Abstract
Background
Recent studies have found that clinical pain is related to cognitive impairment. However, there remains a scarcity of systematic reviews on the influence of acute pain on attention. Laboratory-induced pain is often used to simulate acute pain. The current systematic meta-analysis aimed to evaluate the effect of induced-pain on three components of attention (orienting, alerting, and executive attention) in healthy subjects.
Methods
A systematic search of three databases was performed. Only data from studies that administered laboratory-induced pain and that also included a control group were selected. The effects of experimental pain on orienting attention, alerting attention, and executive attention were analyzed. Two reviewers assessed the studies and extracted relevant data according to the Cochrane Collaboration and Preferred Reporting Items for Systematic Reviews and Meta-Analysis Guidelines.
Results
Eight studies were included in the meta-analysis. Orienting attention was marginally interrupted by pain under the invalid cue and marginally facilitated by pain under the valid cue condition. Performance on alerting attention was decreased by pain. Executive attention was not significantly affected by pain.
Conclusion
There was moderate evidence that experimentally induced pain can produce effects on orienting and alerting attention but not on executive attention. This meta-analysis suggests that experimentally induced pain influences some aspects of attention.
Keywords: experimental pain, attention, meta-analysis
Introduction
Investigations into human cognition have long sought to define attention and to describe the processes involved. Models of attention agree that in addition to cognitive resources, automatic behaviors and thoughts are required. Moreover, there is a consensus that more cognitive resources are allocated to essential information, and multiple tasks are difficult to maintain concurrently. Attention governs our perception of the world and the voluntary control of our thoughts and emotions.1
Recent neuroimaging studies have provided new perspective on the processes involved in attention.2,3 Based on neuroimaging technology, Posner’s Attention Networks has been accepted as a framework for the cognitive underpinnings of attention. Posner and Rothbart suggest that there are three distinct networks for attention: orienting, alerting, and executive attention.1 Orienting involves the selection of information from sensory input, alerting is defined as achieving and maintaining of high sensitivity to approaching stimuli, and executive attention includes a set of mechanisms responsible for monitoring and addressing conflict among feelings, thoughts, and responses.
Pain is a salient signal that demands a high level of cognitive resources. Pain warns an organism of a threat, which may interfere, distract, and demand attention.4 Most of the existing reviews on the psychological mechanisms involved in pain have been conducted with chronic pain patients.5 It is widely accepted that chronic pain leads to attention deficits, predominantly on tasks that require high attentional demands.6 This prior research has contributed greatly to the understanding of pain and has provided avenues for treatment. However, the extent to which and how acute pain interferes with attention remains unclear.
For healthy people, pain is an unpleasant sensory and emotional experience. It is a protective mechanism signaling an immediate response to avoid further injury. Therefore, attention to pain can be positive and adaptive. Despite this, the mechanisms by which acute pain alters attention are not well understand. Prior studies using techniques that mimic pain, such as cold pressure, heat, and electric stimuli, have demonstrated that laboratory-induced pain decreases overall attention performance.7 Some studies have revealed that experimentally induced pain draws attention to its location in healthy individuals.8
However, the effect of experimentally induced pain on attention differs from chronic pain.9,10 Despite frequent reports that laboratory-induced pain leads to poor concentration,11 there have been no systematic reviews of the literature. Our meta-analysis examined the effect of laboratory-induced pain on attention, which is often used to simulate acute pain. Immediate protective responses to acute pain include increased arousal, orientation to the sources of threat, and various safety-seeking behaviors including escape and avoidance. And attention protects the pursuit of current goals, by inhibiting the processing of goal-irrelevant information.12 These three components are more closely related to acute pain. So the current review focuses on the effects of experimentally induced pain on three components of attention: orienting, alerting, and executive.
Gaining a better understanding of attention performance, as well as the processes that underpin it, within the context of experimentally induced pain may provide additional information that may assist in the process of choosing the most beneficial type of intervention for a given client.
