ABSTRACT
Background
For low-carbohydrate diets, a public health approach has focused on the replacement of carbohydrates with unsaturated fats. However, little research exists on the impacts of saturated fat intake on the lipid profile in the context of whole-food-based low-carbohydrate weight-loss diets.
Objectives
The primary aim of this secondary analysis of the DIETFITS weight loss trial was to evaluate the associations between changes in percentage of dietary saturated fatty acid intake (%SFA) and changes in low-density lipoproteins, high-density lipoproteins, and triglyceride concentrations for those following a healthy low-carbohydrate (HLC) diet. The secondary aim was to examine these associations specifically for HLC dieters who had the highest 12-month increases in %SFA.
Methods
In the DIETFITS trial, 609 generally healthy adults, aged 18–50 years, with body mass indices of 28–40 kg/m2 were randomly assigned to a healthy low-fat (HLF) or HLC diet for 12 months. In this analysis, linear regression, both without and with adjustment for potential confounders, was used to measure the association between 12-month change in %SFA and blood lipids in 208 HLC participants with complete diet and blood lipid data.
Results
Participants consumed an average of 12–18% of calories from SFA. An increase of %SFA, without significant changes in absolute saturated fat intake, over 12 months was associated with a statistically significant decrease in triglycerides in the context of a weight-loss study in which participants simultaneously decreased carbohydrate intake. The association between increase in %SFA and decrease in triglycerides was no longer significant when adjusting for 12-month change in carbohydrate intake, suggesting carbohydrate intake may be a mediator of this relationship.
Conclusions
Those on a low-carbohydrate weight-loss diet who increase their percentage intake of dietary saturated fat may improve their overall lipid profile provided they focus on a high-quality diet and lower their intakes of both calories and refined carbohydrates. This trial was registered at clinicaltrials.gov as NCT01826591.
Keywords: saturated fat, low carbohydrate, LDL cholesterol, HDL cholesterol, triglycerides, human study, weight-loss trial, healthy adults, diet quality
Introduction
The 2015 Dietary Guidelines for Americans (DGA) recommend consuming less than 10% of daily calories from saturated fats, but more than 70% of Americans exceed this limit (1). This emphasis on limiting saturated fat intake has continued for decades based on the scientific understanding that dietary intake of saturated fatty acids (SFAs) increases plasma LDL cholesterol, whereas substituting mono- and poly-unsaturated fatty acids for SFAs reduces LDL cholesterol concentrations (2–5). However, evidence on the impact of SFAs on cardiovascular disease (CVD) risk is more nuanced than guidelines suggest. Although replacing dietary saturated fats with unsaturated fats is associated with reduced risk of CVD, substitution of saturated fats with carbohydrates has been associated with either no improvement or worsening of health outcomes (6–8). Furthermore, the effect of particular foods on CVD risk cannot be predicted solely by their SFA content (9). Individual SFAs may have different cardiovascular effects, and major plant- and animal-based SFA food sources contain a wide variety of other compounds that could influence cardiovascular outcomes (10–12).
In parallel with the recent debate around the role of SFAs in CVD risk, low-carbohydrate (low-carb) diets have risen in popularity as challengers to low-fat diets in the context of weight loss studies (13). According to the 2013 American Heart Association/American College of Cardiology/The Obesity Society Guideline for the Management of Overweight and Obesity in Adults, a variety of dietary approaches including low-fat and low-carbohydrate diets can produce weight loss in overweight and obese adults provided a reduction in dietary energy intake is achieved (14, 15). Some studies have reported that low-carbohydrate diets may help people lose weight more quickly and maintain that weight loss longer than, or equally effectively as, low-fat diets (14, 16, 17, 10). There is also evidence of improvement in CVD risk factors that result from a low-carbohydrate weight-loss diet, such as decreases in plasma triglyceride concentrations and increases in HDL cholesterol concentrations (16). These changes in blood lipids may be driven by the reduction in added sugars and refined carbohydrates that typically occurs in parallel with an increase in total dietary fat that occurs in low-carbohydrate diets (14, 16, 18–20).
A public health approach to low-carbohydrate diets has built on this idea and focused on replacing refined carbohydrates with unsaturated fats. Although the limitation of refined carbohydrates is an important part of a high quality diet, this understanding is often coupled with recommendations for limiting percentage daily intake of total and saturated fat. Little research exists on the relation between intake of SFAs and their effects on the lipid profile in the context of a primarily whole-food, low-carbohydrate weight-loss diet. In this secondary analysis, the novel study design of the Diet Intervention Examining The Factors Interacting with Treatment Success (DIETFITS) weight loss trial, focused on high-quality diets, was harnessed to further investigate the potential impacts of differential changes in dietary SFA intake on serum LDL cholesterol, HDL cholesterol, and triglyceride concentrations.
Methods
Study design
Detailed study methods of the DIETFITS trial were reported previously (21). In brief, the study was a randomized controlled weight loss trial of 609 participants that aimed to test whether genetics (3-single nucleotide polymorphisms) or metabolic predispositions (insulin secretion) at baseline could help explain differential weight loss for those assigned to either healthy low-carbohydrate (HLC) or healthy low-fat (HLF) diets. All study procedures followed were in accordance with the ethical standards of the Stanford University Human Subjects Committee and in accordance with the Helsinki Declaration of 1975 as revised in 1983. The study was conducted between January 2013 and May 2016. Participants were generally healthy women and men, aged 18–50 y with BMIs of 28–40 kg/m2. Exclusion criteria included uncontrolled metabolic disease or hypertension; pregnancy or lactation; diabetes; cancer; cardiovascular, renal, or liver disease; or use of medications that affect weight. Both dietary weight loss interventions spanned 12 mo and included 22 group educational sessions with registered dietitian nutritionists and clinical health educators. Data were collected at baseline and months 3, 6, and 12 (21).
