Abstract
Background
Keloid management remains a challenging clinical problem despite numerous therapies reported until now. The efficacy of corticosteroids in the treatment of keloids has been well established. The most commonly used corticosteroid is intralesional triamcinolone. Sporadic reports on the use of intralesional verapamil suggest its efficacy.
Aim
Since there is not sufficient evidence to support the role of intralesional verapamil as an effective alternative modality, it was decided to undertake a randomized study to determine its efficacy as a treatment for keloids.
Methods
A randomized, single-blind, single-group comparison with 15 patients (30 scars) was carried out to compare the effects of intralesional triamcinolone with verapamil injections. Injections were scheduled every 3 weeks accompanied by cryotherapy until complete flattening of the scar or maximum 8 sessions, whichever came earlier. Scar evaluation at each stage was done by serial photographic records as well as by Vancouver scar scale. Statistical analysis was done by Wilcoxon and Mann-Whitney U tests using SPSS version 19.
Results
In both study groups there was a reduction in height and pliability at the end of the study. Better improvement in height and pliability was seen with triamcinolone in comparison with verapamil. However, a desired change in vascularity and pigmentation was not seen with either of the drugs.
Conclusion
Verapamil is not as effective as triamcinolone in the treatment of keloids.
Keywords: keloid, verapamil, triamcinolone, Vancouver scar scale, scar
Introduction
Characterized by firm, tender nodules or plaques, keloids occur more frequently on shoulders, chest, neck, upper arms, and face [1]. They are benign overgrowth of fibrous tissue, usually developing after healing of a skin injury due to trauma, inflammation, surgery, or burns and extend beyond the original defect [2]. The uncontrolled growth of keloids can lead to cosmetic disfigurement and functional impairment, which might adversely affect the quality of life [3].
A variety of treatment modalities such as silicone gel sheeting, intralesional injections, surgical manipulation, laser, and radiotherapy have been used, but no particular treatment has been shown to be effective for all cases [4]. Drugs like bleomycin and 5-fluorouracil have better efficacy, but they are costly and cause severe drug reactions. Surgery and laser therapy have their own limitations, while radiotherapy can cause malignancy [5].
Corticosteroids seem to be effective in the treatment of keloids as they diminish collagen and glycosaminoglycan synthesis, inhibit fibroblast growth, enhance collagen and fibroblast degeneration, and have a powerful anti-inflammatory effect. Triamcinolone acetonide (TAC) is the most commonly used intralesional corticosteroid for keloid treatment. TAC is cost-effective and practical and has become first-line treatment for keloids, in spite of some local adverse effects such as dermal atrophy, telangiectasia, and hypopigmentation [2].
It has been demonstrated that calcium channel blockers decrease extracellular matrix production in scars. Furthermore, they depolymerize actin filaments to modify fibroblast morphology by a consequent increased secretion of pro-collagenase [6]. Intralesional verapamil hydrochloride has already been successfully applied for the treatment of keloids [7].
This study was hence conducted to assess the efficacy of intralesional verapamil in the treatment of keloids by its comparison with the effects of intralesional triamcinolone.
Methods
The study was conducted at the Department of Dermatology, Faghihi Hospital, Shiraz, Iran, from December 2017 to December 2018. This study was approved by the local ethics committee of Shiraz University of Medical Sciences and registered in the Iranian clinical trial registry. All patients signed the informed consent form prior to initiation of the trial. This study is a randomized, single-group, single-blind comparison between triamcinolone and verapamil injection. Inclusion criteria involved patients aged between 18 and 70 years old, with at least 2 scars with duration of less than 2 years. Patients with evidence of any infection (in or near the scar area), those with a history of cardiovascular problems, pregnant women, and patients with a history of prior treatment with any intralesional injections were excluded from the study.
The minimum sample size of 15 patients with at least 2 scars (30 scars) was calculated for this trial, where triamcinolone was considered as the standard treatment and verapamil was regarded as the experimental drug. Fifteen consecutive patients who fulfilled our inclusion criteria entered the study using simple randomization technique.
