Abstract
Background: Decentralization of the sympathetic nervous system in persons with spinal cord injury (SCI) results in impaired vasomotor and sudomotor activity and, subsequently, impaired thermoregulatory capacity during exercise in the heat. Hyperthermia can be life-threatening and, as such, cooling interventions are needed to prevent this sequela. Objectives: To measure change in core temperature (ΔTC) over time during exercise in normothermic and high ambient heat conditions to compare thermoregulatory capacity in persons with varying degrees of intact vasomotor and sudomotor activity and to determine the efficacy of three cooling interventions in mitigating TC rise. Methods: Three persons participated: a 51-year-old with complete (AIS A) tetraplegia (TP), a 32-year-old with AIS A paraplegia (PP), and a 40-year-old without SCI (AB). Each exercised for 30 minutes on a wheelchair treadmill propelled at 30 revolutions per minute under five different conditions: (1) cool (C) = 75°F without cooling, (2) hot (H) = 90°F without cooling, (3) 90°F with cooling vest (CV), (4) 90°F with water spray (WS), and (5) 90°F with ice slurry ingestion (IS). ΔTC was compared for all conditions in all participants. Results: ΔTC in the C and H conditions was proportional to the neurological level of injury, with Tc rising highest in the TP followed by the PP then AB. WS was most efficacious at mitigating rise in TC followed by IS and CV in TP and PP. None of the cooling interventions provided an added TC cooling effect in AB. Conclusion: WS was most efficacious at mitigating rise in TC in TP>PP during exercise in the heat and should be studied in a larger SCI population.
Keywords: core temperature, spinal cord injury, thermoregulation
Maintenance of core body temperature (TC) requires intact afferent temperature input to the hypothalamus and efferent vasomotor and sudomotor autonomic pathways. After spinal cord injury (SCI), disruption in afferent neural feedback and efferent pathways below the level of injury impairs the body's ability to maintain thermoregulatory stability (ie, persons with SCI have difficulty regulating TC when exposed to environmental heat stress and/or physiological heat stress due to dynamic exercise).1–4 Studies in individuals with paraplegia show that, both at rest and during exercise, the effectiveness of thermoregulatory reflex responses varies according to residual sympathetic function, which is a function of lesion level, and may impact the degree to which vasodilation and sweating responses to heat exposure are compromised.5,6 Interestingly, under heat stress, persons with tetraplegia have repeatedly shown concomitant increases in Tc up to hyperthermic levels of >100.4 °C with minimal to no subjective perception of increased thermal strain.7–9 In summary, if the rise in Tc is not constrained by self-perceived discomfort during exposure to hot ambient temperature and/or exercise, hyperthermia may ensue to levels that are life-threatening in persons with SCI. Additionally, it is well established that exercise-induced increases in TC, especially under high ambient temperatures (30–38°C) and/or high relative humidity (rh; 40%–80%) negatively impacts endurance and performance during exercise,1 which may also adversely impact athletes with SCI.
One must also consider that individuals with SCI have an increased prevalence of cardiovascular disease (CVD), diabetes (DM), and obesity compared to the able-bodied population, for which regular exercise is commonly prescribed.10–13 The inability to regulate TC can thus pose a significant impediment to exercise in persons with SCI and, as a result, the incidence of CVD, DM, and obesity may not be adequately mitigated by physical activity.
In summary, the inability to appropriately regulate Tc during heat stress, whether due to increased Tc from increased muscle metabolism during exercise or from exercise in the heat, may have an adverse impact on longevity, physical health, and athletic performance. As such, effective cooling therapies are needed to prevent such adverse sequelae after SCI.
Whole body cooling by immersion (50–75°F), cooling vests (50–68°F), ice vests (<32°F), cold water (38.5°F), ice slurry (<32°F) ingestion, and hand immersion have been shown to decrease TC during heat stress in persons without SCI.14–20 However, evidence documenting efficacy of such cooling interventions in mitigating the rise in Tc during ambient heat exposure or exercise in the SCI population is limited.21
Within the small pool of literature published to date (n = 11 SCI cooling studies), pre-cooling using water immersion and ice vests combined with ice vests and/or water sprays applied during exercise significantly attenuate the rise in Tc during exercise.21,22 However, the strength of this published evidence is low as these protocols were done in of a wide range of study settings (lab vs field), with mixed groups of persons with SCI, including different levels and completeness of injury, differing types of exercise (aerobic vs anaerobic), varied timing (pre-exercise vs during exercise cooling), and differing body regions of cooling application.
