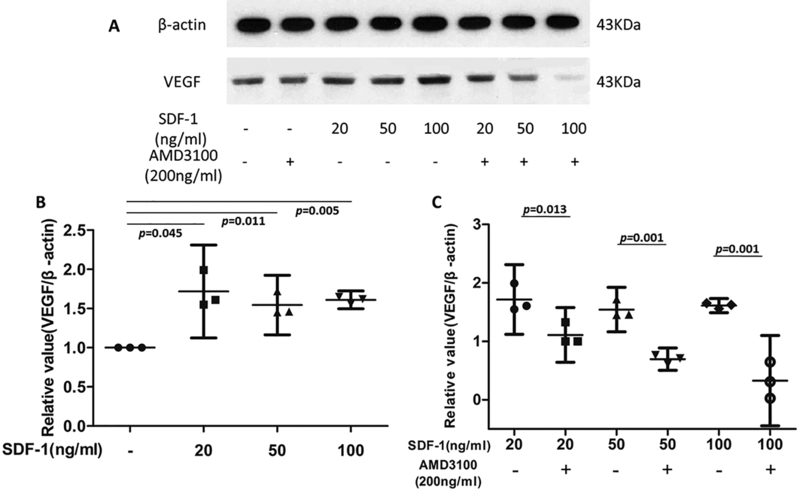
CPCs were treated with SDF-1α (20 ng/ml, 50 ng/ml and 100 ng/ml) with or without pretreatment with AMD3100 (200 ng/ml) for 2 hrs. Western blot results showed that SDF-1α increased VEGF protein expression relative to untreated controls by 1.72-, 1.54-, and 1.61-fold in cells treated with 20, 50, and 100 ng/ml SDF-1α, respectively. The increases were statistically significant (p = 0.045, 0.011 and 0.005 respectively) (Fig.2 A and B). AMD3100 reduced SDF-1α-induced VEGF expression by 34% in the 20 ng/ml group, 55% in the 50 ng/ml SDF-1α group, and 79% in the 100 ng/ml SDF-1α group. These effects were highly significant (p = 0.013, 0.001, and 0.001 for 20, 50, and 100 ng/ml respectively). (Fig.2 A and C).
Figure 2.
Effects of SDF-1α and AMD3100 on VEGF expression by CPCs. Western blot results showed that SDF-1α significantly increased VEGF protein expression relative to untreated controls, CPCs’ VEGF expression was in the same level when treated with 3 concentrations of SDF-1 (20, 50, and 100 ng/ml), around 1.6 fold higher than untreated group (A, B), while AMD3100 dramatically reduced SDF-1α-induced VEGF expression (34%, 55%,and 79% lower in 20, 50, and 100 ng/ml SDF-1 treated groups, respectively) (A, C). β-actin expression was used as an internal loading control. P values indicate the statistical differences between groups.
RT-PCR for VEGF
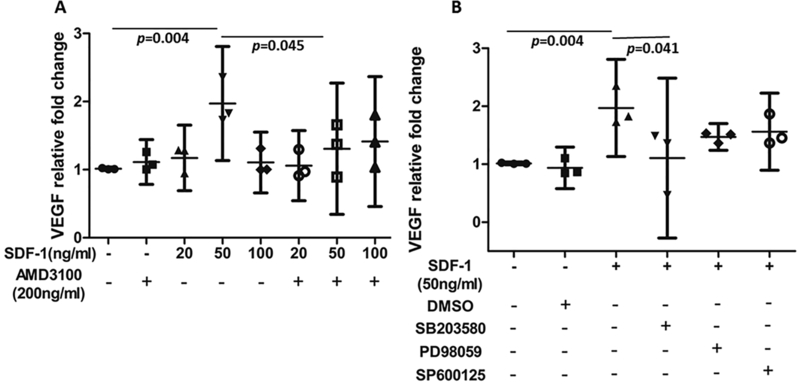
Bovine CPCs were treated with SDF-1α (20 ng/ml, 50 ng/ml and 100 ng/ml) for 24 hrs, RT-PCR indicated that SDF-1α at 50 ng/ml increased mRNA of VEGF expression 1.96-fold (p = 0.004) (Fig.3 A), The CXCR4-specific chemical inhibitor AMD3100 antagonized SDF-1α-induced VEGF mRNA expression (relative fold change: 1.30 vs 1.96, p = 0.045) (Fig.3 A). Although all three kinase inhibitors tended to reduce the SDF-1α response, the effect was only significant for the p38 inhibitor SB203580, which reduced VEGF expression in SDF-1α-treated cultures by 43% ( p = 0.041) (Fig.3 B).