Of the total 609 study participants, 304 were randomly assigned to the HLC group. For this secondary analysis, we included only participants from the HLC group with complete diet and plasma lipid data at baseline and 12 mo (n = 208). The primary objective was to examine the associations between 12-mo changes in percentage SFA intake and changes in lipid profiles (LDL cholesterol, HDL cholesterol, and triglycerides). Participants in the HLC group were not given any instructions regarding target energy or SFA intakes. Instead, they were asked to achieve the lowest net carbohydrate intake that they could maintain long term while focusing on eating a high-quality diet made up primarily of whole foods.
Dietary intake was assessed using 3 unannounced, 24-h dietary recalls within a 2-wk window at each data-collection time point. Dietary recalls were collected using a standardized multiple-pass interview approach (22). Lipids were measured at a laboratory certified by the Centers for Disease Control and Prevention—National Heart, Lung, and Blood Institute lipid standardization program (the Krauss Lab at the Children's Hospital Oakland Research Institute, Oakland, CA) using fasting blood samples collected at each time point (23). Plasma triglycerides and HDL cholesterol were measured by enzymatic endpoint analysis, whereas LDL cholesterol was calculated using the Friedewald equation (24–27). Data collectors were blinded to the assigned diets. Data managers had no role in the conduct of the study; data were maintained using REDCap electronic data-capture tools hosted at Stanford University (28).
Statistical analysis
Descriptive statistics were used to summarize saturated fat intake and demographic information. For each participant at each of up to 3 dietary recalls, saturated fat in calories was calculated as saturated fat intake in grams multiplied by 9. Mean saturated fat was calculated by averaging every available recall, up to 3 recalls at each time point, at baseline and at 12 mo. Percentage SFA was calculated at baseline and 12 mo by dividing mean saturated fat in calories by mean total calories. Finally, percentage SFA change was calculated as the mean percentage SFA at 12 mo minus the mean percentage SFA at baseline. Carbohydrate intake was calculated as the mean carbohydrate intake in grams across up to 3 dietary recalls at each time point. The 12-mo change in carbohydrate intake was calculated as the mean carbohydrate intake at 12 mo minus the mean carbohydrate intake at baseline.
For ease of presentation, participants were divided into tertiles according to the 12-mo change in percentage SFA intake in a preliminary descriptive analysis: those who lowered (lowest tertile), somewhat increased (middle tertile), or most substantially increased (highest tertile) their percentage SFA intake over the 12-mo study period. Pearson and Spearman correlation coefficients and scatter plots were used to examine bivariate associations between percentage SFA change and lipid outcomes. The 12-mo lipid differences between diet groups were determined by t-test for those with available data at 12 mo.
In our primary analysis for each lipid variable, linear regression was used to quantify the association between 12-mo change in SFAs as a percentage of energy intake and 12-mo change in the plasma lipids. This was done using 3 models: 1) linear regression of 12-mo lipid change on 12-mo percentage SFA change with no adjustment (unadjusted); 2) the same model as 1) plus adjustment for age, gender, race and 12-mo change in body weight (Model 1); and 3) the same model as 2) plus additional adjustment for 12-mo change in carbohydrate intake (Model 2) in order to assess whether carbohydrate intake may act as a mediator of the association between percentage SFA intake and lipid outcomes. This modeling approach allowed assessment of the association of a 1% increase in percentage SFA intake with 12-mo lipid changes. These analyses were performed using percentage SFA and plasma lipid data as continuous variables. Statistical significance of the variables included in the models was assessed via a 2-sided Wald test with type I error at 0.05.
For comparison with the HLC analyses described above, all descriptive and primary analyses were repeated for the HLF group. Because the focus of this analysis is on the HLC group, and for the sake of efficiency and space limitations, some of the HLF data and findings are available in the supplemental materials. All statistical analyses were performed using SAS University Edition (SAS Studio 3.6; SAS Institute).
Results
Baseline characteristics of the study population
Retention at 12 mo in the DIETFITS trial (defined as participants who provided any data at 12 mo) was 78% for the HLC group (29). Although 238 out of the total 304 HLC participants contributed at least some 12-mo data for the study, this complete case analysis included only those with complete diet and plasma lipid data at 12 mo (n = 208, 68% of those randomly assigned to the HLC group) (Supplemental Figure 1). Among the tertiles of percentage SFA change in the HLC group, there were no statistically significant baseline differences in age, gender, education, weight, or plasma lipids; however, there were significant differences in race/ethnicity and dietary factors (Table 1). The highest tertile (those with the greatest change in percentage SFA intake) had a slightly higher proportion of non-Hispanic whites relative to the other tertiles. Baseline kilocalories, fats, and protein, but not carbohydrate, were different among tertiles. Baseline demographic characteristics for the HLF group can be found in Supplemental Table 1. Additional HLF diet group results have been provided to represent the full span of 12-mo changes in percentage SFA in a broader context (Tables 2 and 3 and Figures 1 and 2).
TABLE 1.
