Abstract
Background:
Enoxaparin was shown to have a neuroprotective effect in animal models as well as a human study following traumatic brain injury. This study was conducted to assess the effect of enoxaparin on the clinical outcome of severe traumatic brain injury (TBI) and its safety.
Methods:
This study is a randomized double-blinded placebo-controlled trial. The inclusion criteria were age 16–70, a closed head injury, a postresuscitation Glasgow Coma Scale (GCS) between 5 and 8, and a latency time between the injury and entering the study of less than 5 h. The patients were randomized into enoxaparin and placebo groups. In the enoxaparin group, 0.5 mg/kg enoxaparin was injected subcutaneously every 6 h in six total doses. The two groups were compared for the occurrence of intracranial hematoma (ICH) and for clinical neurological outcome, assessed by the Glasgow Outcome Scale.
Results:
Twenty-seven patients were assigned to the placebo group and 26 to the enoxaparin group. The two groups were similar regarding baseline characteristics, including age, sex, postresuscitation GCS, and best motor response. The occurrence of new ICH or an ICH size increase was insignificantly more frequent in the enoxaparin group than the placebo group (26.9% vs. 7.4%, P = 0.076). The favorable outcome rate in the enoxaparin group was significantly higher than in the placebo group (57.7% vs. 25.9%, P = 0.019).
Conclusions:
This study showed that the early administration of enoxaparin could lead to favorable outcomes in severe TBI patients without significantly increasing cerebral hemorrhagic complications.
Keywords: Enoxaparin, intracranial hematoma, outcome, traumatic brain injury
INTRODUCTION
Traffic accidents are a common cause of morbidity and mortality in Iran.[2,7,8] Traumatic brain injury (TBI) is the most common fatal consequence of traffic accidents and the most common cause of death and disability following motor vehicle accidents.[1,20] The primary insult can result in severe brain damage at the time of impact. However, a significant portion of neurological impairments are due to secondary insults, including hypoxia, hypotension, brain edema, intracranial hypertension, cerebral hypoperfusion, seizure, and coagulopathy.[6,9] Following TBI, circulating leukocytes and particularly polymorphonuclear cells are believed to interact with cerebrovascular endothelial cells to migrate out of circulation.[12,14] The recruited leukocytes promote the inflammatory process in the interstitial area, leading to blood–brain barrier permeability and impairments that induce secondary insults as brain swelling.[10,14,24] In addition to their anticoagulant behaviors, heparinoid derivatives, such as enoxaparin, have anti-inflammatory effects by blunting endothelial activation and restoring endothelial cells.[22,26,27] Studies in animal models have shown that the heparinoid derivatives reduce cerebral edema and brain damage following TBI through their anti-inflammatory effects.[12,13,18,25,28]
Recent studies have reported that heparin and low molecular weight heparin (enoxaparin) are safe for the prevention of deep vein thrombosis (DVT) in TBI and can be used even in the early phase in patients with traumatic intracranial hematoma (ICH) without increasing the risk of hemorrhage.[3,4,5,21] A recent human study revealed that the early initiation of DVT prophylaxis can improve radiological and neurological outcomes in patients with severe TBI.[9] We conducted a double-blind randomized study to evaluate the effect of a high dose of enoxaparin on the occurrence of intracranial hemorrhage and neurological outcome in severe TBI.
MATERIALS AND METHODS
This study was a pilot trial to compare the effects of subcutaneous enoxaparin versus placebo in severe TBI. The study design was a randomized double-blinded placebo-controlled clinical trial. The study was approved by the ethics committee of Mashhad Medical Faculty. As the patients had severe TBI, informed consent was obtained from their nearest relatives.
