Abstract
Context
Treatment options for nonalcoholic fatty liver disease (NAFLD) are needed.
Objective
The aim of this review was to systematically assess the effects of omega-3 long-chain polyunsaturated fatty acids (n-3 LC-PUFAs), particularly eicosapentaenoic acid and docosahexaenoic acid, on liver-related and metabolic outcomes in adult and pediatric patients with NAFLD.
Data Sources
The online information service ProQuest Dialog was used to search 8 literature databases.
Study Selection
Controlled intervention studies in which the independent effects of n-3 LC-PUFAs could be isolated were eligible for inclusion.
Data Extraction
The 18 unique studies that met the criteria for inclusion were divided into 2 sets, and data transcriptions and study quality assessments were conducted in duplicate. Each effect size was expressed as the weighted mean difference and 95%CI, using a random-effects model and the inverse of the variance as a weighting factor.
Results
Based on the meta-analyses, supplementation with n-3 LC-PUFAs resulted in statistically significant improvements in 6 of 13 metabolic risk factors, in levels of 2 of 3 liver enzymes, in liver fat content (assessed via magnetic resonance imaging/spectroscopy), and in steatosis score (assessed via ultrasonography). Histological measures of disease [which were assessed only in patients with nonalcoholic steatohepatitis (NASH)] were unaffected by n-3 LC-PUFA supplementation.
Conclusions
Omega-3 LC-PUFAs are useful in the dietary management of patients with NAFLD. Additional trials are needed to better understand the effects of n-3 LC-PUFAs on histological outcomes in patients with NASH.
Systematic Review Registration
PROSPERO CRD42017055951.
Keywords: docosahexaenoic acid, DHA, eicosapentaenoic acid, EPA, nonalcoholic fatty liver disease, NAFLD, omega-3
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is present when the following 2 conditions are met: (1) there is evidence (via imaging or histology) of hepatic steatosis and (2) there are no causes for secondary hepatic fat accumulation (eg, inborn errors of metabolism, Wilson’s disease, excessive alcohol intake, hepatitis, iron toxicity, or hepatotoxic drugs or toxins).1 Histologically, NAFLD is present when ≥ 5% of hepatocytes contain macrovesicular steatosis (reviewed by Petäjä and Yki-Järvinen2). NAFLD encompasses a range of conditions with increasing severity, including nonalcoholic fatty liver (NAFL), nonalcoholic steatohepatitis (NASH) with or without fibrosis, and cirrhosis. With NAFL (also called simple steatosis), there is hepatic steatosis without hepatocellular injury (ie, there is no hepatocyte ballooning or fibrosis). In contrast, with NASH, hepatic steatosis is accompanied by inflammation and hepatocyte injury (ballooning), with or without fibrosis.1 In some individuals, NASH can progress to hepatocellular carcinoma.1,3 Factors that increase the risk of NAFLD include obesity, type 2 diabetes mellitus, dyslipidemia, and metabolic syndrome.1
As reviewed by Neuschwander-Tetri,3 20% to 30% of adults living in Westernized countries have NAFLD, 2% to 5% have NASH, and 1% to 2% have cirrhosis of the liver. The prevalence of NAFLD is reported to be even greater in certain population subgroups. For example, in adults with diabetes, the prevalence of NAFLD was reported to be 46.2% in the United Kingdom and 69.5% in Italy (reviewed by Bellentani4). In children and adolescents in Europe, the prevalence of NAFLD was reported to range from 2% to 12.5%; however, the prevalence among obese children was much higher: 36% in Germany and 44% in Italy.4 In the United States, cirrhosis and hepatocellular carcinoma related to NAFLD have emerged as the second leading cause of liver transplant, and projections show that NAFLD will become the leading indication for liver transplantation in the next 10 years.3,4 Reductions in body weight—achieved via reduced caloric intake and increased physical activity—appear to be effective in the treatment of NAFLD; however, most patients cannot achieve the required degree of weight loss or have trouble maintaining weight loss over the long term.5 Currently, there are no drugs approved for the management of NAFLD.
The efficacy of omega-3 long-chain polyunsaturated fatty acids (n-3 LC-PUFAs), particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), in the dietary management of patients with NAFLD is actively being investigated. Both EPA and DHA are potent modulators of hepatic gene expression, promoting genes involved in hepatic fatty acid β-oxidation and export from the liver and inhibiting genes involved in hepatic fatty acid synthesis and storage.6 Specifically, n-3 LC-PUFAs suppress sterol regulatory element-binding protein 1 (SREBP-1), a transcription factor that regulates both the rate of triglyceride synthesis and its storage in the liver.6–8 Of note, SREBP-1 is stimulated by insulin; thus, the inhibition of SREBP-1 by n-3 LC-PUFAs is of particular importance in patients with NAFLD, as they are often also affected by hyperinsulinemia and insulin resistance and are thus predisposed to insulin-induced stimulation of SREBP-1 and, consequently, the synthesis and accumulation of liver fatty acids. The n-3 LC-PUFAs further act to stimulate both hepatic peroxisome proliferator-activated receptor-α (PPAR-α), thereby increasing fatty acid β-oxidation, and peroxisome proliferator-activated receptor-γ (PPAR-γ), which increases insulin sensitivity.9 Collectively, the effects of n-3 LC-PUFAs on SREBP-1, PPAR-α, and PPAR-γ result in a net reduction in hepatic fat accumulation and a net increase in hepatic fatty acid β-oxidation. Thus, mechanistically, insufficient hepatic levels of EPA and DHA could tip the balance toward hepatic fatty acid lipogenesis, rather than toward hepatic fatty acid β-oxidation. Therefore, it seems biologically plausible that patients with NAFLD may benefit from an increased intake of EPA and DHA.
The clinical efficacy of n-3 LC-PUFAs, primarily EPA and DHA, in the management of adult and pediatric patients with NAFLD has been investigated in several clinical studies. The results of these studies have been critically appraised in 3 systematic reviews and meta-analyses, the first of which was published in 2012.10 Since that publication, many additional clinical studies of the effects of n-3 LC-PUFAs in patients with NAFLD have been published. The remaining systematic reviews did not include studies conducted in children and also did not include studies if the data were not reported in a manner that permitted the inclusion of the study in the meta-analyses.11,12 In the systematic review and meta-analyses presented here, the effects of n-3 LC-PUFA supplementation in adult and pediatric patients with NAFLD on liver-related outcomes and metabolic risk factors are presented. Of importance, if a study did not report data for liver outcomes in a manner that permitted inclusion of the study in the meta-analyses, the corresponding author of the study was contacted a minimum of 3 times to request the data, in order to reduce publication bias and have as robust and inclusionary a data set as possible.
METHODS
The objective of the current review was to systematically assess the effects of n-3 LC-PUFA supplementation on liver-related and metabolic outcomes in adult and pediatric patients with NAFLD. The research question was defined by the PICOS (Population, Intervention, Comparator, Outcomes, Study design) criteria presented in Table 1. The systematic review and meta-analyses were conducted in compliance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.13 This systematic review is registered with PROSPERO (an international prospective register of systematic reviews), registration number CRD42017055951.
Table 1.
PICOS criteria for inclusion of studies
| Data domain | Categories used for data extraction |
|---|---|
| Participants |
|
| Interventions | Supplementation with n-3 LC-PUFAs, predominantly EPA and/or DHA |
| Comparators |
|
| Outcomes |
|
| Study design |
|
Abbreviations: ALT, alanine transaminase; AST, aspartate aminotransferase; BP, blood pressure; BMI, body mass index; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; GGT, γ-glutamyl transferase; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; n-3 LC-PUFA, omega-3 long-chain polyunsaturated fatty acid; LDL-C, low-density lipoprotein cholesterol; NAFL, nonalcoholic fatty liver; NAFLD¸ nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; TC, total cholesterol; TG, triglyceride.
Literature search
To retrieve relevant literature on the effects of n-3 LC-PUFAs in patients with NAFLD, the electronic search tool ProQuest Dialog was used to search the following 8 literature databases: Allied & Complementary Medicine Database (AMED); BIOSIS Previews; CAB Abstracts; Embase; Foodline: Science; FSTA—Food Science and Technology Abstracts; MEDLINE; and, National Technical Information Service (NTIS). Two literature searches were conducted, one on September 26, 2016 (for studies conducted in adults), and the other on October 19, 2016 (for studies conducted in children and adolescents). At least one search term for the exposure [“docosahexaenoic,” “eicosapentaenoic,” “DHA,” “EPA,” “fish oil,” “cod oil,” “krill oil,” “algal oil,” “marine lipid,” “omacor,” “lovaza,” or (“long chain,” “long-chain,” “omega-3,” “n-3,” or “omega 3”) within two words of (“fatty” or “PUFA”)], the study population (“men,” “women,” “man,” “woman,” “human,” “subject,” “participant,” “volunteer*,” “patient,” “elder*,” “senior,” “geriatric,” “older,” “adult,” “people,” “person,” “individual,” “breastfe*,” “breast-fe*,” “breast fe*,” “pediatric,” “paediatric,” “teen*,” “adolescen*,” “child*,” “boy,” “girl,” “toddler*,” “baby,” “babies,” “infant,” or “neonat*”), and the outcome [(“liver” or “hepat*”) AND (“*steat*,” “fibrosis,” “cirrhosis,” “fatty liver,” “non-alcoholic,” “nonalcoholic,” “injury,” “NAFLD,” “NAFL,” “inflammation,” or “NASH”)] had to appear in either the title or the abstract of the article. Furthermore, to limit the search to studies conducted in humans, animal terms (“rat,” “mice,” “mouse,” “dog,” “pig,” “piglet,” “rabbit,” “hamster,” “monkey,” “rodent*,” “chick,” “broiler,” “cow,” “cattle,” “sheep,” “ewe,” “porcine,” “horse,” “equine,” “in vitro,” or “ex vivo”) were required not to appear in the title of the article. The use of an asterisk in some of the above search terms allowed for flexibility in the word ending (eg, “adolescen*” would have resulted in the identification of “adolescents” or “adolescence”). No limitations were placed on the literature search with respect to language; however, the year of publication was restricted to 1960 and onward.
Inclusion and exclusion criteria
Once the search strategy was implemented and the publication titles were retrieved, the eligibility of the publications for inclusion was determined at 3 stages using the titles, abstracts, and, subsequently, the full text of each publication. A study was included if it met all of the following inclusion criteria: (1) it was a full-length article published in a peer-reviewed journal; (2) it was a controlled intervention study conducted in patients (adults or children) with NAFLD (either NAFL or NASH); and (3) the investigational product was composed of n-3 LC-PUFAs (predominantly EPA and/or DHA). A study was excluded if it met one or more of the following exclusion criteria: (1) it was a full-length article published in a non–peer-reviewed source (eg, website, magazine); (2) it was published in abstract form only or as a short communication (eg, letter to the editor, commentary, etc); (3) it was an animal or in vitro study; (4) it was an uncontrolled human intervention study; (5) the investigational product was not composed of n-3 LC-PUFAs or was composed of additional bioactive agents, the independent effects of which could not be isolated; (6) the route of administration was not oral; (7) the study population consisted of individuals with serious diseases, other than NAFLD or diet-related diseases; (8) it was a secondary research paper (eg, narrative review, systematic review, meta-analysis, etc); or (9) the study was a duplicate publication. Although secondary research papers were excluded, the reference lists of these publications were manually screened to ensure all relevant studies were identified.
Data extraction and assessment of study quality
The intervention studies were divided into 2 sets, and for each set, the assessment of study quality and the transcription of data (which were subsequently used in the meta-analyses), were conducted in duplicate.
H.Y.L. and S.F. transcribed data from one-half of the studies, and M.D. and S.W. transcribed data from the other half of the studies. The data were transcribed into Excel spreadsheets and included the units for the outcome measure (eg, triglycerides were presented in either milligrams per deciliter or millimoles per liter, depending on the publication), the number of individuals in each group on which the analysis was based, the baseline measure and its variability (either standard deviation, standard error of the mean, or 95%CI), the end-of-treatment measure and its variability, the change from baseline measure and its variability, and the results of statistical analyses, both within groups and between groups. These data were captured for serological measures of liver function (aspartate aminotransferase [AST], alanine transaminase [ALT], γ-glutamyl transferase [GGT]); measures of liver fat, as assessed via magnetic resonance imaging (MRI) or magnetic resonance spectroscopy (MRS); steatosis score (as assessed via ultrasonography; see Table 2 for scoring14–22); liver biopsy measures (fibrosis score, ballooning score, steatosis score, lobular inflammation score, NAFLD activity score—see Table 2 for scoring23–27); blood lipid levels (total cholesterol, low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C], and triglycerides); measures of glycemic control (fasting blood glucose, fasting insulin, homeostatic model assessment of insulin resistance [HOMA-IR], adiponectin); measures of body mass/composition (body mass index [BMI], body weight, waist circumference); and other metabolic risk factors (systolic and diastolic blood pressure). The data entered by each individual were cross-checked by either H.Y.L. or K.M.V., and discrepancies were resolved by referring to the original publication.
Table 2.