Baseline demographics of the healthy low-carbohydrate weight-loss diet group by tertiles of 12-mo change in percentage dietary saturated fat1
| Tertile2 | |||||
|---|---|---|---|---|---|
| Total (N = 208) | Lowest (n = 69) | Middle (n = 70) | Highest (n = 69) | P value3 | |
| Percentage change in saturated fatty acid2 range (min, max) | 2.9 ± 4.4 (−8.0, 21.5) | −1.9 ± 2.3 (−8.0, 0.8) | 2.9 ± 1.1 (0.9, 4.6) | 7.5 ± 2.9 (4.7, 21.5) | |
| Characteristics | |||||
| Age, y | 40.4 ± 6.6 | 40.1 ± 6.6 | 40.0 ± 6.9 | 41.2 ± 6.1 | 0.50 |
| Sex, n (%) | 0.89 | ||||
| Female | 125 (60) | 43 (62) | 41 (59) | 41 (59) | |
| Male | 83 (40) | 26 (38) | 29 (41) | 28 (41) | |
| Race, n (%) | 0.04 | ||||
| White, non-Hispanic | 132 (63) | 40 (58) | 40 (57) | 52 (75) | |
| Other | 76 (37) | 29 (42) | 30 (43) | 17 (25) | |
| Education, n (%) | 0.70 | ||||
| High school or less | 7 (3) | 1 (1) | 4 (6) | 2 (3) | |
| College graduate | 118 (57) | 39 (57) | 40 (57) | 39 (57) | |
| Postgraduate degree | 83 (40) | 29 (42) | 26 (37) | 28 (41) | |
| Body weight, kg | 94.6 ± 15.5 | 95.5 ± 14.8 | 94.7 ± 17.0 | 93.5 ± 14.9 | 0.76 |
| Baseline diet | |||||
| Energy, kcal | 2199 ± 638 | 2293 ± 686 | 2287 ± 678 | 2015 ± 499 | 0.01 |
| Carbohydrates, g | 243 ± 73 | 243 ± 35 | 254 ± 68 | 233 ± 72 | 0.22 |
| Fat, g | 91 ± 34 | 102 ± 35 | 94 ± 36 | 78 ± 24 | 0.0001 |
| Saturated fat, g | 30 ± 13 | 36 ± 13 | 31 ± 13 | 24 ± 10 | <0.0001 |
| Saturated fat, % | 12 ± 3 | 14 ± 3 | 12 ± 2 | 11 ± 3 | <0.0001 |
| Unsaturated fat, g | 54 ± 20 | 57 ± 22 | 56 ± 23 | 48 ± 15 | 0.01 |
| Protein, g | 92 ± 29 | 99 ± 32 | 93 ± 29 | 85 ± 22 | 0.02 |
| Plasma lipids, mg/dL | |||||
| LDL cholesterol | 116 ± 26 | 116 ± 25 | 113 ± 25 | 119 ± 28 | 0.48 |
| HDL cholesterol | 50 ± 9 | 50 ± 10 | 51 ± 8 | 50 ± 8 | 0.74 |
| Triglycerides | 126 ± 60 | 123 ± 49 | 125 ± 63 | 130 ± 67 | 0.79 |
Data are expressed as means ± SDs unless otherwise indicated.
Tertiles are based on 12-mo change in percentage dietary saturated fat from baseline. See Table 2 for values.
P values from 1-factor ANOVA for continuous variables (age, weight, baseline diet variables) and chi-squared tests for categorical variables (sex, race, education).
TABLE 2.
Mean changes from baseline in diet and weight by tertiles of 12-mo change in percentage dietary saturated fat1
| Tertile | ||||
|---|---|---|---|---|
| 12-mo change | Total (n = 208) | Lowest (n = 69) | Middle (n = 70) | Highest (n = 69) |
| Healthy low-carb | ||||
| Percentage change in saturated fatty acids2 | 2.9 ± 4.4 | −1.9 ± 2.3 | 2.9 ± 1.1 | 7.5 ± 2.9 |
| Range min, max | −8.0, 21.5 | −8.0, 0.8 | 0.9, 4.6 | 4.7, 21.5 |
| Energy, kcal | −506.7 ± 616.5 | −605.5 ± 641.5 | −528.0 ± 627.5 | −386.3 ± 566.5 |
| Carbohydrates, g | −111.9 ± 76.1 | −87.4 ± 71.0 | −115.3 ± 75.4 | −132.9 ± 75.7 |
| Saturated fat, g | −1.9 ± 14.1 | −12.8 ± 11.8 | −1.7 ± 10.0 | 8.9 ± 11.3 |
| Unsaturated fat, g | −3.0 ± 23.8 | −10.9 ± 23.6 | −2.8 ± 22.8 | 4.9 ± 22.6 |
| Body weight, kg | −6.3 ± 6.8 | −4.7 ± 7.3 | −6.1 ± 5.9 | −8.3 ± 6.7 |
| Healthy low-fat | ||||
| Percentage change in saturated fatty acids | −2.6 ± 3.6 | −6.7 ± 1.8 | −2.3 ± 0.9 | 1.2 ± 1.9 |
| Range min, max | −12.6, 6.8 | −12.6, −4.1 | −4.0, −1.1 | −1.0, 6.8 |
| Energy, kcal | −484.3 ± 625.8 | −665.3 ± 534.3 | −428.9 ± 694.4 | −359.4 ± 597.1 |
| Carbohydrates, g | −36.1 ± 81.3 | −30.2 ± 72.9 | −32.1 ± 89.2 | −46.1 ± 80.1 |
| Saturated fat, g | −11.4 ± 12.3 | −22.3 ± 9.4 | −9.7 ± 9.5 | −2.0 ± 8.4 |
| Unsaturated fat, g | −18.6 ± 21.7 | −28.6 ± 20.6 | −16.9 ± 22.1 | −10.3 ± 18.3 |
| Body weight, kg | −5.6 ± 7.3 | −7.3 ± 8.0 | −5.5 ± 7.2 | −3.9 ± 6.4 |
Data are expressed as means ± SDs.
A positive change means an increase in percentage saturated fatty acids from baseline.