Patients with the following criteria were enrolled in the study: age between 16 and 70, closed head injury, postresuscitation Glasgow Coma Scale (GCS) between 5 and 8, and latency time between injury and entering the study of less than 5 h. Patients with the following criteria were excluded from the study: ICH greater than 5 mm in diameter, major extracranial injury, history of coagulopathy, history of anticoagulant or anti-aggregant drugs, abnormal coagulation profile on admission, pregnancy, and admission creatinine of more than 2 mg/dL. Any petechial hemorrhage with a diameter of less than 5 mm, extra-axial hematoma with a diameter of less than 2 mm, small subarachnoid hemorrhage (SAH) in only one cistern or one sulcus, or small intraventricular hemorrhage (IVH) in one ventricle were accepted.
In a randomized balanced fashion, one patient received enoxaparin and one received placebo. The dosage of enoxaparin was 0.5 mg/kg in each subcutaneous injection; the placebo was 2 mL sterile water in each subcutaneous injection. The patients were subjected to six doses every 6 h, and the first dose was administered within 5 h of trauma. The patient, investigators, and evaluators were blinded to the treatment, with the exception of the nurse responsible for randomization and injection.
On admission, the basic characteristics, including age, sex, mechanism of trauma, vital signs, postresuscitation GCS, best motor response (BMR), and associated extracranial injury, were recorded.
On admission, the complete blood count, coagulation profile (prothrombin time, partial thromboplastin time, and international normalized ratio), and brain CT scan were carried out. Then, every 6 h, the above lab tests and CT scan were repeated for 2 days and evaluated before each treatment injection. The laboratory tests were assessed every other day for the next 12 days. The CT scans were repeated at day 4 and before discharge. The CT scan could be repeated as needed in case of neurological changes. On admission and every other day, blood sugar, blood urea nitrogen, and creatinine were measured for 2 weeks. The patient's CT scans were evaluated according to the Marshall Classification.[15] All hematomas, including contusion, petechial hemorrhage, extra-axial hematoma, SAH, or IVH, were considered as ICH. The hematomas were assessed for an increase in size and number. If the ICH size increased to more than 5 mm, the treatment was stopped. The injections were also stopped if the coagulation profile became abnormal during treatment.
The patients were treated and monitored based on the routine guidelines[2] for severe TBI at the neurosurgical ICU. Because of bleeding risk, intracranial pressure monitoring was not carried out. All patients received DVT prophylaxis starting 48 h after admission.
At discharge, the patient's outcome was assessed according to the Glasgow Outcome Scale, that is, good recovery, moderate disability, severe disability, vegetative state, and death. Additionally, the clinical outcome was divided into two groups: favorable outcome, including good recovery and moderate disability; and poor outcome, including severe disability, vegetative state, and death.
The primary end point was the radiological appearance of a new ICH or an increase in the size of the previous ICH. Any ICH change was considered major if it led to neurosurgical intervention or neurological deterioration. The secondary end point was the clinical outcome of the patient at discharge, favorable outcome versus poor outcome, or death.
The statistical analysis was performed using SPSS for Windows, release 13.0 (SPSS Inc., Chicago, IL, USA). The Chi-square test, two-tailed Fisher's exact test, and t-test were used to compare differences between two groups for categorical and continuous variables accordingly. The Mann–Whitney U test was used to compare medians. P < 0.05 was considered to indicate significance.
RESULTS
Basic characteristic
Fifty-four patients were enrolled in the study and randomized. One patient was excluded because he had an ICH larger than 5 mm on admission. Of the remaining 53 patients, 27 were assigned into the placebo group and 26 to the enoxaparin group. The patient characteristics are shown in Table 1. The mean age of the patients was 26.5 with a range of 17–66. Males were predominant. The mechanism of trauma was motor vehicle accident in all patients. The median postresuscitation GCS was 7 with a BMR median of 5. The two groups were similar for baseline characteristics, with the exception of a higher rate of abnormal pupils and poor GCS (GCS 5–6) in the placebo group, which was not significant.
Table 1.