Liver imaging and biopsy scoring
| Measure | Scoring algorithm | Studies in adults | Studies in children |
|---|---|---|---|
| Steatosis score (via ultrasonography) | Scored on a 4-grade scale as 0 (absent steatosis), 1 (mild steatosis), 2 (moderate steatosis), or 3 (severe/advanced steatosis)a | Capanni et al (2006)14; Sofi et al (2010)15; Spadaro et al (2008)16; Zhu et al (2008)17 | Boyraz et al (2015)18; Janczyk et al (2015)19; Nobili et al (2011, 2013)20–22 |
| Liver biopsy measures (NASH-CRN criteria)b,c | |||
| Fibrosis score | Scored on a 5-grade scale as 0 (none), 1 (perisinusoidal or periportal), 2 (perisinusoidal and portal/periportal), 3 (bridging fibrosis), or 4 (cirrhosis) | Argo et al (2015)25; Dasarathy et al (2015)26; Li et al (2015)24; Nogueira et al (2016)27 | None |
| Hepatocellular ballooning score | Scored on a 3-grade scale as 0 (none), 1 (few), or 2 (many) | Argo et al (2015)25; Dasarathy et al (2015)26; Li et al (2015)24; Nogueira et al (2016)27 | None |
| Steatosis score | Scored on a 4-grade scale as 0 (< 5%), 1 (5%–33%), 2 (34%–66%), or 3 (> 66%) | Argo et al (2015)25; Dasarathy et al (2015)26; Li et al (2015)24; Nogueira et al (2016)27 | None |
| Lobular inflammation score | Scored on a 4-grade scale as 0 (none), 1 (< 2), 2 (2–4), or 3 (> 4) | Argo et al (2015)25; Dasarathy et al (2015)26; Nogueira et al (2016)27 | None |
| NAFLD activity score | Scored by summing the scores for hepatocellular ballooning (0–2), steatosis (0–3), and lobular inflammation (0–3), resulting in a possible NAFLD activity score of 0 (best) to 8 (worse) | Argo et al (2015)25; Dasarathy et al (2015)26; Nogueira et al (2016)27 | None |
Abbreviations: NASH-CRN, Nonalcoholic Steatohepatitis Clinical Research Network.
Detailed descriptions of each score are as follows: absent steatosis (grade 0), normal liver echotexture; mild steatosis (grade 1), slight and diffuse increase in fine parenchymal echoes with normal visualization of diaphragm and portal vein borders; moderate steatosis (grade 2), moderate and diffuse increase in fine echoes with slightly impaired visualization of diaphragm and portal vein borders; severe steatosis (grade 3), fine echoes with poor or no visualization of diaphragm, portal vein borders, and posterior portion of the right lobe.
NASH-CRN criteria were defined by Kleiner et al.23
It was not explicitly stated by Li et al24 that the NASH-CRN criteria were used in the grading of the liver biopsy samples for fibrosis, ballooning, and steatosis; however, it appears from the baseline values (and their similarity to those reported for the other 3 studies), that the NASH-CRN criteria were most likely used.
Using the Modified Jadad scale,28 H.Y.L. and S.F. appraised the quality of one-half of the studies, and M.D. and S.W. appraised the quality of the other half of the studies. The quality appraisals were then consolidated by K.M.V., at which point any discrepancies were resolved by referring to the original publication and by discussion and consensus.
Statistical analysis
To determine the effects of n-3 LC-PUFAs on each of the liver-related outcomes and metabolic risk factors, the results of the studies were pooled in a meta-analysis. The effect size calculated for each study was the raw mean difference. The inverse of the variance was used as the weighting factor, and so the pooled effect was the weighted mean difference. For parallel studies, the raw mean difference for each liver-related outcome and metabolic risk factor was calculated as the change from baseline in the control group subtracted from the change from baseline in the n-3 LC-PUFA group. For crossover studies, the raw mean difference in the effect for each liver-related outcome and metabolic risk factor was calculated as the value at the end of the control phase subtracted from the value at the end of the n-3 LC-PUFA phase. In the majority of studies, variances for the raw mean differences were not reported; thus, variances were calculated using information provided in the publication (eg, using CIs or individual variances for the n-3 LC-PUFA and control groups). If, in parallel studies, variances for the changes from baseline were reported separately for the n-3 LC-PUFA and control groups, then a pooled variance for the raw mean difference was calculated. If, for parallel studies, variances were reported only for the baseline and end-of-treatment values, then these were used to calculate the variance for the change from baseline, using a correlation coefficient of 0.8. Similarly, for crossover studies, if variances were reported only for the end-of-treatment values, then the variance for the raw mean difference was calculated using a correlation coefficient of 0.8. The magnitude of the correlation coefficient has no bearing on the size of the pooled effect or the 95%CI; the pooled mean differences and 95%CIs have been demonstrated to be similar, whether the correlation coefficient used was 0.2, 0.5, or 0.829; 0.25, 0.5, or 0.7530; or 0.5, 0.7, or 1.0.31
If, for the liver-related outcomes, data were missing from the publications or were reported in a manner not conducive to pooling in a meta-analysis, then the corresponding author of the paper was contacted a minimum of 3 times, and each time, a formal request for the data was made. For the meta-analyses, a random-effects model was used according to the methods described by DerSimonian and Laird,32 given that random-effects models take into consideration the variability in response both within and between studies.
Some of the identified studies had multiple comparisons (eg, in the study by Chen et al,33 participants were randomly allocated to receive either placebo or 1 of 2 doses of seal oil). Each comparison between seal oil and placebo, hereafter referred to as a stratum, was considered a separate trial; however, the sample size of the control group was divided evenly among the comparisons to avoid inflating the weight of the study, as has been done previously.34
The weighted mean differences and accompanying 95%CIs were determined using Comprehensive Meta-analysis Software (version 2.2.064). A P value of < 0.05 was considered statistically significant, and P values of < 0.10 were considered nearly significant. Publication bias was assessed according to the trim and fill method developed by Duval and Tweedie.35 With this method, asymmetry is searched for within the funnel plot. If the asymmetry is determined to be caused by the presence of small studies (with large variances) in which large effect sizes were reported, with an unbalanced number of small studies showing a small effect, then those “missing” studies are imputed, and the pooled effect size is recalculated.
Subgroup analyses were conducted to evaluate the influence of age (ie, children vs adults), dose (adults only; ie, EPA + DHA < 3 g/d vs ≥ 3 g/d), duration of intervention (ie, ≤ 6 mo vs > 6 mo), and study quality (ie, quality rating using the modified Jadad scale score of < 6 vs ≥ 6) on each of the liver-related outcomes and metabolic risk factors. The subgroup analyses were conducted only if there were at least 3 data points for each comparison; otherwise, insufficient data precluded the subgroup analyses. Per the information registered with PROSPERO, there were plans to also conduct subgroup analyses to evaluate the influence of baseline disease severity (NAFL vs NASH) as well as the presence vs the absence of metabolic syndrome on each of the liver-related outcomes and metabolic risk factors. In most of the studies, a liver biopsy (which is required to differentiate NAFL from NASH) was not conducted, and so it is not known whether the study was comprised predominantly of NAFL patients or NASH patients. Furthermore, there were too few studies in which the participants were defined as having metabolic syndrome, and so these sensitivity analyses were not possible. Assessments of publication bias were not conducted for any of the subgroup analyses, as these were intended to be exploratory only. Forest plots and results of sensitivity analyses for the liver-related outcomes are reported within the manuscript. Forest plots and results of sensitivity analyses for all other outcomes are reported in the Supporting Information online.
RESULTS
Literature search
As shown in Figure 1, the literature search resulted in the identification of 1058 titles, and abstracts were retrieved for 291 records. Of the 291 abstracts, 50 were considered potentially relevant, and their full-length versions were retrieved. One of the 50 publications was published in Chinese33; this article was officially translated, and the publication was determined to meet the eligibility criteria for study inclusion. Of the remaining 49 publications, 26 were excluded; reasons for exclusion are provided in Figure 1. Thus, in total, 24 of the 50 full-text publications were included: 17 were of studies conducted in adults, and 7 were of studies conducted in children. Three publications by Scorletti et al36–38 are kin studies (publications of different results but for the same study population) and were therefore considered collectively as 1 unique study in adults. Likewise, 3 publications by Nobili et al20–22 are kin studies and were also considered collectively as 1 unique study in children. The parallel study in adults by Al-Gayyar et al39 could not be included in any of the meta-analyses because only end-of-treatment measures were provided for the control and n-3 LC-PUFA groups; likewise, the study in children by Spahis et al40 could not be included in any of the meta-analyses because only baseline values were reported. Therefore, the evidence base comprises a total of 22 publications (and 18 unique studies): 16 publications (and 14 unique studies) in adults, and 6 publications (and 4 unique studies) in children.
Figure 1.
Flow diagram of the literature search process. Abbreviations: NAFLD, nonalcoholic fatty liver disease; LC-PUFAS, long-chain polyunsaturated fatty acids.
Overview of studies conducted in adults
The key characteristics of the 14 unique studies conducted in adults are summarized in Table 314–17,24–27,33,36–38,41–44; more detailed summaries can be found in Table S1 in the Supporting Information online. All of the studies were parallel in design, except for the crossover studies by Vega et al44 and Cussons et al.41 Of the 14 controlled intervention studies, 9 were described as randomized, double blinded, and placebo controlled; the placebo administered was soybean oil in 1 study,25 olive oil in 2 studies,36–38,41 corn oil in 2 studies,26,42 and mineral oil in 1 study27; in the remaining 3 randomized, double-blind, placebo-controlled studies, the placebo was not characterized.17,33,43 Of the 5 other controlled intervention studies, 3 were described as randomized but not double blinded,15,16,24 and 2 were described neither as randomized nor double blinded.14,44 In these 5 studies, the control group was not administered any product14,16 or was administered saline,24 non–n-3 LC-PUFA-enriched olive oil,15 or an oil composed of 72% C18:1 trans fatty acid, 10% linoleic acid, and 12% palmitic acid.44 Although the placebo oil in the study by Vega et al44 was described as containing predominantly trans fatty acids, this is believed to be a typographical error.
Table 3.
Key study characteristics of omega-3 long-chain polyunsaturated fatty acid (n-3 LC-PUFA) supplementation trials conducted in adults and children with nonalcoholic fatty liver disease (NAFLD)
| Reference | Country of study | Study design | Duration of study | Study population |
Investigational product |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Health status | No. of patients (gender distribution) |
Mean age (y)a | Mean BMI (kg/m2)b | Control | Active | LC-PUFA dosage (mg/d) |
|||||||
| Initial count | Final count | EPA | DHA | ALA | |||||||||
| Studies in adults | |||||||||||||
| Argo et al (2015)25 | USA | R, DB, PC, P | 1 y | NASH | 41 (NR) | 34 (13 M, 21 F) | 46.8±11.9 | 32.5±7.3 | Soybean oil | Fish oil | 1050 | 750 | NR |
| Capanni et al (2006)14 | Italy | Cc, OP, P | 12 mo | NAFLD | 56 (32 M, 24 F) | 56 (32 M, 24 F) | 57.5±10.5 | 28.5±4.5 | None | EPA+DHA EE | 375 | 625 | NR |
| Chen et al (2008)33 (strata 1 and 2) | China | R, DB, PC, P | 24 wk | NAFLD | 46 (30 M, 16 F) | 46 (30 M, 16 F) | 46.0d | NR | Placebo (NFS) | Seal oil | 1440 or 1800 | 1680 or 2100 | NR |
| Cussons et al (2009)41 | Australia | R, PC, DB, X | 8 wk | NAFLD+PCOS | 12 (12 F) | 12 (12 F) | 35.3±6.7 | 38.2±7.4 | Olive oil | Fish oil | 1080 | 2240 | NR |
| Dasarathy et al (2015)26 | USA | R, DB, PC, P | 48 wk | NASH+T2DM | 37 (8 M, 29 F) | 37 (8 M, 29 F) | 50.6±9.9 | 35.3±6.0 | Corn oil | Fish oil | 2160 | 1440 | NR |
| Li et al (2015)24 | China | R, C, SBe, P | 6 mo | NASH | 78 (70 M, 8 F) | 78 (70 M, 8 F) | 51.9±7.8 | 27.9±1.6 | Saline | PUFAsf (NFS) | NR | NR | NR |
| Nogueira et al (2016)27 | Brazil | R, DB, PC, P | 6 mo | NASH | 60 (NR) | 50 (9 M, 41 F) | 53.2±7.0 | 30.7±4.5 | Mineral oil | n-3 oil (NFS) | 195 | 150 | 600 |
| Qin et al (2015)42 | China | R, DB, PC, P | 3 mo | NAFLD+HyperL | 80 (NR) | 70 (51 M, 19 F) | 45.2±10.8 | 26.2±3.4 | Corn oil | Fish oil | 728 | 516 | NR |
| Sanyal et al (2014)43 (strata 1 and 2) | USA | R, DB, PC, P | 12 mo | NASH | 243 (95 M, 148 F) | 174 (NR) | 48.6±12.0 | 34.9±6.3 | Placebo (NFS) | EPA EE | 1800 or 2700 | NR | NR |
| Scorletti et al (2014, 2014, 2015)36–38 | UK | R, DB, PC, P | 15–18 mo | NAFLD+MetSyn | 103 (60 M, 43 F) | 95 (55 M, 40 F) | 51.3±10.3 | 33.1±5.1 | Olive oil | EPA+DHA EE | 1840 | 1520 | NR |
| Sofi et al (2010)15 | Italy | R, OP, PC, P | 12 mo | NAFLD | 11 (9 M, 2 F) | 11 (9 M, 2 F) | 54.5g | 29.3±4.0 | Olive oil | Enriched olive oil | 470 | 240 | NR |
| Spadaro et al (2008)16 | Italy | R, P, C, OP | 6 mo | NAFLD | 40 (NR) | 36 (19 M, 17 F) | 50.7±11.5 | 30.6±4.1 | None | PUFAs (NFS) | NR | NR | NR |
| Vega et al (2008)44 | USA | PC, OP, X | 4 wk (placebo) 8 wk (n-3 PUFAs) | NAFLD | 22 | 16 (12 M, 4 F) | 50.0±10.0 | 36.2±8.5 | Oil (72% C18:1 trans FA, 10% LA, and 12% PA) | Fish oil | 4626 | 2151 | NR |
| Zhu et al (2008)17 | China | R, DB, PC, P | 24 wk | NAFLD+HyperL | 144 (NR) | 134 (97 M, 37 F) | 44.5±11.1 | 26.2±2.9 | Placebo (NFS) | Seal oil | 2160 | 2520 | NR |
| Studies in children | |||||||||||||
| Boyraz et al (2015)18 | Turkey | R, PC, DB, P | 12 mo | NAFLD+OB | 138 (73 M, 65 F) | 108 (55 M, 53 F) | 13.7±3.6 | 28.5±4.2 | Placebo (NFS) | PUFAs (NFS) | 380 | 200 | NR |
| Janczyk et al (2015)19 | Poland | R, PC, DB, P | 24 wk | NAFLD +OB/OW | 76 (65 M, 11 F) | 64 (NR) | 13.0h | 28.7i | Sunflower oil | Fish oil | 178, 355, or 533j | 267, 534, or 800 | NR |
| Nobili et al (2011, 2013, 2013)20–22 (strata 1 and 2) | Italy | R, PC, DB, P | 24 mo | NAFLD | 60 (25 M, 35 F) | 60 (25 M, 35 F) | 11.7k | 25.7l | Germ oil | Algal oil | NR | 250 or 500 | NR |
| Pacifico et al (2015)45 | Italy | R, PC, DB, P | 6 mo | NAFLD+OB/OW | 58 (NR) | 51 (30 M, 21 F) | 10.9±2.7 | 28.2±4.9 | Germ oil | Algal oil | NR | 250 | NR |
Abbreviations: ALA, α-linolenic acid; BMI, body mass index; C, controlled; DB, double-blinded; DHA, docosahexaenoic acid; EE, ethyl ester; EPA, eicosapentaenoic acid; F, females; FA, fatty acid; HyperL, hyperlipidemia/dyslipidemia; LA, linoleic acid; M, males; MetSyn, metabolic syndrome; NASH, nonalcoholic steatohepatitis; NFS, not further specified; NR, not reported; OB, obese; OP, open label; OW, overweight; P, parallel; PA, palmitic acid; PC, placebo-controlled; PCOS, polycystic ovary syndrome; PUFA = polyunsaturated fatty acid; R, randomized; SB, single-blinded; T2DM, type 2 diabetes mellitus; X, crossover.