TABLE 3.
Linear regression model estimates for the association between a 1% increase in saturated fatty acid intake and 12-mo change in plasma lipids1
| Characteristics | Unadjusted estimate (95% CI) | Model 12 (95% CI) | Model 23 (95% CI) |
|---|---|---|---|
| Healthy low-carbohydrate (n = 208) | |||
| LDL-C, mg/dL | 0.50 (−0.12, 1.13) | 0.42 (−0.22, 1.08) | 0.42 (−0.25, 1.08) |
| P value | 0.11 | 0.19 | 0.22 |
| HDL-C, mg/dL | 0.19 (−0.01, 0.40) | 0.13 (−0.08, 0.34) | 0.13 (−0.08, 0.34) |
| P value | 0.06 | 0.22 | 0.23 |
| Triglycerides, mg/dL | −2.28 (−3.74, −0.83) | −1.52 (−2.95, −0.08) | −1.25 (−2.71, 0.20) |
| P value | 0.002 | 0.04 | 0.09 |
| Healthy low-fat (n = 208) | |||
| LDL-C, mg/dL | 0.94 (0.18, 1.70) | 0.99 (0.20, 1.78) | 1.07 (0.28, 1.86) |
| P value | 0.02 | 0.01 | 0.01 |
| HDL-C, mg/dL | 0.10 (−0.13, 0.33) | 0.11 (−0.13, 0.35) | 0.13 (−0.11, 0.37) |
| P value | 0.39 | 0.38 | 0.29 |
| Triglycerides, mg/dL | 0.76 (−1.34, 2.86) | −0.37 (−2.46, 1.72) | −0.31 (−2.41, 1.80) |
| P value | 0.48 | 0.73 | 0.77 |
The models were conducted via 2-sided Wald tests at a significance level of 0.05. HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol
Adjusted for age, gender, race, and weight change.
Adjusted for age, gender, race, weight change, and 12-mo change in carbohydrate intake.
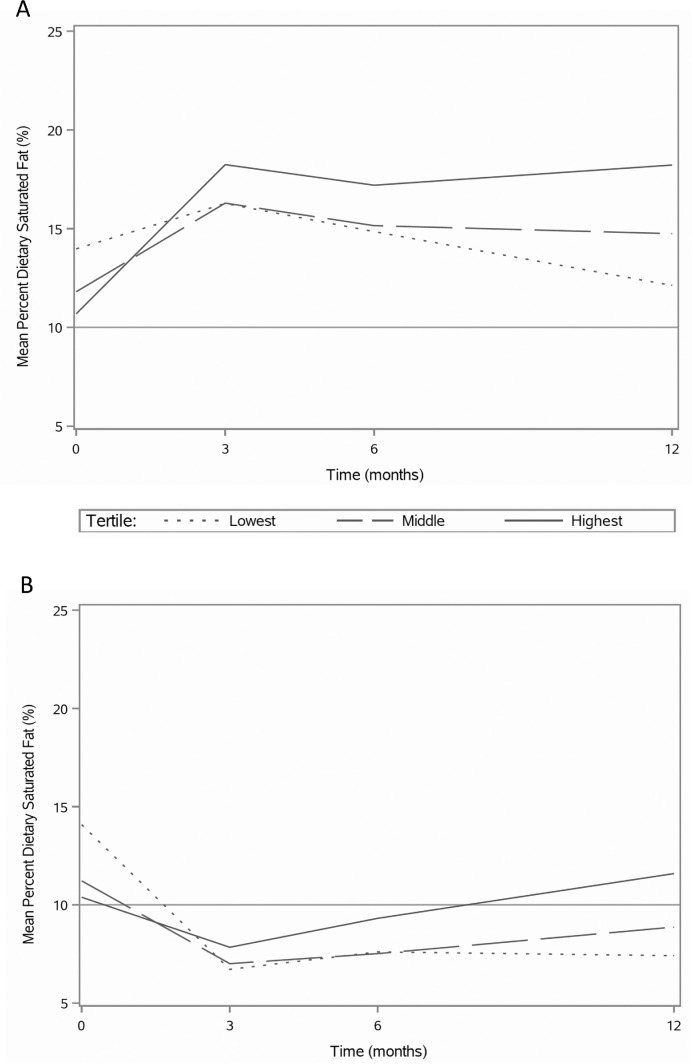
FIGURE 1.
Mean percentage dietary saturated fat over 12 mo by tertiles of 12-mo change percentage dietary saturated fat for the (A) healthy low-carbohydrate and (B) healthy low-fat weight-loss group. N = 208, per group.