Basic patient characteristics
| Total 53 (100%) | Enoxaparin group 26 (100%) | Placebo group 27 (100%) | P | |
|---|---|---|---|---|
| Age (years) | 26.5±11.3 | 27.2±13.2 | 25.9±0.9 | 0.669 |
| Male | 43 (81.1) | 22 (84.6) | 22 (81.5) | 1.000 |
| Motor vehicle accident | 53 (100) | 26 (100) | 27 (100) | |
| Abnormal pupil | 13 (24.5) | 3 (11.5) | 10 (37.0) | 0.054 |
| Postresuscitation GCS | 7 | 7 | 7 | 0.378 |
| BMR | 5 | 5 | 5 | 0.064 |
| GCS 5–6 | 15 (28.3) | 4 (15.4) | 11 (37) | 0.119 |
| Hypotension | 3 (5.7) | 0 (0.0) | 3 (11.1) | 0.236 |
| Hypoxia | 3 (5.7) | 0 (0.0) | 2 (7.4) | 0.491 |
| History of opium abuse | 23 (43.4) | 11 (42.3) | 12 (44.4) | 0.875 |
| Intracranial hematoma | 20 (37.7) | 11 (42.3) | 9 (33.3) | 0.500 |
| Marshall CT grade >1 | 13 (24.5) | 6 (23.1) | 7 (25.9) | 0.810 |
GCS=Glasgow Coma Scale
Primary end point
In the enoxaparin group, seven patients (26.9%) showed new ICH or increasing ICH size compared with two patients (7.4%) in the placebo group [Table 2]. The rate of ICH development was more than twofold of the enoxaparin group; however, this difference did not reach statistical significance (P = 0.076). In the enoxaparin group, three patients developed new small extra-axial hematoma, two patients developed new small contusions, and another two showed an expansion of existing contusions. In the placebo group, small petechial hemorrhage and small IVH were found in the follow-up CT scan of two patients. None was major, and no patients required neurosurgical intervention.
Table 2.
CT changes during hospitalization (primary outcome)
| Total 53 (100%) | Enoxaparin group 26 (100%) | Placebo group 27 (100%) | P | |
|---|---|---|---|---|
| New contusion | 4 (7.5) | 2 (7.7) | 2 (7.4) | 1.000 |
| Contusion expansion | 2 (3.8) | 2 (7.7) | 0 (0.0) | 0.236 |
| New extraaxial hematoma | 3 (5.7) | 3 (11.5) | 0 (0.0) | 0.111 |
| Any ICH change | 9 (17.0) | 7 (26.9) | 2 (7.4) | 0.076 |
| CT grade increase | 4 (7.5) | 2 (7.7) | 2 (7.4) | 1.000 |
| Followup CT sequels* | 9 (19.1) | 2 (8.3%) | 7 (30.4%) | 0.054 |
*Showed among survivals, ICH=Intracranial hematoma
According to Marshall CT grade,[22] four patients (14.8%) showed worsening grade (two patients in each group), which is compatible with increasing edema. Follow-up CT scan showed brain injury sequelae in seven patients (30.4%) in the placebo group compared with two patients (8.3%) in the enoxaparin group.
Secondary end point
On discharge, death occurred in two patients (7.7%) in the enoxaparin group versus four patients (14.8%) in the placebo group; vegetative state occurred in two patients (7.7%) in the enoxaparin group versus nine (33.3%) in the placebo group; severe disability occurred in 26.9% in the enoxaparin group versus 25.9% in the placebo group; moderate disability occurred in 7.7% of the enoxaparin group versus 18.5% in the placebo group; and good recovery occurred in 50% of the enoxaparin group versus 7.4% in the placebo group [Table 3]. As shown in Figure 1, there was a significant tendency toward a better outcome in the enoxaparin group compared with the placebo group (P = 0.006). The favorable outcome rate was significantly higher in the enoxaparin group than in the placebo group (57.7% vs. 25.9%, P = 0.019). Death occurred twice in the placebo group; however, the difference was not significant. The two groups were similar regarding ICU stay and hospital stay. Coagulation abnormalities occurred in 29.6% of the placebo group and 23.1% of the enoxaparin group; however, this difference was not significant (P = 0.589). DVT occurred in three patients, all of which (11.5%) were in the enoxaparin group.
Table 3.