If mean age was reported separately for control and active groups, a weighted average was calculated and standard deviations were pooled.
If mean BMI was reported separately for control and active groups, a weighted average was calculated and standard deviations were pooled.
Patients who met the inclusion criteria but refused treatment were monitored as controls without therapy.
No standard deviation or standard error of the mean was reported.
In the study by Li et al,24 it is specifically stated that “All working staff involved in evaluating parameters were blinded to the information about both groups.” Thus, it appears this study was single blinded, even though it was not explicitly labeled as such.
Participants consumed 50 mL of PUFAs per day with a 1:1 ratio of EPA:DHA in their daily diet; details not further specified.
Age was reported as the median and interquartile range; the median was assumed to approximate the mean, and the weighted mean was calculated.
Age was reported as the median and interquartile range; the median was assumed to approximate the mean, and the weighted mean was calculated.
BMI was reported as the median and interquartile range; the median was assumed to approximate the mean, and the weighted mean was calculated.
The dose of EPA and DHA was dependent on body weight.
Age was reported as the median and interquartile range; the median was assumed to approximate the mean, and the weighted mean was calculated.
BMI was reported as the median and interquartile range; the median was assumed to approximate the mean, and the weighted mean was calculated.
There were some notable differences between the studies that were described as randomized, double blinded, and placebo controlled vs those that were either not randomized or not double blinded. Specifically, the latter studies were associated with smaller sample sizes, and the dose of EPA + DHA administered to the participants was either not reported or was generally lower; furthermore, the average quality rating of the latter studies was approximately half that of the former studies (3.6 vs 6.1), attributable, of course, to the lack of randomization or double blinding (quality ratings are discussed further in the section “Modified Jadad scale scores”).
The studies were conducted in the United States,25,26,43,44 China,17,24,33,42 Italy,14–16 Australia,41 Brazil,27 and the United Kingdom.36–38 All of the studies included both male and female patients, except for the study by Cussons et al,41 which included only female participants (who also had polycystic ovarian syndrome). The participants were described as having NAFLD in 6 studies,14–16,33,41,44 NAFLD and hyperlipidemia/dyslipidemia in 2 studies,17,42 NAFLD and metabolic syndrome in 1 study,36–38 NASH in 4 studies,24,25,27,43 and NASH and type 2 diabetes mellitus in 1 study.26 The effects of n-3 LC-PUFA supplementation on NAFLD were assessed using ultrasonography,14–17,33 biopsy/histology,24,26,27,43 MRS,36–38,41,44 or both biopsy and MRI.25
The number of study participants ranged from 5 to 68 per group among the parallel studies and from 12 to 16 among the crossover studies. The mean age of the participants ranged from 35.3 to 57.5 years. All but 1 study33 provided the mean BMI at baseline. On the basis of the mean BMI values, all study participants were generally overweight or obese. In 5 of the 14 studies, the participants were advised to follow a hypocaloric diet or a heart-healthy diet (eg, the American Heart Association Diet) and to increase their physical activity levels16,17,24–26; in 1 additional study, the participants were said to have been given dietary recommendations, but no further details were provided.15
The source of EPA + DHA was described as fish oil in 5 studies,25,26,41,42,44 seal oil in 2 studies,17,33 an EPA or EPA + DHA ethyl ester in 3 studies,14,36–38,43 an enriched olive oil in 1 study,15 or an n-3 oil that also contained α-linolenic acid in 1 study.27 In 2 studies, the source of the oil was not reported.16,24
There were 16 strata among the 14 studies. A stratum is defined as a set of data from one control group or arm and an n-3 LC-PUFA group or arm. The daily intake of supplemental EPA + DHA was greater than 3 g/d in 7 strata (Chen et al33 strata 1 and 2, Vega et al,44 Zhu et al,17 Cussons et al,41 Scorletti et al,36–38 Dasarathy et al26), between 1 g/d and < 3 g/d in 5 strata (Capanni et al,14 Sanyal et al,43 strata 1 and 2, Argo et al,25 Qin et al42), and less than 1 g/d in 2 strata (Sofi et al,15 Nogueira et al27). In 2 strata, the dose of EPA + DHA was not reported and could not be estimated from the information provided in the publication (Spadaro et al,16 Li et al24). The duration of the supplementation period was 2 months in 2 studies,41,44 3 months in 1 study,42 6 months in 5 studies,16,17,24,27,33 1 year in 5 studies,14,15,25,26,43 and 15 to 18 months in 1 study.36–38
Biological measures of compliance were assessed in 7 of the 14 studies; these included the ratio of n-6 to n-3 fatty acids,14,25 the ratio of EPA to arachidonic acid,43 or levels of EPA and DHA.27,36–38,42,44 These biological measures of compliance were assessed in various tissues, and in different lipid fractions, including plasma,14,27,42 serum,43 plasma phospholipids,44 red blood cells,36–38 and red blood cell phospholipids.25
The outcomes assessed in the studies are classified as liver related, blood lipids, glycemic control, body weight/composition, or other in Table S1 in the Supporting Information online.
Overview of studies conducted in children
The key characteristics of the 4 unique studies conducted in children are summarized in Table 3;18–22,45 more detailed summaries can be found in Table S2 in the Supporting Information online. The 4 studies, described as randomized, double blind, and placebo controlled, were all parallel in design. All 4 studies included both male and female pediatric patients with NAFLD. The effects of n-3 LC-PUFA supplementation on NAFLD were assessed using ultrasonography in the studies by Boyraz et al,18 Janczyk et al,19 and Nobili et al.20–22 Details on the scoring are provided in Table 2. It should be noted that liver biopsies were performed in addition to ultrasonography in the study by Nobili et al,20–22 but only at baseline. Baseline steatosis, hepatocellular ballooning, inflammation, and fibrosis were scored using the Non-alcoholic Steatohepatitis Clinical Research Network (NASH-CRN) criteria.23 The proportion of children with NASH was not reported, but, on the basis of the NASH-CRN scores for ballooning and inflammation, it appears that the majority of children had NASH. In the remaining study,45 the effects of n-3 LC-PUFA supplementation were assessed using MRI; as in the study by Nobili et al,20–22 liver biopsies were performed in addition to MRI, but only at baseline. On the basis of the NASH-CRN scoring criteria,23 65% of the children in the study by Pacifico et al45 had NASH.
The studies were conducted in Turkey,18 Poland,19 and Italy.20–22,45 The children in the studies ranged in age from a mean of 10.9 to 13.7 years and, on the basis of the mean BMI, were generally overweight. The number of children per group ranged from 20 to 56. DHA from algal oil was administered in 2 of the 4 studies at doses of 250 or 500 mg/d for 6 or 24 months.20–22,45 In the remaining 2 studies, fish oil was administered for 6 months, with daily EPA + DHA intakes of 580 mg/d18 or 450 to 1300 mg/d, depending on the child’s body weight.19 Across 3 of the studies, the placebo was either germ oil20–22,45 or sunflower oil19; in 1 study, the placebo was not characterized.18 In all studies, the children were prescribed or were encouraged to follow a calorically restricted diet and increase their energy expenditure. Biological measures of compliance, assessed as the content of DHA in whole blood, were reported in 2 of the 4 studies.20–22,45
The outcomes assessed in the studies are classified as liver related, blood lipids, glycemic control, body weight/composition, or other in Table S2 in the Supporting Information online.
Modified Jadad scale scores
The quality ratings ranged from 2 to 8 among the studies in adults and from 4.5 to 8 among the studies in children (Table 414–22,24–27,88,36–38,41–45). Moreover, only in the studies by Scorletti et al36–38 and Dasarathy et al24 was there evidence of allocation concealment, justification for the selected sample size, and an intention-to-treat analysis. Overall, the studies in children were generally associated with higher quality ratings than the studies in adults.
Table 4.
Results of scoring with the modified Jadad scale
| Reference | Score (/8) | Allocation concealment | Intention-to-treat analysis | Sample size justification |
|---|---|---|---|---|
| Studies in adults | ||||
| Argo et al (2015)25 | 6.5 | Yes | No | Yes |
| Capanni et al (2006)14 | 3.0 | No | Yes | Yes |
| Chen et al (2008)33 | 5.0 | No | Yes | No |
| Cussons et al (2009)41 | 5.0 | No | Yes | Yes |
| Dasarathy et al (2015)26 | 7.0 | Yes | Yes | Yes |
| Li et al (2015)24 | 3.5 | No | Yes | No |
| Nogueira et al (2016)27 | 7.0 | No | No | Yes |
| Qin et al (2015)42 | 7.0 | Yes | No | Yes |
| Sanyal et al (2014)43 | 6.5 | Yes | No | Yes |
| Scorletti et al (2014, 2014, 2015)36–38 | 8.0 | Yes | Yes | Yes |
| Sofi et al (2010)15 | 3.5 | No | No | Yes |
| Spadaro et al (2008)16 | 6.0 | No | No | Yes |
| Vega et al (2008)44 | 2.0 | No | No | No |
| Zhu et al (2008)17 | 3.0 | No | Yes | No |
| Studies in children | ||||
| Boyraz et al (2015)18 | 4.5 | No | No | No |
| Janczyk et al (2015)19 | 7.0 | Yes | No | Yes |
| Nobili et al (2011, 2013, 2013)20–22 | 8.0 | No | Yes | Yes |
| Pacifico et al (2015)45 | 8.0 | No | No | Yes |
Meta-analyses
Liver-related outcomes.
