Abstract
The Hoatzin (Opisthocomus hoazin) is the only extant member of the order Opisthocomiformes. This unique South American bird lives in the riparian lowland vegetation characteristic of the Amazon and Orinoco basins. Hoatzins nest in communal social units close to water bodies; they are strictly folivores being the only bird with pregastric fermentation in the crop. Because of the complex logistics involved in capturing this bird, there is a knowledge gap on its parasites. This study documents two distant lineages of haemosporidian parasites (Plasmodium spp.) in a juvenile and two adults sampled in the Cojedes state, Venezuela. Although negative by microscopy, the parasite identification was possible by using molecular methods. We estimated the phylogenetic relationships on the parasite cytochrome b (cytb, 480 bp) gene and the mitochondrial DNA. We found one of the parasites lineages in two individuals (nestling and adult), and the corresponding fragment of cytb was identical to a one found in Wood Stork (Mycteria americana) from Brazil. The other lineage, found in an adult, has an identity of 469 out of 478 bp (98%) with Plasmodium sp. GAL-2012 (isolate THAMB08) from Brazil. Although a morphological description of these parasites was not possible, this is the first molecular study focusing on Hoatzin haemosporidian parasites and the first documentation of Plasmodium infections in the Hoatzin from Venezuela. Furthermore, we reported microfilaria in two adults as well as hematological parameters for six individuals. Information on hematological parameters could contribute to establishing the necessary baseline to detect underlying conditions, such as infections, in this bird species.
Keywords: Cytochrome b, Avian malaria, Erythrocyte measurements, Microfilaria, Differential white blood cell, Phylogeny
Introduction
The Hoatzin (Opisthocomus hoazin), the only extant species of the Order Opisthocomiformes, is a unique bird native to the Orinoco and the Amazon basins in South America that includes Bolivia, Colombia, Ecuador, Peru, Venezuela, Brazil, and the lowlands of the Guianas. Little is known about its ecology and evolution. The lack of information is due in part to difficulties accessing its habitat, as well as the behavior and size of these birds that makes them hard to capture. In Venezuela, this species is distributed along rivers of the central savannas to the eastern Orinoco River. They live in colonies in riverine and swamp forest, vegetation at edges of ponds, oxbow lakes, and other freshwater wetlands. It is folivore bird that feeds on riverine tree species, and nests in communal social units building their nest close to water bodies (Thomas, 1996). Its phylogenetic relationship remains an enigma (Jarvis et al., 2014; Claramunt & Cracraft, 2015; Prum et al., 2015). However, given that is the only bird with pregastric fermentation in the crop like that in ruminants (Grajal et al., 1989), most of the knowledge about this species is on the crop microorganism’s community structure and ecology (e.g., Godoy-Vitorino et al., 2010; Godoy-Vitorino et al., 2012; Bardele et al., 2017). Indeed, there are only a few studies on ectoparasites (Hernandes & Mironov, 2015; Bauchan et al., 2017) and hemoparasite infections in the Hoatzin (Renjifo, Sanmartin & De Zuleta, 1952; Gabaldon, 1998). Only filarial parasite infections have been reported in the Hoatzin from Colombia (Renjifo, Sanmartin & De Zuleta, 1952), and no haemosporidian parasites had been found before in this species using blood films (Renjifo, Sanmartin & De Zuleta, 1952; Gabaldon, 1998).
In this study, we report two distant molecular lineages of haemosporidian parasites of the genus Plasmodium (Family Plasmodiidae, Order Haemosporida, Phylum Apicomplexa) found in the Hoatzin from the Cojedes River, a tributary of the Orinoco River in central Venezuela that is part of the Orinoquia. Plasmodiidae is a diverse group of vector-borne haemoparasites found in many terrestrial vertebrate hosts (Garnham, 1966; Valkiūnas, 2005; Telford Jr, 2009), including the species of human (Cavalier-Smith, 2014) and avian malaria (e.g., Plasmodium relictum; Bensch, Hellgren & Pérez-Tris, 2009; Atkinson & Samuel, 2010). This is the first evidence of Plasmodium infections in the Hoatzin in South America; additionally, we provided hematological parameters to generate information that may help to assess the health status of this bird species.
Material and Methods
Study area and samples
We caught eight individuals in El Baúl massif along the Cojedes River near the town of El Baúl located at the southwestern of the Cojedes state in the northcentral Venezuela. This is considered part of the Orinoquia region at the north of the Guyana Shield. El Baúl massif is about 720 km2; it is relatively isolated and mountainous with steep topography following a northwest-southeast trend (Viscarret, Wright & Urbani, 2009). The vegetation is typical of the Orinoquia sedimentary and alluvial plains (e.g., vegetation of savannas, gallery forests, palm groves, and semi-deciduous forests). In the context of this study, the birds were captured in the gallery forest, which is moderately intervened and subject to seasonal flooding from the Cojedes River as well as forest fires (González-Fernández et al., 2007).
We trapped two individuals (one adult and one juvenile) in October 2010, and 6 individuals (two adult and four nestlings) in August 2015 in their nest during nighttime using butterfly nets and transported to the field laboratory. From each bird, we obtained blood samples by brachial vein puncture, and then, immediately we prepared three to five thin smears. We preserved the rest of the sample in protein saver cards (Whatman 903, Whatman™, Cardiff, UK) for molecular analysis. We collected the specimens under permit number 0950 issued by the Venezuelan government (Oficina Nacional de Diversidad Biológica, Ministerio del Poder Popular para Ecosocialismo, Hábitat y Vivienda). All the animal protocols were approved by the ethics committees of Instituto Venezolano de Investigaciones Científicas (IVIC, Venezuela) under the number COBIANIM Dir-0885/1517/2014.