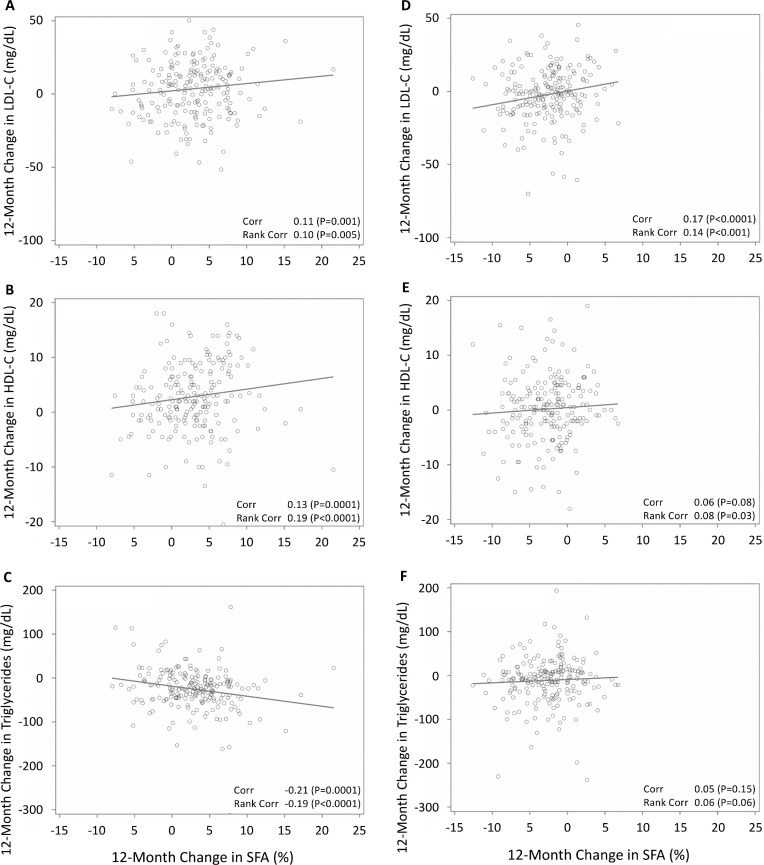
FIGURE 2.
Scatterplots of 12-mo change in percentage SFA with 12-mo changes in lipids. For the healthy low-carbohydrate group: (A) LDL cholesterol, (B) HDL cholesterol, and (C) triglycerides. For the healthy low-fat group: (D) LDL cholesterol, (E) HDL cholesterol, and (F) triglycerides. Pearson and Spearman correlations, using unadjusted data. Corr, correlation; HDL-C, HDL cholesterol; LDL-C, low-density lipoprotein cholesterol; SFA, saturated fatty acid.
There were no statistically significant differences in gender and education between those in this analysis who did and did not complete the study (Supplemental Table 2). Age, baseline weight, race, and baseline LDL cholesterol differed modestly, but significantly, between those in this analysis who did and did not complete the study (P < 0.01), with those missing more likely to be younger (mean age 38.2 ± 6.9 compared with 40.4 ± 6.6 y), heavier (mean weight 99.9 ± 15.5 compared with 94.6 ± 15.5 kg), and have lower LDL cholesterol (109 ± 25.4 compared with 116 ± 26.1 mg/dL).
Saturated fat intake over 12 mo
For all tertiles in the HLC group, at all time points, the mean percentage of calories consumed as SFA was greater than 10%, which is the upper limit intake recommended by the Dietary Guidelines for Americans 2015–2020 (1), with the lowest mean percentage SFA intake occurring for the lowest tertile at 12 mo (12.1%) and the highest intake (18.2%) occurring for the highest tertile at 3 mo (Figure 1A). For the HLF group (Figure 1B), mean percentage SFA intakes for all tertiles were greater than 10% at baseline and dropped below 10% at all post-randomization time points with the exception of the highest tertile at 12 mo (11.6%). For both the HLC and HLF groups, there were differences of approximately 20 g/d between the lowest and highest tertiles for 12-mo change in saturated fat intake (−12.8, 8.9 and −22.3, −2.0, respectively) (Table 2). However, the mean daily intake in total grams of saturated fat for the overall HLC group in this study did not change significantly from baseline to 12 mo. Although 12-mo percentage SFA increased for the HLC participants overall, these subjects concomitantly and substantially reduced their energy (−506.7 ± 616.5 kcal) and carbohydrate intakes (−111.9 ± 76.1 g) while participating in the weight-loss study.
Twelve-month diet group differences in lipids
At 12 mo, lipid differences by diet group were significant for all 3 lipid variables [mean (SD), mg/dL]: HLF had lower LDL cholesterol than HLC—108.5 (28.8) compared with 119.8 (26.8), P < 0.0001; HLC had higher HDL cholesterol than HLF—53.1 (8.9) compared with 49.5 (9.5), P < 0.0001; and HLC had lower triglycerides than HLF—100.6 (59.7) compared with 119.2 (65.5), P = 0.003.
Association between changes in saturated fat intake and lipid profile
Changes in percentage SFA were correlated with changes in LDL cholesterol, HDL cholesterol, and triglycerides: Pearson's r = 0.11 (P = 0.001), 0.13 (P = 0.0001), and −0.21(P = 0.0001), respectively, showing significant positive correlations with LDL cholesterol and HDL cholesterol, and a negative correlation with triglycerides (Supplemental Figure 2A–C). The relations between SFA and lipid outcomes were similar using Spearman correlations for LDL cholesterol, r = 0.10 (P = 0.005), HDL cholesterol, r = 0.19 (P < 0.0001), and triglycerides, r = −0.19 (P < 0.0001). Medians and interquartile ranges (IQRs) for 12-mo changes in blood lipids in order from the lowest to highest tertiles were: for LDL cholesterol, 2.7 (−10.3, 16.1), 2.9 (−12.5, 15.2), and 5.9 (−9.5, 16.9) mg/dL (Figure 2A); for HDL cholesterol, 1.5 (−1.5, 5.0), 2.0 (−1.5, 5.0), and 5.5 (0.5, 10.0) mg/dL (Figure 2B); and for triglycerides, −16.0 (−46.0, 11.0), −24.0 (−40.5, −1.5), and −28.5 (−56.0, −12.5) mg/dL (Figure 2C).
In our primary analysis, every 1% increase in percentage SFA intake from baseline to 12 mo in the HLC group resulted in a statistically significant decrease in triglycerides without any significant changes in LDL cholesterol or HDL cholesterol in both the unadjusted model and after adjustment for age, gender, race, and weight change (Model 1) (Table 3). However, results for triglycerides were no longer statistically significant after further adjustment for 12-mo change in carbohydrate intake (Model 2). Directions of changes for all lipid outcomes in response to each 1% increase in SFA intake remained stable regardless of adjustment. Of the confounders included in the adjusted models, weight change was the only factor that was significantly associated with lipid outcomes (P < 0.05 for HDL cholesterol, P < 0.0001 for triglycerides).