Clinical outcome (secondary outcome) of the patients
| Total 53 (100%) | Enoxaparin group 26 (100%) | Placebo group 27 (100%) | P | |
|---|---|---|---|---|
| GOS | ||||
| Good recovery | 15 (28.3) | 13 (50.0) | 2 (7.4) | 0.006 |
| Moderate disability | 7 (13.2) | 2 (7.7) | 5 (18.5) | |
| Severe disability | 14 (26.4) | 7 (26.9) | 7 (25.9) | |
| Vegetative state | 11 (20.8) | 2 (7.7) | 9 (33.3) | |
| Death | 6 (11.3) | 2 (7.7) | 4 (14.8) | |
| Favorable outcome | 22 (41.5) | 15 (57.7) | 7 (25.9) | 0.019 |
| Hospital stay duration | 27.3±14 | 24.4±12.1 | 30.1±15.4 | 0.138 |
| ICU stay duration | 22.2±13.3 | 19.3±13.2 | 25.0±13.1 | 0.119 |
GOS=Glasgow Outcome Scale
Figure 1.
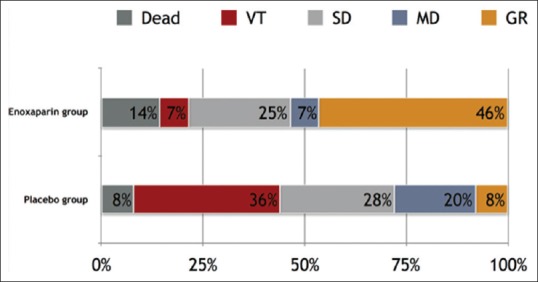
Glasgow Outcome Scale for the enoxaparin and placebo groups
DISCUSSION
This randomized double-blinded placebo-controlled pilot trial compares the effects of a high dose of enoxaparin with placebo regarding the clinical outcomes of patients with severe TBI. We found that a favorable outcome was significantly more common in the enoxaparin group. The rate of hematoma size change was twofold higher in the enoxaparin group; however, this difference was not significant, and none required neurosurgical intervention. To the best of our knowledge, this is the first human trial to assess the effect of low molecular heparin in managing severe TBI. Kim et al.[9] assessed the effect of early DVT prophylaxis on the outcome of patients with severe TBI. In their study, neurological exam improved faster in patients who received DVT prophylaxis started in the first 3 days compared with the patients with later DVT prophylaxis. In addition, brain injury occurred more slowly in the early group radiologically. Kim et al. suggested that a clinical trial could answer many questions regarding the neuroprotective effects of heparin derivatives and their safety.
Animal studies showed that enoxaparin reduced brain edema and secondary brain injury following TBI due to its anti-inflammatory effects.[12,13,18,25,28] The anti-inflammatory effects of enoxaparin occurred in various ways. Enoxaparin could blunt oxidative lipid and protein damage, COX-2 overexpression, and reactive astrocytosis in the hypothalamus of rats following TBI.[29] Enoxaparin consequently reduced hypothalamus damage. Enoxaparin has also been shown to reduce the cerebrovascular interaction between circulating leukocytes and endothelial cells.[12,13,18,25,28] By reducing brain edema involving the parietal cortex and hippocampus, enoxaparin improved the functional outcome in rats in the context of experimental TBI and cerebral ischemia.[16,25,28] In addition to reducing the brain edema and improving the neurological outcome following TBI, enoxaparin did not increase the cerebral contusion size even with a dose as high as 1 mg/kg every 6 h.[12,13] Enoxaparin was also hypothesized to prevent thrombosis in cerebral microcirculation and to reduce related damage in another study.[28] This study did not find any evidence for reducing brain edema in a serial brain CT scan. In other words, the Marshall grading did not differ between the enoxaparin and placebo groups. However, the follow-up and late CT scans showed the less abnormalities, including brain atrophy and hypodensities, in the enoxaparin group compared with the placebo group, which may be consistent with reduced brain damage due to secondary brain injury. The better outcome in the enoxaparin group in this study supported the neuroprotective effects of enoxaparin shown in the other studies.