In studying cooling techniques in persons with SCI, it is important to consider that thermoregulatory responses are proportional to the neurological level of SCI.2,5,6,23,24 Evaporative cooling via sweating is the main method of body cooling during exercise in the heat.25 In the SCI population, persons with complete (American Spinal Cord Injury Impairment Scale [AIS] A) paraplegia below levels T8 can elicit sweat rate responses similar to persons without SCI and thus can attenuate TC elevations under heat stress conditions to some extent, whereas persons with complete tetraplegia demonstrate little to no sweating.26–28 Thus, when comparing cooling interventions in persons with SCI, it is important to stratify outcome data by level and completeness of injury to determine the efficacy of the interventions. A recent review on cooling techniques in SCI athletes recommended that “future studies need to be as closely matched as possible.”21 In summary, it is clear that additional controlled studies of cooling interventions are needed to be able to provide SCI clinicians with optimal cooling techniques for use in this unique population.
The aims of this pilot study were to determine the thermoregulatory capacity under heat stress during exercise with no cooling intervention in persons with varying levels of complete (AIS A) SCI and to compare this response to changes in Tc under conditions of (1) a cooling vest, (2) water spray, and (3) ice slurry. Because evaporative cooling is the main homeostatic mechanism by which Tc is maintained during heat stress, we hypothesized that the water spray intervention (ie, artificial sweat) would be most effective. Results from this pilot protocol will help determine which cooling intervention is most efficacious in maintaining TC during exercise under heat stress, which will serve as a guide for a larger clinical trial.
Methods
Subjects
Two persons with complete (AIS A) SCI were recruited and enrolled. Levels of injury were classified into tetraplegia (TP) and paraplegia (PP). One age- and sex-matched able-bodied (AB) male was also recruited. None of these persons regularly performed scheduled exercise activities in thermoneutral or warm environments so heat acclimation (which requires 7–14 consecutive days of exercise) was not likely to be a confounder. Each participant was asked to fast for 3 hours prior to exercise and not exercise the day before or of the study. None of the participants were taking anticholinergics, which may have the potential to alter sweating.
Intervention
At least 3 hours prior to the study, each subject ingested a US Food and Drug Administration (FDA)–approved thermometer pill (CorTemp) to measure Tc at 5-minute intervals throughout the study. On arrival to the lab, participants were asked to empty their bladder to prevent any interruption to the protocol and limit the occurrence of autonomic dysreflexia in the TP subject who would be at risk. The only clothing worn was shorts to allow for the maximum amount of ambient heat input through the skin while at the same time enabling optimal heat dissipation through evaporative or conductive heat loss. After entering an air-tight sealed room heated to 90°F via space heaters with continuous temperature monitoring via sling psychrometer, participants rested for 10 minutes to acclimate to the ambient temperature. Participants then began to exercise on a wheelchair treadmill for 30 minutes at a speed of 30 revolutions per minute counted by a metronome. This protocol was conducted under five different conditions, which were administered in a random order on five different days: (1) cool (C) = 75°F without cooling intervention; (2) hot (H) = 90°F without cooling intervention; (3) 90°F wearing a cooling vest (CV); (4) 90°F applying a water spray (WS) every 5 minutes over face, neck, arms and legs; and (5) 90°F drinking an ice slurry (IS) every 5 minutes. Each exercise session was conducted at the same time of day to control for circadian variation in Tc with 7 days between sessions to avoid heat acclimation. By controlling the ambient environment, randomizing order of sessions, and using a repeated measures design in persons with motor complete (AIS A) injury, we aimed to objectively compare the efficacy of each intervention in a rigorously designed protocol in persons with distinctly varied degrees of intact central sympathetic control, that is, connection from the hypothalamic thermoregulatory center to the efferent sympathetic chain.
Ethical considerations
This study was approved by the local institutional review board. All subjects provided informed consent to participate.