Examination of blood films
Smears were air-dried immediately after preparation, fixed in absolute methanol for 5 min, and then stained with Giemsa (pH 7.2) for 45 min. Using a Leica DM750e microscope (Leica Microsystems, Heerbrugg, Switzerland), we first examined blood slides at ×400 for 10 min and then at ×1,000 for 20 min. For the capture of digital images, we scanned entirety those slides with hemoparasites using a Leica EC3 digital camera and processed with the LAS EZ (Leica Microsystems Suiza Limited, 2012). Then, we estimated the intensity of infection as No. of parasites/10,000 erythrocytes from erythrocyte counts with an increase of ×1,000, focusing on areas where blood cells formed a monolayer (Muñoz et al., 1999). In addition, using ImageJ software (Schneider, Rasband & Eliceiri, 2012), we performed morphometric analyses of the erythrocytes. We measured the maximum cell width and length, as well as nuclear width and length for 30 erythrocytes per slide and per individual, following Hartman & Lessler (1963). To estimate the percentage of each type of white blood cell present in blood, we also measured a differential white blood cell (WBC) count per 100 cells using the blood samples collected in 2015 following the protocol by Clark, Boardman & Raidal (2009). We obtained all these measures for only those individuals caught in 2015 (N = 6) because of the better quality of their blood films.
Molecular diagnostic of haemosporidian parasites
We extracted genomic DNA from whole blood using QIAamp® DNA Micro Kit (Qiagen GmbH, Hilden, Germany). We screened each sample for haemosporidian parasites by using a nested polymerase chain reaction (PCR) protocol that targets the parasite mitochondrial cytochrome b (cytb, 1,131 bp) gene using the primers described in Pacheco et al. (2011); Pacheco et al. (2018). The cytb external primers were forward AE298 5′-TGT AAT GCC TAG ACG TAT TCC 3′ and reverse AE299 5′-GT CAA WCA AAC ATG AAT ATA GAC 3′, and the internal primers were forward AE064 5′-T CTA TTA ATT TAG YWA AAG CAC 3′ and reverse AE066 5′-G CTT GGG AGC TGT AAT CAT AAT 3′. The primary PCR amplifications were carried out in 50 µl volume reaction using 5-8µl of total genomic DNA, 2.5 mM MgCl2, 1 × PCR buffer, 1.25 mM of each deoxynucleoside triphosphate, 0.4 mM of each primer, and 0.03 U/µl AmpliTaq polymerase (Applied Biosystems, Roche-USA). The primary PCR conditions were: A partial denaturation at 94 °C for 4 min and 36 cycles with 1 min at 94 °C, 1 min at 53 °C and 2 min extension at 72 °C, and we added a final extension of 10 min at 72 °C in the last cycle. Then, the nested PCRs were also made in 50 µl volume reaction using only 1 µl of the primary PCRs, 2.5 mM MgCl2, 1 × PCR buffer, 1.25 mM of each deoxynucleoside triphosphate, 0.4 mM of each primer, and 0.03 U/µl AmpliTaq polymerase. The nested PCR conditions were: A partial denaturation at 94 °C for 4 min and 25 cycles with 1 min at 94 °C, 1 min at 56 °C and 2 min extension at 72 °C, and we also added a final extension of 10 min at 72 °C in the last cycle. Both strands for all the cytb fragments were directly sequenced using an Applied Biosystems 3730 capillary sequencer. We identified all the cytb fragments obtained here as Plasmodium using BLAST (Altschul et al., 1997).
For those samples that were positive using the cytb PCR protocol, we amplified between 5,515 to 5,838 bp of the parasite mitochondrial genomes (mtDNA) using a nested PCR with Takara LA Taq™ Polymerase (TaKaRa Takara Mirus Bio) following manufacturers’ directions. This fragment of the mtDNA included the three nonprotein coding regions between the ORFs (fragmented SSU rRNA and LSU rRNA) and the three protein-coding genes (Cox3, Cox1 and Cytb) so only three tRNAs (7, 11, and 14) and two fragments of small subunit ribosomal RNAs (5 and 7) are missing. Oligos forward AE170 5′ GAGGATTCTCTCCACACTT CAATTCGTACTTC 3′ and reverse AE171 5′ CAGGAAAATWA TAGACCGAACCTTGGACTC 3′ were used for the primary PCR and internal oligos forward AE176 5′ TTTCATCCTTAAATCTCGTAAC 3′ and AE136 reverse 5′ GACCGAA CCTTGGACTCTT 3′ for the inner PCR. The PCR conditions were a partial denaturation at 94 °C for 1 min and 30 cycles with 30 s at 94 °C and 7 min at 68 °C and a final extension of 10 min at 72 °C. Then, we excised two independent PCR products (50 ul) from the gel (bands of approximately 6 kbp) and purified using QIAquick® Gel extraction kit (Qiagen, GmbH, Hilden, Germany). We cloned at least two independent PCR products using pGEM®-T Easy Vector Systems (Promega, Madison, WI, USA), and we sequenced four clones from each individual. We sequenced both strands for PCR products and clones using an Applied Biosystems 3730 capillary sequencer. Given that the cytb partial sequences and the cytb gene from the mtDNA genome were 100% identical for each sample, we only deposited the mtDNA genome sequences in GenBank under the accession numbers KY653749 to KY653751.