Figure 3.
Effects of SDF-1α and AMD3100 on VEGF mRNA levels in CPCs. The RT-PCR results indicated that SDF-1α (50 ng/ml) substantially upregulated mRNA expression of VEGF comparing to untreated CPCs (1.96 fold higher) and AMD3100 significantly decreased VEGF expression in 50 ng/ml group by 34% (A), in addition, p38 inhibitor (SB203580) tended to significantly reduce the SDF-1α response while mild decreases were also observed in other two groups treated with different kinase inhibitors (PD98059 and SP600125) (B). β-actin expression was used as an internal loading control. P values indicate the statistical differences between groups.
Effect of SDF-1α on mitogen-activated protein kinases (MAPK) signaling pathway in chondrogenic progenitor cells
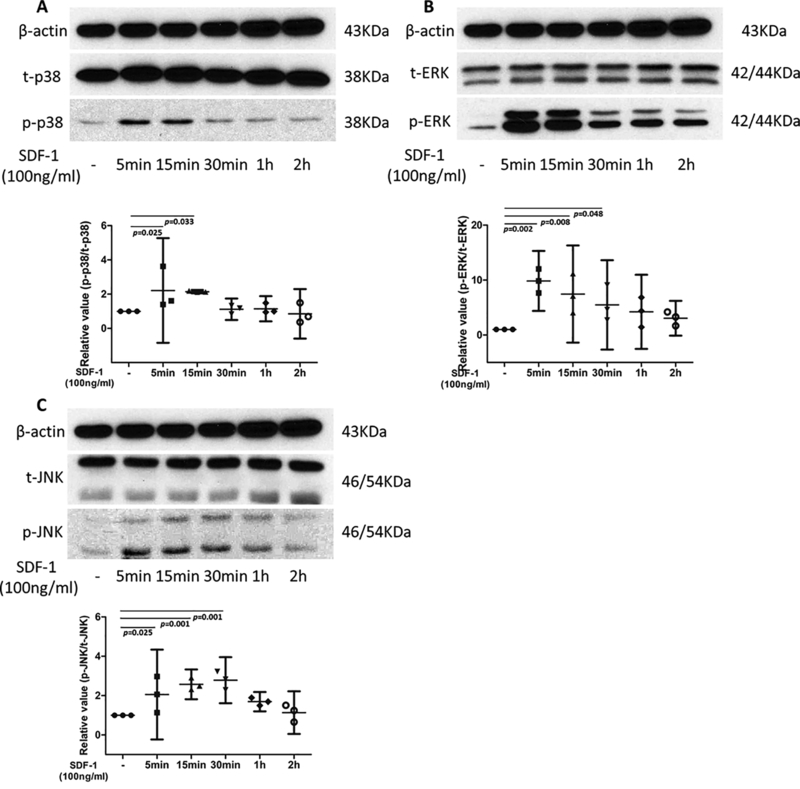
The levels of total and phosphorylated p38, ERK and JNK were measured by western blot analysis to determine which MAPK signaling pathway was activated by SDF-1α, The relative absorbance ratio of p-p38 to t-p38 in the cells treated with SDF-1α for 5min was 2.21 (Fig.4 A), while for the cells treated with SDF-1α for 15min, the relative ratio was 2.06. The expression levels of p-p38 between blank, SDF-1α treated for 5min and SDF-1α treated for 15min were identified to be significantly different (p = 0.025 and 0.033, respectively; Fig.4 A). The relative mean absorbance ratio of p-ERK1/2 over t-ERK1/2 in the SDF-1α treated for 5min, 15min and 30min were 9.85, 7.46 and 5.48, respectively, significant differences were observed between these three groups and the untreated group (p = 0.002, 0.008 and 0.048, respectively) (Fig. 4 B). Similarly, p-JNK to t-JNK was low in control group, increased progressively from 5min after SDF-1α treatment, peaked at 30min, and then declined gradually. Relative value of p-JNK to t-JNK of CPC after treated with SDF-1α 5min, 15 min and 30min were 2.06, 2.57 and 2.78, respectively, along with significant differences with untreated group (p=0.025, 0.001 and 0.001, respectively) (Fig.4 C). These results indicated that the above proteins were activated following SDF-1α treatment for 5min, and that activation was sustained for up to 30 min
Figure 4.
