Abstract
Mesolimbic dopaminergic function influences addiction through effects on reinforcement learning, decision-making, and impulsivity. This review covers sex differences in dopaminergic neurochemistry, their hormonal and genetic determinants, and how differences in dopaminergic tone interact with sex and/or ovarian hormone status to affect cognitive functions. Findings from research on cigarette smoking reveal sex differences in striatal and midbrain dopamine D2-type receptor availability and striatal dopamine release that suggest mechanisms of nicotine dependence, and stronger subjective responses to nicotine and efficacy of nicotine replacement therapies in male smokers than in their female counterparts. Opportunities exist to extend such efforts in studies of how sex and hormone status influence other addictions.
II. Introduction: Sex differences and Dopaminergic Signaling in Drug Use Disorders
The prevalence, course, and consequences of substance use disorders differ in men and women. Although men are approximately 70% more likely than women to have a substance use disorder, more women than men (12–49 years of age) recently initiated the use of marijuana, stimulants, heroin, phencyclidine, and alcohol [1]. Women tend to escalate from initiation of drug use to addiction more rapidly than men [2,3], and are more likely than men to relapse after initiating abstinence [4]. Because substance use increases maternal and perinatal morbidity, women experience addiction-related health consequences that do not affect men [5]. They also are disproportionately affected by other untoward effects of drug use, exhibiting a higher relative risk of smoking-related cardiovascular disease [6], and occurrence of cardiovascular [7] and other alcohol-related physical illnesses at lower levels of drinking than men [8].
The National Institutes of Health now has mandated that sex be considered as a biological variable in all biomedical research [9], and sex differences have been evaluated in animal models of addiction and human addiction. This review extends previous syntheses of relevant literature [10,11] on sex differences (and in some cases, similarities) in dopaminergic neurochemistry (for review, see [12]) and addiction. Involvement of dopaminergic systems in the effects of cigarette smoking are highlighted because recent developments and expert recommendations in this research area illustrate an exemplary role for studies of other addictions.
Dopaminergic signaling is central to the etiology and maintenance of substance use disorders by influencing the motivational processes underlying the learning and execution of goal-directed behaviors, as indicated by electrophysiological recordings from midbrain dopamine neurons in monkeys [13,14]. Numerous studies of animal models have implicated striatal dopamine D2-type receptors in vulnerability as well as resilience to addiction [15–22]. These include findings that striatal D2-type receptor availability influences subsequent cocaine self-administration and that striatal D2-type receptors and dopamine transporters show long-lasting neuroadaptations to stimulant administration in nonhuman primates [23,24]. Human studies reinforcing this view show below control striatal dopamine D2-type receptor availability in individuals with addictions to various substances, including stimulants, heroin, alcohol, and tobacco (for reviews, see [25–27]), and (as detailed below) associations of striatal D2-type receptor availability with cognitive functions that affect addiction.
III. Dopamine Signaling, Cognition and Responses to Drugs: Effects of Ovarian Hormones
Cognitive control and adaptive decision-making, functions that are linked to dopaminergic neurochemical markers, influence the course of addictions [28,29]. Impaired cognitive control, measured as impulsivity in personality inventories, has been implicated in the initiation, maintenance, and relapse of drug-seeking behaviors [32]. Stimulant users are more impulsive than healthy control subjects, and show a negative correlation between impulsivity and striatal D2-type receptor availability (e.g., [33,34]). Conversely, cognitive control, measured in tests of motor response inhibition [35–37] and cognitive flexibility [38,39], show weaknesses in substance abusers, and positive correlations of striatal D2-type receptor availability with performance on relevant tests in healthy controls is disrupted in stimulant users [40,41].
Maladaptive decision-making also is linked to drug addiction and to dopaminergic function. Individuals with substance use disorders of various types discount monetary rewards as a function of their delay more than healthy individuals [42–45]. Stimulant users exhibit correlation between the steepness of the discounting rate and striatal D2-type receptor availability that is not seen in healthy controls [46]. Methamphetamine users also make maladaptive choices and fail to show the relationship between risk and activation in the right dorsolateral prefrontal cortex that is exhibited by healthy control subjects during risk taking in a reward-based laboratory test; instead, they show a linear relationship between riskiness of choice and ventral striatal activation [47]. Moreover, association of response in the dorsolateral prefrontal cortex with riskiness of a decision is highly negatively correlated with striatal D2-type receptor availability in controls [48]. Thus, dopaminergic function, especially signaling through D2-type receptors, can influence addiction by promoting disadvantageous behaviors leading to continued drug use.
Circulating levels of ovarian hormones may influence both striatal dopamine signaling and cognitive control. Estradiol levels are correlated with longer reaction time in motor response inhibition [49] and Stroop task performance [50], suggesting that inhibitory control is weaker when estrogen levels are high. This effect may reflect estrogenic effects on striatal dopamine in that local administration of estrogen into the nucleus accumbens decreases K+-stimulated dopamine release [51], and estrogen administration to the caudal striatum reduces dopamine D2-type receptor density [52]. Circulating progesterone levels also affect inhibitory control, attenuating impulsive action for sucrose pellets in a Go/No-Go task (130) and reducing impulsive action for cocaine in a sex-dependent manner (129; 131). Positron emission tomography (PET) imaging in humans has provided only indirect evidence that ovarian hormones may influence striatal DA receptor availability, specifically that D2-type receptor availability was lower in the putamen during the luteal phase of the menstrual cycle, when estradiol and progesterone levels are relatively elevated [53].
Inasmuch as striatal dopaminergic neurotransmission is an important mechanism of subjective response to drugs, associations of hormone status with responses to drugs of abuse also suggest that the hormones influence striatal dopaminergic function. The subjective responses to a number of drugs - most prominently stimulants - differ as a function of hormone levels in women. As reviewed in [23], subjective responses to cocaine tend to be higher during the follicular phase, when hormone levels are low (except for a brief estrogen surge at the end of the follicular phase), compared to the luteal phase, when estrogen and progesterone are elevated. Consistent with that observation, progesterone administration decreases positive subjective effects of cocaine.
Similar investigations in smokers have also revealed effects of ovarian hormones on effects of nicotine and smoking although the direction of the relationship between ovarian hormones and smoking behaviors is less clear. A systematic review and meta-analysis found that higher progesterone levels were associated with both increased negative and decreased positive subjective effects of nicotine. The same review reported higher levels of withdrawal, and a trend toward higher levels of craving, during the luteal (high progesterone) versus the follicular (low progesterone) phase [54]. In oral contraceptive (OC) users, a high dose (0.25 μg) of norgestimate, a synthetic progesterone analog, reduced smoking satisfaction more than a lower dose (0.18 μg), but high levels of endogenous progesterone were positively related to smoking satisfaction in naturally-cycling women [55]. Studies of abstinence-related symptoms in women using a variety of OCs produced mixed findings, with these women reporting less [56] or more [57] craving than those who were not using OCs. Together, these data suggest a likely relationship between ovarian hormones and smoking-related behaviors that may potentially be mediated by hormonal effects on the dopamine system, but a great deal more research is needed to confirm or disconfirm this link and to identify the direction of the relationship. Such investigations may help to clarify the mechanism by which sex differences in dopaminergic signaling and addiction emerge.