Phylogenetic analysis of the cytb fragment and mtDNA genome
We performed two different nucleotide alignments by using ClustalX v2.0.12 and Muscle as implemented in SeaView v4.3.5 (Gouy, Guindon & Gascuel, 2010) with manual editing. The first alignment was constructed with 45 cytb partial sequences (480 bp) belonging to three genera (Leucocytozoon, Haemoproteus, and Plasmodium). This alignment included the sequences obtained in this study as well as sequences from well-known parasite species based on morphology (Valkiūnas & Iezhova, 2018) that were available in GenBank (Benson et al., 2012) and MalAvi (Bensch, Hellgren & Pérez-Tris, 2009) databases at the time of this study. Sequences that showed a similarity >95% using BLAST (Altschul et al., 1997) were also included even when they are not clearly linked to the described species. The second alignment (5,286 bp excluding gaps) was done using 31 mtDNA genome sequences belonging also to the three genera, including the sequences obtained in this study and sequences from well-known parasite species (using morphology) available in GenBank. Subsequently, the alignment was divided into six partitions corresponding to the three non-protein coding regions between the ORFs (fragmented SSU rRNA and LSU rRNA) and the three protein-coding genes, keeping their order in the mtDNA genome (nonprotein coding, cox3, nonprotein coding, cox1, cytb, nonprotein coding).
Then, we inferred phylogenetic hypotheses based on the first (partial cytb gene) and second (mtDNA genome) alignments using the Bayesian methods implemented in MrBayes v3.2.6 with the default priors (Ronquist & Huelsenbeck, 2003). To estimate the phylogenetic hypothesis that best fit the data, we used the general time reversible model with gamma-distributed substitution rates and a proportion of invariant sites (GTR +Γ + I) on the cytb alignment and for each partition in the mtDNA genome alignment. This model was the one with the lowest Bayesian Information Criterion (BIC) scores for both alignments and each partition as estimated by MEGA v7.0.14 (Kumar, Stecher & Tamura, 2016). We inferred Bayesian support for the nodes in MrBayes by sampling every 1,000 generations from two independent chains lasting 4 × 106 Markov Chain Monte Carlo (MCMC) steps. The chains were assumed to have converged once the value of the potential scale reduction factor (PSRF) was between 1.00 and 1.02 and the average SD of the posterior probability was < 0.01 (Ronquist & Huelsenbeck, 2003). Then, we discarded 25% of the sample once convergence was reached as a “burn-in”. For both phylogenies, we used Leucocytozoon species as out-group. Genbank accession numbers for all sequences used in the analyses are given in the phylogenetic trees.
Results
In this study, one sample was positive for haemosporidian parasites by microscopy (adult caught in 2015). The individual had only Plasmodium sp. trophozoites in its blood films (Fig. 1), so a morphological description of this parasite was not possible. Indeed, the parasitemia was very low (<0.01), consistent with a subpatent infection. In addition, the two adults caught in 2015 were infected with microfilariae, so one of the adults has a coinfection of filarial parasites and Plasmodium sp. (Fig. 1). Unfortunately, the identification of nematode microfilariae at species level is difficult given their high degree of morphological and morphometric similarities (McKeand, 1998). None of the nestlings were infected with hemoparasites. Morphometry of the uninfected erythrocytes is shown in Table 1. We compared these measurements with those from the erythrocytes of other avian species with similar body size. In addition, we provided differential white blood cell (WBC) count profiles in Table 2.
Figure 1. Microfilaria and Plasmodium sp. blood stage infecting the Hoatzins (O. hoazin) caught in August 2015 at the Cojedes River, Venezuela.

(A) Hoatzin individual infected only with filarial parasites. (B*), (C*), and (D&) Hoatzin individual infected with filarial parasites and Plasmodium sp. *Microfilarial stages of the filarial parasite (Scale bar = 20 µm) and &Plasmodium sp. trophozoite (Scale bar = 10 µm). We indicated the hemoparasite stages by the black arrows.
Table 1. Comparison of erythrocyte measurements from different bird orders including the values for Hoatzin.
The values for Hoatzin (O. hoazin) are from six individuals caught in August 2015 at the Cojedes River, Venezuela.
| Cytosome | Nucleus | |||||
|---|---|---|---|---|---|---|
| Length (µm) | Width (µm) | Ratio (L/W) | Length (µm) | Width (µm) | Ratio (L/W) | |
| Opisthocomiformes | ||||||
| O. hoazin | 15.3 ± 0.85 | 8.7 ± 0.71 | 1.76 | 6.2 ± 0.61 | 3.4 ± 0.47 | 1.82 |
| Gruiformesa | ||||||
| Rallus elegans | 14.5 ± 0.32 | 7.7 ± 0.17 | 1.89 | 5.7 ± 0.13 | 2.9 ± 0.12 | 1.97 |
| Aramides cajanea | 12.9 ± 0.78 | 7.2 ± 0.50 | 1.79 | 5.5 ± 0.40 | 3.3 ± 0.37 | 1.67 |
| Fulica americana | 11.4 ± 0.26 | 7.5 ± 0.22 | 1.51 | 4.2 ± 0.06 | 2.3 ± 0.07 | 1.83 |
| Charadriiformesa | ||||||
| Jacana spinosa | 13.7 ± 0.79 | 7.5 ± 0.52 | 1.83 | 5.9 ± 0.64 | 3.7 ± 0.33 | 1.59 |
| Charadrius wilsonia | 12.8 ± 0.27 | 7.3 ± 0.13 | 1.76 | 5.8 ± 0.11 | 2.4 ± 0.05 | 2.41 |
| Himantopus mexicanus | 12.8 ± 0.16 | 6.9 ± 0.18 | 1.85 | 5.8 ± 0.20 | 2.5 ± 0.99 | 2.32 |
| Accipitriformesa | ||||||
| Accipiter cooperii | 14.3 ± 0.27 | 8.1 ± 0.13 | 1.77 | 6.2 ± 0.14 | 2.4 ± 0.11 | 2.58 |
| Buteo platypterus | 13.4 ± 0.51 | 7.6 ± 0.34 | 1.77 | 6.2 ± 0.31 | 3.0 ± 0.32 | 2.07 |
| Rupornis magnirostrisb | 13.1 ± 0.72 | 7.4 ± 0.36 | 1.78 | 6.83 ± 0.38 | 2.84 ± 0.18 | 2.4 |
Table 2. Differential white blood cell (WBC) counts and H:L ratio in the Hoatzins (N = 6) caught in August 2015 at the Cojedes River, Venezuela.