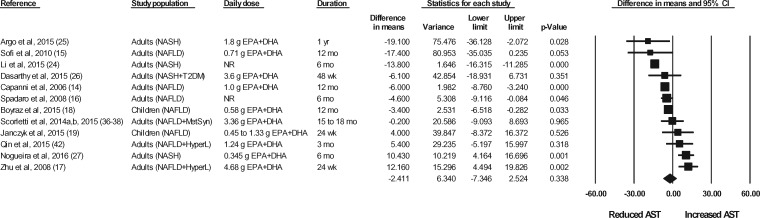
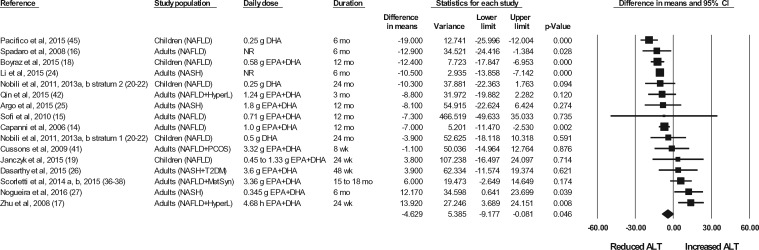
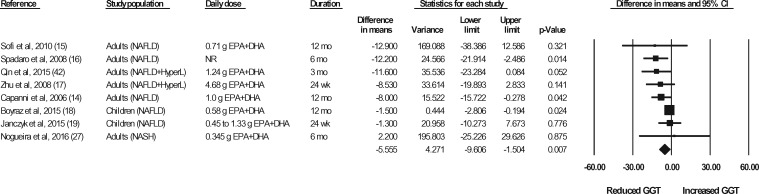
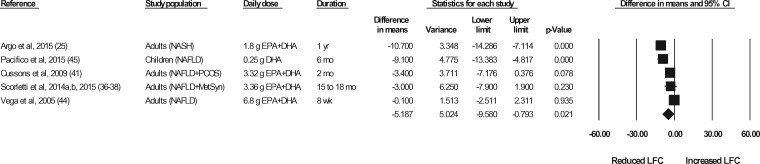
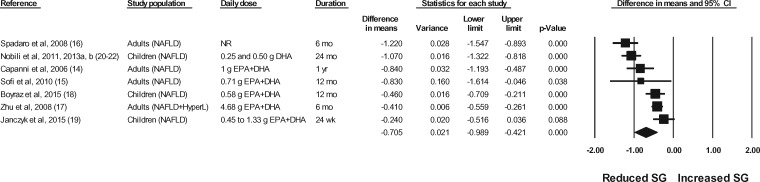
The effects of n-3 LC-PUFA supplementation on liver enzymes (ie, AST, ALT, and GGT), liver fat content (assessed via MRI or MRS), and steatosis score (assessed via ultrasonography), corrected for the effects in the control group, are shown in Figures 2 through 6. When all of the available data were pooled, there were statistically significant reductions in ALT, GGT, liver fat content, and steatosis score. For AST, the pooled reduction was statistically significant only when results from studies longer than 6 months were pooled (Table 535). The pooled reduction in ALT was statistically significant when data from the children (but not the adult) studies were pooled, when data from the higher-dose (but not the lower-dose) adult studies were pooled, and when data from the longer-duration (but not the shorter-duration) studies were pooled (Table 5). The pooled reduction in GGT was statistically or nearly statistically significant when data from the adult studies were pooled (there were insufficient data points to conduct a separate analysis for children), when results from the lower-dose adult studies were pooled (there were insufficient data points to permit an analysis of the higher-dose adult studies), when the results from the shorter-duration (but not the longer-duration) studies were pooled, and irrespective of the quality rating of the study (Table 5). The pooled reduction in liver fat content was nearly statistically significant when data from the adult studies were pooled (there were insufficient data points to permit an analysis of the studies in children) and when data from the higher-quality studies were pooled (Table 5). For steatosis score, there were statistically significant reductions when the results for adults and children were pooled separately, when the results of shorter-duration and longer-duration studies were pooled separately, and when the results of studies with lower- and higher-quality ratings were pooled separately (Table 5).
Figure 2.
Effects of n-3 LC-PUFAs vs a control on AST levels in patients with NAFLD. A random-effects model was used to calculate the pooled estimate of the differences in means and the accompanying 95%CI. Studies were weighted by the inverse of their variance; the area of each symbol is proportional to the weight of the study. The diamond represents the pooled effect. The pooled change from baseline in serum AST levels with intake of n-3 LC-PUFAs, corrected for changes from baseline in the control group, is −2.41 IU/L (95%CI, −7.35 to 2.52 IU/L; P=0.338). Using the trim and fill method of Duval and Tweedie,35 1 study was found to be missing to the right of the mean effect. With this study imputed, the pooled effect is −1.56 IU/L (95%CI, −6.47 to 3.35 IU/L). Abbreviations: AST, aspartate aminotransferase; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HyperL, hyperlipidemia/dyslipidemia; n-3 LC-PUFAs, omega-3 long-chain polyunsaturated fatty acids; MetSyn, metabolic syndrome; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; NR, not reported; T2DM, type 2 diabetes mellitus.
Table 5.
Sensitivity analyses: liver enzymes and imaging measuresa
| Liver enzymes |
Imaging measures |
|||||
|---|---|---|---|---|---|---|
| Aspartate aminotransferase (IU/L) | Alanine transaminase (IU/L) | γ-Glutamyl transferase (IU/L) | Liver fat content (assessed by MRI or MRS) | Steatosis score (assessed by ultrasonography) | ||
| All strata | n = 12 | n = 16 | n = 8 | n = 5 | n = 7 | |
| −1.56 (−6.47 to 3.35)b | −4.63 (−9.18 to −0.08) | −5.16 (−8.92 to −1.40)b | −5.19 (−9.58 to −0.79) | −0.71 (−0.99 to −0.42) | ||
| NS | P = 0.046 | P < 0.05 | P = 0.021 | P < 0.001 | ||
| Study population | Adults | n = 10 | n = 11 | n = 6 | n = 4 | n = 4 |
| −2.89 (−8.93 to 3.14) | −1.79 (−7.50 to 3.92) | −9.52 (−14.22 to −4.82) | −4.25 (−9.16 to 0.65) | −0.81 (−1.26 to −0.36) | ||
| P = 0.348 | P = 0.538 | P < 0.001 | P = 0.089 | P < 0.001 | ||
| Children | n = 2; NA | n = 5 | n = 2; NA | n = 1; NA | n = 3 | |
| −11.69 (−17.66 to −5.73) | −0.59 (−1.08 to −0.11) | |||||
| P < 0.001 | P = 0.017 | |||||
| EPA + DHA dose (g/d), adult studies only | < 3 g/d | n = 5 | n = 5 | n = 4 | n = 1; NA | n = 2; NA |
| −3.38 (−13.51 to 6.75) | −3.41 (−11.63 to 4.81) | −8.76 (−14.85 to −2.67) | ||||
| P = 0.513 | P = 0.416 | P = 0.005 | ||||
| ≥ 3 g/d | n = 3 | n = 4 | n = 1; NA | n = 3 | n = 1; NA | |
| 2.77 (−7.95 to 13.49) | 6.85 (0.98 to 12.73) | −1.57 (−3.84 to 0.70) | ||||
| P = 0.613 | P = 0.022 | P = 0.174 | ||||
| Supplementation duration (months) | ≤ 6 mo | n = 6 | n = 8 | n = 5 | n = 3 | n = 3 |
| 1.90 (−8.17 to 11.96) | −3.66 (−11.76 to 4.45) | −7.45 (−12.48 to −2.41) | −3.96 (−9.06 to 1.13) | −0.61 (−1.09 to −0.13) | ||
| P = 0.712 | P = 0.377 | P = 0.004 | P = 0.127 | P = 0.014 | ||
| > 6 mo | n = 6 | n = 8 | n = 3 | n = 2; NA | n = 4 | |
| −5.17 (−8.20 to −2.14) | −5.42 (−10.58 to −0.27) | −3.78 (−9.04 to 1.48) | −0.79 (−1.13 to −0.46) | |||
| P = 0.001 | P = 0.039 | P = 0.159 | P < 0.001 | |||
| Jadad score | < 6/8 | n = 5 | n = 6 | n = 4 | n = 2; NA | n = 4 |
| −4.89 (−11.78 to 2.01) | −5.13 (−11.38 to 1.11) | −4.32 (−9.0 to 0.367) | −0.54 (−0.74 to −0.34) | |||
| P = 0.165 | P = 0.107 | P = 0.071 | P < 0.001 | |||
| ≥ 6/8 | n = 7 | n = 10 | n = 4 | n = 3 | n = 3 | |
| −0.20 (−6.68 to 6.29) | −4.25 (−11.55 to 3.06) | −7.25 (−13.69 to −0.81) | −7.04 (−14.58 to 0.50) | −0.84 (−1.44 to −0.25) | ||
| P = 0.952 | P = 0.254 | P = 0.027 | P = 0.067 | P = 0.006 | ||
Abbreviations: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; n, number of strata; NA, not applicable; NS, not significant.
Results in boldface type are statistically or nearly statistically significant (P < 0.10).
Using the trim and fill method of Duval and Tweedie,35 1 study was found to be missing to the right of the mean effect. The missing study was imputed. The results presented are adjusted for publication bias.
Figure 3.
Effects of n-3 LC-PUFAs vs a control on ALT levels in patients with NAFLD. A random-effects model was used to calculate the pooled estimate of the differences in means and the accompanying 95%CI. Studies were weighted by the inverse of their variance; the area of each symbol is proportional to the weight of the study. The diamond represents the pooled effect. The pooled change from baseline in serum ALT levels with intake of n-3 LC-PUFAs, corrected for changes from baseline in the control group, is −4.63 IU/L (95%CI, −9.18 to −0.08 IU/L; P=0.046). Using the trim and fill method of Duval and Tweedie,35 no studies were found to be missing to the right of the mean effect. Abbreviations: ALT, alanine aminotransferase; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HyperL, hyperlipidemia/dyslipidemia; n-3 LC-PUFAs, omega-3 long-chain polyunsaturated fatty acids; MetSyn, metabolic syndrome; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; NR, not reported; PCOS, polycystic ovarian syndrome; T2DM, type 2 diabetes mellitus.
Figure 4.
Effects of n-3 LC-PUFAs vs a control on GGT levels in patients with NAFLD. A random-effects model was used to calculate the pooled estimate of the differences in means and the accompanying 95%CI. Studies were weighted by the inverse of their variance; the area of each symbol is proportional to the weight of the study. The diamond represents the pooled effect. The pooled change from baseline in serum GGT with intake of n-3 LC-PUFAs, corrected for changes from baseline in the control group, is −5.56 IU/L (95%CI, −9.61 to −1.50 IU/L; P=0.007). Using the trim and fill method of Duval and Tweedie,35 1 study was found to be missing to the right of the mean effect. With this study imputed, the pooled reduction in serum GGT levels with n-3 LC-PUFAs vs a control is −5.16 IU/L (95%CI, −8.92 to −1.40 IU/L). Abbreviations: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; GGT, γ-glutamyl transferase; HyperL, hyperlipidemia/dyslipidemia; n-3 LC-PUFAs, omega-3 long-chain polyunsaturated fatty acids; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; NR, not reported.
Figure 5.
Effects of n-3 LC-PUFAs vs a control on liver fat content, assessed using magnetic resonance imaging or magnetic resonance spectroscopy, in patients with NAFLD. A random-effects model was used to calculate the pooled estimate of the differences in means and the accompanying 95%CI. Studies were weighted by the inverse of their variance; the area of each symbol is proportional to the weight of the study. The diamond represents the pooled effect. The pooled change from baseline in liver fat content with intake of n-3 LC-PUFAs, corrected for changes from baseline in the control group, is −5.19% (95%CI, −9.58 to −0.79%; P=0.021). Using the trim and fill method of Duval and Tweedie,35 no studies were found to be missing to the right of the mean effect. Abbreviations: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; n-3 LC-PUFAs, omega-3 long-chain polyunsaturated fatty acids; LFC, liver fat content; MetSyn, metabolic syndrome; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; PCOS, polycystic ovarian syndrome.
Figure 6.
Effects of n-3 LC-PUFAs vs a control on the grade of steatosis, assessed using ultrasonography, in patients with NAFLD. A random-effects model was used to calculate the pooled estimate of the differences in means and the accompanying 95%CI. Studies were weighted by the inverse of their variance; the area of each symbol is proportional to the weight of the study. The diamond represents the pooled effect. The pooled change from baseline in the grade of steatosis with intake of n-3 LC-PUFAs, corrected for changes from baseline in the control group, is −0.71 (95%CI, −0.99 to −0.42; P<0.001). Using the trim and fill method of Duval and Tweedie,35 no studies were found to be missing to the right of the pooled effect. Abbreviations: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HyperL, hyperlipidemia/dyslipidemia; n-3 LC-PUFAs, omega-3 long-chain polyunsaturated fatty acids; NAFLD, nonalcoholic fatty liver disease; NR, not reported; SG, steatosis grade.
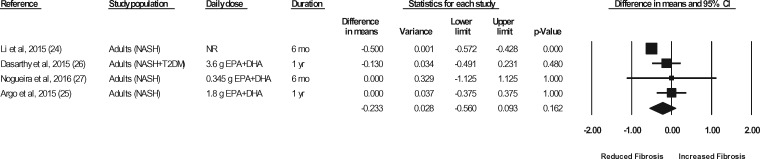
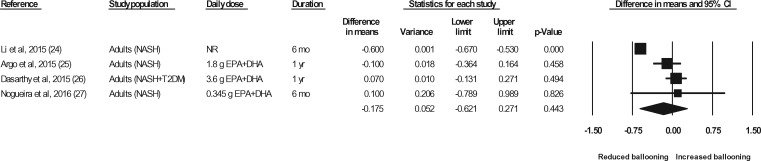
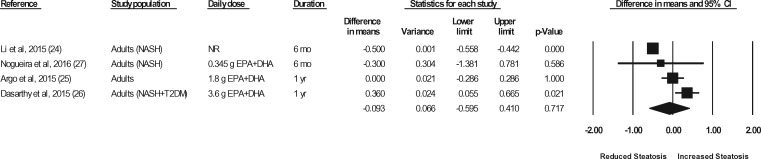
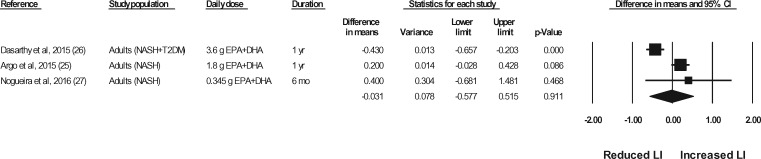
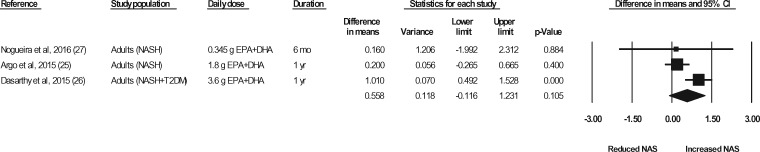
Liver biopsies in NASH patients were conducted at baseline and at the end of treatment in a total of 5 studies (representing 6 strata) (Sanyal et al,43 strata 1 and 2, Argo et al,25 Dasarathy et al,26 Li et al,24 Nogueira et al27). In the studies by Argo et al25 and Nogueira et al,27 the end-of-treatment values were not reported in the publication; however, the authors were contacted for the data, and the data were made available for the meta-analyses. In the study by Sanyal et al43 (strata 1 and 2), the liver biopsy results were not reported in a manner that permitted their inclusion in the meta-analyses. The authors were contacted a minimum of 3 times, and requests for means and standard deviations were made, but the required data were not provided. Thus, data for 4 of the studies (representing 4 strata) were available for pooling in meta-analyses.24–27 As shown in Figures 7 through 11, relative to a placebo, n-3 LC-PUFA supplementation had no effect on the liver fibrosis score, hepatocellular ballooning score, steatosis score, lobular inflammation score, or the NAFLD activity score. Most of the sensitivity analyses could not be conducted because there were too few data sets (Table 6). Of note, all studies in which a liver biopsy was conducted at baseline and at end of treatment included adults with NASH.