Several studies reported the safety of enoxaparin for preventing DVT and pulmonary embolism in severe TBI.[11,17,19,23] Chemical DVT prophylaxis could be started in the early phase of severe TBI without increasing the occurrence of ICH or increasing their size.[3,21] According to these studies, we started enoxaparin within 6 h of trauma. The next issue was the dose of enoxaparin. All human studies reported a prophylaxis dose of enoxaparin. However, the animal studies administered different doses from as low as 0.5 mg/kg in the initial dose followed by 0.5 mg/kg every 6 h to as high as 0.5 mg/kg in the initial dose and 2 mg/kg every 6 h. We chose the lowest dose in the animal studies, which was equal to the treatment dose for DVT. We used four doses of 0.5 mg/kg/day instead of two doses of 1 mg/kg/day to reduce the risk of potential hemorrhage in earlier treatment. Similar to studies with a prophylaxis dose, our results confirmed that a higher dose does not significantly increase the risk of hemorrhage. In addition, this study showed that the initiation of a high dose of enoxaparin in the very early phase of trauma, that is, within 6 h of trauma, did not increase major ICH.
There are several limitations to this study. First, the number of participating subjects was limited, and a larger number could strengthen the results and lead to stronger conclusions. Second, the patients in the placebo group had slightly worse GCS on admission compared with those in the enoxaparin group, which could impact the results. Third, due to the risk of hemorrhage, we did not use intracranial pressure monitoring, which could have more accurately assessed brain edema and the effects of enoxaparin.
CONCLUSION
This study revealed that the treatment dose of enoxaparin is safe in the early phase of severe nonhemorrhagic TBI and that it did not significantly increase major cerebral hemorrhagic complications. Enoxaparin could also increase favorable outcomes in these patients. Double-blind randomized multicenter studies with larger cases could better evaluate the safety and effect of enoxaparin in severe TBI.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Contributor Information
Humain Baharvahdat, Email: humainbv@yahoo.fr.
Babak Ganjeifar, Email: humainbv@yahoo.fr.
Hamid Etemadrezaie, Email: humainbv@yahoo.fr.
Mohammad Farajirad, Email: humainbv@yahoo.fr.
Samira Zabihyan, Email: humainbv@yahoo.fr.
Ashkan Mowla, Email: amowla@mednet.ucla.edu.
REFERENCES
- 1.Abbasi HR, Mousavi SM, Taheri Akerdi A, Niakan MH, Bolandparvaz S, Paydar S. Pattern of traumatic injuries and injury severity score in a major trauma center in Shiraz, Southern Iran. Bull Emerg Trauma. 2013;1:81–5. [PMC free article] [PubMed] [Google Scholar]
- 2.Brain Trauma Foundation, American Association of Neurological S, Congress of Neurological S. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(Suppl 1):S1–106. doi: 10.1089/neu.2007.9999. [DOI] [PubMed] [Google Scholar]
- 3.Byrne JP, Mason SA, Gomez D, Hoeft C, Subacius H, Xiong W, et al. Timing of pharmacologic venous thromboembolism prophylaxis in severe traumatic brain injury: A propensity-matched cohort study. J Am Coll Surg. 2016;223:621–31.e5. doi: 10.1016/j.jamcollsurg.2016.06.382. [DOI] [PubMed] [Google Scholar]
- 4.Farooqui A, Hiser B, Barnes SL, Litofsky NS. Safety and efficacy of early thromboembolism chemoprophylaxis after intracranial hemorrhage from traumatic brain injury. J Neurosurg. 2013;119:1576–82. doi: 10.3171/2013.8.JNS13424. [DOI] [PubMed] [Google Scholar]