Results
Cool condition
Change in Tc (ΔTC) from 0 to 30 minutes was 0.56, 0.28, and 0.27°F in the TP, PP, and AB participant, respectively. As expected, the TP participant demonstrated twice the ΔTC as compared to the PP and AB participants. Comparison of the results demonstrating ΔTC for H, CV, WS, and IS interventions are presented in Figure 1.
Figure 1.
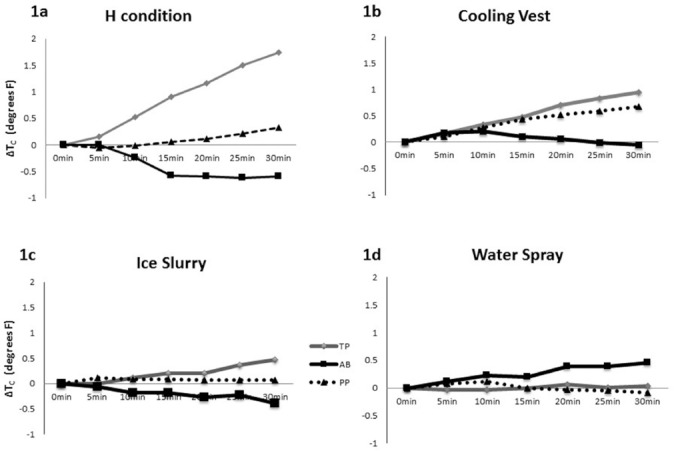
Change in core temperature (ΔTC) from 0 to 30 minutes of heat stress with four conditions: (a) hot (H), (b) cooling vest (CV), (c), ice slurry (IS), and (d) water spray (WS).
Heat stress condition
ΔTC of the H condition of all participants is depicted in Figure 1a. A progressive rise in TC was noted in the TP participant (ΔTC of 1.74°F), whereas there was no appreciable change in Tc in the PP participant (ΔTC of 0.32°F) and a small lowering of Tc in the AB participant (ΔTC of −0.6°F). No sweat responses were observed over the entire body surface area of TP, whereas sweating over the abdomen and legs was seen in PP.
Cooling vest condition
ΔTC in the CV condition is depicted in Figure 1b. Interestingly, TC increased over the course of study in both the TP (ΔTC of 0.95°F) and PP (ΔTC of 0.68°F) participants, whereas Tc was initially increased and then returned to baseline levels in the AB participant.
Ice slurry condition
ΔTC in the IS intervention is depicted in Figure 1c. The rise in TC appeared to be attenuated under the condition of IS in the TP participant compared to the H condition (ΔTC of 0.5°F vs 1.74°F, respectively), whereas Tc continuously fell during the IS condition in the AB participant and was fairly well maintained throughout the IS intervention in the PP participant.
Water spray condition
ΔTC in the WS cooling intervention is depicted in Figure 1d. Unlike all the other conditions where ΔTC was highest in persons with SCI, in this condition, ΔTC was highest in the AB at 0.45°F. Meanwhile, ΔTC of TP and PP remained essentially unchanged at 0.03°F and −0.09°F, respectively.
Comparison of ΔTC
ΔTC over all conditions by participants is presented in Figure 2. As anticipated, the participant with TP had the greatest degree of thermoregulatory dysfunction in the H condition. All three cooling interventions mitigated the rise in TC in the TP subject, whereas only WS and IS mitigated TC rise in the PP subject. Meanwhile, none of the cooling interventions provided an additive core temperature cooling effect in AB.
Figure 2.
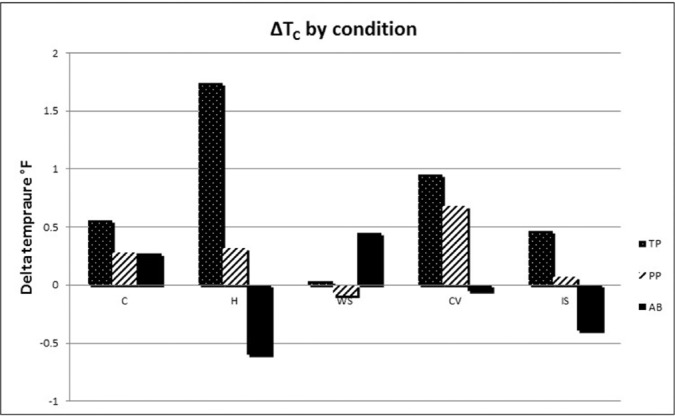
Change in core temperature (ΔTC) over all conditions by participants. AB = able-bodied; CV = cooling vest; H = hot; IS = ice slurry; PP = paraplegia; TP = tetraplegia; WS = water spray.