IV. Sex differences in the functional genetics of dopamine synthesis and degradation
Sex differences in the physiology of the dopamine system can be traced back to chromosomal differences between men and women. The male-determining gene Sry, located on the Y chromosome and found only in (phenotypic) males, is expressed in dopaminergic neurons of the substantia nigra, and influences expression of tyrosine hydroxylase (TH), the rate-limiting enzyme in the biosynthetic pathway to dopamine [58,59]. Estrogen is a regulator of TH activity [60,61], suggesting that different pathways to a similar endpoint are important for regulation of dopaminergic function in males and females.
Catechol-O-methyltransferase (COMT) catalyzes the degradation of dopamine (and other catechols), and a single nucleotide polymorphism at codon 158 encodes either valine (Val) or methionine (Met), the latter of which produces COMT that is more thermolabile. COMT activity in the prefrontal cortex of Met158 homozygotes, therefore, is 35–50% lower than in Val158 homozygotes [62], and Met158 carriers have greater concentrations of extracellular cortical dopamine than Val158 homozygotes. This is not the case regarding striatal dopamine [63], where the dopamine transporter rather than COMT determines intrasynaptic dopamine concentrations. Independent of genotype, women have significantly lower COMT activity in the dorsolateral prefrontal cortex [62], and higher D2-type receptor availability in the frontal cortex compared to men [64]. These findings broadly suggest that women have greater cortical dopaminergic tone than men.
Recent investigations have capitalized on the discovery that COMT influences prefrontal dopaminergic tone to investigate dopamine / behavior relationships, and many have revealed significant sex differences in effects of COMT genotype (see Table 1).
Table 1:
Sex differences in the effect of COMT polymorphisms on behavior. Depending on task demands and participant sex, additional cortical dopaminergic tone provided by val/met polymorphisms may improve or impair performance.
| Reference | Task | Finding |
|---|---|---|
| Gurvich and Rossell, 2015 | Continuous performance task | Female: val/val > val/met > met/met Male: val/val = val/met = met/met |
| Costa et al., 2016 | Iowa Gambling Task (risk but not ambiguity trials) | Female: val/val > val/met = met/met Male: met/met = val/met = met/met |
| Lamb et al., 2016 | Facial recognition | Female: val/val = val/met = met/met Male: val/val = val/met < met/met |
<InlineShape1>Estrogen levels interact with COMT genotype to affect cognition in women. Higher estrogen improves working memory, measured by the N-back task, in val/val homozygotes, but impairs this ability in met/met homozygotes [65], and a significant negative correlation between change in estradiol and change in delay discounting is observed only in Val carriers [66]. While these findings provide behavioral evidence that estrogen shifts cortical and/or striatal dopaminergic tone, such a shift has not yet been demonstrated directly in humans. A model describing the effects of sex and estrogen levels on dopaminergic tone and cognitive task performance is depicted in Figure 1, building on previous evidence [67] and models [68], and describing an inverted-U-shaped relationship between dopaminergic tone and cognitive performance. The model proposes that cortical dopaminergic tone is higher in women than men, and under conditions of high vs. low estrogen.
Figure 1. COMT genotype, sex, and estrogen (E) effects on cortical dopaminergic tone and cognitive task performance.
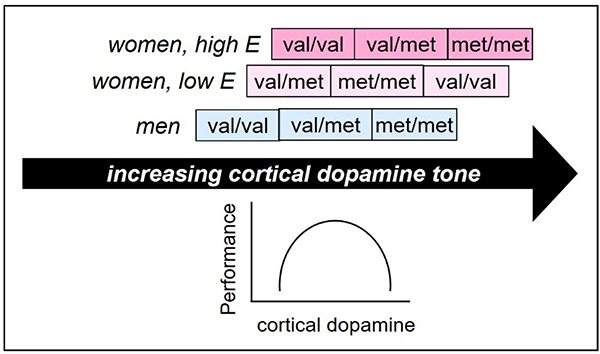
Cortical dopamine tone is higher in women than men, and in individuals with the met/met vs. val/met, and val/met vs. val/val genotypes. Cortical dopamine levels affect task performance in a parabolic, task-dependent manner: performance improves with increases in dopamine tone up to an optimum that depends on task demands, and then declines.
Variance introduced by normal hormonal fluctuations may partially explain a finding of a stronger relationship between genotype and brain function in men compared to women. A composite “gene score” reflecting additive effects of five genes related to dopamine function (DRD2, DRD3, DRD4, DAT1, and COMT) was negatively related to the modulation of activation of the left dorsolateral prefrontal cortex during the decision to take risk in a laboratory decision-making task, but the relationship was significantly stronger in male compared to female participants, and hormone levels were not measured [48].
Dopamine signaling plays an important role in nicotine addiction (for review, see [69]), and predictably, COMT genotype influences responses to nicotine and smoking. Compared to heterozygotes and met/met homozygotes, val/val homozygotes are more likely to be smokers [70], to show greater brain activation to a reward processing task while undergoing transdermal nicotine administration [71], and to experience greater negative subjective effects in response to intravenous nicotine [72]. A reasonable interpretation is that val/val homozygotes have greater cortical COMT activity, less tonic dopaminergic activity, and therefore a greater response to phasic release of dopamine in the striatum. The next section discusses sex differences in dopamine signaling, smoking and links between the two.
V. Sex Differences in Striatal Dopamine: Cigarette Smoking and Implications for Addiction
One area of research that has recently provided mechanistic information regarding dopaminergic function concerns Tobacco Use Disorder. Among its other well-known actions, nicotine is an aromatase inhibitor [73], reducing the aromatization of testosterone to estrogens both peripherally [74] and in the brain [75]. Accordingly, female smokers have higher levels of adrenal androgens than nonsmoker females, and experience symptoms of estrogen deficiency, including early menopause [76].
Some evidence suggests that smoking is driven more by affect in women than in men, although these findings are somewhat equivocal. Women experience stronger negative affect than men during abstinence, and unlike men, show improvement in affect upon resumption of smoking [77,78]. However, studies of smokers who were not asked to remain abstinent have produced somewhat different findings. When affect was decomposed into more specific domains, sadness was more closely associated with smoking in men vs. women, and the association between happiness and smoking was stronger in women vs. men [79]. Similarly, ecological momentary assessment of smoking did not find a stronger link between negative affect and smoking in women than in men [80].
Male smokers are more sensitive to the rewarding effects of nicotine than women [81], in whom smoking may theoretically be sustained more by conditioned reinforcement from smoking-related cues [82,83] - although notably, men are more influenced by social smoking cues than women [84]. Consistent with these predictions, in a study of cigarettes in which nicotine levels varied but other smoking-related sensorimotor cues did not, men but not women reported liking cigarettes more with higher nicotine yields, and reported greater relief of craving from cigarettes with higher nicotine yields, whereas women reported no dose effects on any self-report measures of craving, withdrawal, affect, or cigarette characteristics [77].
