Abstract
In this observational study, ranolazine was associated with a statistically significant decrease in HbA1c among veterans with diabetes mellitus.
Diabetes mellitus (DM) is a risk factor for cardiovascular disease(CVD).1–4 Death rates from heart disease are 2- to 4-times higher among adults with DM compared with those of adults without DM. In the US, it is estimated that 21.1 million adults have diagnosed DM, 8.1 million adults have undiagnosed DM, and 80.8 million adults have prediabetes.3 The American Heart Association has identified an untreated fasting blood glucose level < 100 mg/dL as a component of ideal cardiovascular health.3
Although the use of antidiabetic agents has been shown to reduce the risks of micro-vascular complications among patients with DM, a cardiovascular benefit has not been consistently demonstrated with all available agents, and some used in the treatment of DM are associated with cardiovascular harm.5 In addition, some cardiovascular medications may contribute to the development of DM or may mask the symptoms of hypoglycemia.6 Given the risk for CVD among patients with DM, a medication with utility in both DM and CVD could be beneficial.
EVIDENCE FOR USE OF RANOLAZINE
Ranolazine is indicated for the treatment of chronic angina.7 In clinical trials, ranolazine also was found to decrease hemoglobin A1c (HbA1c).8–15 The possible mechanisms for lowering HbA1c with ranolazine include preservation of pancreatic β-cells and an increase in glucose-dependent insulin secretion. 6 The most common adverse effects associated with ranolazine include dizziness, headache, constipation, and nausea.7
Ranolazine has been shown to be efficacious and safe in the reduction of angina symptoms among patients with and without DM.8–12 In addition to improving symptoms of angina, studies have demonstrated a reduction in HbA1c among patients taking ranolazine.9,13–15 In an open-label extension of the Combination Assessment of Ranolazine in Stable Angina (CARISA) trial, ranolazine 750 mg twice daily and 1,000 mg twice daily led to a greater reduction in HbA1c when each was compared with placebo (−0.48% HbA1c, P = .008; and −0.70% HbA1c, P = .001, respectively).9
Among the 5,576 patients enrolled in the Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST-Elevation Acute Coronary Syndromes—Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) trial with a baseline HbA1c, ranolazine significantly reduced HbA1c at 4 months when compared with placebo among patients with and without DM.13 In addition, patients with DM who were treated with ranolazine were more likely to achieve a HbA1c < 7% at 4 months when compared with placebo (59% vs 49%; P < .001). Ranolazine was not found to increase the incidence of hypoglycemia.
A subgroup analysis of MERLIN-TIMI 36 evaluated the effects of ranolazine compared with those of placebo on fasting plasma glucose and HbA1c in patients with moderate DM (HbA1c ≥ 6% but < 8%, fasting plasma glucose < 250 mg/dL) and severe DM (A1c ≥ 8%, fasting plasma glucose 150–400 mg/dL).14 A significant reduction in HbA1c with ranolazine in addition to standard of care antidiabetes treatment was observed among both groups. The placebo-corrected decrease in HbA1c in the moderate DM group was 0.28% (95% confidence interval [CI] −0.55 to 0; P = .045) and in the severe DM group was 0.59% (95% CI −0.99 to −0.20; P < .001).
In a trial designed to evaluate change in HbA1c in patients taking ranolazine 1,000 mg twice daily compared with that of placebo, ranolazine led to a greater decrease in HbA1c compared with that of placebo (placebo corrected change in HbA1c −0.56%, P = .001).15 In addition, a higher percentage of patients achieved HbA1c < 7% at 24 weeks in the ranolazine group compared with that of placebo (41.2% vs 25.7%; P = .001). No patient experienced severe hypoglycemia or had documented hypoglycemia in this study.
These trials suggest that ranolazine, in addition to decreasing anginal events, is potentially beneficial in achieving better control of DM. However, more studies are needed to determine this benefit. In addition, no trials have examined the 500-mg twice daily dose of ranolazine in HbA1c reduction.
The purpose of this study was to evaluate the change in HbA1c among veterans with DM after the initiation of ranolazine. The study compared the percentage of veterans achieving HbA1c < 7% or < 8% after initiation of ranolazine with the baseline, to determine whether there is a dose-related change in HbA1c among different ranolazine regimens and to determine whether there is a change in the incidence of hypoglycemia after the initiation of ranolazine.
METHODS
This was a multicenter, retrospective study. The institutional review board and research and development committee for 3 Veterans Affairs medical centers (VAMCs) approved this study and waived informed consent. Additionally, this study was approved for access to national patient information through the Corporate Data Warehouse (CDW).