| Uninfected nestlings | Infected adults | ||
|---|---|---|---|
| (N = 4) | microfilaria (N = 1) | microfilaria/Plasmodium (N = 1) | |
| Heterophils | 64.25 ± 2.75 | 67 | 53 |
| Lymphocytes | 27 ± 1.83 | 17 | 35 |
| Monocytes | 5 ± 0.82 | 8 | 5 |
| Eosinophils | 3.25 ± 0.5 | 7 | 5 |
| Basophils | 0.5 ± 0.58 | 1 | 1 |
| H:L | 2.4 | 3.9 | 1.5 |
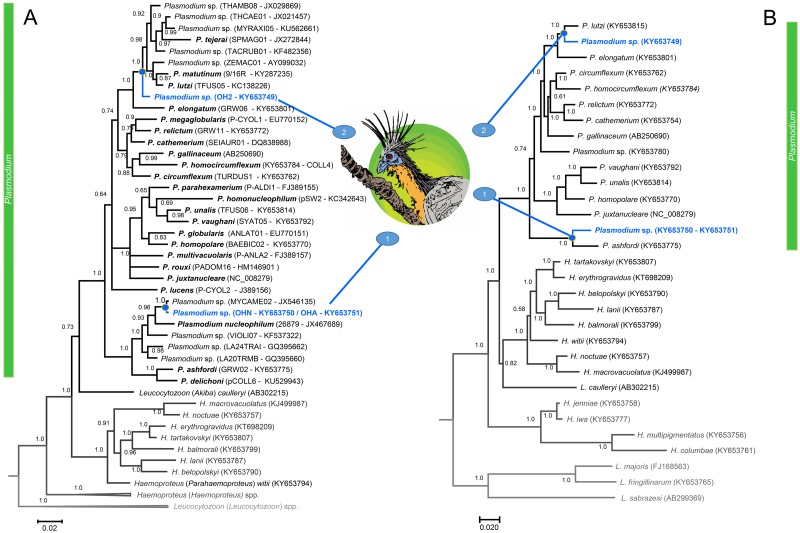
The molecular diagnostic detected that the two individuals caught in 2010 (one adult and one juvenile) and one of the adults caught in 2015 (the one positive by microscopy) were positive by nested PCR (3/8, 37.5%). In order to characterize these Plasmodium species, for those individuals (N = 3) that were positive by nested PCR, we obtained the parasite mtDNA genome sequences. We further examined these sequences by using phylogenetic analyses (Fig. 2) yielding two estimated gene trees, one just with partial cytb sequences commonly used to identify haemosporidia parasites (Bensch, Hellgren & Pérez-Tris, 2009; Pacheco et al., 2018) and the other with mtDNA genome (Pacheco et al., 2018). These phylogenies have similar topologies. We found two lineages of Plasmodium (identified as 1 and 2 in Fig. 2) in three individuals of Hoatzin. The individuals caught in 2010 (one adult and one juvenile) were infected with the same Plasmodium lineage (KY653750–KY653751), and their mtDNA genome sequences were 100% identical.
Figure 2. A Bayesian phylogenetic hypothesis of Plasmodium parasites infecting the Hoatzins (O. hoazin) caught at the Cojedes River, Venezuela.
We constructed phylogenetic trees based on parasites (A) partial sequences of the cytb gene (45 sequences and 480 bp excluding gaps) and mtDNA genomes (31 sequences and 5286 bp excluding gaps). The values above branches are posterior probabilities (see “Material and methods”). Leucocytozoon genus (outgroup) is indicated in grey. We provided in parentheses both lineages (as deposited in the MalAvi database) and their Genbank accession numbers for all the sequences used in the analyses.
Discussion
This is the first report of Plasmodium species in this bird and the first record of microfilarias in Hoatzins from Venezuela. We have no sign of pathogenesis associated with these infections. However, as numbers of heterophils and lymphocytes could be affected by stress such as a parasitic infection, the ratio of one to the other (H:L) is commonly used as a stress indicator (Gross & Siegel, 1983; Maxwell, 1993). Thus, we reported the H:L ratio since it is expected to increase in response to stressors such as infectious diseases (Davis, Maney & Maerz, 2008), especially in birds with high parasitemia (Granthon & Williams, 2017). Here, the bird infected with filarial parasites and Plasmodium sp. had an increase in the number of lymphocytes and a decrease in the number of heterophils in comparation with the haemosporidian negative birds (infected only with microfilaria and uninfected individuals), and so a lower H:L ratio (1.5 vs. 2.4, Table 2). The opposite occurred in the individual infected only with filarial parasites (H:L = 3.9 vs. 2.4, Table 2). WBC counts are difficult to interpret within and between species (e.g., Ricklefs & Sheldon, 2007) so these results should be taken with caution especially considering that these are a few individuals from a small sample. Considering the paucity of data from this species, we provided the WBC count measures for comparison in future studies.