- 5.Frisoli F, Huang PP, Frangos S. 180 Early deep vein thrombosis chemoprophylaxis in traumatic brain injury. Neurosurgery. 2016;63(Suppl 1):171–2. [Google Scholar]
- 6.Haddad SH, Arabi YM. Critical care management of severe traumatic brain injury in adults. Scand J Trauma Resusc Emerg Med. 2012;20:12. doi: 10.1186/1757-7241-20-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heydari ST, Hoseinzadeh A, Ghaffarpasand F, Hedjazi A, Zarenezhad M, Moafian G, et al. Epidemiological characteristics of fatal traffic accidents in Fars province, Iran: A community-based survey. Public Health. 2013;127:704–9. doi: 10.1016/j.puhe.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Khalili H, Sadraei N, Niakan A, Ghaffarpasand F, Sadraei A. Role of intracranial pressure monitoring in management of patients with severe traumatic brain injury: Results of a large level I trauma center in Southern Iran. World Neurosurg. 2016;94:120–5. doi: 10.1016/j.wneu.2016.06.122. [DOI] [PubMed] [Google Scholar]
- 9.Kim L, Schuster J, Holena DN, Sims CA, Levine J, Pascual JL. Early initiation of prophylactic heparin in severe traumatic brain injury is associated with accelerated improvement on brain imaging. J Emerg Trauma Shock. 2014;7:141–8. doi: 10.4103/0974-2700.136846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumasaka K, Marks JA, Eisenstadt R, Murcy MA, Samadi D, Li S, et al. In vivo leukocyte-mediated brain microcirculatory inflammation: A comparison of osmotherapies and progesterone in severe traumatic brain injury. Am J Surg. 2014;208:961. doi: 10.1016/j.amjsurg.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurtoglu M, Yanar H, Bilsel Y, Guloglu R, Kizilirmak S, Buyukkurt D, et al. Venous thromboembolism prophylaxis after head and spinal trauma: Intermittent pneumatic compression devices versus low molecular weight heparin. World J Surg. 2004;28:807–11. doi: 10.1007/s00268-004-7295-6. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Eisenstadt R, Kumasaka K, Johnson VE, Marks J, Nagata K, et al. Does enoxaparin interfere with HMGB1 signaling after TBI?. A potential mechanism for reduced cerebral edema and neurologic recovery. J Trauma Acute Care Surg. 2016;80:381. doi: 10.1097/TA.0000000000000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S, Marks JA, Eisenstadt R, Kumasaka K, Samadi D, Johnson VE, et al. Enoxaparin ameliorates post-traumatic brain injury edema and neurologic recovery, reducing cerebral leukocyte endothelial interactions and vessel permeability in vivo. J Trauma Acute Care Surg. 2015;79:78–84. doi: 10.1097/TA.0000000000000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S, Marks J, Sanati P, Eisenstadt R, Gong W, Browne K, et al. In Vivo evolution of microvascular inflammation after traumatic brain injury: An intravital microscopy study. Crit Care Med. 2011;39:1. [Google Scholar]
- 15.Marshall LF, Marshall SB, Klauber MR, Van Berkum Clark M, Eisenberg H, Jane JA, et al. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma. 1992;9(Suppl 1):S287–92. [PubMed] [Google Scholar]
- 16.Mary V, Wahl F, Uzan A, Stutzmann JM. Enoxaparin in experimental stroke: Neuroprotection and therapeutic window of opportunity. Stroke. 2001;32:993–9. doi: 10.1161/01.str.32.4.993. [DOI] [PubMed] [Google Scholar]
- 17.Minshall CT, Eriksson EA, Leon SM, Doben AR, McKinzie BP, Fakhry SM. Safety and efficacy of heparin or enoxaparin prophylaxis in blunt trauma patients with a head abbreviated injury severity score>2. J Trauma. 2011;71:396. doi: 10.1097/TA.0b013e31822734c9. [DOI] [PubMed] [Google Scholar]