Figure 3 depicts that the slope of the WS condition was the least of all conditions, indicating it be most efficacious at attenuating ΔTC in TP.
Figure 3.
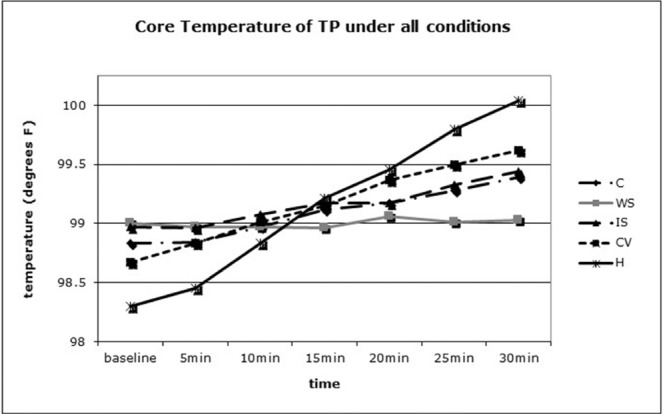
Comparison of core temperature (TC) rise over time in the tetraplegic (TP) participant under each condition. C = cool; CV = cooling vest; H = hot; IS = ice slurry; WS = water spray.
Discussion
This is the first study to compare efficacy of more than two cooling interventions during exercise under heat stress in a controlled setting in persons with complete (AIS A) cervical and thoracic injury compared to an intact individual.
Thermoregulatory responses without cooling intervention
Not surprisingly, the person with TP had the largest rise in TC during exercise under thermoneutral (75°F) and heat stress (90°F) conditions without any cooling intervention. The degree of TC rise from baseline was highest in TP followed by PP and AB, respectively, which confirms previous findings that the balance of heat gain and heat loss via sweating and vasodilation is proportional to the neurological level of SCI.5,23,27
Thermoregulatory responses with various cooling interventions
Cooling vest
Heat energy is constantly transferred from hotter sources to cooler ones (ie, down a thermal gradient). Conductive cooling involves the transfer of heat by direct contact with the skin. Physiologically, when wearing a topical garment that is cooler than the skin temperature, conductive cooling will occur. This response may be enhanced by the increased skin blood flow as occurs with dynamic exercise, thus cooling the peripheral blood and attenuating TC increases. In persons without SCI, ice vests (<32°F) are comparably more efficacious than cooling vests (50–68°F).14
In our study, CV was overall the least effective cooling intervention among the three subjects tested (Figure 3). Most notably, the ΔTC of PP and AB was higher with CV than in the H condition, suggesting the CV worsened heat dissipation. We hypothesize that (1) CV may attenuate or fully prevent evaporative cooling over skin surface area it covers; and/or (2) local cutaneous vasoconstriction of skin surface areas in direct contact with the CV may reduce delivery of heat to the body surface and hence reduce whole body cooling via conduction. CV only mitigated the rise in TC in the TP participant. For this reason, future studies documenting the effectiveness of cooling devices in the SCI population should stratify by lesion level (ie, TP vs PP).
Two studies have investigated the efficacy of the cooling vests (50–68°F) in persons with SCI. A randomized crossover study of 10 men with motor complete (AIS A and B) paraplegia (levels T4-T12) exercising in ambient temperatures with CV demonstrated that the vest reduced skin temperature and perceived thermal sensation but did not affect TC.29 A subsequent experiment of exercise in a normothermic field-based setting (ie, wheelchair rugby and basketball) further showed no significant decrease in TC with use of a cooling vest in a mixed group of persons with tetraplegia and paraplegia of varying levels of completeness.7
In contrast, ice vests (<32°F) in a group of individuals with tetraplegia with varying degrees of completeness of injury have been shown to reduce TC when applied during or before intermittent sprint exercise in the heat.30,31
Ice slurry
Internal heat loss occurs when a cold (<50°F) fluid is ingested and equilibrates toward TC. Heat loss is not magnified with ingestion of IS during heat stress in persons without SCI, as it transiently decreases evaporative and convective cooling through sweating and vasodilation, respectively.32 However, in persons who have physiological disruptions in sweating, such as those with SCI, it may be hypothesized that the cooling effects of the IS are “disproportionally greater.”32 A recent follow-up study reported that cold water ingestion improves heat tolerance in a persons with multiple sclerosis (MS) whose vasomotor and sudomotor activity is impaired from neurological disruption of the thermoregulatory pathways.33
In the present study, IS provided an additive cooling benefit compared to H condition in the persons with SCI (TP and PP), but not the AB, which supports the aforementioned phenomena and parallels the MS study.