MAPK phosphorylation in CPCs treated with SDF-1α. CPCs were incubated with SDF-1α (100 ng/ml) for the indicated times. Blots were probed with antibodies detecting total (t-) or phosphorylated (p-) p38, ERK, or JNK. Compared to untreated group, the relative absorbance ratios of p-p38 to t-p38 were significantly elevated in groups of 5mins and 15mins (2.21 and 2.06 fold higher, respectively), no significant differences were found in groups treated with longer SDF-1 (A). The relative absorbance ratio of p-ERK to t-ERK was peaked at group of 5mins (9.85 fold higher), then gradually decreased for longer time periods (7.46 and 5.48 fold higher for 15mins and 30mins, respectively), no significant differences were found in groups of 1hrs and 2hrs (B). An significantly increasing trend of relative absorbance ratios of p-JNK to t-JNK was identified in the CPC groups treated with SDF-1 for first 30 mins (2.06, 2.57, and 2.78 fold higher for 5mins, 15mins, and 30mins, respectively). no significant differences were found in 1hrs and 2hrs groups. β-actin expression was used as an internal loading control. P values indicate the statistical differences between groups.
Effects of CPC conditioned media on chondrocyte VEGF expression
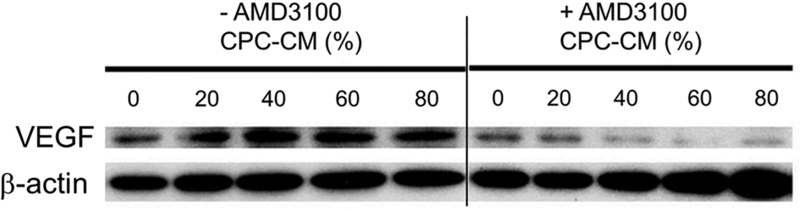
Chondrocytes were cultured in media containing varying proportions of CPC-conditioned media (20%, 40%, 60%, and 80%). Half of the cultures were treated with AMD3100. VEGF expression was assessed by western blot analysis of chondrocyte extracts (Fig. 5). The analysis revealed that CPC-conditioned media had dose-dependent stimulatory effects on chondrocyte VEGF expression, and that AMD3100 pretreatment completely suppressed this response. At higher doses of conditioned media (>40%), VEGF expression was driven below baseline.
Figure 5.
CPC-condition media stimulates chondrocyte VEGF expression. In chondrocytes that were not treated with AMD3100 (Lanes 1–5), VEGF expression increased as the proportion of CPC-conditioned media increased from 0 to 20%. Expression peaked at 40% and declined slightly at the highest CPC-CM doses (60%, 80%). In contrast, CPC-CM had no effect on VEGF expression in chondrocytes treated with AMD3100 (Lanes 6–10). β-actin expression was used as an internal loading control. (CPC-CM: CPC-condition media)
DISCUSSION
We hypothesized that CPCs, which are known to be present in significant numbers in damaged and degenerating cartilage, may contribute significantly to the pathogenic accumulation of VEGF in joints. We found that CPCs simultaneously over-expressed VEGF and SDF-1α, and that medium conditioned by CPCs stimulated VEGF expression in chondrocytes. CPCs responded to SDF-1α with increased VEGF synthesis, an effect that depended on CXCR4 and p38 activation. The latter observations suggest that persistent overexpression of VEGF by CPCs may be supported by sustained autocrine/paracrine stimulation of the SDF-1α pathway. Taken as a whole these findings support the possibility that CPCs may help to drive the increase in VEGF seen in fluids from injured and osteoarthritic joints.
Immunohistochemical staining showed that SDF-1α increased VEGF expression in normal cartilage, especially in the superficial zone, a finding that was consistent with a previously published study [27]. Furthermore, RT-PCR and western blot analysis showed that SDF-1α up-regulated VEGF expression in isolated CPCs, indicating that CPCs and superficial chondrocytes both contribute to the pool of VEGF in joint fluids. As expected, AMD3100, an SDF-1α antagonist that blocks the activation of the CXCR4 receptor, inhibited the SDF-1α-stimulated increase in VEGF expression by both cell types [17, 28].
Downstream signaling kinases activated by SDF-1α-CXCR4 binding in other cell types include p38, JNK, and ERK MAPKs, and PI3K. These in turn have been shown to up-regulate the expression of VEGF, MMPs, and IL-8, which all play significant roles in OA progression [20]. Phospho-specific immunoblots confirmed that SDF-1α treatment activated all 3 MAPKs in CPCs, but VEGF expression was substantially blocked only by a p38-specific inhibitor, and not by ERK- and JNK-specific inhibitors. These findings suggest that other factors that activate p38, including pro-inflammatory cytokines, may synergize with SDF-1α in stimulating VEGF expression.