Discussion
In this secondary analysis of a randomized controlled weight loss trial for generally healthy adults, participants who increased their percentage of energy from saturated fat improved their overall lipid profiles in the context of reducing their overall calorie and carbohydrate intakes and eating a healthy, low-carbohydrate weight-loss diet focused on whole and plant-based foods. Within the HLC group, mean 12-mo changes in LDL cholesterol concentrations were not associated with changes in percentage SFA intake. Moreover, the tertile that displayed the largest increase in percentage SFA (the highest tertile) experienced a significant decrease in triglycerides and a modest increase, albeit insignificant, in HDL cholesterol. These findings suggest that the context of the relatively high percentage of saturated fat intake is important to consider when balancing guidance for weight loss and related metabolic parameters.
These post hoc analyses also contribute to the scientific debate on the issue of dietary fat and its association with both CVD and obesity. For several decades, national dietary guidelines have recommended that Americans follow dietary patterns in which a maximum of 30% of energy is obtained from total fat, and a maximum of 10% is obtained from saturated fat. These explicit numbers have stayed largely unchanged since the USDA and the US Department of Health and Human Services first established quantitative recommendations for dietary fat and saturated fat intakes in the 1990 Dietary Guidelines for Americans (DGA) (30). These fat-limiting recommendations have largely been driven by 2 separate but related health concerns—CVD and obesity.
Indicators that the controversy around total dietary fat recommendations has largely subsided in recent years include the raising of the recommended upper limit of total fat intake from 30% to 35% of energy intake in the 2010 DGA, and then the removal of this upper limit entirely in the 2015 DGA (31). However, the national DGA continue to recommend a 10% SFA limit, and other organizations have placed even stricter limitations on saturated fat intake (1). The 2013 American Heart Association/American College of Cardiologists Guideline on Lifestyle Management to Reduce Cardiovascular Risk recommends that for patients who would benefit from lowering their LDL cholesterol, only 5–6% of energy intake should come from saturated fat (32). Notably, the mean percentage SFA intake for the entire HLC group in this secondary analysis was 15% at 12 mo, and all 3 tertiles of 12-mo percentage SFA change had mean percentage SFA intakes greater than 10% at all study data-collection time points.
In comparison with the HLC group, the percentage SFA-LDL relation appears stronger (greater in magnitude) for the HLF group (Table 3). One interpretation of this is that the percentage change in total energy intake from saturated fat could have a larger effect on LDL cholesterol for those who consume lower levels of either saturated or total fat. Meanwhile, both HDL cholesterol and triglycerides improved at a greater magnitude for the HLC group than for the HLF group. Furthermore, the increase in percentage SFA in the HLC group was primarily driven by a substantial decrease in carbohydrate intake and corresponded to only a modest change in absolute grams of saturated fat. In line with this observation, the association between 12-mo change in percentage SFA and 12-mo change in triglycerides was no longer significant after adjusting for 12-mo change in total carbohydrate intake (g). Research shows that decreasing intake of refined carbohydrates is associated with decreases in triglyceride levels (33, 34). The findings of this analysis further suggest that more research is needed to determine whether and how much this decrease in triglycerides is independently affected by an increase in percentage SFA intake.
The DIETFITS trial and this secondary analysis have many strengths. The DIETFITS study had a large sample size (N = 609) that included roughly equal proportions of women and men, good retention, and a sufficient duration to evaluate long-term effects of the dietary interventions. These factors, in addition to relatively comparable baseline characteristics between both the participants included in this analysis and those missing, minimize the potential for withdrawal bias—a factor that could decrease generalizability. Because this study included generally healthy overweight and obese adults, it has potential generalizability to a large segment of the US population.
Inherent to the nature of a secondary analysis, the main limitation of this study is that participants were not randomly assigned to different concentrations of saturated fat intake; this is essentially an observational substudy nested within a randomized control trial. Another limitation is the inability to differentiate the independent contributions of concomitant changes occurring in the diets, such as the parallel increase in percentage SFA with the decrease in grams of carbohydrate intake. This has been addressed in the current analysis that suggests a diminished relation between 12-mo change in percentage SFA and lipid outcomes when additionally adjusting for 12-mo change in carbohydrate intake (Table 3, Model 2). Although the change in fat intake was accompanied by a commensurate 12-mo change in carbohydrate intake for the HLC group, this corresponding increase and decrease in different diet components is common in all diet studies of this nature and is difficult to isolate. A third limitation is that by measuring blood lipid outcomes as 12-mo change, we may have masked short-term effects of dietary intake on blood lipids. However, reporting blood lipid outcomes as 12-mo changes takes advantage of the longest-term data available that are likely most relevant to clinicians counseling patients in the real world. A final limitation involves the approach to identifying the tertiles of groups that had the smallest compared with the largest changes in percentage SFA. The tertile groups were dissimilar at baseline (Figure 1), and as might be expected, the tertile that had the largest increase by 12 mo started with the lowest level of percentage SFA at baseline (i.e., likely involving some baseline under-reporting and having the most opportunity for increase). Conversely, the tertile that had the smallest increase by 12 mo had the highest level of percentage SFA at baseline (i.e., likely involving some baseline over-reporting and having the most opportunity for decrease). Despite the likelihood of the role of regression to the mean, the tertile that increased the most in percentage SFA over time did end up with substantially higher percentage SFA than the other 2 tertiles at 12 mo.