Figure 7.
Effects of n-3 LC-PUFAs vs a control on the fibrosis score, assessed histologically, in patients with NASH. A random-effects model was used to calculate the pooled estimate of the differences in means and the accompanying 95%CI. Studies were weighted by the inverse of their variance; the area of each symbol is proportional to the weight of the study. The diamond represents the pooled effect. The pooled change from baseline in the fibrosis score with intake of n-3 LC-PUFAs, corrected for changes from baseline in the control group, is −0.23 (95%CI, −0.56 to 0.093; P=0.162). Using the trim and fill method of Duval and Tweedie,35 no studies were found to be missing to the right of the mean effect. Abbreviations: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; n-3 LC-PUFAs, omega-3 long-chain polyunsaturated fatty acids; NASH, nonalcoholic steatohepatitis; NR, not reported; T2DM, type 2 diabetes mellitus.
Table 6.
Sensitivity analyses: liver biopsy measures
| Fibrosis | Hepatocellular ballooning score | Steatosis score | Lobular inflammation score | NAFLD activity score | ||
|---|---|---|---|---|---|---|
| All strata | n = 4 | n = 4 | n = 4 | n = 3 | n = 3 | |
| −0.23 (−0.56 to 0.093) | −0.18 (−0.62 to 0.27) | −0.09 (−0.60 to 0.41) | −0.03 (−0.58 to 0.52) | 0.56 (−0.12 to 1.23) | ||
| P = 0.162 | P = 0.443 | P = 0.717 | P = 0.911 | P = 0.105 | ||
| Study population | Adults | n = 4 | n = 4 | n = 4 | n = 3 | n = 3 |
| −0.23 (−0.56 to 0.093) | −0.18 (−0.62 to 0.27) | −0.09 (−0.60 to 0.41) | −0.03 (−0.58 to 0.52) | 0.56 (−0.12 to 1.23) | ||
| P = 0.162 | P = 0.443 | P = 0.717 | P = 0.911 | P = 0.105 | ||
| Children | n = 0; NA | n = 0; NA | n = 0; NA | n = 0; NA | n = 0; NA | |
| EPA + DHA dose (g/d), adult studies only | < 3 g/d | n = 2; NA | n = 2; NA | n = 2; NA | n = 2; NA | n = 2; NA |
| ≥ 3 g/d | n = 1; NA | n = 1; NA | n = 1; NA | n = 1; NA | n = 1; NA | |
| Supplementation duration (months) | ≤ 6 mo | n = 2; NA | n = 2; NA | n = 2; NA | n = 1, NA | n = 1, NA |
| > 6 mo | n = 2; NA | n = 2; NA | n = 2; NA | n = 2; NA | n = 2; NA | |
| Jadad score | < 6/8 | n = 1, NA | n = 1, NA | n = 1, NA | n = 0; NA | n = 0; NA |
| ≥ 6/8 | n = 3 | n = 3 | n = 3 | n = 3 | n = 3 | |
| −0.06 (−0.32 to 0.19) | 0.01 (−0.15 to 0.17) P = 0.895 | 0.14 (−0.17 to 0.45) | −0.03 (−0.58 to 0.52) | 0.56 (−0.12 to 1.23) | ||
| P = 0.620 | P = 0.379 | P = 0.911 | P = 0.105 | |||
Abbreviations: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; n, number of strata; NA, not applicable.
Figure 8.
Effects of n-3 LC-PUFAs vs a control on the hepatocellular ballooning score, assessed histologically, in patients with NASH. A random-effects model was used to calculate the pooled estimate of the differences in means and the accompanying 95%CI. Studies were weighted by the inverse of their variance; the area of each symbol is proportional to the weight of the study. The diamond represents the pooled effect. The pooled change from baseline in the hepatocellular ballooning score with intake of n-3 LC-PUFAs, corrected for changes from baseline in the control group, is −0.18 (95%CI, −0.62 to 0.27; P=0.443). Using the trim and fill method of Duval and Tweedie,35 no studies were found to be missing to the right of the mean effect. Abbreviations: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; n-3 LC-PUFAs, omega-3 long-chain polyunsaturated fatty acids; NASH, nonalcoholic steatohepatitis; NR, not reported; T2DM, type 2 diabetes mellitus.
Figure 9.
Effects of n-3 LC-PUFAs vs a control on the steatosis score, assessed histologically, in patients with NASH. A random-effects model was used to calculate the pooled estimate of the differences in means and the accompanying 95%CI. Studies were weighted by the inverse of their variance; the area of each symbol is proportional to the weight of the study. The diamond represents the pooled effect. The pooled change from baseline in the steatosis score with intake of n-3 LC-PUFAs, corrected for changes from baseline in the control group, is −0.09 (95%CI, −0.60 to 0.41; P=0.717). Using the trim and fill method of Duval and Tweedie,35 no studies were found to be missing to the right of the mean effect. Abbreviations: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; n-3 LC-PUFAs, omega-3 long-chain polyunsaturated fatty acids; NASH, nonalcoholic steatohepatitis; SS, steatosis score; T2DM, type 2 diabetes mellitus.
Figure 10.
Effects of n-3 LC-PUFAs vs a control on the lobular inflammation score, assessed histologically, in patients with NASH. A random-effects model was used to calculate the pooled estimate of the differences in means and the accompanying 95%CI. Studies were weighted by the inverse of their variance; the area of each symbol is proportional to the weight of the study. The diamond represents the pooled effect. The pooled change from baseline in the lobular inflammation score with intake of n-3 LC-PUFAs, corrected for changes from baseline in the control group, is −0.03 (95%CI, −0.58 to 0.52; P=0.911). Using the trim and fill method of Duval and Tweedie,35 no studies were found to be missing to the right of the mean effect. Abbreviations: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; n-3 LC-PUFAs, omega-3 long-chain polyunsaturated fatty acids; LI, lobular inflammation; NASH, nonalcoholic steatohepatitis; T2DM, type 2 diabetes mellitus.
Figure 11.
Effects of n-3 LC-PUFAs vs a control on NAS, assessed histologically, in patients with NASH. A random-effects model was used to calculate the pooled estimate of the differences in means and the accompanying 95%CI. Studies were weighted by the inverse of their variance; the area of each symbol is proportional to the weight of the study. The diamond represents the pooled effect. The pooled change from baseline in NAS with intake of n-3 LC-PUFAs, corrected for changes from baseline in the control group, is 0.56 (95%CI, −0.12 to 1.23; P=0.105). Using the trim and fill method of Duval and Tweedie,35 no studies were found to be missing to the right of the mean effect. Abbreviations: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; n-3 LC-PUFAs, omega-3 long-chain polyunsaturated fatty acids; NAS, nonalcoholic fatty liver disease activity score; NASH, nonalcoholic steatohepatitis; T2DM, type 2 diabetes mellitus.
Blood lipids.
Changes from baseline in total cholesterol, LDL-C, HDL-C, and triglycerides with n-3 LC-PUFA supplementation, corrected for changes from baseline in the control group, are presented in Figures S1 through S4, respectively, in the Supporting Information online. Significant improvements with n-3 LC-PUFA supplementation were noted for all of the blood lipid parameters. Based on the sensitivity analyses (Table S3 in the Supporting Information online), all of the pooled effects were favorable (ie, reductions in total cholesterol, LDL-C, and triglycerides and an increase in HDL-C), though they varied with regard to their statistical significance. All the improvements were significant when the analyses were restricted to studies conducted in adults; only for triglycerides was it possible to conduct an analysis restricted to studies conducted in children. When data from the 3 children strata were pooled, the reduction in triglycerides was of borderline significance (P = 0.056). When lipid data from studies 6 months or shorter in duration were pooled, the pooled effects were favorable and statistically significant for total cholesterol, LDL-C, HDL-C, and triglycerides; in contrast, when lipid data from studies longer than 6 months were pooled, the pooled effects were significantly favorable only for LDL-C and HDL-C. In general, statistically significant or near-significant improvements in blood lipid levels were observed, whether pooling the results of studies with a low-quality or a high-quality rating.
Glycemic control.
Fasting blood glucose, fasting insulin, and adiponectin levels were unaffected by the supplementation of NAFLD patients with n-3 LC-PUFAs (Figures S5–S7 in the Supporting Information online, respectively). These observations generally persisted in the sensitivity analyses (Table S4 in the Supporting Information online), except that fasting insulin was significantly reduced when the results from studies with a lower quality rating were pooled (there were insufficient data points to conduct a separate analysis for higher-quality studies). Insulin resistance was significantly improved from baseline with the supplementation of NAFLD patients with n-3 LC-PUFAs relative to the change from baseline in the control/placebo group (−0.54; 95%CI, −0.93 to −0.14; P = 0.008; Figure S8 in the Supporting Information online). This result was based on the pooling of data from 8 strata (5 of which were from studies in adults and 3 of which were from studies in children), with a supplementation duration ranging from 8 weeks to 24 months, and an EPA + DHA intake of 0.25 to 3.6 g/d. Based on the sensitivity analyses (Table S4 in the Supporting Information online), the improvement in HOMA-IR was evident in children, but not in adults, and in the studies with a lower (but not a higher) quality rating.
Body weight/composition and other metabolic risk factors.
Body mass index was significantly reduced from baseline with the supplementation of NAFLD patients with n-3 LC-PUFAs relative to the change from baseline in the control/placebo group (−0.85 kg/m2; 95%CI, −1.44 to −0.26 kg/m2; P = 0.005; Figure S9 in the Supporting Information online). Supplementation with n-3 LC-PUFAs had no significant effects on body weight, waist circumference, systolic blood pressure, or diastolic blood pressure (Figures S10–S13, respectively, in the Supporting Information online), and these null effects persisted in all of the sensitivity analyses. It should be noted that the BMI meta-analysis was based on 10 strata (8 strata from studies in adults and 2 strata from studies in children), while the body weight meta-analysis was based on 6 strata (4 strata from studies in adults and 2 strata from studies in children). The reductions in BMI were generally the greatest for the 2 strata from the studies in children, and without these strata, the pooled effect on BMI was not statistically significant (P = 0.059) (Table S5 in the Supporting Information online).
An overall summary of the meta-analysis results is provided in Table 7.25,35
Table 7.
Effects of omega-3 long-chain polyunsaturated fatty acids on liver-related outcomes and metabolic risk factors in patients with nonalcoholic fatty liver disease (NAFLD): results of meta-analysesa
| Parameter | No. of strata | No. of participants |
Pooled effect | Publication bias | Adjusted pooled effect | ||
|---|---|---|---|---|---|---|---|
| Adults | Children | ||||||
| Liver-related outcomes | |||||||
| Serological measures | AST | 12 | 600 | 172 | −2.41 IU/L (95%CI, −7.35 to 2.52 IU/L; P = 0.338) | Yesb | −1.56 IU/L (95%CI, −6.47 to 3.35 IU/L) |
| ALT | 16 | 612 | 283 | −4.63 IU/L (95%CI, −9.18 to −0.08 IU/L; P = 0.046) | No | NA | |
| GGT | 8 | 356 | 172 | −5.55 IU/L (95%CI, −9.61 to −1.50 IU/L; P = 0.007) | Yesb | −5.16 IU/L (95%CI, −8.92 to −1.40 IU/L) | |
| Imaging measures | Liver fat contentc | 5 | 153 | 51 | −5.19% (95%CI, −9.58 to −0.79; P = 0.021) | No | NA |
| Steatosis scored | 7 | 237 | 225 | −0.71 (95%CI, −0.99 to −0.42; P < 0.001) | No | NA | |
| Biopsy measurese | Fibrosis score | 4 | 199 | 0 | −0.23 (95%CI, −0.56 to 0.093; P = 0.162) | No | NA |
| Hepatocellular ballooning score | 3 | 199 | 0 | −0.18 (95%CI, −0.62 to 0.27; P = 0.443) | No | NA | |
| Steatosis score | 4 | 199 | 0 | −0.09 (95%CI, −0.60 to 0.41; P = 0.717) | No | NA | |
| Lobular inflammation score | 3 | 121 | 0 | −0.03 (95%CI, −0.58 to 0.52; P = 0.911) | No | NA | |
| NAFLD activity score | 3 | 121 | 0 | 0.56 (95%CI, −0.12 to 1.23; P = 0.105) | No | NA | |
| Blood lipids | |||||||
| Total cholesterol | 11 | 507 | 159 | −8.2 mg/dL (95%CI, −15.8 to −0.60 mg/dL; P = 0.035) | No | NA | |
| LDL-C | 9 | 447 | 108 | −7.2 mg/dL (95%CI, −11.4 to −3.1 mg/dL; P = 0.001) | No | NA | |
| HDL-C | 11 | 486 | 159 | 3.1 mg/dL (95%CI, 1.5 to 4.8 mg/dL; P < 0.001) | No | NA | |
| Triglycerides | 15 | 563 | 168 | −24.8 mg/dL (95%CI, −36.7 to −12.9 mg/dL; P < 0.001) | Yesb | −23.4 mg/dL (95%CI, −35.1 to −11.7 mg/dL) | |
| Glycemic control | |||||||
| Fasting blood glucose | 9 | 298 | 159 | −0.80 mg/dL (95%CI, −2.78 to 1.18 mg/dL; P = 0.427) | No | NA | |
| Fasting insulin | 5 | 94 | 108 | −1.48 mIU/L (95%CI, −4.68 to 1.72 mIU/L; P = 0.364) | No | NA | |
| HOMA-IR | 8 | 130 | 168 | −0.54 (95%CI, −0.93 to −0.14; P = 0.008) | No | NA | |
| Adiponectin | 3 | 115 | 0 | 0.49 µg/mL (95%CI, −0.30 to 1.27 µg/mL; P = 0.223) | No | NA | |
| Body weight, body composition | |||||||
| BMI | 10 | 418 | 159 | −0.85 kg/m2 (95%CI, −1.44 to −0.26 kg/m2; P = 0.005) | No | NA | |
| Body weight | 6 | 236 | 159 | −0.64 kg (95%CI, −1.59 to 0.31 kg; P = 0.184) | Yesb | −0.57 kg (95%CI, −1.51 to 0.37 kg) | |
| Waist circumference | 5 | 236 | 51 | −1.5 cm (95%CI, −3.5 to 0.5 cm; P = 0.133) | Yesf | −0.39 cm (95%CI, −2.1 to 1.3 cm) | |
| Other | |||||||
| Systolic BP | 5 | 177 | 159 | −4.0 mmHg (95%CI, −15.0 to 7.0 mmHg; P = 0.476) | No | NA | |
| Diastolic BP | 5 | 177 | 159 | −0.43 mmHg (95%CI, −2.4 to 1.5 mmHg; P = 0.668) | No | NA | |
Abbreviations: ALT, alanine transaminase; AST, aspartate aminotransferase; BMI, body mass index; BP, blood pressure; GGT, γ-glutamyl transaminase; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model of assessment–estimated insulin resistance; LDL-C, low-density lipoprotein cholesterol; NA, not applicable.