Two similar keloid scars of each patient were randomly selected, using simple randomization by random number table. Deciding on putting which lesion into which therapeutic arm was randomly made by flipping a coin. A lidocaine 2 injection with ring block technique was used to anaesthetize the site of injection before starting the treatment. The injections were made with an insulin syringe, 27-gauge needle.
One of the scars received intralesional TAC (Exir Pharmaceutical Co, Borujerd, Iran), while the other received intralesional verapamil hydrochloride (Verahexal, Knoll AG, Ludwigshafen, Germany) every 3 weeks for a maximum of 8 sessions or until complete flattening of the scar. Each intralesional session was preceded by cryotherapy using cryospray technique for 20 seconds at 1 cm distance from the lesion. The maximum volume of triamcinolone (20 mg/mL) and verapamil (2.5 mg/mL) at each session was 1.5 cc. We used multiple intralesional injections until the lesion was blanched. Detailed history and demographic parameters including age, sex, duration of the scar, and prior treatments were recorded.
Scar evaluation at each stage was done by Vancouver scar scale (VSS) [8]. The mentioned scale scores the scars on 4 parameters: height, vascularity, pliability, and pigmentation. Scar height was accurately measured with a ruler in millimeters. Scar vascularity and pigmentation were assessed by visual inspection. Scar pliability was subjectively assessed by palpation. For study parameters in each group, the mean value and standard deviation (SD) were calculated. The decreasing values reflect the clinical improvement of the scar. The Wilcoxon test was used to test the significant improvement of VSS parameters in each group. The VSS scores were compared between the 2 groups using Mann-Whitney U test. P value <0.05 was considered to be statistically significant. Statistical analysis was done using SPSS version 19.
Results
Thirty scars were studied in 15 randomly selected patients who met our inclusion criteria (15 scars treated with verapamil; 15 scars treated with triamcinolone). Among 15 participants, 14 were female and 1 was male. The mean age of the patients was 31.53 ± 12.58 years (mean ± SD) and the mean duration of the disease was 11.46 ± 7.06 months.
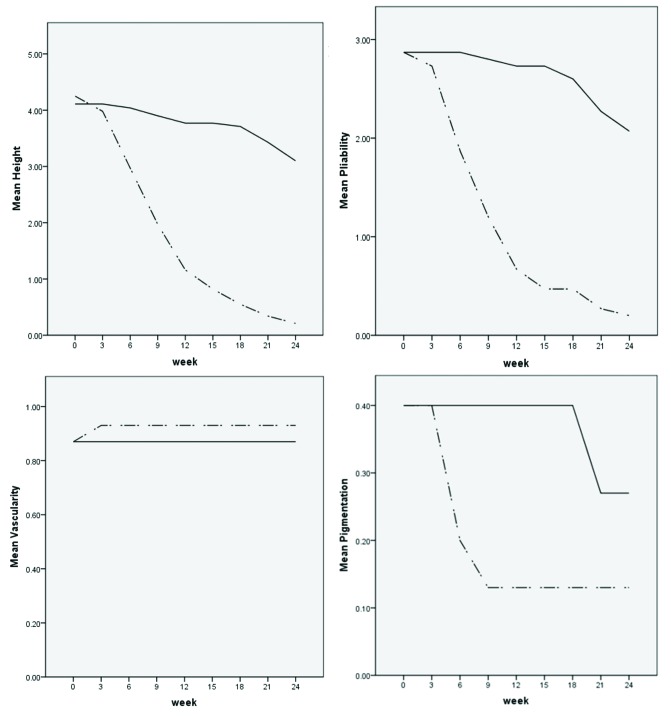
The VSS parameters for both treatment groups are presented in Table 1. At the beginning of the study, there was no significant difference in parameters of the 2 groups (P > 0.05). In both study groups, there was a reduction in height and pliability at the end of the study as determined by Wilcoxon test (Table 2). Using Mann-Whitney U test, statistically better improvement in height and pliability was observed in the triamcinolone-receiving group compared with the verapamil-receiving group (P < 0.001). However, a desired change in vascularity and pigmentation was not seen with either of the drugs (Table 2). Scar vascularity became worse in 1 out of 15 scars in the triamcinolone group. It can be observed that 12 out of 15 scars in both groups had normal pigmentation at the start of the study. After 24 weeks, 1 out of 3 hyperpigmented scars in the verapamil group regained normal pigmentation while the other 2 scars remained hyperpigmented. However, out of 3 hyperpigmented scars in the triamcinolone group, 1 regained normal pigmentation while the other 2 scars became hypopigmented at the end of our trial (Table 3). No significant difference was detected in vascularity and pigmentation in the 2 groups (P > 0.05).