Water spray
With evaporative cooling, the heat required to effect a phase change from liquid to vapor is lost to the environment, thus sweat evaporation is responsible for removing heat from the skin surface. Evaporative cooling is the main method of heat dissipation during exercise in high ambient heat in persons without SCI.25 In persons with SCI, previous studies have demonstrated that persons with lesions above T6-T8, with intact sweat responses on more than 50% of total body skin surface area, are able to effectively maintain TC during heat stress nearly as effectively as persons without SCI.26,27 This is consistent with literature from persons without SCI that defines a minimum cutoff of 40% skin surface area of body cooling that is required to prevent rise on TC during heat stress.34 Furthermore, whole body sweat rates in some individuals with paraplegia are similar to matched persons without SCI, which may also explain this ability to maintain TC during high heat strain conditions.35,36 We hypothesize that, if WS is applied to skin surface areas that do not sweat, WS would cause evaporative cooling and attenuate the rise in TC during exercise or high ambient heat exposure. As such, providing artificial sweat in the form of WS to persons with high paraplegia (above T6) may be a promising intervention.
In our study, WS applied to all exposed skin surface areas (ie, face, chest, abdomen, arms, and legs) was most effective at maintaining TC during exercise in the heat in both participants with SCI, but the most profound benefit was in the TP participant who demonstrated no sweating responses over any skin surface area. Meanwhile, WS caused a rise in Tc in the AB participant, who had innate sweating capacity and whose Tc dropped most in the intervention without cooling (ie, H). This suggests no additional Tc cooling benefit of WS applied to skin surface areas with intact endogenous sweating responses, which, of note, has been previously suggested by other human and animal studies.17,37,38
Only one study to date has tested the efficacy of WS in a mixed group (complete and incomplete injuries) of individuals with paraplegia (T3-T12) under heat stress.39 WS was “ineffective in attenuating the onset of heat strain during high-intensity arm exercise in a comfortable environment.”39 It should be noted that WS was applied to the back of head, neck, forearm, and face, which accounts for ~15% body surface area; this may have been too small an area, as mentioned earlier. Furthermore, only persons with paraplegia were studied. This group typically has intact sweating responses over these specific skin surface areas, and it is unknown if applying additional WS in regions with intact sweat responses would have an additive cooling effect, as previously mentioned. Moreover, outcome measures were not stratified by level of paraplegia such as high paraplegic (at/above T6) versus low paraplegic (below T6) to evaluate for differences between these groups with varying thermoregulatory capacities; this might have revealed a main effect of level of injury. Furthermore, as our data and past data suggest, persons with paraplegia do not struggle with progressive increases in TC during exercise, so it may be harder to detect a significant cooling effect, regardless of the intervention, in this population.
Griggs et al combined precooling with an ice vest followed by WS during a 60-minute intermittent sprint exercise protocol and found the combination of precooling with an ice vest and WS significantly attenuated the rise in TC in persons with tetraplegia. When compared to precooling with the ice vest without the WS during exercise, the attenuation in rise of TC was less.40 Thus, WS as an efficient cooling technique for persons with tetraplegia appears to hold promise.
Anecdotally, in SCI athletic events, WS is observed to be the most frequently used external cooling intervention; possibly because it reproduces the evaporative cooling of endogenous sweat that is impaired in persons with levels of injury above T6.
To my knowledge, there are no reported studies examining effects of WS alone on TC and exercise capacity in persons with TP, who have the greatest thermoregulatory dysfunction. This promising approach should be tested.