Methods
Data sources
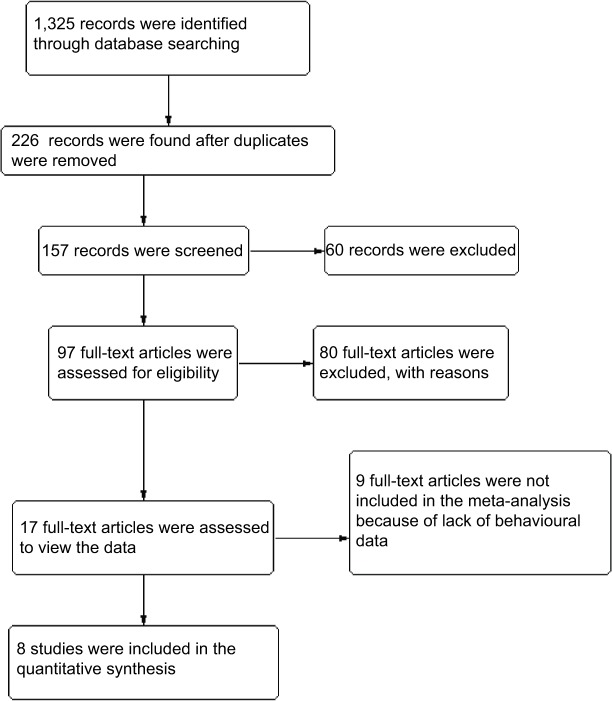
The current meta-analysis was conducted following the Cochrane Collaboration and Preferred Reporting Items for Systematic Review and Meta-Analyses (“PRISMA”) Statement guidelines. Searches were performed in the following databases: PubMed, Web of Science, and PsychINFO. The following keywords were used as search items: orienting, alerting, executive attention, spatial cue task, continuous performance, Stroop, go/no-go, attention, and intersected with pain. The search results were limited to studies using human participants and studies that are related to the interruptive effect of experimental pain. Only articles written in English were included. Studies related to alerting, orienting, executive attention, and experimentally induced pain were exported to Endnote X7. Full-text articles published in the area of induced pain and attention, which were identified through background reading and systematic searching were retrieved. All the articles were hand-searched for citations containing original data. Figure 1 shows the search and selection process.
Figure 1.
Flowchart of the article search and inclusion process.
Study selection
Studies related to the effect of experimentally induced pain on orienting,10,13–16 alerting,10,17 and executive attention18,19 were selected. Each of the components of attention were measured by well-known paradigms, such as spatial cue test, continuous performance task (CPT), Stroop test,18 and go/no-go test.19 Selection criteria were also established, and irrelevant studies were removed.
The spatial cue task, in which a cue signals either a correct (valid cue) or incorrect (invalid cue) location of a target, is used to investigate orienting of attention. Orienting is facilitated when the target appears at the same location as the cue (valid cue). It is inhibited when the target appears at a different location than the cue (invalid cue).20 The difference in reaction times (RTs) between a painful and a nonpainful conditions signifies an effect on orienting. Studies that use pain as a cue were included.13–16 In the spatial cue task, a cue was presented on left/right side of the screen, and the cue (or the other location) is replaced by a probe stimulus. Participants are instructed to respond as quickly as possible to the probe. During the orientation, participants respond to the probe as the location of the cue. Pain as a cue would capture participants’ attention, which affects the response to the probe. So, pain as a cue is also an immediate effect on orienting. Considering the use of pain as a cue may lead to shift the attention to the location of the painful cues, which would affect the orienting, we singled out these four studies using pain as a cue (see the “Results” section).
Achieving and maintaining a high sensitivity to approach stimuli1 during an extended task is necessary for alerting attention. Alertness is measured by the CPT where participants are asked to attend to a subject or location during the task and respond to the target. The difference in RT between a painful and a nonpainful conditions signifies an effect on alerting.
Executive attention involves mechanisms for monitoring and resolving conflicts among thoughts, feelings, and responses. Two tests were selected for the analysis of executive attention: the Stroop test and the go/no-go test. The former measures the ability to resolve cognitive conflicts, the latter measures the ability to resolve response conflicts. The difference in RT between painful and nonpainful conditions signifies an effect of pain on executive attention.