Table 1.
Mean VSS Scores During 24 Weeks of Follow-up
| Intralesional Injection Interval in Weeks With Mean VSS Scores ± SD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VSS Parameters | Drug | 0 wks | 3 wks | 6 wks | 9 wks | 12 wks | 15 wks | 18 wks | 21 wks | 24 wks |
| Height | V | 4.11±1.90 | 4.11±1.90 | 4.04±1.92 | 3.90±1.95 | 3.77±1.94 | 3.77±1.94 | 3.71±1.99 | 3.43±2.04 | 3.10±1.85 |
| T | 4.25±1.94 | 3.98±1.92 | 2.97±2.07 | 1.97±2.06 | 1.16±1.91 | 0.82±1.95 | 0.55±1.12 | 0.34±0.90 | 0.21±0.56 | |
| Vascularity | V | 0.87±0.74 | 0.87±0.74 | 0.87±0.74 | 0.87±0.74 | 0.87±0.74 | 0.87±0.74 | 0.87±0.74 | 0.87±0.74 | 0.87±0.74 |
| T | 0.87±0.74 | 0.93±0.70 | 0.93±0.70 | 0.93±0.70 | 0.93±0.70 | 0.93±0.70 | 0.93±0.70 | 0.93±0.70 | 0.93±0.70 | |
| Pliability | V | 2.87±0.35 | 2.87±0.35 | 2.87±0.35 | 2.80±0.41 | 2.73±0.46 | 2.73±0.46 | 2.60±0.51 | 2.27±0.46 | 2.07±0.26 |
| T | 2.87±0.35 | 2.73±0.46 | 1.93±0.70 | 1.20±1.01 | 0.67±0.90 | 0.47±0.83 | 0.47±0.83 | 0.27±0.59 | 0.20±0.41 | |
| Pigmentation | V | 0.40±0.83 | 0.40±0.83 | 0.40±0.83 | 0.40±0.83 | 0.40±0.83 | 0.40±0.83 | 0.40±0.83 | 0.27±0.70 | 0.27±0.70 |
| T | 0.40±0.83 | 0.40±0.83 | 0.20±0.56 | 0.13±0.35 | 0.13±0.35 | 0.13±0.35 | 0.13±0.35 | 0.13±0.35 | 0.13±0.35 | |
SD = standard deviation; T = triamcinolone; V = verapamil.
Table 2.
Mean VSS Scores ± SD Before and After Treatment in Triamcinolone and Verapamil Groups
| VSS Parameters | Drug | Week 0 | Week 24 | P Value |
|---|---|---|---|---|
| Height | V | 4.11±1.90 | 3.10±1.85 | <0.001 |
| T | 4.25±1.94 | 0.21±0.56 | <0.001 | |
| Vascularity | V | 0.87±0.74 | 0.87±0.74 | 1 |
| T | 0.87±0.74 | 0.93±0.70 | 0.32 | |
| Pliability | V | 2.87±0.35 | 2.07±0.26 | <0.001 |
| T | 2.87±0.35 | 0.20±0.41 | <0.001 | |
| Pigmentation | V | 0.40±0.83 | 0.27±0.70 | 0.32 |
| T | 0.40±0.83 | 0.13±0.35 | 0.10 |
SD = standard deviation; T = triamcinolone; V = verapamil.