Further research is needed to assess the role of LDL particle size in saturated fat's effect on CVD risk. The ability of SFAs to increase LDL cholesterol concentrations and, presumably, CVD risk may be due in part to differential effects of saturated fat on different LDL subclass concentrations. In particular, small, dense LDL particles (pattern A) are more strongly associated with CVD risk than large LDL particles (pattern B) (32, 35–38). Furthermore, recent clinical trial evidence suggests that moderately restricted carbohydrate diets rich in plant-based fat improve plasma lipid profiles and cardiovascular outcomes (11, 10). This study did not separate the analysis between plant- and animal-based saturated fat. Thus, the differential effects of plant- and animal-based sources of saturated fat on cardiovascular outcomes should be investigated further.
These findings may have clinical relevance for physicians in terms of filling a gap in evidence available to guide counseling regarding percentage saturated fat intake in the context of promoting successful weight loss by following a high-quality, whole-food, low-carbohydrate diet. Specifically, these secondary analyses support shifting the focus from concern about percentage SFA intake on the overall lipid profile to instead aiming to maintain a relatively stable absolute level of saturated fat intake while focusing on improving the quality of the overall diet by incorporating more whole foods and decreasing processed carbohydrates as much as possible.
Supplementary Material
Acknowledgements
We thank the research team members for their extraordinary work. Dr Jennifer Robinson and Antonella Dewell served as study coordinators. The team of health educators included Rise Cherin, Susan Kirkpatrick, Jae Berman, Dalia Perelman, and Mandy Murphy Carroll. The diet assessment team included Sarah Farzinkhou, Valerie Alaimo, Margaret Shimer, and Diane Demis. Various other important study roles in recruitment, screening, blood sample management, innovation, and other tasks were played by Josephine Hau, Erin Avery, Alexandra Rossi, Katherine Dotter, and Dr Sarah Mummah. Beyond our coauthors from the Quantitative Sciences Unit, additional statistical support was provided by Ariadna Garcia, FeiFei Qin, and Vidhya Balasubramanian. Additional postdoctoral research fellows who were involved in various phases of the study included Jennifer Hartle, Lisa Offringa, Kenji Nagao, Marily Oppezzo, Ben Chrisinger, and Michael Stanton.
The authors’ responsibilities were as follows—CWS, MEH, LA, JR, and CDG: designed the research (project conception, development of overall research plan, and study oversight) and wrote the paper; CWS and CDG: conducted the research (hands-on conduct of the experiments and data collection); CWS: analyzed the data, performed statistical analyses, and had primary responsibility for the final content; and all authors: took responsibility for the final content and read and approved the final manuscript. None of the authors reports a conflict of interest related to research presented in this article.
Notes
The study received support from The European Union’s Horizon 2020 Research and Innovation Programme (grant/award number 701,944). This investigation was supported by the National Institute of Diabetes and Digestive and Kidney Diseases NIH 1R01DK091831, the Nutrition Science Initiative, the National Heart, Lung, and Blood Institute NIH T32HL007034, and the Stanford Clinical and Translational Science Award to Spectrum NIH UL1 TR001085.
Supplemental Figures 1 and 2 and Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: CVD, cardiovascular disease; DGA, Dietary Guidelines for Americans; DIETFITS, Diet Intervention Examining The Factors Interacting with Treatment Success; HLC, healthy low-carb; HLF, healthy low-fat.
References
- 1. U.S. Department of Health and Human Services, U.S. Department of Agriculture. Dietary Guidelines for Americans, 2015–2020 [Internet]. 8th ed 2015. Available from: https://health.gov/dietaryguidelines/2015/guidelines/ [Google Scholar]
- 2. Keys A, Anderson J, Grande F. Prediction of serum-cholesterol responses of main to changes in fats in the diet. The Lancet [Internet]. 1957;270(7003):959–66. [DOI] [PubMed] [Google Scholar]
- 3. Hegsted DM, Mcgandy RB, Myers ML, Stare FJ. Quantitative effects of dietary fat on serum cholesterol in man. Am J Clin Nutr [Internet]. 1965;17(5):281–95. [DOI] [PubMed] [Google Scholar]
- 4. Hegsted DM, Ausman LM, Johnson JA, Dallal GE. Dietary fat and serum lipids: An evaluation of the experimental data. Am J Clin Nutr [Internet]. 1993;57(6):875–83. [DOI] [PubMed] [Google Scholar]
- 5. Mensink RP, Zock PL, Kester ADM, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77(5):1146–55. [DOI] [PubMed] [Google Scholar]
- 6. Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fatty acids and risk of coronary heart disease: Modulation by replacement nutrients. Curr Atheroscler Rep [Internet]. 2010;12(6):384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hu FB. Are refined carbohydrates worse than saturated fat? Am J Clin Nutr [Internet]. 2010;91(6):1541–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, Miller M, Rimm EB, Rudel LL, Robinson JG et al.. Dietary fats and cardiovascular disease: A presidential advisory from the American Heart Association. Circulation. 2017;136(3):e1–23. [DOI] [PubMed] [Google Scholar]
- 9. Astrup A, Dyerberg J, Elwood P, Hermansen K, Hu FB, Jakobsen MU, Kok FJ, Krauss RM, Lecerf JM, LeGrand P et al.. The role of reducing intakes of saturated fat in the prevention of cardiovascular disease: Where does the evidence stand in 2010?. Am J Clin Nutr. 2011;93(4):684–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Halton TL, Willett WC, Liu S, Manson JE, Albert CM, Rexrode K, Hu FB. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med. 2006;355(19):1991–2002. [DOI] [PubMed] [Google Scholar]