All studies included in the meta-analysis compared attention performance under an experimentally induced pain condition with a non-pain condition. Titles and abstracts of articles were screened by two authors (WG and LF). Studies were included if they evaluated orienting, alerting, or executive attention and used spatial cue test, CPT, Stroop test, or go/no-go test. Irrelevant articles were removed. Studies that used pain-related (eg, pain-related images or words) information instead of the sensory experience of pain were excluded because this topic has been previously studied.5 Other exclusion criteria were: 1) child participants; 2) studies that included participants with traumatic brain injury or any disease that would be expected to impair attention. The full texts of potentially eligible studies were retrieved and then formally assessed for eligibility by the two reviewers, using the aforementioned criteria.
Risk of bias assessment
Risk of bias was assessed by two reviewers using a customized risk of bias form. The form was developed a priori and was based on relevant items from a previous meta-analysis that used the Cochrane Collaboration Risk of Bias Tool.21 Any differences were resolved by discussion between two reviewers.
Data extraction
Two independent reviewers used a previously piloted, customized form to extract data.21 RT results were compared. The rate of correct answers was not analyzed as the tasks were very simple. Extracted data included group-specific data (type of induced pain, sample size in each group, gender, and mean age ± SD); attention test data (name of attention test), cognitive process evaluated (where it was specified), type of outcome measure of test (RT), interpretation of test (group-specific outcomes on attentional test results; eg, mean differences) (Table 1).
Table 1.
Characteristics of included studies
| Study | Pain stimulation | Matched or tested for baseline in characteristics | Participants | |||||
|---|---|---|---|---|---|---|---|---|
| Induced pain | Non-pain | |||||||
| Age mean ± (SD) | Gender | Number | Age mean ± (SD) | Gender | Number | |||
| Babiloni et al (2004)19 | Electrical stimulation | Within-group | 25 (not described) | Not described | 12 | 25 (not described) | Not described | 12 |
| Cheng et al (2017)18 | TENS (painful transcutaneous electrical nerve stimuli) | Within-group | 20–31 | 25 M, 26 F | 51 | 20–31 | 25 M, 26 F | 51 |
| Kurita et al (2015)17 | Cuff | Within-group | 23.5 (2.1) | All M | 22 | 23.5 (2.1) | All M | 22 |
| Koster et al (2005)13 | An aversive white noise burst CS | Within-group | 19.32 (2.36) | 55 F, 11 M | 66 | 19.32 (2.36) | 55 F, 11 M | 66 |
| Moore et al (2012),10 E1 | Thermal pain (ATS) | Within-group | 23.5 (3.86) | 13 F, 7 M | 20 | 23.5 (3.86) | 13 F, 7 M | 20 |
| Moore et al (2012),10 E3 | Thermal pain (ATS) | Within-group | 24.5 (5.59) | 16 F, 4 M | 20 | 24.5 (5.59) | 16 F, 4 M | 20 |
| Moore et al (2012),10 E5 | Thermal pain (ATS) | Within-group | 27.7 (8.76) | 14 F, 6 M | 20 | 27.7 (8.76) | 14 F, 6 M | 20 |
| Van Damme et al (2004)14 | Transcutaneous electrocutaneous stimulus CS | Within-group | 19.04 (not described) | 44 F, 8 M | 52 | 19.04 (not described) | 44 F, 8 M | 52 |
| Van Damme et al (2006)15 | Electrocutaneous stimulus CS | Within-group | 19.3 (1.38) | 15 F, 12 M | 27 | 19.3 (1.38) | 15 F, 12 M | 27 |
| Van Damme et al (2012)16 | Electrocutaneous stimulus | Between-group | (Not described) | (Not described) | 51 (E1) 50 (E2) | (Not described) | (Not described) | 51 (E1) 50 (E2) |
Abbreviations: ATS, advanced thermal stimulator; CS, conditioned stimulus; E, experiment; F, female; M, male.