Subjects were eligible for inclusion in this study if they were aged ≥ 18 years, had a diagnosis of type 2 DM, and received their first prescription of ranolazine at a VAMC from January 1, 2008 through March 31, 2015. Exclusion criteria included subjects with no baseline HbA1c (defined as the HbA1c result closest to the ranolazine initiation date and within 90 days before to 14 days after ranolazine initiation), no follow-up HbA1c (defined as the first HbA1c result within 60 to 180 days after the ranolazine initiation date), any change to their DM medication regimen during the follow-up period, or who discontinued ranolazine prior to collection of the follow-up HbA1c.
Data were collected from the electronic health record (EHR) for each subject from 6 months prior to the ranolazine initiation date through 6 months after the ranolazine initiation date. The ranolazine initiation date was defined as the date ranolazine was picked up in person at a VAMC pharmacy or 7 days after the date filled for medications sent by mail. Progress notes, laboratory values, and pharmacy records were evaluated for this time frame, and the following data were collected: ranolazine dose and initiation date, ranolazine possession ratio (total numbers of days patient was in possession of ranolazine between initiation date and follow-up HbA1c divided by total number of days between ranolazine initiation date and follow-up HbA1c), baseline HbA1c, follow-up HbA1c, hypoglycemia incidence before and after the initiation of ranolazine, concomitant DM medications and interacting medications, patient age and sex, and creatinine clearance at baseline.
The primary endpoint of this study was the change in HbA1c after ranolazine initiation. The secondary endpoint was the percentage of study subjects achieving HbA1c < 7% and < 8% before and after the initiation of ranolazine.
To achieve 80% power to detect a change in HbA1c of 0.4%, a sample size of 52 patients was required. For the primary endpoint, a paired t test was used to test for statistical significance. The McNemar test was used to evaluate for a significant change in subjects achieving an HbA1c < 7% and HbA1c < 8% with the initiation of ranolazine.
RESULTS
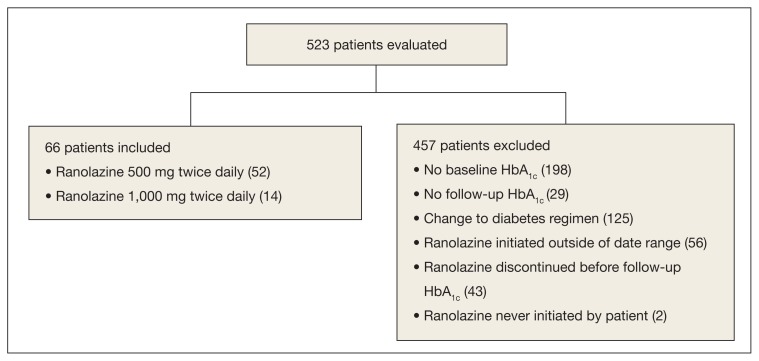
A total of 523 patients were evaluated for study inclusion, of which 66 patients were included (Figure). The most common reasons for exclusion included no HbA1c at baseline and changes to the DM medication regimen during follow-up. At baseline, the average age was 73.4 years, the patient population was 100% male, patients took an average of 1.3 antihyperglycemic agents at baseline, and the average HbA1c was 6.9%. About 80% of patients were prescribed ranolazine at a dose of 500 mg twice daily (Table 1).
Figure.
Inclusion and Exclusion Criteria
Abbreviation: HbA1c, hemaglobin A1c.
Table 1.
Baseline Characteristics (n = 66)
| Variable | Total |
|---|---|
| Age, mean (SD), y | 73.4 (9.3) |
|
| |
| Male, No. (%) | 66 (100) |
|
| |
| HbA1c, mean (SD), % | 6.9 (0.9) |
|
| |
| Diabetes mellitus agents, No. (%) | |
| Average No. of agents | 1.3 |
| Metformin | 33 (50) |
| Sulfonylurea | 24 (36.4) |
| DPP-4 inhibitor | 0 (0) |
| GLP-1 agonist | 0 (0) |
| Thiazolidinedione | 1 (1.5) |
| Insulin glargine | 3 (4.5) |
| Insulin detemir | 0 (0) |
| Insulin NPH | 15 (22.7) |
| Insulin 70/30 | 5 (7.6) |
| Insulin regular | 5 (7.6) |
| Insulin aspart | 3 (4.5) |
|
| |
| Interacting medication (diltiazem, erythromycin, fluconazole, verapamil), No. (%) | 0 (0) |
|
| |
| Ranolazine dose, No. (%) | |
| 500 mg twice daily | 52 (78.8) |
| 1,000 mg twice daily | 14 (21.2) |
|
| |
| Ranolazine possession ratio, %a | 80.4 |
Abbreviations: DPP-4, dipeptidyl pedtidase-4; GLP-1, glucagon-like peptide-1; HbA1C, hemoglobin A1C; NPH, neutral protomine Hagedorn.