Pooled effects represent the changes from baseline in the omega-3 long-chain polyunsaturated fatty acids group, corrected for the corresponding changes from baseline in the control group. Results in boldface type are statistically significant (P < 0.05).
One study was found to be missing to the right of the pooled effect, using the trim and fill method of Duval and Tweedie.35.
All study participants were NAFLD patients.
All study participants were NAFLD patients, except for those in the study by Argo et al,25 which included NASH patients.
All study participants were NASH patients.
Three studies were found to be missing to the right of the pooled effect., using the trim and fill method of Duval and Tweedie.35
DISCUSSION
Nonalcoholic fatty liver disease has become a disease of public health significance in Westernized countries, afflicting both adults and children. Currently, there is no pharmacological therapy for NAFLD, and diet and exercise represent the first line of treatment in NAFLD management. Intrahepatic fat content is significantly improved with exercise (either aerobic or resistance), even in the absence of weight loss.46–50 Weight loss also is effective in improving NAFLD, with benefits dependent on the amount of weight lost: with a loss in body weight of 3% to 5%, steatosis is improved; with a loss of 5% to 7%, hepatic inflammation is improved; and with a loss of 7% to 10%, there may be a regression of fibrosis and a remission of NAFLD/NASH.5,51 Most patients, however, have difficulty with long-term changes in exercise and diet. For example, Pugh et al52 noted significant improvements in liver fat content in patients with NAFLD following a 16-week supervised exercise program; however, 12 months after the cessation of exercise supervision, all exercise-induced benefits, including the reduction in liver fat content, had reverted to baseline. Likewise, Dudekula et al53 reported that most patients cannot achieve the required degree of weight loss or have trouble with the long-term maintenance of body weight after weight loss.
Adjunctive treatments to exercise and weight loss are needed for the management of NAFLD. The meta-analysis results presented herein demonstrate the usefulness of n-3 LC-PUFAs in the dietary management of patients with NAFLD.
Effects of n-3 LC-PUFA supplementation on liver enzymes and on liver fat content and steatosis score assessed via imaging in patients with NAFLD
Based on the meta-analyses, n-3 LC-PUFA supplementation of patients with NAFLD is associated with significant reductions in both serum ALT and serum GGT (favorable effects), with no effects on serum AST, findings that are generally consistent with the meta-analyses conducted by He et al.11 Further, based on the meta-analyses, liver fat content and steatosis score, assessed via MRI/MRS or ultrasonography, respectively, were both significantly improved with n-3 LC-PUFA supplementation. The pooled reduction in liver fat content of approximately 5.2% is clinically meaningful; likewise, the pooled reduction of approximately 0.7 in steatosis score also is clinically meaningful. As steatosis is scored using a 4-grade scale, the pooled reduction equates to an improvement approaching an entire grade. The improvements in steatosis score were very robust—pooled reductions were statistically significant when data from all studies were pooled as well as when data from studies in adults and children, from longer and shorter studies, and from higher-quality and lower-quality studies were pooled separately.
Effects of n-3 LC-PUFA supplementation on liver histological outcomes in patients with NAFLD
On the basis of the meta-analysis results, liver histology parameters (ie, fibrosis score, hepatocellular ballooning score, steatosis score, lobular inflammation score, and NAFLD activity score) are unaffected by supplementation with n-3 LC-PUFAs. Most of the liver histology meta-analyses were based on 4 clinical studies in NASH patients,24–27 3 of which showed no benefit of n-3 LC-PUFA supplementation on any of the liver histology outcomes assessed.25–27 It is difficult to draw definitive conclusions from these studies, given that the dose of EPA + DHA administered was very low (0.345 g/d),27 the studies were small,25,26 measures of compliance were not assessed,26 physical activity levels were not assessed,26,27 or there were unexpected improvements in the placebo group in NAFLD activity score and steatosis score, implicating changes in diet or exercise as potential confounding variables.26 In the study by Argo et al25 the patients in the n-3 LC-PUFA group tended to have more severe NAFLD relative to patients in the placebo group at baseline; moreover, baseline intakes of fructose were significantly greater in the n-3 LC-PUFA group relative to the placebo group (P = 0.01). The latter observation is notable, given that high intakes of dietary fructose negatively affect the progression of NAFLD.54 The disconnect between the higher-quality ratings of these studies (scores ranged from 6.5 to 8) and their methodological limitations illustrates the challenges in using a quality-appraisal tool in assessing study quality.
Only in the study by Li et al24 was the n-3 LC-PUFA supplementation (50 mL/d with a 1:1 ratio of EPA to DHA) of patients with NASH associated with significant improvements in histological outcomes (ie, steatosis score, necroinflammatory score, fibrosis score, and hepatocellular ballooning score) when compared with a placebo. This study was larger than those by Argo et al25 and Dasarathy et al,26 and participants in the placebo and n-3 LC-PUFA groups were well matched at baseline. The patients in the study by Li et al24 were instructed to consume a low-fat, low-cholesterol, low-carbohydrate diet and to engage in moderate physical exercise (30 min/d, 5 d/wk). Dietary intakes were not monitored, and there were no measures of biological compliance; however, physical activity levels were monitored and were demonstrated to increase to the same degree in both groups.
Sanyal et al43 (strata 1 and 2) evaluated the effects of EPA (administered at either 1.8 or 2.7 g/d in ethyl ester form for 12 mo) on histological outcomes in NASH patients, but this study could not be included in the meta-analyses because the data were not reported in a manner that permitted their inclusion in the meta-analysis and the research group failed to provide the requested data after at least 3 requests. No improvements in any of the histological outcomes were noted. However, in no other study was EPA administered in isolation. In the studies in children by Pacifico et al45 and Nobili et al,20–22 DHA, when administered alone, was shown to improve liver fat content (assessed via imaging) in populations that included NASH patients, indicating that DHA may be required for efficacy. Likewise, Scorletti et al36–38 demonstrated that the reduction in liver fat content (assessed via imaging) was a function of the enrichment of red blood cells with DHA but not EPA. In an analysis of the total patient population (irrespective of the treatment received), Nogueira et al27 demonstrated that lobular inflammation score (assessed histologically) was significantly and inversely correlated with plasma DHA. Thus, it appears that DHA may be required for efficacy. If this is indeed the case, then it is reasonable that the study by Sanyal et al43 was a null study, in that only EPA was administered.
In the study in children conducted by Nobili et al,20–22 NAFLD was graded via liver histology in all children at baseline20–22 and again after 18 months, but only in the 20 children randomized to receive DHA at 250 mg/d.55 Because histological analysis was not conducted in the context of a controlled intervention study, the results reported by Nobili et al55 could not be included in the liver histology meta-analyses; however, the results are relevant for discussion, particularly since reports of histological evaluation of pediatric patients before and after n-3 LC-PUFA supplementation are limited. Histological analysis was performed by a single, blinded pathologist. Among the 20 children with NAFLD—12 (60%) of whom had NASH—there were significant improvements after DHA supplementation in several histological outcomes, including steatosis score, hepatocellular ballooning score, lobular inflammation score, NAFLD activity score, and a pediatric-specific histology score, the Pediatric NAFLD Histological Score. Only fibrosis was unaffected by DHA supplementation. All children in the Nobili et al20–22,55 study were prescribed an energy-reduced diet and encouraged to exercise. Without liver biopsy results for the placebo group, it is not possible to definitively attribute the improvements in liver histology to DHA supplementation. However, steatosis grade, assessed via ultrasonography, was significantly improved with DHA supplementation (either 250 or 500 mg/d) vs the placebo by 6 months and remained so at 24 months (Nobili et al20), indicating that improvements from baseline observed in liver histology in the 250 mg/d DHA group (Nobili et al55) are likely attributable to DHA supplementation and not just to changes in diet or exercise.
As it stands, there is evidence from only 2 studies that n-3 LC-PUFA supplementation improves histological outcomes in patients with NASH (Nobili et al,55 Li et al24). Unfortunately, the study by Li et al24 has a lower quality rating of 3.5 out of 8, and in the study by Nobili et al,55 the liver histology assessments were not conducted in a controlled manner (ie, histology was assessed before and after n-3 LC-PUFA supplementation, rather than before and after n-3 LC-PUFA vs placebo supplementation). Thus, based on the available studies, no definitive conclusions on the effects of n-3 LC-PUFA supplementation on histological outcomes in patients with NAFLD can be made. It should be noted, however, that of all the studies in which effects of n-3 LC-PUFAs on histological outcomes were assessed, only in the studies by Li et al24 and Nobili et al20,55 were significant benefits on triglyceride levels noted, in favor of the n-3 LC-PUFA groups. Since n-3 LC-PUFAs are used pharmacologically to treat hypertriglyceridemia, it is surprising that effects on triglyceride levels were not observed in any of the other studies, particularly those by Argo et al,25 Dasarathy et al,26 and Sanyal et al,43 in which the doses administered were within the therapeutic range for triglyceride lowering (in the study by Sanyal et al,43 the EPA ethyl ester used had previously been demonstrated to improve hyperlipidemia, including hypertriglyceridemia and hypercholesterolemia, in Japanese populations and is still used for this purpose in Japan).
Although histological examination by biopsy remains the gold standard in the grading of NAFLD, it is losing popularity, given that a liver biopsy is invasive, provides information on only 1/50 000 of the liver, and is associated with potential (and sometimes life-threatening) complications to the patient, errors in sampling, and complexity in pathological interpretation (reviewed by Golabi et al46). Indeed, the study by Scorletti et al36–38 was planned to include a liver biopsy at the end of supplementation period; however, this plan was changed because 2 liver biopsy–associated deaths (unrelated to the study) occurred in the hospital that year. In the studies in children by Nobili et al20–22 and Pacifico et al45, it was noted that liver biopsies are ethical when conducted for diagnostic purposes, but of questionable ethics for the subsequent monitoring of NAFLD.
Imaging via MRI/MRS and ultrasonography is increasingly being used to assess liver fat content and steatosis score, respectively, in patients with NAFLD. The MRI method used by Pacifico et al45 is similar to that used by Argo et al25 and has been previously validated against histological findings confirming hepatic steatosis; specifically, strong and statistically significant correlations were reported between the hepatic fat fraction (assessed via MRI) and the histological grade of steatosis, such that mild (grade 1), moderate (grade 2), and severe (grade 3) steatosis were associated with hepatic fat fractions of 8.7% (95%CI, 6.0% to 11.6%), 21.6% (95%CI, 15.3% to 27.0%), and 39.7% (95%CI, 34.4% to 45.0%), respectively.56 Moreover, the degree of fibrosis and inflammation had no impact on the accuracy of MRI in the assessment of the degree of steatosis. Likewise, MRS is a noninvasive and well-validated method for quantifying liver fat content, and results of MRS correlate well with findings of steatosis assessed via liver histology.57,58 Ultrasonography is the most economical and well-tolerated imaging technique that permits the grading of steatosis across all severities (mild to severe).59,60 As reviewed here, there is strong evidence that hepatic fat content (assessed via MRI or MRS) and steatosis score (assessed via ultrasonography) are significantly improved with n-3 LC-PUFA supplementation.