- 18.Nagata K, Kumasaka K, Browne KD, Li S, St-Pierre J, Cognetti J, et al. Unfractionated heparin after TBI reduces in vivo cerebrovascular inflammation, brain edema and accelerates cognitive recovery. J Trauma Acute Care Surg. 2016;81:1088–94. doi: 10.1097/TA.0000000000001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norwood SH, McAuley CE, Berne JD, Vallina VL, Kerns DB, Grahm TW, et al. Prospective evaluation of the safety of enoxaparin prophylaxis for venous thromboembolism in patients with intracranial hemorrhagic injuries. Arch Surg. 2002;137:696. doi: 10.1001/archsurg.137.6.696. [DOI] [PubMed] [Google Scholar]
- 20.Paydar S, Shokrollahi S, Jahanabadi S, Ghaffarpasand F, Malekmohammadi Z, Akbarzadeh A, et al. Emergency operating room workload pattern: A single center experience from southern Iran. Bull Emerg Trauma. 2013;1:38–42. [PMC free article] [PubMed] [Google Scholar]
- 21.Phelan HA, Wolf SE, Norwood SH, Aldy K, Brakenridge SC, Eastman AL, et al. A randomized, double-blinded, placebo-controlled pilot trial of anticoagulation in low-risk traumatic brain injury: The Delayed Versus Early Enoxaparin Prophylaxis I (DEEP I) study. J Trauma Acute Care Surg. 2012;73:1434–41. doi: 10.1097/TA.0b013e31825ac49e. [DOI] [PubMed] [Google Scholar]
- 22.Rana MW, Singh G, Wang P, Ayala A, Zhou M, Chaudry IH. Protective effects of preheparinization on the microvasculature during and after hemorrhagic shock. J Trauma. 1992;32:420–6. doi: 10.1097/00005373-199204000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Saadeh Y, Gohil K, Bill C, Smith C, Morrison C, Mosher B, et al. Chemical venous thromboembolic prophylaxis is safe and effective for patients with traumatic brain injury when started 24 hours after the absence of hemorrhage progression on head CT. J Trauma Acute Care Surg. 2012;73:426–30. doi: 10.1097/TA.0b013e31825a758b. [DOI] [PubMed] [Google Scholar]
- 24.Schoettle RJ, Kochanek PM, Magargee MJ, Uhl MW, Nemoto EM. Early polymorphonuclear leukocyte accumulation correlates with the development of posttraumatic cerebral edema in rats. J Neurotrauma. 1990;7:207–17. doi: 10.1089/neu.1990.7.207. [DOI] [PubMed] [Google Scholar]
- 25.Stutzmann JM, Mary V, Wahl F, Grosjean-Piot O, Uzan A, Pratt J. Neuroprotective profile of enoxaparin, a low molecular weight heparin, in in vivo models of cerebral ischemia or traumatic brain injury in rats: A review. CNS Drug Rev. 2002;8:1–30. doi: 10.1111/j.1527-3458.2002.tb00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wahl F, Grosjean-Piot O, Bareyre F, Uzan A, Stutzmann JM. Enoxaparin reduces brain edema, cerebral lesions, and improves motor and cognitive impairments induced by a traumatic brain injury in rats. J Neurotrauma. 2000;17:1055–65. doi: 10.1089/neu.2000.17.1055. [DOI] [PubMed] [Google Scholar]
- 27.Wang P, Ba ZF, Chaudry IH. Endothelial cell dysfunction occurs very early following trauma-hemorrhage and persists despite fluid resuscitation. Am J Physiol. 1993;265:H973–9. doi: 10.1152/ajpheart.1993.265.3.H973. [DOI] [PubMed] [Google Scholar]
- 28.Wang P, Singh G, Rana MW, Ba ZF, Chaudry IH. Preheparinization improves organ function after hemorrhage and resuscitation. Am J Physiol. 1990;259:R645–50. doi: 10.1152/ajpregu.1990.259.3.R645. [DOI] [PubMed] [Google Scholar]
- 29.Zupan Z, Pilipovic K, Dangubic B, Frkovic V, Sustic A, Zupan G. Effects of enoxaparin in the rat hippocampus following traumatic brain injury. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1846–56. doi: 10.1016/j.pnpbp.2011.08.005. [DOI] [PubMed] [Google Scholar]