Total number of days patient was in possession of ranolazine between initiation date and follow-up HbA1c ÷ total number of days between ranolazine initiation date and follow-up HbA1c.
Table 2.
Change in HbA1c
| Baseline, % | Follow-up, % | Change in HbA1c, % | P Value | |
|---|---|---|---|---|
| All doses ranolazine (n = 66) | 6.9 | 6.6 | −0.3 | < .001 |
| Ranolazine 500 mg twice daily (n = 52) | 6.9 | 6.6 | −0.3 | .001 |
| Ranolazine 1,000 mg twice daily (n = 14) | 6.9 | 6.5 | −0.4 | .09 |
Abbreiviation: HbA1c, hemoglobin A1c.
Table 3.
HbA1c Goals Achieved
| Variable | Baseline (Pre-Ranolazine Initiation), No. (%) | Follow-up (Post-Ranolazine Initiation), No. (%) | P Value |
|---|---|---|---|
| All doses ranolazine (n = 66) | |||
| HbA1c < 7% | 30 (45.5) | 49 (74.2) | < .001 |
| HbA1c < 8% | 56 (84.8) | 60 (90.9) | .221 |
|
| |||
| Ranolazine 500 mg twice daily (n = 52) | |||
| HbA1c < 7% | 22 (42.3) | 38 (73.1) | .001 |
| HbA1c < 8% | 43 (82.7) | 47 (90.4) | .371 |
|
| |||
| Ranolazine 1,000 mg twice daily (n = 14) | |||
| HbA1c < 7% | 8 (57.1) | 11 (78.6) | .248 |
| HbA1c < 8% | 13 (92.8) | 13 (92.8) | NS |
Abbreiviations: HbA1c, hemoglobin A1c; NS, not significant.
Ranolazine at any dose was associated with a change in HbA1c of −0.3% (P < .001). In addition, the percentage of veterans achieving HbA1c < 7% was significantly higher after the initiation of ranolazine (P < .001). More veterans achieved HbA1c < 8% after the initiation of ranolazine, although this result was not statistically significant (P = .22).
A dose of 500 mg ranolazine twice daily also was associated with a significant decrease in HbA1c by 0.3% (P = .001). A significant increase in veterans achieving HbA1c < 7% after ranolazine initiation was observed (42.3% before ranolazine initiation vs 73.1% after ranolazine initiation; P = .001), and a nonsignificant increase in veterans achieving HbA1c < 8% was observed (82.7% before ranolazine initiation vs 90.4% after ranolazine initiation, P = .37).
At a dose of 1,000 mg twice daily, a 0.4% decrease in HbA1c was observed. However, this result was not found to be statistically significant (P = .09), and the study was underpowered to detect a significant change in HbA1c at this dose. A nonsignificant increase in veterans achieving HbA1c < 7% was observed after ranolazine initiation (57.1% before ranolazine initiation vs 78.6% after ranolazine initiation, P = .25), but no difference was found in veterans achieving HbA1c < 8%.
Hypoglycemia was not reported in a majority of study patient progress notes; thus, it was not evaluated further.
DISCUSSION
In this study of a veteran population, ranolazine was associated with an HbA1c decrease of 0.3%. This change is less than that observed in previous studies, which may be related to a lower baseline HbA1c for the patients in this study. In addition, a greater percentage of veterans achieved an HbA1c < 7% after initiation of ranolazine compared with that of the baseline.
To the authors’ knowledge, this is the first study evaluating ranolazine and HbA1c in a veteran population. It also is the first study to demonstrate an association between HbA1c lowering and ranolazine at a dose of 500 mg twice daily. These results suggest that in patients with chronic angina and type 2 DM, ranolazine could potentially play a dual role in therapy.
Limitations
The authors recognize several limitations in this study. Given its observational design, it cannot be definitively concluded that the decrease in HbA1c was due to the initiation of ranolazine. While excluding patients with changes to their antidiabetic medication regimen was done in an effort to minimize confounding factors, it is possible that other factors, such as lifestyle, also could explain changes in HbA1c. It is possible that changes to the DM medication regimen were made but not documented in the EHR. In addition, information on hypoglycemia was not readily available; thus, the safety of ranolazine among patients with DM could not be evaluated fully. Finally, the patient population characteristics may limit external validity.