Effects of n-3 LC-PUFAs on cardiometabolic risk factors in patients with NAFLD
Based on the results of the meta-analyses presented herein, n-3 LC-PUFA supplementation of patients with NAFLD is associated with significant improvements in total cholesterol, LDL-C, HDL-C, and triglycerides. These findings are aligned with the results of a previous meta-analysis of 7 randomized controlled studies evaluating the effects of dietary n-3 LC-PUFAs in adults with NAFLD.11 The current meta-analysis includes all 7 of the randomized controlled trials15–17,24,25,33,36–38 included in the earlier meta-analysis by He et al11 as well as another 4 randomized controlled trials in adults,26,27,41,42 2 nonrandomized controlled trials in adults,14,44 and 4 randomized controlled trials in children.18–22,45
No effects of n-3 LC-PUFA supplementation on fasting blood glucose, fasting insulin, or adiponectin were observed; however, a significant reduction in HOMA-IR (−0.54; 95%CI, −0.93 to −0.14; P = 0.008) was found. When data from adult and pediatric studies were analyzed separately, the significant reduction in HOMA-IR was evident only for children. There was also a significant reduction in BMI (−0.85 kg/m2; 95%CI, −1.44 to −0.26 kg/m2; P = 0.005) but no significant effects on body weight, waist circumference, systolic blood pressure, or diastolic blood pressure. It should be noted that, once the studies in children were removed from the BMI meta-analysis, the reduction in BMI was attenuated and was no longer significant, indicating that the pooled reduction in BMI when all studies were included may have been driven by a reduction in BMI in children, who, owing to increases in height during the study, may have experienced reductions in BMI.
Increased requirement for n-3 LC-PUFAs in patients with NAFLD
It has been demonstrated in several observational studies that patients with NAFLD have reduced relative hepatic levels of EPA and DHA compared with individuals who do not have NAFLD.61–63 Of note, Elizondo et al63 observed that, compared with controls, patients with NAFLD had significantly elevated relative levels of Osbond acid in hepatic and red blood cell phospholipids. Osbond acid, or docosapentaenoic acid of the n-6 series (C22:5n-6), increases only in states of DHA deficiency.64,65 The production of Osbond acid is believed to be a compensatory mechanism for conserving the fluidity of membrane phospholipids when there is insufficient DHA.66 Thus, there is some indication from the study by Elizondo et al63 that patients with NAFLD may be deficient in DHA.
The etiology of the depressed hepatic levels of EPA and DHA in patients with NAFLD is unclear. The activity of Δ5 desaturase has been found to be significantly lower in patients with NAFLD than in controls.40,67–70 A reduction in the activity of D5D essentially means that EPA (20:5n-3) and, therefore, by default, DHA (22:6n-3) cannot be adequately synthesized from their precursor, 20:4n-3, thereby increasing the requirement for preformed dietary sources of EPA and DHA. Puri et al71 suggested there may be derangements in peroxisomal function in patients with NAFLD, given that DHA synthesis occurs in peroxisomes. Araya et al62 observed that levels of elaidic acid (trans 18:1n-9) in adipose tissue were significantly increased in NAFLD patients vs controls. Trans fatty acids are potent inhibitors of Δ6 desaturase,72 a rate-limiting enzyme in the α-linolenic acid desaturation/elongation pathway. It is unclear why levels of trans fatty acids are increased in patients with NAFLD; while dietary intakes of these fatty acids could be greater in patients with NAFLD, the possibility of de novo synthesis of trans fatty acids by the human microbiome in NAFLD patients has never been investigated and cannot be ruled out. Finally, allelic variants in the genes that code for the desaturase enzymes have been strongly associated with NAFLD risk.73
Both EPA and DHA are important modulators of hepatic gene expression, and, via their interactions with SREBP-1 and PPAR, they have a role in reducing hepatic lipogenesis and increasing hepatic fatty acid β-oxidation (reviewed in the Introduction). They also undergo metabolism in the cytochrome P450, cyclooxygenase, and lipoxygenase pathways (in direct competition with arachidonic acid) to eicosanoids such as prostaglandins, leukotrienes, thromboxanes, protectins, and resolvins.74,75 Without sufficient levels of EPA and DHA, the balance in the liver is tipped in favor of lipogenesis over lipolysis, and there may be inadequate production of anti-inflammatory mediators via the cytochrome P450, cyclooxygenase, and lipoxygenase pathways.74,75
CONCLUSION
Given the results reported here of significant improvements in patients with NAFLD in liver fat content, steatosis score, and several cardiometabolic risk factors following supplementation with n-3 LC-PUFAs, adult and pediatric patients with NAFLD should be encouraged to increase their intakes of n-3 LC-PUFAs. Supplementation with n-3 LC-PUFAs should be encouraged in the context of increased physical activity and caloric restriction, which effectively reduce the progression of NAFLD and currently constitute the first line of treatment for patients with NAFLD.46,51
In children with NAFLD, dietary and lifestyle interventions are particularly important, given that pharmacological interventions to treat NAFLD, when or if they become available, may be less desirable because of potential adverse effects. In addition, since NAFLD is a progressive disease most often associated with comorbidities, it is particularly concerning when the disease manifests in children. Despite the limited number of studies conducted specifically in children with NAFLD, it is clear from the sensitivity analyses reported herein that n-3 LC-PUFA supplementation in children significantly reduces serum ALT, steatosis score, triglycerides, and HOMA-IR. Liver fat content was assessed in only 1 controlled study in children.45 In that study, liver biopsies were conducted at baseline, and MRI was used to assess liver fat content at baseline and at the end of the 6-month DHA (250 mg/d) intervention phase. There was a significant reduction in liver fat content in DHA-supplemented children vs controls; of note, 65% of the children had NASH. Other cardiometabolic risk factors may also be favorably impacted by n-3 LC-PUFA supplementation, but there were simply too few strata to conduct pooled analyses of results for these variables specifically in children with NAFLD.
Based on the dosages administered across the studies, effective daily intakes appear to be 250 mg of DHA in children and approximately 3.0 g of EPA + DHA in adults. The minimum effective intake of n-3 LC-PUFAs is not known, nor is it clear whether EPA is even necessary for clinical efficacy, given that efficacy has been reported in children with supplements containing only DHA. Additional studies are required to resolve these important questions. It is strongly recommended that diet and physical activity be closely monitored in future studies to ensure results are not confounded by unbalanced changes in these variables. Moreover, it is imperative that biological measures of compliance be assessed to ensure participants in the active intervention group consume the n-3 LC-PUFA supplements and those in the control group do not increase their intakes of n-3 LC-PUFAs (eg, from marine sources).
Supplementary Material
Acknowledgments
The authors thank Judy Vowles for her assistance with the formatting of the manuscript; Judith Hill for her assistance in preparing the list of references; and Barbara Danielewska-Nikiel for providing editorial feedback.
Author contributions. K.M.V., H.Y.L., and C.V. generated the literature strategy and filtered through the identified studies. H.Y.L., M.D., S.F., and S.W. evaluated study quality (each study was appraised in duplicate), with final quality scores consolidated with the input of K.M.V. Study tabulations were carried out by S.F. and S.W., with final review by K.M.V. Study results were entered in duplicate by H.Y.L., M.D., S.F., and S.W., with results validated and discrepancies resolved by either K.M.V. or H.Y.L. K.M.V. ran the meta-analyses and wrote the manuscript. R.S. provided critical feedback on the manuscript.
Funding/support. Financial support for the scientific review was provided by Pronova BioPharma Norge AS (Lysaker, Norway), part of BASF. All aspects of the scientific review, from the literature identification to the assimilation, reporting, and interpretation of the study results and the composition of the manuscript, were completed independently by the named authors.
Declaration of interest. All of the authors are employees of Intertek Scientific & Regulatory Consultancy. Pronova BioPharma Norge AS (Lysaker, Norway), part of BASF AS, is a client of Intertek Scientific & Regulatory Consultancy.
Supporting Information
The following Supporting Information is available through the online version of this article at the publisher’s website.
Appendix S1 PRISMA checklist
Table S1 Key study characteristics of intervention studies in which the effects of n-3 LC-PUFAs in adults with NAFLD were assessed
Table S2 Key study characteristics of intervention studies in which the effects of long-chain n-3 LC-PUFA supplementation in children with NAFLD were assessed
Table S3 Sensitivity analyses—blood lipids
Table S4 Sensitivity analyses—measures of glycemic control
Table S5 Sensitivity analyses—other metabolic risk factors
Figure S1 The effects of n-3 LC-PUFAs vs a control on total cholesterol (TC) levels in patients with NAFLD
Figure S2 The effects of n-3 LC-PUFAs vs a control on LDL-C levels in patients with NAFLD
Figure S3 The effects of n-3 LC-PUFAs vs a control on HDL-C levels in patients with NAFLD
Figure S4 The effects of n-3 LC-PUFAs vs a control on triglyceride (TG) levels in patients with NAFLD
Figure S5 The effects of n-3 LC-PUFAs vs a control on fasting blood glucose (FBG) levels in patients with NAFLD
Figure S6 The effects of n-3 LC-PUFAs vs a control on fasting insulin (FIN) levels in patients with NAFLD
Figure S7 The effects of n-3 LC-PUFAs vs a control on adiponectin levels in patients with NAFLD
Figure S8 The effects of n-3 LC-PUFAs vs a control on HOMA-IR in patients with NAFLD
Figure S9 The effects of n-3 LC-PUFAs vs a control on BMI in patients with NAFLD
Figure S10 The effects of n-3 LC-PUFAs vs a control on body weight (BW) in patients with NAFLD
Figure S11 The effects of n-3 LC-PUFAs vs a control on waist circumference (WC) in patients with NAFLD
Figure S12 The effects of n-3 LC-PUFAs vs a control on systolic blood pressure (SBP) in patients with NAFLD
Figure S13 The effects of n-3 LC-PUFAs vs a control on diastolic blood pressure (DBP) in patients with NAFLD
References
- 1. Chalasani N, Younossi Z, Lavine JE et al. , . The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol. 2012;107:811–826. [DOI] [PubMed] [Google Scholar]
- 2. Petäjä EM, Yki-Järvinen H.. Definitions of normal liver fat and the association of insulin sensitivity with acquired and genetic NAFLD—a systematic review. Int J Mol Sci. 2016;17:633. doi:10.3390/ijms17050633 [16pp]. doi: 10.3390/ijms1705063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neuschwander-Tetri BA. Non-alcoholic fatty liver disease. BMC Med. 2017;15:45. doi:10.1186/s12916-017-0806-8 [6pp]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bellentani S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017;37(suppl 1):81–84. [DOI] [PubMed] [Google Scholar]
- 5. Marchesini G, Petta S, Dalle Grave R.. Diet, weight loss, and liver health in nonalcoholic fatty liver disease: pathophysiology, evidence, and practice. Hepatology. 2016;63:2032–2043. [DOI] [PubMed] [Google Scholar]
- 6. Clarke SD. Nonalcoholic steatosis and steatohepatitis. I. Molecular mechanism for polyunsaturated fatty acid regulation of gene transcription. Am J Physiol Gastrointest Liver Physiol. 2001;281:G865–G869. [DOI] [PubMed] [Google Scholar]
- 7. Shimomura I, Bashmakov Y, Horton JD.. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem. 1999;274:30028–30032. [DOI] [PubMed] [Google Scholar]
- 8. Shimomura I, Matsuda M, Hammer RE et al. , . Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell. 2000;6:77–86. [PubMed] [Google Scholar]
- 9. Krey G, Braissant O, L'Horset F et al. , . Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol. 1997;11:779–791. [DOI] [PubMed] [Google Scholar]
- 10. Parker HM, Johnson NA, Burdon CA et al. , . Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;56:944–951. [DOI] [PubMed] [Google Scholar]
- 11. He XX, Wu XL, Chen RP et al. , . Effectiveness of omega-3 polyunsaturated fatty acids in non-alcoholic fatty liver disease: a meta-analysis of randomized controlled trials. PLoS One. 2016;11:e0162368. doi:10.1371/journal.pone.0162368 [22pp]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lu W, Li S, Li J et al. , . Effects of omega-3 fatty acid in nonalcoholic fatty liver disease: a meta-analysis. Gastroenterol Res Pract. 2016;2016:1459790. doi:.10.1155/2016/1459790 [11pp]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J et al. , . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement [The PRISMA Group]. PLoS Med. 2009;6:e1000097. [6pp]. doi:10.1371/journal.pmed1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Capanni M, Calella F, Biagini MR et al. , . Prolonged n-3 polyunsaturated fatty acid supplementation ameliorates hepatic steatosis in patients with non-alcoholic fatty liver disease: a pilot study. Aliment Pharmacol Ther. 2006;23:1143–1151. [DOI] [PubMed] [Google Scholar]
- 15. Sofi F, Giangrandi I, Cesari F et al. , . Effects of a 1-year dietary intervention with n-3 polyunsaturated fatty acid-enriched olive oil on non-alcoholic fatty liver disease patients: a preliminary study. Int J Food Sci Nutr. 2010;61:792–802. [DOI] [PubMed] [Google Scholar]