Exercise intensity considerations
Intensity was not strictly controlled, however all participants propelled the wheelchair at a rate of 30 revolutions per second. Although it is intuitive that higher energy expenditure/intensity would likely increase heat generation, two recent studies have shown the level of injury to play a larger role in rising TC during exercise. Griggs et al in 2015 concluded, despite similar external work (in TP vs PP), a marked increase in TC in TP during exercise and recovery signifies that “thermoregulatory differences between the groups were predominantly due to differences in heat loss.”24(p469) In 2016 Griggs did a study to directly test whether a player's physical impairment or activity profile determined the amount of thermal train experienced during wheelchair rugby match play.41 Players with tetraplegia were under greater thermal strain during wheelchair rugby match play (compared AB persons) “as a result of their reduced heat loss capacity, due to their physical impairment and not because of their activity profile.”41(p177) Despite these data, uncontrolled exercise intensity could still be considered a limitation in this study and should be noted.
Conclusion
This study is the first to compare three cooling interventions during exercise in a controlled heated setting among three persons with varying degrees of neurological control of sudomotor and vasomotor activity. WS attenuated the rise in TC during heat stress most effectively when compared to the CV and IS interventions in both persons with SCI. These findings provide a rationale for a larger study in persons with SCI under exercise-induced heat stress using WS, being sure to cover skin surface areas that do not sweat. Given that those with complete (AIS A) TP demonstrate most thermal dysregulation, this population should be studied first to be able to detect an effect.
Footnotes
Conflicts of Interest
The author has no conflicts of interest to disclose.
REFERENCES
- 1.Price MJ. Preparation of paralympic athletes; environmental concerns and heat acclimation. Front Physiol. 2015;6:415. doi: 10.3389/fphys.2015.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Price MJ. Thermoregulation during exercise in individuals with spinal cord injuries. Sports Med. 2006;36(10):863–879. doi: 10.2165/00007256-200636100-00005. [DOI] [PubMed] [Google Scholar]
- 3.Handrakis JP, Trbovich M, Hagen EM, Price M. Thermodysregulation in persons with spinal cord injury: Case series on use of the autonomic standards. Spinal Cord Ser Cases. 2017;3:17086. doi: 10.1038/s41394-017-0026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawka MN, Latzka WA, Pandolf KB. Temperature regulation during upper body exercise: Able-bodied and spinal cord injured. Med Sci Sports Exerc. 1989;21(5 suppl):S132–140. [PubMed] [Google Scholar]
- 5.Price MJ, Campbell IG. Effects of spinal cord lesion level upon thermoregulation during exercise in the heat. Med Sci Sports Exerc. 2003;35(7):1100–1107. doi: 10.1249/01.MSS.0000074655.76321.D7. [DOI] [PubMed] [Google Scholar]
- 6.Popa C, Popa F, Grigorian VT et al. Vascular dysfunctions following spinal cord injury. J Med Life. 2010;3(3):275–285. [PMC free article] [PubMed] [Google Scholar]
- 7.Trbovich M, Ortega C, Schroeder J, Fredrickson M. Effect of a cooling vest on core temperature in athletes with and without spinal cord injury. Top Spinal Cord Inj Rehabil. 2014;20(1):70–80. doi: 10.1310/sci2001-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goosey-Tolfrey VL, Diaper NJ, Crosland J, Tolfrey K. Fluid intake during wheelchair exercise in the heat: Effects of localized cooling garments. Int J Sports Physiol Perform. 2008;3(2):145–156. doi: 10.1123/ijspp.3.2.145. [DOI] [PubMed] [Google Scholar]