It has been hypothesized an association between erythrocyte size and the species body size as result of differences in their metabolic rates (Hartman & Lessler, 1963). As expected, we found that erythrocyte size from Hoatzins was comparable to those reported from putative sister taxa like Gruiiformes (Jarvis et al., 2014; Claramunt & Cracraft, 2015), Charadriiformes (Claramunt & Cracraft, 2015), and Accipitriformes (Prum et al., 2015) (Table 1). Furthermore, differential white blood cell (WBC) count profiles (Table 2) from non-infected individuals showed marked similitude with those reported in nonpasserine birds such as cranes, raptors, and vultures (Davis, 2009).
Wherever there are subpatent infections, it is difficult to determine the prevalence of haemosporidian parasites since those infections are usually submicroscopic. In such cases, parasites can only be detected by polymerase chain reaction using genes with high copy number like cytb (Pacheco et al., 2018). The molecular diagnostic detected two lineages of Plasmodium genus (Fig. 2) in these three individuals of Hoatzin. Interesting, the lineage found in the individuals caught in 2010 (one adult and one juvenile) has been only reported so far in Brazil (Villar et al., 2013; Ferreira Jr et al., 2017; Tostes et al., 2017) suggesting that it is distributed solely in South America. In particular, its partial cytb sequence match 100% with a parasite lineage reported in several bird species: MYCAME02 isolated from Wood Storks nestlings found in the northern region of Brazil (Mycteria Americana, Pelecaniformes)(Villar et al., 2013), H2 isolate from Streaked Flycatcher found in Southeastern Brazil (Myiodynastes maculatus, Passeriformes) (Ferreira Jr et al., 2017), and lineages reported from birds belonging to the orders Strigiformes, Accipitriformes, and Falconiformes kept in captivity in Southeaster Brazil (Tostes et al., 2017). In the phylogenetic analysis, this lineage appears as the sister taxon of Plasmodium nucleophilum, parasite isolated from an Egyptian Goose in São Paulo Zoo, Brazil (Chagas et al., 2013). Both parasites, the lineage reported here and P. nucleophilum, are part of a monophyletic group that includes Plasmodium ashfordi and Plasmodium delichoni. This monophyletic group is at the base of the mitochondrial phylogeny of the known Avian Plasmodium parasites. Given the lack of gametocyte data, it is possible that the Plasmodium infections in these two Hoatzin individuals were abortive, but the fact that the two individuals (caught together) were infected with the same parasite suggests that its transmission is occurring, and the parasite lineage is circulating in Cojedes State, Venezuela. Tostes et al. (2017) re-described the parasite linked to this cytb lineage as Plasmodium (Novyella) paranucloephilum, a species originally described by Manwell & Sessler (1971) in a South American tanager of uncertain species likely from northern Brazil. However, given the absence of morphological data in this study, and that there is no cytb neither other mtDNA genome sequences belonging to the original parasite description, we consider that it is premature to identify the lineage found in this study as P. paranucloephilum. The identity of this lineage could be established when more samples from birds with high parasitemia of this haemosporidian parasite become available.
Regarding the second lineage found infecting hoatzin (KY653749, Fig. 2), it appears as the common ancestor of a clade that includes Plasmodium tejerai, Plasmodium matutinum and Plasmodium lutzi. This clade forms a monophyletic group with P. elongatum (Fig. 2); one of the most pathogenic and generalist avian malaria parasite worldwide (Palinauskas et al., 2016). Given that we did not find any sequence with 100% similarity with our lineage in the available databases, this result indicates that likely a new parasite is circulating in the area. Considering the difficulty of catching this bird species, it is worth noticing that even with this small sample size (only eight individuals including four nestlings) we found two Plasmodium lineages infecting the Hoatzins.
Riparian zones from the Orinoco and Amazon basins are considered important in terms of their biodiversity since they result from variable flood regimes, geographically unique channel processes, altitudinal climate shifts, and upland influences on fluvial corridors. Furthermore, these areas are treated as critical habitat to several endangered bird species, refuges to the fauna inhabiting adjacent areas and, in some cases, hotspots and corridors for bird migration and dispersal (Naiman & Decamps, 1997; Franchin et al., 2009). In the Amazonia, the ability of avian malaria parasites to disperse geographically and shift among avian hosts have been played a role in their radiation and have shaped their current distributions and diversity (Sebaio et al., 2012; Fecchio et al., 2018a; Fecchio et al., 2018b). Thus, the fact that one of the lineages reported in the Hoatzin has been found also in species in Brazil is consistent with this notion of broad geographic distribution and multiple hosts. This finding also indicate that the parasite communities in the Orinoquia and the Amazon basin share species so, at this point, we can only speculate that similar processes may shape the parasite communities in both areas.
Riparian ecosystems in the Orinoquia are in a state of dynamic flux due to human interventions, seasonal flooding, and fires. In addition, these areas are suitable for insects that could act as vectors. Considering all these factors, the Orinoquia should be given special priority for future research in order to document its parasite-avian host ecology and biodiversity.
Acknowledgments
The authors express their sincere gratitude to the local personnel at the study sites that helped with trapping the birds. We thank Ariana Cristina Pacheco for the silhouettes design and the DNA laboratory at the School of Life Sciences (Arizona State University) for their technical support. We thank the Editor and the reviewers for their valuable comments.
Funding Statement
This research was supported in part by Temple University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
M. Andreína Pacheco, Email: tug00270@temple.edu, Maria.Pacheco@temple.edu.
Ananias A. Escalante, Email: ananias.escalante@temple.edu.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
M. Andreína Pacheco conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
M. Alexandra García-Amado performed the experiments, contributed reagents/materials/analysis tools, approved the final draft.