These sex differences in responses to smoking and nicotine sensitivity presumably reflect underlying differences in the primary and downstream neurochemical targets affected by smoking. Male but not female smokers have greater availability of β2-containing nicotinic acetylcholine receptors (nAChRs) in the striatum, cortex, and cerebellum compared to non-smokers [85], consistent with animal studies finding nAChR upregulation in response to nicotine administration in male rats only [86]. These findings suggest that males are more susceptible than females to upregulation of nAChRs. Inasmuch as nicotine promotes dopamine release [87,88] and other indices of dopaminergic function, greater upregulation of nAChRs in men may promote greater reliance on nicotine in a cigarette through an enhancement of the dopaminergic response to smoking. In this regard, women show significantly less ventral and more dorsal striatal dopamine release in response to smoking than do male smokers [89].
Moreover, sex differences in dopaminergic signaling in the brain can influence the response to nicotine in cigarettes and replacement therapies. Although D2-type receptor availability is similar in healthy men and women, evidence suggests that striatal dopaminergic tone is higher in women [12]. Lower striatal D2-type receptor availability measured using [11C]raclopride may reflect higher competing endogenous dopamine concentrations in women compared to men [90], and higher striatal [18F]fluorodopa uptake has suggested greater dopamine synthesis capacity in women vs. men [91].
Although male smokers have lower striatal D2-type receptor availability than corresponding nonsmokers, female smokers do not [92,93]. This difference may reflect a protective effect of midbrain dopamine D2-type autoreceptors. Female smokers have higher D2-type receptor binding potential in the midbrain (substantia nigra/ventral tegmental area), where autoreceptors may contribute to reduced dopamine release and therefore reduced D2-type receptor downregulation in female vs. male smokers [94]. Reduced dopamine release in the striatum also can contribute to a relatively smaller subjective response to nicotine. Midbrain D2-type receptor binding potential is negatively correlated with nicotine dependence in women only, suggesting a protective mechanism: higher densities of midbrain autoreceptors may prevent striatal D2 receptor downregulation, thereby reducing nicotine dependence in women [94].
Another influence on intrasynaptic dopamine and thereby on response to dopamine release is the dopamine transporter (DAT). A preliminary study using single photon emission computed tomography indicated that regardless of smoking status, uptake of [123I]beta-CIT was higher in the striatum, diencephalon, and brainstem in females than in males [95]. Higher DAT concentration can limit the smoking-induced change in intrasynaptic dopamine, thereby reducing both the subjective response to smoking and the downregulation of D2-type receptors by nicotine (or other abused drugs). In addition to the aforementioned note of a sex difference in striatal dopamine release due to smoking [89], men show more dopamine release than women in the ventral striatum in response to amphetamine challenge [53], and throughout the striatum in response to drinking alcohol [96]. These findings suggest that in men, addressing the rewarding effects of drugs may be a more important therapeutic target than in women.
VI. Implications, Future Directions, and Conclusions
Despite temptation to speculate that sex differences in behavior affect reproductive activities only, there are sex differences in dopaminergic signaling that are related to addiction, a maladaptive disorder that does not explicitly facilitate reproduction except insofar as sexual engagement may be a byproduct of drug taking (e.g., links between methamphetamine use and risky sex [97]). Therefore, sex differences related to addiction generally are not adaptations to facilitate reproduction. Genetic and hormonal differences between men and women produce significant differences in dopamine function, which may alter the course of drug consumption and cessation. As initially reviewed in [12], “higher dopaminergic tone in women may protect against the development of ... diseases with established disturbances in dopamine function.” Research in the past decade has supported this observation, especially with respect to substance use disorders, which are less prevalent in women than in men.
Clinical trials that fail to consider male and female patients independently may obscure the value of interventions that would succeed in one sex but not the other. Reducing the nicotine content in cigarettes to non-addictive levels is being considered as a national policy to improve public health [98], an action supported by a large-scale clinical trial [99]. Low nicotine cigarettes have also been tested as a smoking cessation aid [4,100–104]; however, a secondary analysis of one such trial indicated greater efficacy in women than men [105]. Conversely, nicotine replacement therapies show lower efficacy in smoking cessation for women quitting smoking compared to men ([83,106]; but see also [107]). These findings are consistent with evidence from two key studies, demonstrating that women are less sensitive to nicotine than men [77,108]. Faulkner et al. (2017) found that women do not report differences in perceived nicotine levels after smoking cigarettes that ranged in doses of 0.027 to 0.763 mg nicotine delivered, a difference of 28X. Similarly, Jensen et al. (2016) found a statistically significant dose-dependence in intravenous nicotine self-administration in men (doses ranging from 0.1 to 0.4 mg), while women self-administered all doses at the same rate.
Upregulation of D2-type receptors has been suggested as a potential therapeutic target for addictive disorders [26,109], and a recent study found D2 upregulation in female but not male rats following treatment with methylphenidate, which blocks DAT [110]. Varenicline, a partial agonist of α4β2 nicotinic acetylcholine receptors (nAChRs) and full agonist at α7 nAChRs, currently FDA-approved as a smoking cessation therapy, upregulates striatal D2-type receptors in rats [111,112], and is a more effective smoking cessation aid for women than men in the intermediate (24-week) term of abstinence [113]. Sex-specific analyses also indicate that varenicline is a more effective smoking cessation aid for women than bupropion or transdermal nicotine [114]. However, whether the sex difference in varenicline efficacy is related to sex differences in dopaminergic signaling is unknown, and is an important question for future investigations.
Future directions may include a focus on how neuroendocrine factors that differ between men and women may influence addiction. For instance, high endogenous progesterone is associated with better efficacy of transdermal nicotine patch replacement therapy for relief of smoking urges [115], and administration of exogenous progesterone reduces positive subjective effects of smoking cigarettes [116] and cocaine [117]. Smoking cessation therapies may potentially be able to leverage menstrual cycle phase to reduce relapse by identifying the optimal cycle window in which to initiate abstinence; however, findings to date have been mixed, with some studies showing no effect of cycle phase on cessation [118–120], others showing better outcomes when cessation is initiated in the low progesterone follicular phase [121,122], and still others showing better outcomes when cessation is initiated in the luteal phase [123–125] or when progesterone levels are high [126,127]. Expert recommendations to clarify these findings have been made [128], and can be adapted from the smoking literature to inform evaluations of ovarian hormone effects in other substance use disorders.
Gold-standard brain imaging studies to determine effects of ovarian hormone status on striatal dopamine receptor availability and release are also needed, as existing findings are mixed. In the first such investigation, Wong et al. (1988) reported a trend toward lower D2-type receptor binding potential measured with 3-N-[11C]-methylspiperone during the follicular phase compared to the luteal phase [137], and Munro et al. (2006) reported lower binding potential measured with [11C]raclopride in the putamen during the luteal compared to follicular phase [52]. However, Czoty et al. (2009) found higher binding potential measured with [18F]fluoroclebopride in cynomolgus monkeys [135], and Nordstrom et al. (1998) found no statistically significant effect of menstrual phase on binding potential in five women who were scanned twice during varying menstrual cycle phases [136]. All studies involved small Ns (<10 per group), wide menstrual phase windows, and in some cases, a cross-sectional design. Evidently, a larger study with careful monitoring of menstrual phase and a longitudinal design to control for inter-individual variation is needed.