- 11. Jenkins DJA, Wong JMW, Kendall CWC, Esfahani A, Ng VWY, Leong TCK, Faulkner DA, Vidgen E, Greaves KA, Paul G et al.. The effect of a plant-based low-carbohydrate (“Eco-Atkins”) diet on body weight and blood lipid concentrations in hyperlipidemic subjects. Arch Intern Med [Internet]. 2009;169(11):1046–54. [DOI] [PubMed] [Google Scholar]
- 12. de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, Uleryk E, Budylowski P, Schünemann H, Beyene J et al.. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ [Internet]. 2015;351:h3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malhotra A, Redberg RF, Meier P. Saturated fat does not clog the arteries: Coronary heart disease is a chronic inflammatory condition, the risk of which can be effectively reduced from healthy lifestyle interventions. Br J Sports Med [Internet]. 2017;51(15):1111–12. [DOI] [PubMed] [Google Scholar]
- 14. Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N et al.. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360(9):859–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF et al.. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985–3023. [DOI] [PubMed] [Google Scholar]
- 16. Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348(21):2082–90. [DOI] [PubMed] [Google Scholar]
- 17. Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams T, Williams M, Gracely EJ, Stern L. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348(21):2074–81. [DOI] [PubMed] [Google Scholar]
- 18. Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, Kraemer HC, King AC. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: A randomized trial. JAMA [Internet]. 2007;297(9):969–77. [DOI] [PubMed] [Google Scholar]
- 19. Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293(1):43–53. [DOI] [PubMed] [Google Scholar]
- 20. Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, Golan R, Fraser D, Bolotin A, Vardi H et al.. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359(3):229–41. [DOI] [PubMed] [Google Scholar]
- 21. Stanton MV, Robinson JL, Kirkpatrick SM, Farzinkhou S, Avery EC, Rigdon J, Offringa LC, Trepanowski JF, Hauser ME, Hartle JC et al.. DIETFITS study (diet intervention examining the factors interacting with treatment success)—Study design and methods. Contemp Clin Trials. 2016;53:151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnson RK, Driscoll P, Goran MI. Comparison of multiple-pass 24-hour recall estimates of energy intake with total energy expenditure determined by the doubly labeled water method in young children. J Am Diet Assoc [Internet]. 1996;96(11):1140–44. [DOI] [PubMed] [Google Scholar]
- 23. Myers GL, Cooper GR, Winn CL, Smith SJ. The Centers for Disease Control-National Heart, Lung and Blood Institute Lipid Standardization Program. An approach to accurate and precise lipid measurements. Clin Lab Med. 1989;9(1):105–35. [PubMed] [Google Scholar]
- 24. Allain CC, Poon LS, Chan CSG, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem [Internet]. 1974;20(4):470–5. [PubMed] [Google Scholar]
- 25. Nägele U, Hägele EO, Sauer G, Wiedemann E, Lehmann P, Wahlefeld AW, Gruber W. Reagent for the enzymatic determination of serum total triglycerides with improved lipolytic efficiency. J Clin Chem Clin Biochem Z Klin Chem Klin Biochem. 1984;22(2):165–74. [DOI] [PubMed] [Google Scholar]
- 26. Warnick GR, Nguyen T, Albers AA. Comparison of improved precipitation methods for quantification of high-density lipoprotein cholesterol. Clin Chem [Internet]. 1985;31(2):217–22. [PubMed] [Google Scholar]
- 27. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 28. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gardner CD, Trepanowski JF, Del Gobbo LC, Hauser ME, Rigdon J, Ioannidis JPA, Desai M, King AC. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion. JAMA. 2018;319(7):667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. US Department of Health and Human Services, USDA. Dietary Guidelines for Americans, 1990 [Internet]. Available from: https://health.gov/dietaryguidelines/1990.asp. [Google Scholar]
- 31. US Department of Health and Human Services, USDA. Dietary Guidelines for Americans, 2010 [Internet]. 7th ed 2010. Available from: https://health.gov/dietaryguidelines/2010/. [Google Scholar]
- 32. Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE et al.. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S76–99. [DOI] [PubMed] [Google Scholar]
- 33. Krauss RM, Blanche PJ, Rawlings RS, Fernstrom HS, Williams PT. Separate effects of reduced carbohydrate intake and weight loss on atherogenic dyslipidemia. Am J Clin Nutr [Internet]. 2006;83(5):1025–31. [DOI] [PubMed] [Google Scholar]
- 34. Yancy WS, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: A randomized, controlled trial. Ann Intern Med [Internet]. 2004;140(10):769. [DOI] [PubMed] [Google Scholar]
- 35. St-Pierre AC, Cantin B, Dagenais GR, Mauriège P, Bernard P-M, Després J-P, Lamarche B. Low-density lipoprotein subfractions and the long-term risk of ischemic heart disease in men: 13-year follow-up data from the Québec Cardiovascular Study. Arterioscler Thromb Vasc Biol. 2005;25(3):553–59. [DOI] [PubMed] [Google Scholar]
- 36. Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119(7):931–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Musunuru K, Orho-Melander M, Caulfield MP, Li S, Salameh WA, Reitz RE, Berglund G, Hedblad B, Engström G, Williams PT et al.. Ion mobility analysis of lipoprotein subfractions identifies three independent axes of cardiovascular risk. Arterioscler Thromb Vasc Biol. 2009;29(11):1975–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Williams PT, Zhao X-Q, Marcovina SM, Otvos JD, Brown BG, Krauss RM. Comparison of four methods of analysis of lipoprotein particle subfractions for their association with angiographic progression of coronary artery disease. Atherosclerosis. 2014;233(2):713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.