- 9.Trbovich MB, Kiratli JB, Price MJ. The effects of a heat acclimation protocol in persons with spinal cord injury. J Therm Biol. 2016;62(Pt A):56–62. doi: 10.1016/j.jtherbio.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Cragg JJ, Noonan VK, Krassioukov A, Borisoff J. Cardiovascular disease and spinal cord injury: Results from a national population health survey. Neurology. 2013;81(8):723–728. doi: 10.1212/WNL.0b013e3182a1aa68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cragg JJ, Noonan VK, Dvorak M, Krassioukov A, Mancini GB, Borisoff JF. Spinal cord injury and type 2 diabetes: Results from a population health survey. Neurology. 2013;81(21):1864–1868. doi: 10.1212/01.wnl.0000436074.98534.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: An overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil. 2007;86(2):142–152. doi: 10.1097/PHM.0b013e31802f0247. [DOI] [PubMed] [Google Scholar]
- 13.Gater DR., Jr. Obesity after spinal cord injury. Phys Med Rehabil Clin N Am. 2007;18(2):333–351. vii. doi: 10.1016/j.pmr.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Bongers CC, Hopman MT, Eijsvogels TM. Cooling interventions for athletes: An overview of effectiveness, physiological mechanisms, and practical considerations. Temperature (Austin) 2017;4(1):60–78. doi: 10.1080/23328940.2016.1277003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barr D, Reilly T, Gregson W. The impact of different cooling modalities on the physiological responses in firefighters during strenuous work performed in high environmental temperatures. Eur J Appl Physiol. 2011;111(6):959–967. doi: 10.1007/s00421-010-1714-1. [DOI] [PubMed] [Google Scholar]
- 16.Ruddock A, Robbins B, Tew G, Bourke L, Purvis A. Practical cooling strategies during continuous exercise in hot environments: A systematic review and meta-analysis. Sports Med. 2017;47(3):517–532. doi: 10.1007/s40279-016-0592-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens CJ, Kittel A, Sculley DV, Callister R, Taylor L, Dascombe BJ. Running performance in the heat is improved by similar magnitude with pre-exercise cold-water immersion and mid-exercise facial water spray. J Sports Sci. 2017;35(8):798–805. doi: 10.1080/02640414.2016.1192294. [DOI] [PubMed] [Google Scholar]
- 18.Yazdanirad S, Dehghan H. Designing of the cooling vest from paraffin compounds and evaluation of its impact under laboratory hot conditions. Int J Prev Med. 2016;7:47. doi: 10.4103/2008-7802.177890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mejuto G, Chalmers S, Gilbert S, Bentley D. The effect of ice slurry ingestion on body temperature and cycling performance in competitive athletes. J Therm Biol. 2018;72:143–147. doi: 10.1016/j.jtherbio.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Khomenok GA, Hadid A, Preiss-Bloom O et al. Hand immersion in cold water alleviating physiological strain and increasing tolerance to uncompensable heat stress. Eur J Appl Physiol. 2008;104(2):303–309. doi: 10.1007/s00421-008-0693-y. [DOI] [PubMed] [Google Scholar]
- 21.Griggs KE, Price MJ, Goosey-Tolfrey VL. Cooling athletes with a spinal cord injury. Sports Med. 2015;45(1):9–21. doi: 10.1007/s40279-014-0241-3. [DOI] [PubMed] [Google Scholar]
- 22.Forsyth P, Pumpa K, Knight E, Miller J. Physiological and perceptual effects of precooling in wheelchair basketball athletes. J Spinal Cord Med. 2016;39(6):671–678. doi: 10.1080/10790268.2016.1180098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Normell LA. Distribution of impaired cutaneous vasomotor and sudomotor function in paraplegic man. Scand J Clin Lab Invest Suppl. 1974;138:25–41. [PubMed] [Google Scholar]
- 24.Griggs KE, Leicht CA, Price MJ, Goosey-Tolfrey VL. Thermoregulation during intermittent exercise in athletes with a spinal-cord injury. Int J Sports Physiol Perform. 2015;10(4):469–475. doi: 10.1123/ijspp.2014-0361. [DOI] [PubMed] [Google Scholar]
- 25.Casa DJ, Guskiewicz KM, Anderson SA et al. National athletic Trainers' Association position statement: Preventing sudden death in sports. J Athl Train. 2012;47(1):96–118. doi: 10.4085/1062-6050-47.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Downey JA, Huckaba CE, Kelley PS, Tam HS, Darling RC, Cheh HY. Sweating responses to central and peripheral heating in spinal man. J Appl Physiol. 1976;40(5):701–706. doi: 10.1152/jappl.1976.40.5.701. [DOI] [PubMed] [Google Scholar]