Table 3.
Improvement in the Pigmentation of Scars in Both Groups
| 0 Week | 24 Weeks | |||||
|---|---|---|---|---|---|---|
| Pigmentation | Normal | Hypo- | Hyper- | Normal | Hypo- | Hyper- |
| Verapamil | 12 | 0 | 3 | 13 | 0 | 2 |
| Triamcinolone | 12 | 0 | 3 | 13 | 2 | 0 |
The changes in VSS score parameters within 24 weeks of follow-up in both groups are shown in Figure 1.
Figure 1.
Line charts for scar height, pliability, vascularity and pigmentation in both groups. [Copyright: ©2019 Saki et al.]
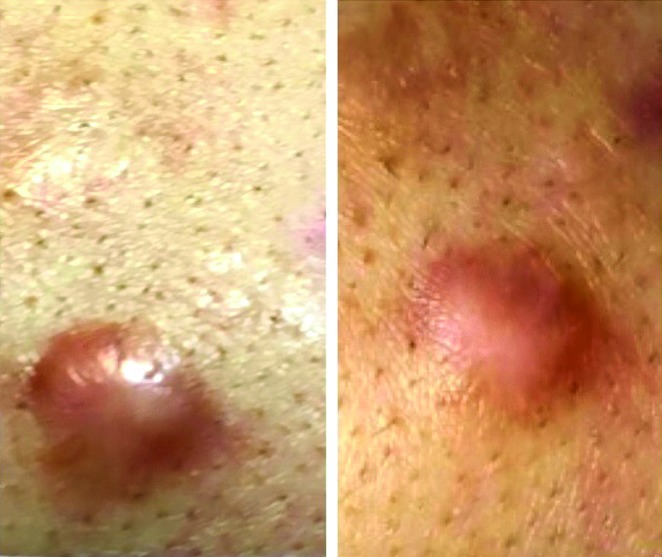
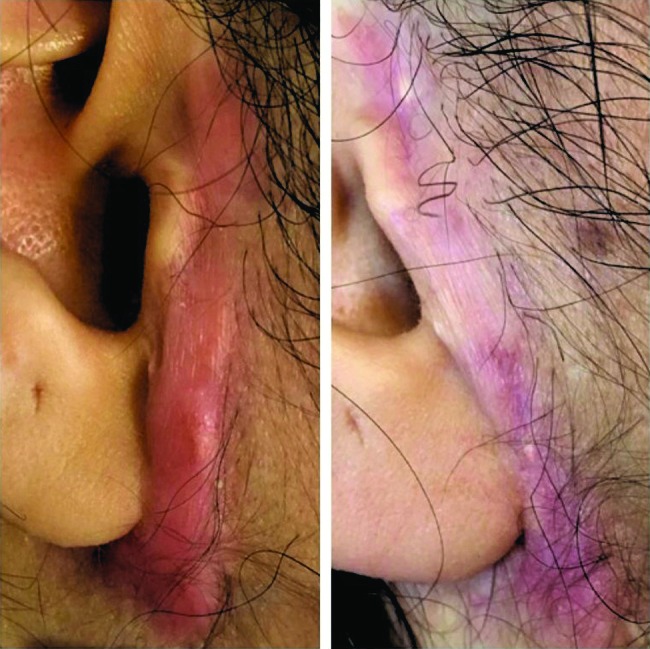
Figures 2 and 3 represent the before-after photographs of a patient in the triamcinolone and verapamil groups, respectively.
Figure 2.
Before and after photographs of a patient in the triamcinolone group. [Copyright: ©2019 Saki et al.]
Figure 3.