In addition to the focus on sex differences recommended above, attention to endogenous hormone levels, exogenous hormone administration, and genotype may improve efficacy of pharmacological and other therapies that target the dopamine system. Findings from the smoking literature may be useful in developing new hypotheses to evaluate sex differences in dopamine function, and ultimately in tailoring treatments to improve abstinence rates across all addictive disorders.
HIGHLIGHTS:
Sex differences in substance use disorders are related to dopaminergic dysfunction.
Genes underlying dopamine function produce different behaviors in men and women.
Hormone interactions with the dopamine system are a priority for future investigations.
Smoking research provides insight into sex differences in therapeutic targets.
Acknowledgements:
This work was supported by endowments from the Thomas P. and Katherine K. Pike Chair in Addiction Studies (EDL), the Marjorie M. Greene Trust, and the National Institutes of Health (NP) [F32 DA039715]. The funding sources had no role in the review and interpretation of literature, in preparing this manuscript, or in the decision to submit it for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.SAMHSA (2016) Results from the 2016 National Survey on Drug Use and Health: Detailed Tables.
- 2.Fattore L, Altea S, Fratta W (2008). Sex differences in drug addiction: a review of animal and human studies. Womens Health (Lond), 4, 51–65. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez-Avila CA, Rounsaville BJ, Kranzler HR (2004). Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend, 74(3), 265–272. [DOI] [PubMed] [Google Scholar]
- 4.Becker JB, Hu M (2008). Sex differences in drug abuse. Front Neuroendocrinol, 29(1), 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Creanga AA, Berg CJ, Ko JY, Farr SL, Tong VT, Bruce FC, Callaghan WM (2014). Maternal mortality and morbidity in the United States: where are we now? J Womens Health (Larchmt), 23(1), 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Appelman Y, van Rijn BB, Ten Haaf ME, Boersma E, Peters SA (2015). Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis, 241(1), 211–218. [DOI] [PubMed] [Google Scholar]
- 7.Hanna EZ, Chou SP, Grant BF (1997). The relationship between drinking and heart disease morbidity in the United States: results from the National Health Interview Survey. Alcohol Clin Exp Res, 21(1), 111–118. [PubMed] [Google Scholar]
- 8.Nolen-Hoeksema S (2004). Gender differences in risk factors and consequences for alcohol use and problems. Clin Psychol Rev, 24(8), 981–1010. [DOI] [PubMed] [Google Scholar]
- 9.Clayton JA (2016). Studying both sexes: a guiding principle for biomedicine. FASEB J, 30(2), 519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker JB, Koob GF (2016). Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev, 68(2), 242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fattore L, Fadda P, Fratta W (2009). Sex differences in the self-administration of cannabinoids and other drugs of abuse. Psychoneuroendocrinology, 34 Suppl 1, S227–236. [DOI] [PubMed] [Google Scholar]
- 12.Cosgrove KP, Mazure CM, Staley JK (2007). Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry, 62(8), 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schultz W (2006). Behavioral theories and the neurophysiology of reward. Annu Rev Psychol, 57, 87–115. [DOI] [PubMed] [Google Scholar]
- 14.Schultz W (2015). Neuronal Reward and Decision Signals: From Theories to Data. Physiol Rev, 95(3), 853–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW (2007). Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science, 315(5816), 1267–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobbs LK, Lemos JC, Alvarez VA (2017). Restructuring of basal ganglia circuitry and associated behaviors triggered by low striatal D2 receptor expression: implications for substance use disorders. Genes Brain Behav, 16(1), 56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maldonado R, Saiardi A, Valverde O, Samad TA, Roques BP, Borrelli E (1997). Absence of opiate rewarding effects in mice lacking dopamine D2 receptors. Nature, 388(6642), 586–589. [DOI] [PubMed] [Google Scholar]
- 18.Nader MA, Czoty PW (2005). PET imaging of dopamine D2 receptors in monkey models of cocaine abuse: genetic predisposition versus environmental modulation. Am J Psychiatry, 162(8), 1473–1482. [DOI] [PubMed] [Google Scholar]
- 19.Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH (2006). PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci, 9(8), 1050–1056. [DOI] [PubMed] [Google Scholar]
- 20.Phillips TJ, Brown KJ, Burkhart-Kasch S, Wenger CD, Kelly MA, Rubinstein M, Grandy DK, Low MJ (1998). Alcohol preference and sensitivity are markedly reduced in mice lacking dopamine D2 receptors. Nat Neurosci, 1(7), 610–615. [DOI] [PubMed] [Google Scholar]
- 21.Spealman RD (1990). Antagonism of behavioral effects of cocaine by selective dopamine receptor blockers. Psychopharmacology (Berl), 101(1), 142–145. [DOI] [PubMed] [Google Scholar]
- 22.Thanos PK, Volkow ND, Freimuth P, Umegaki H, Ikari H, Roth G, Ingram DK, Hitzemann R (2001). Overexpression of dopamine D2 receptors reduces alcohol self-administration. J Neurochem, 78(5), 1094–1103. [DOI] [PubMed] [Google Scholar]
- 23.Gould RW, Duke AN, Nader MA (2014). PET studies in nonhuman primate models of cocaine abuse: translational research related to vulnerability and neuroadaptations. Neuropharmacology, 84, 138–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groman SM, Lee B, Seu E, James AS, Feiler K, Mandelkern MA, London ED, Jentsch JD (2012). Dysregulation of D(2)-mediated dopamine transmission in monkeys after chronic escalating methamphetamine exposure. J Neurosci, 32(17), 5843–5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volkow ND, Fowler JS, Wang GJ (2003). The addicted human brain: insights from imaging studies. J Clin Invest, 111(10), 1444–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volkow ND, Fowler JS, Wang GJ, Swanson JM (2004). Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry, 9(6), 557–569. [DOI] [PubMed] [Google Scholar]
- 27.Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F (2007). Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol, 64(11), 1575–1579. [DOI] [PubMed] [Google Scholar]
- 28.Bechara A (2005). Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci, 8(11), 1458–1463. [DOI] [PubMed] [Google Scholar]
- 29.Grant S, Contoreggi C, London ED (2000). Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia, 38(8), 1180–1187. [DOI] [PubMed] [Google Scholar]
- 30.Groman SM, Lee B, London ED, Mandelkern MA, James AS, Feiler K, Rivera R, Dahlbom M, Sossi V, Vandervoort E, Jentsch JD (2011). Dorsal striatal D2-like receptor availability covaries with sensitivity to positive reinforcement during discrimination learning. J Neurosci, 31(20), 7291–7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee B, Groman S, London ED, Jentsch JD (2007). Dopamine D2/D3 receptors play a specific role in the reversal of a learned visual discrimination in monkeys. Neuropsychopharmacology, 32(10), 2125–2134. [DOI] [PubMed] [Google Scholar]