Jaime Manzano performed the experiments, contributed reagents/materials/analysis tools, prepared figures and/or tables, approved the final draft.
Nubia E. Matta contributed reagents/materials/analysis tools, approved the final draft.
Ananias A. Escalante conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
All the animal protocols were approved by the ethics committees of Instituto Venezolano de Investigaciones Científicas (IVIC, Venezuela) under the number COBIANIM No. Dir-0885/1517/2014.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
We collected the specimens under permit number 0950 issued by the Venezuelan government (“Oficina Nacional de Diversidad Biológica, Ministerio del Poder Popular para Ecosocialismo, Hábitat y Vivienda”).
Data Availability
References
- Altschul et al. (1997).Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research. 1997;125:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson & Samuel (2010).Atkinson CT, Samuel MD. Avian malaria Plasmodium relictum in native Hawaiian forest birds: epizootiology and demographic impacts on ‘apapane Himatione sanguinea. Journal of Avian Biology. 2010;41:357–366. doi: 10.1111/j.1600-048X.2009.04915.x. [DOI] [Google Scholar]
- Bardele et al. (2017).Bardele CF, Schultheiß S, Lynn DH, Wright AG, Dominguez-Bello MG, Obispo NE. Aviisotricha hoazini n. gen., n. sp., the morphology and molecular phylogeny of an anaerobic ciliate from the crop of the Hoatzin (Opisthocomus hoazin), the cow among the birds. Protist. 2017;168:335–351. doi: 10.1016/j.protis.2017.02.002. [DOI] [PubMed] [Google Scholar]
- Bauchan et al. (2017).Bauchan GR, Ochoa R, Hernandes FA, Bauchan GR. New and little known feather mites (Acariformes: Astigmata) analysed with low-temperature scanning electron microscopy. International Journal of Acarology. 2017;43:499–517. doi: 10.1080/01647954.2017.1367032. [DOI] [Google Scholar]
- Bensch, Hellgren & Pérez-Tris (2009).Bensch S, Hellgren O, Pérez-Tris J. MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Molecular Ecology Resources. 2009;9:1353–1358. doi: 10.1111/j.1755-0998.2009.02692.x. [DOI] [PubMed] [Google Scholar]
- Benson et al. (2012).Benson DA, Cavanaugh M, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. GenBank. Nucleic Acids Research. 2012;41:D36–D42. doi: 10.1093/nar/gks1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier-Smith (2014).Cavalier-Smith T. Gregarine site-heterogeneous 18S rDNA trees, revision of gregarine higher classification, and the evolutionary diversification of Sporozoa. European Journal of Protistology. 2014;50:472–495. doi: 10.1016/j.ejop.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Chagas et al. (2013).Chagas CR, Valkiūnas G, Nery CV, Henrique PC, Gonzalez IH, Monteiro EF, Guimarães Lde O, Romano CM, Kirchgatter K. Plasmodium (Novyella) nucleophilum from an Egyptian Goose in São Paulo Zoo, Brazil: microscopic confirmation and molecular characterization. International Journal for Parasitology: Parasites and Wildlife. 2013;2:286–291. doi: 10.1016/j.ijppaw.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claramunt & Cracraft (2015).Claramunt S, Cracraft J. A new time tree reveals Earth history’s imprint on the evolution of modern birds. Science Advances. 2015;1:e1501005. doi: 10.1126/sciadv.1501005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, Boardman & Raidal (2009).Clark P, Boardman W, Raidal SR. Atlas of clinical avian hematology. Wiley-Blackwell; Oxford: 2009. [Google Scholar]
- Davis (2009).Davis AK. The wildlife leukocytes webpage: the ecologist’s source for information about leukocytes of wildlife species. 2009. http://wildlifehematology.uga.edu http://wildlifehematology.uga.edu
- Davis, Maney & Maerz (2008).Davis AK, Maney DL, Maerz JC. The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Functional Ecology. 2008;22:760–772. doi: 10.1111/j.1365-2435.2008.01467.x. [DOI] [Google Scholar]
- Fecchio et al. (2018a).Fecchio A, Bell JA, Collins MD, Farias IP, Trisos CH, Tobias JA, Tkach VV, Weckstein JD, Ricklefs RE, Batalha-Filho H. Diversification by host switching and dispersal shaped the diversity and distribution of avian malaria parasites in Amazonia. Oikos. 2018a;127:1233–1242. doi: 10.1111/oik.05115. [DOI] [Google Scholar]
- Fecchio et al. (2018b).Fecchio A, Pinheiro R, Felix G, Faria IP, Pinho JB, Lacorte GA, Braga EM, Farias IP, Aleixo A, Tkach VV, Collins MD, Bell JA, Weckstein JD. Host community similarity and geography shape the diversity and distribution of haemosporidian parasites in Amazonian birds. Ecography. 2018b;41:505–515. doi: 10.1111/ecog.03058. [DOI] [Google Scholar]