Conditioned medium from CPCs stimulated a dose-dependent increase in VEGF expression by chondrocytes, an effect that was completely blocked by AMD3100. These results confirmed that SDF-1α was the main VEGF-inducing factor in conditioned medium, and was present in sufficient quantities to promote chondrocyte VEGF expression. These data support the hypothesis that CPCs elicit VEGF expression in other cell types primarily via SDF-1α secretion. However, we cannot rule-out the possibility that other factors secreted by CPCs influenced the results.
Our results indicate that CPCs are capable of expressing high levels of VEGF themselves, and of amplifying VEGF expression in chondrocytes. Although these data do not demonstrate that CPCs contribute to the overabundance of VEGF in synovial fluids, they do endorse an in vivo investigation of the impact of PCCs on the pathogenesis of OA. Indeed, our plan forward is to test CPC inhibitors and activators that we identified in the explant model for disease-modifying activity in established animal models of PTOA.
ACKNOWLEDGMENTS:
This work was funded by the US DHHS, National Institutes of Health/NIAMS (5 P50 AR055533), and by the University of Iowa Department of Orthopedics and Rehabilitation. We sincerely thank Barbara Laughlin and John Bierman for the acquisition of the cow stifles and osteochondral explants.
ROLE OF THE FUNDING SOURCE
This work was funded by the US DHHS, National Institutes of Health/NIAMS (5 P50 AR055533), and by the University of Iowa Department of Orthopedics and Rehabilitation.
Footnotes
CONFLICT OF INTEREST
No conflicts of interest were declared.
REFERENCES
- 1.Guilbert JJ. The world health report 2002 - reducing risks, promoting healthy life. Educ Health (Abingdon) 2003; 16: 230. [DOI] [PubMed] [Google Scholar]
- 2.Ludin A, Sela JJ, Schroeder A, Samuni Y, Nitzan DW, Amir G. Injection of vascular endothelial growth factor into knee joints induces osteoarthritis in mice. Osteoarthritis Cartilage 2013; 21: 491–497. [DOI] [PubMed] [Google Scholar]
- 3.Pesesse L, Sanchez C, Delcour JP, Bellahcene A, Baudouin C, Msika P, et al. Consequences of chondrocyte hypertrophy on osteoarthritic cartilage: potential effect on angiogenesis. Osteoarthritis Cartilage 2013; 21: 1913–1923. [DOI] [PubMed] [Google Scholar]
- 4.Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology 2002; 39: 237–246. [PubMed] [Google Scholar]
- 5.Chim SM, Tickner J, Chow ST, Kuek V, Guo B, Zhang G, et al. Angiogenic factors in bone local environment. Cytokine Growth Factor Rev 2013; 24: 297–310. [DOI] [PubMed] [Google Scholar]
- 6.Walsh DA, Bonnet CS, Turner EL, Wilson D, Situ M, McWilliams DF. Angiogenesis in the synovium and at the osteochondral junction in osteoarthritis. Osteoarthritis Cartilage 2007; 15: 743–751. [DOI] [PubMed] [Google Scholar]
- 7.Suri S, Gill SE, Massena de Camin S, Wilson D, McWilliams DF, Walsh DA. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann Rheum Dis 2007; 66: 1423–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev 2004; 56: 549–580. [DOI] [PubMed] [Google Scholar]
- 9.Pufe T, Lemke A, Kurz B, Petersen W, Tillmann B, Grodzinsky AJ, et al. Mechanical overload induces VEGF in cartilage discs via hypoxia-inducible factor. Am J Pathol 2004; 164: 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumoto T, Cooper GM, Gharaibeh B, Meszaros LB, Li G, Usas A, et al. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1. Arthritis Rheum 2009; 60: 1390–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubo S, Cooper GM, Matsumoto T, Phillippi JA, Corsi KA, Usas A, et al. Blocking vascular endothelial growth factor with soluble Flt-1 improves the chondrogenic potential of mouse skeletal muscle-derived stem cells. Arthritis Rheum 2009; 60: 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashimoto S, Creighton-Achermann L, Takahashi K, Amiel D, Coutts RD, Lotz M. Development and regulation of osteophyte formation during experimental osteoarthritis. Osteoarthritis Cartilage 2002; 10: 180–187. [DOI] [PubMed] [Google Scholar]