- 32.Argyriou E, Um M, Carron C, Cyders MA (2018). Age and impulsive behavior in drug addiction: A review of past research and future directions. Pharmacol Biochem Behav, 164, 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.London ED, Kohno M, Morales AM, Ballard ME (2015). Chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging. Brain Res, 1628(Pt A), 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trifilieff P, Martinez D (2014). Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity. Neuropharmacology, 76 Pt B, 498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monterosso JR, Aron AR, Cordova X, Xu J, London ED (2005). Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend, 79(2), 273–277. [DOI] [PubMed] [Google Scholar]
- 36.Sion A, Jurado-Barba R, Alonso MJ, Rubio-Valladolid G (2017). Inhibitory capacity assessment in alcohol dependent patients: translation from a modified stop signal task. Actas Esp Psiquiatr, 45(1), 21–31. [PubMed] [Google Scholar]
- 37.Zeng H, Su D, Jiang X, Zhu L, Ye H (2016). The similarities and differences in impulsivity and cognitive ability among ketamine, methadone, and non-drug users. Psychiatry Res, 243, 109–114. [DOI] [PubMed] [Google Scholar]
- 38.Moreno-Lopez L, Perales JC, van Son D, Albein-Urios N, Soriano-Mas C, Martinez-Gonzalez JM, Wiers RW, Verdejo-Garcia A (2015). Cocaine use severity and cerebellar gray matter are associated with reversal learning deficits in cocaine-dependent individuals. Addict Biol, 20(3), 546–556. [DOI] [PubMed] [Google Scholar]
- 39.Patzelt EH, Kurth-Nelson Z, Lim KO, MacDonald AW 3rd, (2014). Excessive state switching underlies reversal learning deficits in cocaine users. Drug Alcohol Depend, 134, 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghahremani DG, Lee B, Robertson CL, Tabibnia G, Morgan AT, De Shetler N, Brown AK, Monterosso JR, Aron AR, Mandelkern MA, Poldrack RA, London ED (2012). Striatal dopamine D(2)/D(3) receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. J Neurosci, 32(21), 7316–7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robertson CL, Ishibashi K, Mandelkern MA, Brown AK, Ghahremani DG, Sabb F, Bilder R, Cannon T, Borg J, London ED (2015). Striatal D1 - and D2-type dopamine receptors are linked to motor response inhibition in human subjects. J Neurosci, 35(15), 5990–5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bickel WK, Marsch LA (2001). Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction, 96(1), 73–86. [DOI] [PubMed] [Google Scholar]
- 43.Hofmeyr A, Monterosso J, Dean AC, Morales AM, Bilder RM, Sabb FW, London ED (2017). Mixture models of delay discounting and smoking behavior. Am J Drug Alcohol Abuse, 43(3), 271–280. [DOI] [PubMed] [Google Scholar]
- 44.Kirby KN, Petry NM, Bickel WK (1999). Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen, 128(1), 78–87. [DOI] [PubMed] [Google Scholar]
- 45.Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED (2007). Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp, 28(5), 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ballard ME, Mandelken MA, Monterosso JR, Hsu E, Robertson CL, Ishibashi K, Dean AC, London ED (2015). Low Dopamine D2/D3 Receptor Availability is Associated with Steep Discounting of Delayed Rewards in Methamphetamine Dependence. Int J Neuropsychopharmacol, 18(7), pyu119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kohno M, Morales AM, Ghahremani DG, Hellemann G, London ED (2014). Risky decision making, prefrontal cortex, and mesocorticolimbic functional connectivity in methamphetamine dependence. JAMA Psychiatry, 71(7), 812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kohno M, Nurmi EL, Laughlin CP, Morales AM, Gail EH, Hellemann GS, London ED (2016). Functional Genetic Variation in Dopamine Signaling Moderates Prefrontal Cortical Activity During Risky Decision Making. Neuropsychopharmacology, 41(3), 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colzato LS, Hertsig G, van den Wildenberg WP, Hommel B (2010). Estrogen modulates inhibitory control in healthy human females: evidence from the stop-signal paradigm. Neuroscience, 167(3), 709–715. [DOI] [PubMed] [Google Scholar]
- 50.Hatta T, Nagaya K (2009). Menstrual cycle phase effects on memory and Stroop task performance. Arch Sex Behav, 38(5), 821–827. [DOI] [PubMed] [Google Scholar]
- 51.Thompson TL, Moss RL (1994). Estrogen regulation of dopamine release in the nucleus accumbens: genomic- and nongenomic-mediated effects. J Neurochem, 62(5), 1750–1756. [DOI] [PubMed] [Google Scholar]
- 52.Bazzett TJ, Becker JB (1994). Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res, 637(1–2), 163–172. [DOI] [PubMed] [Google Scholar]
- 53.Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, Kuwabara H, Kumar A, Alexander M, Ye W, Wand GS (2006). Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry, 59(10), 966–974. [DOI] [PubMed] [Google Scholar]
- 54.Weinberger AH, Smith PH, Allen SS, Cosgrove KP, Saladin ME, Gray KM, Mazure CM, Wetherington CL, McKee SA (2015). Systematic and meta-analytic review of research examining the impact of menstrual cycle phase and ovarian hormones on smoking and cessation. Nicotine Tob Res, 17(4), 407–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hinderaker K, Allen AM, Tosun N, al’Absi M, Hatsukami D, Allen SS (2015). The effect of combination oral contraceptives on smoking-related symptomatology during short-term smoking abstinence. Addict Behav, 41, 148–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dickmann PJ, Mooney ME, Allen SS, Hanson K, Hatsukami DK (2009). Nicotine withdrawal and craving in adolescents: effects of sex and hormonal contraceptive use. Addict Behav, 34(6–7), 620–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Allen AM, Carlson S, Eberly LE, Hatsukami D, Piper ME (2018). Else of hormonal contraceptives and smoking cessation: A preliminary report. Addict Behav, 76, 236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dewing P, Chiang CW, Sinchak K, Sim H, Fernagut PO, Kelly S, Chesselet MF, Micevych PE, Albrecht KH, Harley VR, Vilain E (2006). Direct regulation of adult brain function by the male-specific factor SRY. Curr Biol, 16(4), 415–420. [DOI] [PubMed] [Google Scholar]
- 59.Milsted A, Serova L, Sabban EL, Dunphy G, Turner ME, Ely DL (2004). Regulation of tyrosine hydroxylase gene transcription by Sry. Neurosci Lett, 369(3), 203–207. [DOI] [PubMed] [Google Scholar]
- 60.Blum M, McEwen BS, Roberts JL (1987). Transcriptional analysis of tyrosine hydroxylase gene expression in the tuberoinfundibular dopaminergic neurons of the rat arcuate nucleus after estrogen treatment. J Biol Chem, 262(2), 817–821. [PubMed] [Google Scholar]