- Ferreira Jr et al. (2017).Ferreira Jr FC, Rodrigues RA, Ellis VA, Leite LO, Borges MAZ, Braga ÉM. Habitat modification and seasonality influence avian haemosporidian parasite distributions in southeastern Brazil. PLOS ONE. 2017;12(6):e0178791. doi: 10.1371/journal.pone.0178791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchin et al. (2009).Franchin AG, De Freitas Juliano R, Kanegae M, Marçal Jr O. Birds in the tropical savannas. In: Del Claro K, Oliveira PS, Rico-Gray V, editors. Tropical biology and conservation management—volume X: savanna ecosystems. Eolss Publishers Co. Ltd.; Oxford: 2009. pp. 160–197. [Google Scholar]
- Gabaldon (1998).Gabaldon A. Malaria aviaria en un país de la región neotropical Venezuela. Fundación Venezolana para la Salud; Caracas, Venezuela: 1998. [Google Scholar]
- Garnham (1966).Garnham PCC. Malaria parasites and other Haemosporidia. Blackwell Scientific Publications; Oxford: 1966. [Google Scholar]
- Godoy-Vitorino et al. (2010).Godoy-Vitorino F, Goldfarb KC, Brodie EL, Garcia-Amado MA, Michelangeli F, Domínguez-Bello MG. Developmental microbial ecology of the crop of the folivorous hoatzin. The ISME Journal. 2010;4:611–620. doi: 10.1038/ismej.2009.147. [DOI] [PubMed] [Google Scholar]
- Godoy-Vitorino et al. (2012).Godoy-Vitorino F, Leal SJ, Díaz WA, Rosales J, Goldfarb KC, García-Amado MA, Michelangeli F, Brodie EL, Domínguez-Bello MG. Differences in crop bacterial community structure between hoatzins from different geographical locations. Research in Microbiology. 2012;163:211–220. doi: 10.1016/j.resmic.2012.01.001. [DOI] [PubMed] [Google Scholar]
- González-Fernández et al. (2007).González-Fernández AJ, González-Fernández ME, Méndez G, Campo-Zambrano MA, González-Fernández MJ, González-Fernández JF, Fernández-Badillo EA. Biodiversidad del Macizo Rocoso de El Baúl, Estado Cojedes, Venezuela. El Baúl, VenezuelaMANFAUNA, UNELLEZ, MinAmb y UCV Informe del Proyecto de Investigación FONACIT No 98003375. 2007:292 pp.
- Gouy, Guindon & Gascuel (2010).Gouy M, Guindon S, Gascuel O. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Molecular Biology and Evolution. 2010;272:221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- Grajal et al. (1989).Grajal A, Strahl SD, Parra R, Gloria Dominguez M, Neher A. Foregut fermentation in the hoatzin, a neotropical leaf-eating bird. Science. 1989;245:1236–1238. doi: 10.1126/science.245.4923.1236. [DOI] [PubMed] [Google Scholar]
- Granthon & Williams (2017).Granthon C, Williams DA. Avian malaria, body condition, and blood parameters in four species of songbirds. The Wilson Journal of Ornithology. 2017;129:492–508. doi: 10.1676/16-060.1. [DOI] [Google Scholar]
- Gross & Siegel (1983).Gross WB, Siegel HS. Evaluation of the heterophil/lymphocyte ratio as a measure of stress in chickens. Avian Diseases. 1983;27:972–979. [PubMed] [Google Scholar]
- Hartman & Lessler (1963).Hartman FA, Lessler MA. Erythrocyte measurements in birds. Auk. 1963;80:467–473. doi: 10.2307/4082852. [DOI] [Google Scholar]
- Hernandes & Mironov (2015).Hernandes FA, Mironov SV. The feather mites of the hoatzin Opisthocomus hoazin (Müller) (Aves: Opisthocomiformes), with the description of two new genera and six new species (Acari: Analgoidea, Pterolichoidea) Zootaxa. 2015;4034:401–444. doi: 10.11646/zootaxa.4034.3.1. [DOI] [PubMed] [Google Scholar]
- Jarvis et al. (2014).Jarvis ED, Mirarab S, Aberer AJ, Li B, Houde P, Li C, Ho SY, Faircloth BC, Nabholz B, Howard JT, Suh A, Weber CC, Da Fonseca RR, Li J, Zhang F, Li H, Zhou L, Narula N, Liu L, Ganapathy G, Boussau B, Bayzid MS, Zavidovych V, Subramanian S, Gabaldón T, Capella-Gutiérrez S, Huerta-Cepas J, Rekepalli B, Munch K, Schierup M, Lindow B, Warren WC, Ray D, Green RE, Bruford MW, Zhan X, Dixon A, Li S, Li N, Huang Y, Derryberry EP, Bertelsen MF, Sheldon FH, Brumfield RT, Mello CV, Lovell PV, Wirthlin M, Schneider MP, Prosdocimi F, Samaniego JA, Vargas Velazquez AM, Alfaro-Núñez A, Campos PF, Petersen B, Sicheritz-Ponten T, Pas A, Bailey T, Scofield P, Bunce M, Lambert DM, Zhou Q, Perelman P, Driskell AC, Shapiro B, Xiong Z, Zeng Y, Liu S, Li Z, Liu B, Wu K, Xiao J, Yinqi X, Zheng Q, Zhang Y, Yang H, Wang J, Smeds L, Rheindt FE, Braun M, Fjeldsa J, Orlando L, Barker FK, Jønsson KA, Johnson W, Koepfli KP, O’Brien S, Haussler D, Ryder OA, Rahbek C, Willerslev E, Graves GR, Glenn TC, McCormack J, Burt D, Ellegren H, Alström P, Edwards SV, Stamatakis A, Mindell DP, Cracraft J, Braun EL, Warnow T, Jun W, Gilbert MT, Zhang G. Whole-genome analyses resolve early branches in the tree of life of modern birds. Science. 2014;346:1320–1331. doi: 10.1126/science.1253451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, Stecher & Tamura (2016).Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution. 2016;337:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwell & Sessler (1971).Manwell RD, Sessler GJ. Plasmodium paranucleophilum n. sp. from a south american tanager. The Journal of Protozoology. 1971;18:629–632. doi: 10.1111/j.1550-7408.1971.tb03386.x. [DOI] [PubMed] [Google Scholar]