- 27.Guttmann L, Silver J, Wyndham CH. Thermoregulation in spinal man. J Physiol. 1958;142(3):406–419. doi: 10.1113/jphysiol.1958.sp006026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trbovich M, Wu Y, Kellogg DL. Vasomotor and sudomotor activity during heat stress in persons with spinal cord injury. Poster presented at American Spinal Injury Assocation Annual Meeting; 2018; http://meetings.asia-spinalinjury.org/meetings/2018/guide/posters/uploads/70--P-99.pdf. [Google Scholar]
- 29.Bongers CC, Eijsvogels TM, van Nes IJ, Hopman MT, Thijssen DH. Effects of cooling during exercise on thermoregulatory responses of men with paraplegia. Phys Ther. 2016;96(5):650–658. doi: 10.2522/ptj.20150266. [DOI] [PubMed] [Google Scholar]
- 30.Webborn N, Price MJ, Castle P, Goosey-Tolfrey VL. Cooling strategies improve intermittent sprint performance in the heat of athletes with tetraplegia. Br J Sports Med. 2010;44(6):455–460. doi: 10.1136/bjsm.2007.043687. [DOI] [PubMed] [Google Scholar]
- 31.Webborn N, Price MJ, Castle PC, Goosey-Tolfrey VL. Effects of two cooling strategies on thermoregulatory responses of tetraplegic athletes during repeated intermittent exercise in the heat. J Appl Physiol (1985) 2005;98(6):2101–2107. doi: 10.1152/japplphysiol.00784.2004. [DOI] [PubMed] [Google Scholar]
- 32.Jay O, Morris NB. Does cold water or ice slurry ingestion during exercise elicit a net body cooling effect in the heat? Sports Med. 2018;48(suppl 1):17–29. doi: 10.1007/s40279-017-0842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaseling GK, Filingeri D, Barnett M, Hoang P, Davis SL, Jay O. Cold water ingestion improves exercise tolerance of heat-sensitive people with MS. Med Sci Sports Exerc. 2018;50(4):643–648. doi: 10.1249/MSS.0000000000001496. [DOI] [PubMed] [Google Scholar]
- 34.Kume M, Yoshida T, Tsuneoka H, Kimura N, Ito T. Relationship between body surface cooling area, cooling capacity, and thermoregulatory responses wearing water perfused suits during exercise in humans. Japan J Phys Fitness Sports Med. 2009;58(1):109–122. [Google Scholar]
- 35.Price MJ, Campbell IG. Thermoregulatory responses of paraplegic and able-bodied athletes at rest and during prolonged upper body exercise and passive recovery. Eur J Appl Physiol Occup Physiol. 1997;76(6):552–560. doi: 10.1007/s004210050289. [DOI] [PubMed] [Google Scholar]
- 36.Dawson B, Bridle J, Lockwood RJ. Thermoregulation of paraplegic and able bodied men during prolonged exercise in hot and cool climates. Paraplegia. 1994;32(12):860–870. doi: 10.1038/sc.1994.132. [DOI] [PubMed] [Google Scholar]
- 37.Chen JM, Schutz KE, Tucker CB. Cooling cows efficiently with sprinklers: Physiological responses to water spray. J Dairy Sci. 2015;98(10):6925–6938. doi: 10.3168/jds.2015-9434. [DOI] [PubMed] [Google Scholar]
- 38.Ansley L, Marvin G, Sharma A, Kendall MJ, Jones DA, Bridge MW. The effects of head cooling on endurance and neuroendocrine responses to exercise in warm conditions. Physiol Res. 2008;57(6):863–872. [PubMed] [Google Scholar]
- 39.Pritchett RC, Bishop PA, Yang Z, Pritchett KL. Evaluation of artificial sweat in athletes with spinal cord injuries. Eur J Appl Physiol. 2010;109:125–131. doi: 10.1007/s00421-010-1371-4. [DOI] [PubMed] [Google Scholar]
- 40.Griggs KE, Havenith G, Paulson TAW, M JP, Goosey-Tolfrey VL. Effects of cooling before and during simulated match play on thermoregulatory responses of athletes with tetraplegia. J Sci Med Sport. 2017;20(9):819–824. doi: 10.1016/j.jsams.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 41.Griggs KE, Havenith G, Price MJ, Mason B, Goosey-Tolfrey VL. Thermoregulatory responses during competitive wheelchair rugby match play. Int J Sports Med. 2017;38:177–183. doi: 10.1055/s-0042-121263. [DOI] [PubMed] [Google Scholar]