- 61.Lloyd T, Weisz J (1978). Direct inhibition of tyrosine hydroxylase activity by catechol estrogens. J Biol Chem, 253(14), 4841–4843. [PubMed] [Google Scholar]
- 62.Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR (2004). Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet, 75(5), 807–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Slifstein M, Kolachana B, Simpson EH, Tabares P, Cheng B, Duvall M, Frankie WG, Weinberger DR, Laruelle M, Abi-Dargham A (2008). COMT genotype predicts cortical-limbic D1 receptor availability measured with [11CJNNC112 and PET. Mol Psychiatry, 13(8), 821–827. [DOI] [PubMed] [Google Scholar]
- 64.Kaasinen V, Nagren K, Hietala J, Farde L, Rinne JO (2001). Sex differences in extrastriatal dopamine d(2)-like receptors in the human brain. Am J Psychiatry, 158(2), 308–311. [DOI] [PubMed] [Google Scholar]
- 65.Jacobs E, D’Esposito M (2011). Estrogen shapes dopamine-dependent cognitive processes: implications for women’s health. J Neurosci, 31(14), 5286–5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith CT, Sierra Y, Oppler SH, Boettiger CA (2014). Ovarian cycle effects on immediate reward selection bias in humans: a role for estradiol. J Neurosci, 34(16), 5468–5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gjedde A, Kumakura Y, Cumming P, Linnet J, Moller A (2010). Inverted-U-shaped correlation between dopamine receptor availability in striatum and sensation seeking. Proc Natl Acad Sci U S A, 107(8), 3870–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cools R, D’Esposito M (2011). Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry, 69(12), e113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dani JA (2003). Roles of dopamine signaling in nicotine addiction. Mol Psychiatry, 8(3), 255– 256. [DOI] [PubMed] [Google Scholar]
- 70.Nedic G, Nikolac M, Borovecki F, Hajnsek S, Muck-Seler D, Pivac N (2010). Association study of a functional catechol-O-methyltransferase polymorphism and smoking in healthy Caucasian subjects. Neurosci Lett, 473(3), 216–219. [DOI] [PubMed] [Google Scholar]
- 71.Lee MR, Gallen CL, Ross TJ, Kurup P, Salmeron BJ, Hodgkinson CA, Goldman D, Stein EA, Enoch MA (2013). A preliminary study suggests that nicotine and prefrontal dopamine affect cortico-striatal areas in smokers with performance feedback. Genes Brain Behav, 12(5), 554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herman AI, Jatlow PI, Gelernter J, Listman JB, Sofuoglu M (2013). COMT Val158Met modulates subjective responses to intravenous nicotine and cognitive performance in abstinent smokers. Pharmacogenomics J, 13(6), 490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barbieri RL, Gochberg J, Ryan KJ (1986). Nicotine, cotinine, and anabasine inhibit aromatase in human trophoblast in vitro. J Clin Invest, 77(6), 1727–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Biegon A (2016). In vivo visualization of aromatase in animals and humans. Front Neuroendocrinol, 40, 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Biegon A, Kim SW, Alexoff DL, Jayne M, Carter P, Hubbard B, King P, Logan J, Muench L, Pareto D, Schlyer D, Shea C, Telang F, Wang GJ, Xu Y, Fowler JS (2010). Unique distribution of aromatase in the human brain: in vivo studies with PET and [N-methyl-11]vorozole. Synapse, 64(11), 801–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baron JA, La Vecchia C, Levi F (1990). The antiestrogenic effect of cigarette smoking in women. Am J Obstet Gynecol, 162(2), 502–514. [DOI] [PubMed] [Google Scholar]
- 77.*Faulkner P, Petersen N, Ghahremani DG, Cox CM, Tyndale RF, Hellemann GS, London ED (2017). Sex differences in tobacco withdrawal and responses to smoking reduced-nicotine cigarettes in young smokers. Psychopharmacology (Berl).In this experiment, young male and female smokers evaluated their subjective responses to cigarettes with reduced nicotine content. Women reported equal relief of craving, withdrawal, and negative affect in response to smoking any cigarette, even those with approximately l/25th as much nicotine as conventional cigarettes.
- 78.Xu J, Azizian A, Monterosso J, Domier CP, Brody AL, Fong TW, London ED (2008). Gender effects on mood and cigarette craving during early abstinence and resumption of smoking. Nicotine Tob Res, 10(11), 1653–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Delfino RJ, Jamner LD, Whalen CK (2001). Temporal analysis of the relationship of smoking behavior and urges to mood states in men versus women. Nicotine Tob Res, 3(3), 235–248. [DOI] [PubMed] [Google Scholar]
- 80.Shiffman S, Rathbun SL (2011). Point process analyses of variations in smoking rate by setting, mood, gender, and dependence. Psychol Addict Behav, 25(3), 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perkins KA, Doyle T, Ciccocioppo M, Conklin C, Sayette M, Caggiula A (2006). Sex differences in the influence of nicotine dose instructions on the reinforcing and self-reported rewarding effects of smoking. Psychopharmacology (Berl), 184(3–4), 600–607. [DOI] [PubMed] [Google Scholar]
- 82.Perkins KA, Gerlach D, Vender J, Grobe J, Meeker J, Hutchison S (2001). Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine Tob Res, 3(2), 141–150. [DOI] [PubMed] [Google Scholar]
- 83.Perkins KA, Scott J (2008). Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res, 10(7), 1245–1250. [DOI] [PubMed] [Google Scholar]
- 84.Ferguson SG, Frandsen M, Dunbar MS, Shiffman S (2015). Gender and stimulus control of smoking behavior. Nicotine Tob Res, 17(4), 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cosgrove KP, Esterlis I, McKee SA, Bois F, Seibyl JP, Mazure CM, Krishnan-Sarin S, Staley JK, Picciotto MR, O’Malley SS (2012). Sex differences in availability of beta2*-nicotinic acetylcholine receptors in recently abstinent tobacco smokers. Arch Gen Psychiatry, 69(4), 418– 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koylu E, Demirgoren S, London ED, Pogun S (1997). Sex difference in up-regulation of nicotinic acetylcholine receptors in rat brain. Life Sci, 61(12), PL 185–190. [DOI] [PubMed] [Google Scholar]
- 87.Imperato A, Mulas A, Di Chiara G (1986). Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Ear J Pharmacol, 132(2–3), 337–338. [DOI] [PubMed] [Google Scholar]
- 88.Zhang H, Sulzer D (2004). Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci, 7(6), 581–582. [DOI] [PubMed] [Google Scholar]
- 89.**Cosgrove KP, Wang S, Kim SJ, McGovern E, Nabulsi N, Gao H, Labaree D, Tagare HD, Sullivan JM, Morris ED (2014). Sex differences in the brain’s dopamine signature of cigarette smoking. J Neurosci, 34(50), 16851–16855.Using a novel and sophisticated image analysis technique (lp-ntPET), Cosgrove et al. measured brief and regionally specific dopamine release as male and female smokers engaged in smoking. Consistent with ample previous behavioral evidence, these data showed greater ventral striatal dopamine release in response to smoking in men, and faster dorsal striatal dopamine release in response to smoking in women.