- Maxwell (1993).Maxwell M. Avian blood leucocyte responses to stress. World’s Poultry Science Journal. 1993;49:34–43. doi: 10.1079/WPS19930004. [DOI] [Google Scholar]
- McKeand (1998).McKeand JB. Molecular diagnosis of parasitic nematodes. Parasitology. 1998;117:S87–S96. doi: 10.1017/s0031182099004096. [DOI] [PubMed] [Google Scholar]
- Muñoz et al. (1999).Muñoz E, Ferrer D, Molina R, Adlard RD. Prevalence of haematozoa in birds of prey in Catalonia, north-east Spain. Veterinary Record. 1999;144:632–636. doi: 10.1136/vr.144.23.632. [DOI] [PubMed] [Google Scholar]
- Naiman & Decamps (1997).Naiman RJ, Decamps H. The ecology of interfaces: Riparian zones. Annual Review of Ecology, Evolution, and Systematics. 1997;28:621–658. doi: 10.1146/annurev.ecolsys.28.1.621. [DOI] [Google Scholar]
- Pacheco et al. (2011).Pacheco MA, Battistuzzi FU, Junge RE, Cornejo OE, Williams CV, Landau I, Rabetafika L, Snounou G, Jones-Engel L, Escalante AA. Timing the origin of human malarias: the lemur puzzle. BMC Evolutionary Biology. 2011;11:299. doi: 10.1186/1471-2148-11-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco et al. (2018).Pacheco MA, Cepeda AS, Bernotiene R, Lotta IA, Matta NE, Valkiūnas G, Escalante AA. Primers targeting mitochondrial genes of avian haemosporidians: PCR detection and differential DNA amplification of parasites belonging to different genera. International Journal for Parasitology. 2018;48:657–670. doi: 10.1016/j.ijpara.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinauskas et al. (2016).Palinauskas V, Žiegyte R, Iezhova TA, Ilgūnas M, Bernotiene R, Valkiūnas G. Description, molecular characterisation, diagnostics and life cycle of Plasmodium elongatum (lineage pERIRUB01), the virulent avian malaria parasite. International Journal for Parasitology. 2016;46:697–707. doi: 10.1016/j.ijpara.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Prum et al. (2015).Prum RO, Berv JS, Dornburg A, Field DJ, Townsend JP, Lemmon EM, Lemmon AR. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature. 2015;526:569–573. doi: 10.1038/nature15697. [DOI] [PubMed] [Google Scholar]
- Renjifo, Sanmartin & De Zuleta (1952).Renjifo S, Sanmartin C, De Zuleta J. A survey of the blood parasites of vertebrates in eastern Colombia. Acta Tropica. 1952;9:121–169. [PubMed] [Google Scholar]
- Ricklefs & Sheldon (2007).Ricklefs RE, Sheldon KS. Malaria prevalence and white-blood-cell response to infection in a tropical and in a temperate thrush. Auk. 2007;124:1254–1266. doi: 10.1642/0004-8038(2007)124[1254:MPAWRT]2.0.CO;2. [DOI] [Google Scholar]
- Ronquist & Huelsenbeck (2003).Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;1912:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Schneider, Rasband & Eliceiri (2012).Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebaio et al. (2012).Sebaio F, Braga EM, Branquinho F, Fecchio A, Marini MÂ. Blood parasites in passerine birds from the Brazilian Atlantic Forest. The Revista Brasileira de Parasitologia Veterinária. 2012;21:7–15. doi: 10.1590/S1984-29612012000100003. [DOI] [PubMed] [Google Scholar]
- Telford Jr (2009).Telford Jr SR. Hemoparasites of the Reptilia: color atlas and text. Taylor & Francis; Boca Raton: 2009. [Google Scholar]
- Thomas (1996).Thomas BT. Family Opisthocomidae (Hoatzin) In: Del Hoyo J, Elliott A, Sargatal J, editors. Handbook of the birds of the world. vol. 3. Lynx Edicions; Barcelona: 1996. pp. 24–32. (Hoatzin to Auks). [Google Scholar]
- Tostes et al. (2017).Tostes R, Dias RJP, Martinele I, Senra MVX, D’Agosto M, Massard CL. Multidisciplinary re-description of Plasmodium (Novyella) paranucleophilum in Brazilian wild birds of the Atlantic Forest kept in captivity. Parasitology Research. 2017;116:1887–1897. doi: 10.1007/s00436-017-5465-3. [DOI] [PubMed] [Google Scholar]
- Valkiūnas (2005).Valkiūnas G. Avian malaria parasites and other haemosporidia. Boca de Raton: CRC press; 2005. [Google Scholar]
- Valkiūnas & Iezhova (2018).Valkiūnas G, Iezhova TA. Keys to the avian malaria parasites. Malaria Journal. 2018;17(1):212. doi: 10.1186/s12936-018-2359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar et al. (2013).Villar CM, Bryan Jr AL, Lance SL, Braga EM, Congrains C, Del Lama SN. Blood parasites in nestlings of wood stork populations from three regions of the American continent. Journal of Parasitology. 2013;99:522–527. doi: 10.1645/12-73.1. [DOI] [PubMed] [Google Scholar]
- Viscarret, Wright & Urbani (2009).Viscarret P, Wright J, Urbani F. New U-Pb zircon ages of El Baul Massif, Cojedes State, Venezuela. Revista Tecnica de la Facultad de Ingenieria Universidad del Zulia. 2009;32:210–221. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
The mtDNA genome sequences described here are accessible via GenBank accession numbers KY653749 to KY653751.