Before and after photographs of a patient in the verapamil group. [Copyright: ©2019 Saki et al.]
Discussion
Despite numerous developed therapies, keloid treatment has remained a challenging clinical problem. This might be due to the fact that the mechanisms of development of keloids have not been completely understood [9]. Laser therapy, surgical removal, radiation therapy, silicone gel, cryosurgery, intralesional injection of various agents, and occlusive dressing have all been used either alone or in various combinations [10]. However, evolution of different therapies has not significantly improved their success rates. In addition, each method has its own limitations such as high cost, poor efficacy, recurrence, and adverse effects (such as malignancy).
Keloids are overgrowth of dense fibrous tissue developing after trauma to the skin [11]. Although the basis of keloid formation has not been fully understood, an imbalance of matrix degradation and collagen biosynthesis, which could result in excess accumulation of collagen in wound, has been considered the primary biochemical feature of these skin lesions [10]. Furthermore, inflammation or alteration of growth factors may contribute to keloid formation [11].
As cellular secretion of macromolecules is known to be a calcium-dependent process, Lee and Ping in 1990 examined the effects of calcium antagonists on extracellular matrix production. They suggested that calcium antagonists depolymerize actin filaments and alter the shape of fibroblast cells from bipolar to spherical, which may result in increased pro-collagenase production [12].
Shanthi et al showed a reduction in vascularity, pliability, and height of the scars with both triamcinolone and verapamil injections [5]. It has also been found that this reduction is faster by triamcinolone injection. However, a desired change in pigmentation was not observed with either of the drugs. They found that similar to triamcinolone, verapamil significantly improved the clinical parameters of the scars; hence, it can be a suitable alternative to triamcinolone for treatment of hypertrophic scars and keloids [5]. Ahuja and Chatterjee showed that a faster rate of improvement in scar height, vascularity, and pliability is achievable with triamcinolone. However, the difference in the rate of pigmentation change by the 2 agents was not statistically significant [6].
The present study was a randomized, single-blind clinical trial comparing the efficacy of intralesional verapamil with intralesional triamcinolone in the treatment of keloids. A significant improvement was observed in the height and pliability of the scars in both groups. In agreement with the literature, the improvement of these parameters was significantly higher in the scars treated with triamcinolone compared with those treated with verapamil. In contrast to studies of Shanthi et al [5] and Ahuja and Chatterjee [6], the scars of both groups in our study did not reach complete flattening and normal pliability at the end of the study. The possible reason could be the higher initial mean scores of height and pliability in both groups relative to the mentioned studies. However, no significant difference was found in the pigmentation of the scars before and after treatment in both groups. It could be due to the fact that most of the scars had normal pigmentation at the beginning of our study. This finding is compatible with previous studies. However, in contrast to previous works, scar vascularity did not show significant difference with both treatments at the end of our study [5,6].
The results of the present study suggest that verapamil can be considered a safe treatment for patients with keloids, but it is not as effective as triamcinolone. To date, corticosteroid injection is the core treatment available for keloid management. Corticosteroids suppress keloid formation by 3 different mechanisms. First, they suppress inflammation by inhibition of leukocyte and monocyte migration and phagocytosis. Second, corticosteroids are potent vasoconstrictors that reduce delivery of oxygen and nutrients to the wound. Third, the antimitotic effect of corticosteroids inhibits proliferation of keratinocytes and fibroblasts, slowing re-epithelialization and new collagen formation. Corticosteroid injection decreases collagen and glycosaminoglycan synthesis by several mechanisms including decline of inflammatory process in the wound, decreasing fibroblast proliferation, and hypoxia enhancement. They also lead to decreased levels of endogenous vascular endothelial growth factor (VEGF), transforming growth factor beta (TGF-β), and interleukin-1 (IL-1), which play important roles in the process of keloid formation [13]. However, calcium antagonists merely affect this process by reduction of collagen production in the extracellular matrix and stimulation of collagenase synthesis, which will decrease fibrous tissue production [7].