- 90.Pohjalainen T, Rinne JO, Nagren K, Syvalahti E, Hietala J (1998). Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. Am J Psychiatry, 155(6), 768–773. [DOI] [PubMed] [Google Scholar]
- 91.Laakso A, Vilkman H, Bergman J, Haaparanta M, Solin O, Syvalahti E, Salokangas RK, Hietala J (2002). Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Biol Psychiatry, 52(7), 759–763. [DOI] [PubMed] [Google Scholar]
- 92.Brown AK, Mandelkern MA, Farahi J, Robertson C, Ghahremani DG, Sumerel B, Moallem N, London ED (2012). Sex differences in striatal dopamine D2/D3 receptor availability in smokers and non-smokers. Int J Neuropsychopharmacol, 15(7), 989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fehr C, Yakushev I, Hohmann N, Buchholz HG, Landvogt C, Deckers H, Eberhardt A, Klager M, Smolka MN, Scheurich A, Dielentheis T, Schmidt LG, Rosch F, Bartenstein P, Grunder G, Schreckenberger M (2008). Association of low striatal dopamine d2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. Am J Psychiatry, 165(4), 507– 514. [DOI] [PubMed] [Google Scholar]
- 94.*Okita K, Petersen N, Robertson CL, Dean AC, Mandelkern MA, London ED (2016). Sex Differences in Midbrain Dopamine D2-Type Receptor Availability and Association with Nicotine Dependence. Neuropsychopharmacology, 41(12), 2913–2919.Using [18F]fallypride PET imaging, the authors discovered higher midbrain dopamine D2-type receptor binding in female smokers compared to non-smokers, which correlated negatively with level of nicotine dependence in women only. This suggests a potential sex-specific protective mechanism against nicotine dependence.
- 95.Staley JK, Krishnan-Sarin S, Zoghbi S, Tamagnan G, Fujita M, Seibyl JP, Maciejewski PK, O’Malley S, Innis RB (2001). Sex differences in [123I]beta-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse, 41(4), 275– 284. [DOI] [PubMed] [Google Scholar]
- 96.Urban NB, Kegeles LS, Slifstein M, Xu X, Martinez D, Sakr E, Castillo F, Moadel T, O’Malley SS, Krystal JH, Abi-Dargham A (2010). Sex differences in striatal dopamine release in young adults after oral alcohol challenge: a positron emission tomography imaging study with [(l)(l)C]raclopride. Biol Psychiatry, 68(8), 689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Iritani BJ, Hallfors DD, Bauer DJ (2007). Crystal methamphetamine use among young adults in the USA. Addiction, 102(7), 1102–1113. [DOI] [PubMed] [Google Scholar]
- 98.Apelberg BJ, Feirman SP, Salazar E, Corey CG, Ambrose BK, Paredes A, Richman E, Verzi SJ, Vugrin ED, Brodsky NS, Rostron BL (2018). Potential Public Health Effects of Reducing Nicotine Levels in Cigarettes in the United States. N Engl J Med. [DOI] [PubMed] [Google Scholar]
- 99.Donny EC, Denlinger RL, Tidey JW, Koopmeiners JS, Benowitz NL, Vandrey RG, al’Absi M, Carmella SG, Cinciripini PM, Dermody SS, Drobes DJ, Hecht SS, Jensen J, Lane T, Le CT, McClernon FJ, Montoya ID, Murphy SE, Robinson ID, Stitzer ML, Strasser AA, Tindle H, Hatsukami DK (2015). Randomized Trial of Reduced-Nicotine Standards for Cigarettes. N Engl J Med, 373(14), 1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hatsukami DK, Kotlyar M, Hertsgaard LA, Zhang Y, Carmella SG, Jensen JA, Allen SS, Shields PG, Murphy SE, Stepanov I, Hecht SS (2010). Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction, 105(2), 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rezaishiraz H, Hyland A, Mahoney MC, O’Connor RJ, Cummings KM (2007). Treating smokers before the quit date: can nicotine patches and denicotinized cigarettes reduce cravings? Nicotine Tob Res, 9(11), 1139–1146. [DOI] [PubMed] [Google Scholar]
- 102.Rose JE, Behm FM, Westman EC, Kukovich P (2006). Precessation treatment with nicotine skin patch facilitates smoking cessation. Nicotine Tob Res, 8(1), 89–101. [DOI] [PubMed] [Google Scholar]
- 103.Walker N, Bullen C, McRobbie H (2009). Reduced-nicotine content cigarettes: Is there potential to aid smoking cessation? Nicotine Tob Res, 11(11), 1274–1279. [DOI] [PubMed] [Google Scholar]
- 104.Walker N, Howe C, Bullen C, Grigg M, Glover M, McRobbie H, Laugesen M, Parag V, Whittaker R (2012). The combined effect of very low nicotine content cigarettes, used as an adjunct to usual Quitline care (nicotine replacement therapy and behavioural support), on smoking cessation: a randomized controlled trial. Addiction, 107(10), 1857–1867. [DOI] [PubMed] [Google Scholar]
- 105.Vogel RI, Hertsgaard LA, Dermody SS, Luo X, Moua L, Allen S, al’Absi M, Hatsukami DK (2014). Sex differences in response to reduced nicotine content cigarettes. Addict Behav, 39(7), 1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cepeda-Benito A, Reynoso JT, Erath S (2004). Meta-analysis of the efficacy of nicotine replacement therapy for smoking cessation: differences between men and women. J Consult Clin Psychol, 72(4), 712–722. [DOI] [PubMed] [Google Scholar]
- 107.Smith PH, Kasza KA, Hyland A, Fong GT, Borland R, Brady K, Carpenter MJ, Hartwell K, Cummings KM, McKee SA (2015). Gender differences in medication use and cigarette smoking cessation: results from the International Tobacco Control Four Country Survey. Nicotine Tob Res, 17(4), 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jensen KP, DeVito EE, Valentine G, Gueorguieva R, Sofuoglu M (2016). Intravenous Nicotine Self-Administration in Smokers: Dose-Response Function and Sex Differences. Neuropsychopharmacology, 41(8), 2034–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Okita K, Mandelkern MA, London ED (2016). Cigarette Use and Striatal Dopamine D2/3 Receptors: Possible Role in the Link between Smoking and Nicotine Dependence. Int J Neuropsychopharmacol, 19(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Izquierdo A, Pozos H, Torre Ade L, DeShields S, Cevallos J, Rodriguez J, Stolyarova A (2016). Sex differences, learning flexibility, and striatal dopamine D1 and D2 following adolescent drug exposure in rats. Behav Brain Res, 308, 104–114.Rats administered methylphenidate or saline during adolescence were later tested on discrimination and reversal learning. Female but not male rats who received methylphenidate had higher D2 receptor expression in adulthood compared to saline-treated animals.