- 13.Saetan N, Honsawek S, Tanavalee A, Yuktanandana P, Meknavin S, Ngarmukos S, et al. Relationship of plasma and synovial fluid vascular endothelial growth factor with radiographic severity in primary knee osteoarthritis. Int Orthop 2014; 38: 1099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Endres M, Andreas K, Kalwitz G, Freymann U, Neumann K, Ringe J, et al. Chemokine profile of synovial fluid from normal, osteoarthritis and rheumatoid arthritis patients: CCL25, CXCL10 and XCL1 recruit human subchondral mesenchymal progenitor cells. Osteoarthritis Cartilage 2010; 18: 1458–1466. [DOI] [PubMed] [Google Scholar]
- 15.Kanbe K, Takemura T, Takeuchi K, Chen Q, Takagishi K, Inoue K. Synovectomy reduces stromal-cell-derived factor-1 (SDF-1) which is involved in the destruction of cartilage in osteoarthritis and rheumatoid arthritis. J Bone Joint Surg Br 2004; 86: 296–300. [DOI] [PubMed] [Google Scholar]
- 16.Chiu YC, Yang RS, Hsieh KH, Fong YC, Way TD, Lee TS, et al. Stromal cell-derived factor-1 induces matrix metalloprotease-13 expression in human chondrocytes. Mol Pharmacol 2007; 72: 695–703. [DOI] [PubMed] [Google Scholar]
- 17.Chen HT, Tsou HK, Hsu CJ, Tsai CH, Kao CH, Fong YC, et al. Stromal cell-derived factor-1/CXCR4 promotes IL-6 production in human synovial fibroblasts. J Cell Biochem 2011; 112: 1219–1227. [DOI] [PubMed] [Google Scholar]
- 18.Mazzetti I, Magagnoli G, Paoletti S, Uguccioni M, Olivotto E, Vitellozzi R, et al. A role for chemokines in the induction of chondrocyte phenotype modulation. Arthritis Rheum 2004; 50: 112–122. [DOI] [PubMed] [Google Scholar]
- 19.Kijowski J, Baj-Krzyworzeka M, Majka M, Reca R, Marquez LA, Christofidou-Solomidou M, et al. The SDF-1-CXCR4 axis stimulates VEGF secretion and activates integrins but does not affect proliferation and survival in lymphohematopoietic cells. Stem Cells 2001; 19: 453–466. [DOI] [PubMed] [Google Scholar]
- 20.Tavor S, Petit I. Can inhibition of the SDF-1/CXCR4 axis eradicate acute leukemia? Semin Cancer Biol 2010; 20: 178–185. [DOI] [PubMed] [Google Scholar]
- 21.Kanbe K, Takagishi K, Chen Q. Stimulation of matrix metalloprotease 3 release from human chondrocytes by the interaction of stromal cell-derived factor 1 and CXC chemokine receptor 4. Arthritis Rheum 2002; 46: 130–137. [DOI] [PubMed] [Google Scholar]
- 22.Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJ, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci 2004; 117: 889–897. [DOI] [PubMed] [Google Scholar]
- 23.Hattori S, Oxford C, Reddi AH. Identification of superficial zone articular chondrocyte stem/progenitor cells. Biochem Biophys Res Commun 2007; 358: 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koelling S, Kruegel J, Irmer M, Path JR, Sadowski B, Miro X, et al. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell 2009; 4: 324–335. [DOI] [PubMed] [Google Scholar]
- 25.Seol D, McCabe DJ, Choe H, Zheng H, Yu Y, Jang K, et al. Chondrogenic progenitor cells respond to cartilage injury. Arthritis Rheum 2012; 64: 3626–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou C, Zheng H, Seol D, Yu Y, Martin JA. Gene expression profiles reveal that chondrogenic progenitor cells and synovial cells are closely related. J Orthop Res 2014; 32: 981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lingaraj K, Poh CK, Wang W. Vascular endothelial growth factor (VEGF) is expressed during articular cartilage growth and re-expressed in osteoarthritis. Ann Acad Med Singapore 2010; 39: 399–403. [PubMed] [Google Scholar]
- 28.Wei F, Moore DC, Li Y, Zhang G, Wei X, Lee JK, et al. Attenuation of osteoarthritis via blockade of the SDF-1/CXCR4 signaling pathway. Arthritis Res Ther 2012; 14: R177. [DOI] [PMC free article] [PubMed] [Google Scholar]