- 16. Spadaro L, Magliocco O, Spampinato D et al. , . Effects of n-3 polyunsaturated fatty acids in subjects with nonalcoholic fatty liver disease. Dig Liver Dis. 2008;40:194–199. [DOI] [PubMed] [Google Scholar]
- 17. Zhu FS, Liu S, Chen XM et al. , . Effects of n-3 polyunsaturated fatty acids from seal oils on nonalcoholic fatty liver disease associated with hyperlipidemia. World J Gastroenterol. 2008;14:6395–6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boyraz M, Pirgon Ö, Dündar B et al. , . Long-term treatment with n-3 polyunsaturated fatty acids as a monotherapy in children with nonalcoholic fatty liver disease. J Clin Res Pediatr Endocrinol. 2015;7:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Janczyk W, Lebensztejn D, Wierzbicka-Rucinska A et al. , . Omega-3 fatty acids therapy in children with nonalcoholic fatty liver disease: a randomized controlled trial. J Pediatr. 2015;166:1358–1363.e3. [DOI] [PubMed] [Google Scholar]
- 20. Nobili V, Bedogni G, Alisi A et al. , . Docosahexaenoic acid supplementation decreases liver fat content in children with non-alcoholic fatty liver disease: double-blind randomised controlled clinical trial. Arch Dis Child. 2011;96:350–353. [DOI] [PubMed] [Google Scholar]
- 21. Nobili V, Alisi A, Della Corte C et al. , . Docosahexaenoic acid for the treatment of fatty liver: randomised controlled trial in children. Nutr Metab Cardiovasc Dis. 2013;23:1066–1070. [DOI] [PubMed] [Google Scholar]
- 22. Nobili V, Bedogni G, Donati B et al. , . The I148M variant of PNPLA3 reduces the response to docosahexaenoic acid in children with non-alcoholic fatty liver disease. J Med Food. 2013;16:957–960. [DOI] [PubMed] [Google Scholar]
- 23. Kleiner DE, Brunt EM, Van NM et al. , . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. [DOI] [PubMed] [Google Scholar]
- 24. Li Y-H, Yang L-H, Sha K-H et al. , . Efficacy of poly-unsaturated fatty acid therapy on patients with nonalcoholic steatohepatitis. World J Gastroenterol. 2015;21:7008–7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Argo CK, Patrie JT, Lackner C et al. , . Effects of n-3 fish oil on metabolic and histological parameters in NASH: a double-blind, randomized, placebo-controlled trial. J Hepatol. 2015;62:190–197. [plus supplementary data]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dasarathy S, Dasarathy J, Khiyami A et al. , . Double-blind randomized placebo-controlled clinical trial of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. J Clin Gastroenterol. 2015;49:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nogueira MA, Pinto Oliveira C, Ferreira Alves VA et al. , . Omega-3 polyunsaturated fatty acids in treating non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2016;35:578–586. [DOI] [PubMed] [Google Scholar]
- 28. Pichora-Fuller MK, Santaguida P, Hammill A et al. , . Appendix B (Data extraction forms. Modified Jadad) In: Evaluation and Treatment of Tinnitus: Comparative Effectiveness. Rockville, MD: Agency for Healthcare Research and Quality; 2013. https://www.ncbi.nlm.nih.gov/books/NBK158959/. Comparative Effectiveness Reviews, No. 122, Report No.: 13-EHC110-EF. Accessed May 10, 2018. [PubMed] [Google Scholar]
- 29. Khalesi S, Irwin C, Schubert M.. Flaxseed consumption may reduce blood pressure: a systematic review and meta-analysis of controlled trials [published correction appears in J Nutr 2015;145:2633]. J Nutr. 2015;145:758–765. [DOI] [PubMed] [Google Scholar]
- 30. Marventano S, Vetrani C, Vitale M et al. , . Whole grain intake and glycaemic control in healthy subjects: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2017;9:769. doi:10.3390/nu9070769 [doi: 10.3390/nu9070769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rohner A, Ried K, Sobenin IA et al. , . A systematic review and metaanalysis on the effects of garlic preparations on blood pressure in individuals with hypertension. Am J Hypertens. 2015;28:414–423. [DOI] [PubMed] [Google Scholar]
- 32. DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 33. Chen R, Guo Q, Zhu W-J et al. , . Therapeutic efficacy of omega-3 polyunsaturated fatty acid capsule in treatment of patients with non-alcoholic fatty liver disease [in Chinese; English abstract]. Shi Jie Hua Ren Xiao Hua Za Zhi. 2008;16:2002–2006. [Google Scholar]
- 34. Phung OJ, Makanji SS, White CM et al. , . Almonds have a neutral effect on serum lipid profiles: a meta-analysis of randomized trials. J Am Diet Assoc. 2009;109:865–873. [DOI] [PubMed] [Google Scholar]
- 35. Duval S, Tweedie R.. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 36. Scorletti E, Bhatia L, McCormick KG et al. , . Design and rationale of the WELCOME trial: a randomised, placebo controlled study to test the efficacy of purified long chain omega-3 fatty acid treatment in non-alcoholic fatty liver disease [published correction appears in Contemp Clin Trials. 2014,38:156]. Contemp Clin Trials. 2014;37:301–311. [DOI] [PubMed] [Google Scholar]
- 37. Scorletti E, Bhatia L, McCormick KG et al. , . Effects of purified eicosapentaenoic and docosahexaenoic acids in nonalcoholic fatty liver disease: results from the Welcome* study. Hepatology 2014;60:1211–1221. [DOI] [PubMed] [Google Scholar]
- 38. Scorletti E, West AL, Bhatia L et al. , . Treating liver fat and serum triglyceride levels in NAFLD, effects of PNPLA3 and TM6SF2 genotypes: results from the WELCOME trial. J Hepatol. 2015;63:1476–1483. [DOI] [PubMed] [Google Scholar]
- 39. Al-Gayyar MMH, Shams MEE, Barakat EAME.. Fish oil improves lipid metabolism and ameliorates inflammation in patients with metabolic syndrome: impact of nonalcoholic fatty liver disease. Pharm Biol. 2012;50:297–303. [DOI] [PubMed] [Google Scholar]
- 40. Spahis S, Alvarez F, Dubois J et al. , . Plasma fatty acid composition in French-Canadian children with non-alcoholic fatty liver disease: effect of n-3 PUFA supplementation. Prostaglandins Leukot Essent Fatty Acids. 2015;99:25–34. [DOI] [PubMed] [Google Scholar]
- 41. Cussons AJ, Watts GF, Mori TA et al. , . Omega-3 fatty acid supplementation decreases liver fat content in polycystic ovary syndrome: a randomized controlled trial employing proton magnetic resonance spectroscopy. J Clin Endocrinol Metab. 2009;94:3842–3848. [DOI] [PubMed] [Google Scholar]
- 42. Qin Y, Zhou Y, Chen S-H et al. , . Fish oil supplements lower serum lipids and glucose in correlation with a reduction in plasma fibroblast growth factor 21 and prostaglandin E2 in nonalcoholic fatty liver disease associated with hyperlipidemia: a randomized clinical trial. PLoS One. 2015;10:e0133496. doi:10.1371/journal.pone.0133496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sanyal AJ, Abdelmalek MF, Suzuki A et al. , . No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 2014;147:377.e1–384.e1. [DOI] [PubMed] [Google Scholar]
- 44. Vega GL, Chandalia M, Szczepaniak LS et al. , . Effects of n-3 fatty acids on hepatic triglyceride content in humans. J Investig Med. 2008;56:780–785. [DOI] [PubMed] [Google Scholar]
- 45. Pacifico L, Bonci E, Di Martino M et al. , . A double-blind, placebo-controlled randomized trial to evaluate the efficacy of docosahexaenoic acid supplementation on hepatic fat and associated cardiovascular risk factors in overweight children with nonalcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2015;25:734–741. [DOI] [PubMed] [Google Scholar]
- 46. Golabi P, Locklear CT, Austin P et al. , . Effectiveness of exercise in hepatic fat mobilization in non-alcoholic fatty liver disease: systematic review. World J Gastroenterol. 2016;22:6318–6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Keating S, Hackett D, George J et al. , . Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157–166. [DOI] [PubMed] [Google Scholar]
- 48. Keating SE, George J, Johnson NA.. The benefits of exercise for patients with non-alcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2015;9:1247–1250. [DOI] [PubMed] [Google Scholar]
- 49. Sullivan S, Kirk EP, Mittendorfer B et al. , . Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology. 2012;55:1738–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bacchi E, Negri C, Targher G et al. , . Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 randomized trial). Hepatology. 2013;58:1287–1295. [DOI] [PubMed] [Google Scholar]
- 51. Hannah WN Jr, Harrison SA.. Effect of weight loss, diet, exercise, and bariatric surgery on nonalcoholic fatty liver disease. Clin Liver Dis. 2016;20:339–350. [DOI] [PubMed] [Google Scholar]
- 52. Pugh CJ, Sprung VS, Jones H et al. , . Exercise-induced improvements in liver fat and endothelial function are not sustained 12 months following cessation of exercise supervision in nonalcoholic fatty liver disease. Int J Obes. 2016;40:1927–1930. [DOI] [PubMed] [Google Scholar]
- 53. Dudekula A, Rachakonda V, Shaik B et al. , . Weight loss in nonalcoholic fatty liver disease patients in an ambulatory care setting is largely unsuccessful but correlates with frequency of clinic visits. PLoS One. 2014;9:e111808. doi:10.1371/journal.pone.0111808 [7pp]. doi:10.1371/journal.pone.0111808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jegatheesan P, De Bandt JP.. Fructose and NAFLD: the multifaceted aspects of fructose metabolism. Nutrients. 2017;9:230. doi:10.3390/nu9030230 [13pp]. [Google Scholar]
- 55. Nobili V, Carpino G, Alisi A et al. , . Role of docosahexaenoic acid treatment in improving liver histology in pediatric nonalcoholic fatty liver disease. PLoS ONE. 2014;9:e88005. doi:10.1371/journal.pone.0088005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pacifico L, Martino MD, Catalano C et al. , . T1-weighted dual-echo MRI for fat quantification in pediatric nonalcoholic fatty liver disease. World J Gastroenterol. 2011;17:3012–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Szczepaniak LS, Nurenberg P, Leonard D et al. , . Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. [DOI] [PubMed] [Google Scholar]
- 58. Clark JM, Brancati FL, Diehl AM.. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–1657. [DOI] [PubMed] [Google Scholar]
- 59. Magalotti D, Marchesini G, Ramilli S et al. , . Splanchnic haemodynamics in non-alcoholic fatty liver disease: effect of a dietary/pharmacological treatment. A pilot study. Dig Liver Dis. 2004;36:406–411. [DOI] [PubMed] [Google Scholar]
- 60. Joy D, Scott BB.. To perform or not to perform liver biopsy: an alternative view [letter]. Gut. 2003;52:610.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Allard JP, Aghdassi E, Mohammed S et al. , . Nutritional assessment and hepatic fatty acid composition in non-alcoholic fatty liver disease (NAFLD): a cross-sectional study. J Hepatol. 2008;48:300–307. [DOI] [PubMed] [Google Scholar]
- 62. Araya J, Rodrigo R, Videla LA et al. , . Increase in long-chain polyunsaturated fatty acid n−6/n−3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin Sci. 2004;106:635–643. [DOI] [PubMed] [Google Scholar]
- 63. Elizondo A, Araya J, Rodrigo R et al. , . Polyunsaturated fatty acid pattern in liver and erythrocyte phospholipids from obese patients. Obesity (Silver Spring). 2007;15:24–31. [DOI] [PubMed] [Google Scholar]
- 64. Igarashi M, DeMar JC, Ma K et al. , . Upregulated liver conversion of α-linolenic acid to docosahexaenoic acid in rats on a 15 week n-3 PUFA-deficient diet. J Lipid Res. 2007;48:152–164. [DOI] [PubMed] [Google Scholar]
- 65. Igarashi M, Kim H-W, Gao F et al. , . Fifteen weeks of dietary n-3 polyunsaturated fatty acid deprivation increases turnover of n-6 docosapentaenoic acid in rat-brain phospholipids. Biochim Biophys Acta. 2012;1821:1235–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Moriguchi T, Loewke J, Garrison M et al. , . Reversal of docosahexaenoic acid deficiency in the rat brain, retina, liver, and serum. J Lipid Res. 2001;42:419–427. [PubMed] [Google Scholar]
- 67. Araya J, Rodrigo R, Pettinelli P et al. , . Decreased liver fatty acid Δ-6 and Δ-5 desaturase activity in obese patients. Obesity (Silver Spring). 2010;18:1460–1463. [DOI] [PubMed] [Google Scholar]
- 68. Park H, Hasegawa G, Shima T et al. , . The fatty acid composition of plasma cholesteryl esters and estimated desaturase activities in patients with nonalcoholic fatty liver disease and the effect of long-term ezetimibe therapy on these levels. Clin Chim Acta. 2010;411:1735–1740. [DOI] [PubMed] [Google Scholar]
- 69. Walle P, Takkunen M, Mannistö V et al. , . Fatty acid metabolism is altered in non-alcoholic steatohepatitis independent of obesity. Metabolism. 2016;65:655–666. [DOI] [PubMed] [Google Scholar]
- 70. Zheng J-S, Xu A, Huang T et al. , . Low docosahexaenoic acid content in plasma phospholipids is associated with increased non-alcoholic fatty liver disease in China. Lipids. 2012;47:549–556. [DOI] [PubMed] [Google Scholar]
- 71. Puri P, Wiest MM, Cheung O et al. , . The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology. 2009;50:1827–1838. [plus supplementary table]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Larqué E, Pérez-Llamas F, Puerta V et al. , . Dietary trans fatty acids affect docosahexaenoic acid concentrations in plasma and liver but not brain of pregnant and fetal rats. Pediatr Res. 2000;47:278–283. [DOI] [PubMed] [Google Scholar]
- 73. Vernekar M, Amarapurkar D, Joshi K et al. , . Gene polymorphisms of desaturase enzymes of polyunsaturated fatty acid metabolism and adiponutrin and the increased risk of nonalcoholic fatty liver disease. Meta Gene. 2017;11:152–156. [Google Scholar]
- 74. Kang JX, Wan J-B, He C.. Concise review: regulation of stem cell proliferation and differentiation by essential fatty acids and their metabolites. Stem Cells. 2014;32:1092–1098. [DOI] [PubMed] [Google Scholar]
- 75. James MJ, Gibson RA, Cleland LG.. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71(suppl 1):343S–348S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.