- 111.Crunelle CL, de Wit TC, de Bruin K, Ramakers RM, van der Have F, Beekman FJ, van den Brink W, Booij J (2012). Varenicline increases in vivo striatal dopamine D2/3 receptor binding: an ultra-high-resolution pinhole [123I]IBZM SPECT study in rats. Nucl Med Biol, 39(5), 640–644. [DOI] [PubMed] [Google Scholar]
- 112.Crunelle CL, Schulz S, de Bruin K, Miller ML, van den Brink W, Booij J (2011). Dose-dependent and sustained effects of varenicline on dopamine D2/3 receptor availability in rats. Eur Neuropsychopharmacol, 21(2), 205–210. [DOI] [PubMed] [Google Scholar]
- 113.McKee SA, Smith PH, Kaufman M, Mazure CM, Weinberger AH (2016). Sex Differences in Varenicline Efficacy for Smoking Cessation: A Meta-Analysis. Nicotine Tob Res, 18(5), 1002– 1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Smith PH, Weinberger AH, Zhang J, Emme E, Mazure CM, McKee SA (2017). Sex Differences in Smoking Cessation Pharmacotherapy Comparative Efficacy: A Network Meta-analysis. Nicotine Tob Res, 19(3), 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pang RD, Liautaud MM, Kirkpatrick MG, Huh J, Monterosso J, Leventhal AM (2017). Ovarian Hormones and Transdermal Nicotine Administration Independently and Synergistically Suppress Tobacco Withdrawal Symptoms and Smoking Reinstatement in the Human Laboratory. Neuropsychopharmacology.In this experiment, female smokers were randomized to receive transdermal nicotine or placebo, and ovarian hormone levels were measured. Transdermal nicotine reduced smoking urges more when progesterone levels were high than low. This rigorous study provides an example for future investigations of neuroendocrine influences on addiction therapies.
- 116.Sofuoglu M, Babb DA, Hatsukami DK (2001). Progesterone treatment during the early follicular phase of the menstrual cycle: effects on smoking behavior in women. Pharmacol Biochem Behav, 69(1–2), 299–304. [DOI] [PubMed] [Google Scholar]
- 117.Evans SM, Foltin RW (2006). Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology, 31(3), 659–674. [DOI] [PubMed] [Google Scholar]
- 118.Allen AM, Allen SS, Widenmier J, Al’absi M (2009). Patterns of cortisol and craving by menstrual phase in women attempting to quit smoking. Addict Behav, 34(8), 632–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Allen SS, Allen AM, Lunos S, Hatsukami DK (2009). Patterns of self-selected smoking cessation attempts and relapse by menstrual phase. Addict Behav, 34(11), 928–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Epperson CN, Toll B, Wu R, Amin Z, Czarkowski KA, Jatlow P, Mazure CM, O’Malley SS (2010). Exploring the impact of gender and reproductive status on outcomes in a randomized clinical trial of naltrexone augmentation of nicotine patch. Drug Alcohol Depend, 112(1–2), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Carpenter MJ, Saladin ME, Leinbach AS, Larowe SD, Upadhyaya HP (2008). Menstrual phase effects on smoking cessation: a pilot feasibility study. J Womens Health (Larchmt), 17(2), 293– 301. [DOI] [PubMed] [Google Scholar]
- 122.Franklin TR, Ehrman R, Lynch KG, Harper D, Sciortino N, O’Brien CP, Childress AR (2008). Menstrual cycle phase at quit date predicts smoking status in an NRT treatment trial: a retrospective analysis. J Womens Health (Larchmt), 17(2), 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Allen SS, Allen AM, Pomerleau CS (2009). Influence of phase-related variability in premenstrual symptomatology, mood, smoking withdrawal, and smoking behavior during ad libitum smoking, on smoking cessation outcome. Addict Behav, 34(1), 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Allen SS, Bade T, Center B, Finstad D, Hatsukami D (2008). Menstrual phase effects on smoking relapse. Addiction, 103(5), 809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mazure CM, Toll B, McKee SA, Wu R, O’Malley SS (2011). Menstrual cycle phase at quit date and smoking abstinence at 6 weeks in an open label trial of bupropion. Drug Alcohol Depend, 114(1), 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Forray A, Gilstad-Hayden K, Suppies C, Bogen D, Sofuoglu M, Yonkers KA (2017). Progesterone for smoking relapse prevention following delivery: A pilot, randomized, doubleblind study. Psychoneuroendocrinology, 86, 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Saladin ME, McClure EA, Baker NL, Carpenter MJ, Ramakrishnan V, Hartwell KJ, Gray KM (2015). Increasing progesterone levels are associated with smoking abstinence among free-cycling women smokers who receive brief pharmacotherapy. Nicotine Tob Res, 17(4), 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wetherill RR, Franklin TR, Allen SS (2016). Ovarian hormones, menstrual cycle phase, and smoking: a review with recommendations for future studies. Curr Addict Rep, 3(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Smethells JR, Swalve NL, Eberly LE, Carroll ME (2016) Sex differences in the reduction of impulsive choice (delay discounting) for cocaine in rats with atomoxetine and progesterone. Psychopharmacology, 233,2999–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Swalve N, Smethells JR Carroll ME (2016). Progesterone attenuates impulsive action in a Go/No-Go task for sucrose pellets in female and male rats. Horm Behav. 85, 43–47.Male and female rats who were administered progesterone subcutaneously showed less impulsive responding in a Go/No-Go task in which the active lever in the task produced sucrose pellets.
- 131.Swalve N, Smethells JR Younk R Mitchell J, Dougen B, Carroll ME. (2018). Sex-specific attenuation of impulsive action by progesterone in a go/no-go task for cocaine in rats. Psychopharmacology (Berl). 235(1), 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gurvich C, & Rossell SL. (2015). Dopamine and cognitive control: Sex-by-genotype interactions influence the capacity to switch attention. Behav Brain Res, 281:96–101.In this large sample of 415 healthy participants, the val/val genotype, associated with relatively (compared to other genotypes) low cortical dopamine levels, was associated with improved attention switching in women but not men.
- 133.Costa DS, Bechara A, de Paula JJ, Romano-Silva MA, Correa H, Lage GM, Miranda DM, Malloy-Diniz LF (2016). Influence of COMT Val158Met polymorphism on emotional decision-making: A sex-dependent relationship? Psychiatry Res, 246: 650–655. [DOI] [PubMed] [Google Scholar]
- 134.Lamb YN, McKay NS, Singh SS, Waldie KE, & Kirk IJ (2016). Catechol-O-methyltransferase val(158)met Polymorphism Interacts with Sex to Affect Face Recognition Ability. Front Psychol, 7:965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Czoty PW, Riddick NV, Gage HD, Sandridge M, Nader SH, Garg S, Bounds M, Garg PK, & Nader MA (2009). Effect of Menstrual Cycle Phase on Dopamine D2 Receptor Availability in Female Cynomolgus Monkeys. Neuropsychopharm, 34: 548–554. [DOI] [PubMed] [Google Scholar]
- 136.Nordstrom A, Olsson H, & Halldin C (1998). A PET study of D2 dopamine receptor density at different phases of the menstrual cycle. Psychiatry Research: Neuroimaging, 83(1): 1–6. [DOI] [PubMed] [Google Scholar]
- 137.Wong DF, Brousselle EP, Wand G, Villemagne V, Dannals RF, Links JM, Zacur HA, Harris J, Naidu S, Braestrup C, Wagner ΗN, & Gjedde A (1988). In Vivo Measurement of Dopamine Receptors in Human Brain by Positron Emission Tomography. Annals New York Academy of Sciences, 515: 203–214. [DOI] [PubMed] [Google Scholar]