CONCLUSION
In this observational study, ranolazine was associated with a statistically significant decrease in HbA1c among veterans with DM, which supports previously published literature. 9, 13–15 However, no randomized controlled trials have been performed specifically studying the impact of ranolazine on HbA1c among patient with DM. Ideally, future prospective, randomized placebo-controlled studies will take place to further evaluate the association between ranolazine use and HbA1c lowering.
Footnotes
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
REFERENCES
- 1.Kannel WB, McGee DL. Diabetes and cardiovascular disease—the Framingham study. JAMA. 1979;241(19):2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 2.Selvin E, Coresh J, Golden SH, Boland LL, Brancati FL, Steffes MW Atherosclerosis risk in communities study. Glycemic control, atherosclerosis, and risk factors for cardiovascular disease in individuals with diabetes: the atherosclerosis risk in communities study. Diabetes Care. 2005;28(8):1965–1973. doi: 10.2337/diacare.28.8.1965. [DOI] [PubMed] [Google Scholar]
- 3.Writing Group Members; Mozaffarian D, Benjamion EJ, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 4.Conaway DG, O’Keefe JH, Reid KJ, Spertus J. Frequency of undiagnosed diabetes mellitus in patients with acute coronary syndrome. Am J Cardiol. 2005;96(3):363–365. doi: 10.1016/j.amjcard.2005.03.076. [DOI] [PubMed] [Google Scholar]
- 5.Hiatt WR, Kaul S, Smith RJ. The cardiovascular safety of diabetes drugs—insights from the rosiglitazone experience. N Engl J Med. 2013;369(14):1285–1287. doi: 10.1056/NEJMp1309610. [DOI] [PubMed] [Google Scholar]
- 6.Ning Y, Zhen W, Fu Z, et al. Ranolazine increases β-cell survival and improves glucose homeostasis in low-dose streptozotocin-induced diabetes in mice. J Pharmacol Exp Ther. 2011;337(1):50–58. doi: 10.1124/jpet.110.176396. [DOI] [PubMed] [Google Scholar]
- 7.Ranexa [package insert] Foster City, CA: Gilead Sciences Inc; 2016. [Google Scholar]
- 8.Chaitman BR, Pepine CJ, Parker JO, et al. Combination Assessment of Ranolazine In Stable Angina (CARISA) Investigators. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA. 2004;291(3):309–316. doi: 10.1001/jama.291.3.309. [DOI] [PubMed] [Google Scholar]
- 9.Timmis AD, Chaitman BR, Crager M. Effects of ranolazine on exercise tolerance and HbA1c in patients with chronic angina and diabetes. Eur Heart J. 2006;27(1):42–48. doi: 10.1093/eurheartj/ehi495. [DOI] [PubMed] [Google Scholar]
- 10.Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, et al. MERLIN-TIMI 36 Trial Investigators. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA. 2007;297(16):1775–1783. doi: 10.1001/jama.297.16.1775. [DOI] [PubMed] [Google Scholar]
- 11.Kosiborod M, Arnold SV, Spertus JA, et al. Evaluation of ranolazine in patients with type 2 diabetes mellitus and chronic stable angina: results from the TERISA randomized clinical trial (Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina) J Am Coll Cardiol. 2013;61(20):2038–2045. doi: 10.1016/j.jacc.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Arnold SV, McGuire DK, Spertus JA, et al. Effectiveness of ranolazine in patients with type 2 diabetes mellitus and chronic stable angina according to baseline hemoglobin A1c. Am Heart J. 2014;168(4):457–465.e2. doi: 10.1016/j.ahj.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 13.Morrow DA, Scirica BM, Chaitman BR, et al. MERLIN-TIMI 36 Trial Investigators. Evaluation of the glyco-metabolic effects of ranolazine patients with and without diabetes mellitus in the MERLIN-TIMI 36 randomized controlled trial. Circulation. 2009;119(15):2032–2039. doi: 10.1161/CIRCULATIONAHA.107.763912. [DOI] [PubMed] [Google Scholar]
- 14.Chisholm JW, Goldfine AB, Dhalla AK, et al. Effect of ranolazine on A1c and glucose levels in hyperglycemic patients with non-ST elevation acute coronary syndrome. Diabetes Care. 2010;33(6):1163–1168. doi: 10.2337/dc09-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckel RH, Henry RR, Yue P, et al. Effect of ranolazine monotherapy on glycemic control in subjects with type 2 diabetes. Diabetes Care. 2015;38(7):1189–1196. doi: 10.2337/dc14-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]