Conclusions
In conclusion, given the anti-inflammatory and antimitotic effects of triamcinolone plus its vasoconstrictor properties, triamcinolone would be an effective treatment for keloids in comparison to verapamil. However, further studies involving a higher number of participants with a longer period of observation are encouraged to shed more light on this subject.
Acknowledgement
This study was extracted from the thesis written by Dr. R. Mokhtari and was financially supported by the Shiraz University of Medical Sciences.
Footnotes
Funding: This study was financially supported by the Shiraz University of Medical Sciences.
Competing interests: The authors have no conflicts of interest to disclose.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Lee SS, Yosipovitch G, Chan YH, Goh CL. Pruritus, pain, and small nerve fiber function in keloids: a controlled study. J Am Acad Dermatol. 2004;51(6):1002–1006. doi: 10.1016/j.jaad.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 2.Garg AM, Shah YM, Garg A, et al. The efficacy of intralesional triamcinolone acetonide (20mg/ml) in the treatment of keloid. Int Surg J. 2018;5(3):868–872. [Google Scholar]
- 3.Robles DT, Berg D. Abnormal wound healing: keloids. Clin Dermatol. 2007;25(1):26–32. doi: 10.1016/j.clindermatol.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17(1–2):113. doi: 10.2119/molmed.2009.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shanthi FM, Ernest K, Dhanraj P. Comparison of intralesional verapamil with intralesional triamcinolone in the treatment of hypertrophic scars and keloids. Indian J Dermatol Venereol Leprol. 2008;74(4):343. doi: 10.4103/0378-6323.42899. [DOI] [PubMed] [Google Scholar]
- 6.Ahuja RB, Chatterjee P. Comparative efficacy of intralesional verapamil hydrochloride and triamcinolone acetonide in hypertrophic scars and keloids. Burns. 2014;40(4):583–588. doi: 10.1016/j.burns.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 7.D’Andrea F, Brongo S, Ferraro G, Baroni A. Prevention and treatment of keloids with intralesional verapamil. Dermatology. 2002;204(1):60–62. doi: 10.1159/000051812. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan Ta, Smith J, Kermode J, Mclver E, Courtemanche D. Rating the burn scar. J Burn Care Rehabil. 1990;11(3):256–260. doi: 10.1097/00004630-199005000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Mofikoya BO, Adeyemo WL, Abdus-salam AA. Keloid and hypertrophic scars: a review of recent developments in pathogenesis and management. Nig Q J Hosp Med. 2007;17(4):134–139. doi: 10.4314/nqjhm.v17i4.12693. [DOI] [PubMed] [Google Scholar]
- 10.Manuskiatti W, Fitzpatrick RE. Treatment response of keloidal and hypertrophic sternotomy scars: comparison among intralesional corticosteroid, 5-fluorouracil, and 585-nm flashlamp-pumped pulsed-dye laser treatments. Arch Dermatol. 2002;138(9):1149–1155. doi: 10.1001/archderm.138.9.1149. [DOI] [PubMed] [Google Scholar]
- 11.Leventhal D, Furr M, Reiter D. Treatment of keloids and hypertrophic scars: a meta-analysis and review of the literature. Arch Facial Plast Surg. 2006;8(6):362–368. doi: 10.1001/archfaci.8.6.362. [DOI] [PubMed] [Google Scholar]
- 12.Lee RC, Ping JA. Calcium antagonists retard extracellular matrix production in connective tissue equivalent. J Surg Res. 1990;49(5):463–466. doi: 10.1016/0022-4804(90)90197-a. [DOI] [PubMed] [Google Scholar]
- 13.Roques C, Téot L. The use of corticosteroids to treat keloids: a review. Int J Low Extrem Wounds. 2008;7(3):137–145. doi: 10.1177/1534734608320786. [DOI] [PubMed] [Google Scholar]