Data analysis
Data were analyzed using Review Manager (Version 5.3). If sufficient data were not reported, the study was not included in the analysis.22–29 Studies lacking a control group were excluded from analysis.8 Heterogeneity was deemed significant on the basis of χ2 P<0.01 and substantial on the basis of I2 >60%.
Evaluation of attention was evaluated – tests and test outcomes
From the 1,325 records identified by the search methods, the full texts of 96 studies were retrieved. Of the 96 studies, 17 reported effects of experimentally induced pain on attention but nine of the 17 reported no behavioral data or control groups,8,22–29 leading to a total of eight that met all inclusion criteria. In total, 26 different test outcomes resulted from tests of eight different attention constructs. The eight included studies reported outcomes from tests that are commonly used to investigate orienting, alerting, and executive attention: the spatial cue test,10,13–16 the CPT,10,17 the Stroop task,18 and the go/no-go task.19
Orienting
Five studies were included in the meta-analysis on orienting attention.10,13–16 All these studies used the spatial cue task to measure RT under valid and invalid conditions. The statistical results of these studies were analyzed separately for each condition. Four of the studies13–16 used cues that were modulated by classical conditioning as follows. The conditioned cue was followed by a nociceptive stimulus, thus becoming a signal for pain. These data (pain preceded by a conditioned cue) were included in the pain condition in the meta-analysis. Data from the conditioned cue that was not followed by nociceptive stimulus were included in the no-pain condition in the meta-analysis.10,13–16
Alerting
Seven comparisons in two studies were included in the alerting meta-analysis. Two studies used CPT.10,17 One of the studies conducted CPT under a pain condition.10 The other study using CPT was performed under both mild and moderate pain. RT was summarized by percentiles: 10th (fastest RT), 50th (plotted in), and 90th (slowest RT).17
Executive attention
Three comparisons in two studies were included in the executive attention meta-analysis. One study used the go/no-go test,19 and the other one used a numerical Stroop test.18 Using the Stroop test, Cheng et al18 characterized individuals as A-type (attention dominates) or P-type (pain dominates) based on how pain interfered with task performance. Both groups were included in the meta-analysis.
Results
Risk of bias
All studies included in the meta-analysis were judged to have a high risk of bias, primarily due to ambiguous recruitment methods. None of the included studies reported a priori calculation of the sample size required for adequate power. None of the included study used a double-blind experimental design. Risk of bias data are summarized in Table 2.
Table 2.
Risk of bias assessment
| Study | Random sequence generation (selection bias) | Sample size was calculated a priori | Blinding of participants and personnel | Cases diagnosed were according to accepted criteria | Confounding variables were controlled | All outcomes and groups were reported |
|---|---|---|---|---|---|---|
| Babiloni et al (2004)19 | NA | N | N | Y | Y | Y |
| Cheng et al (2017)18 | NA | N | N | Y | Y | Y |
| Kurita et al (2015)17 | NA | N | N | Y | Y | Y |
| Koster et al (2005)13 | NA | N | N | Y | Y | Y |
| Moore et al (2012),10 E1 | NA | N | NA | Y | Y | Y |
| Moore et al (2012),10 E3 | NA | N | NA | Y | Y | Y |
| Moore et al (2012),10 E5 | NA | N | NA | Y | Y | Y |
| Van Damme et al (2004)14 | NA | N | NA | Y | Y | Y |
| Van Damme et al (2006)15 | NA | N | N | Y | Y | Y |
| Van Damme et al (2012)16 | NA | N | N | Y | Y | Y |
Abbreviations: N, no; NA, not applicable; Y, yes.
Behavioral outcomes
All the included studies reported on RT in one or more tests of attention. The eight studies presented 26 outcomes for RT, across the three components of attention: orienting, alerting, and executive attention. Table 3 summarizes the outcomes, and Table 4 summarizes the comparisons for three components of attention.
Table 3.
The outcomes for three components of attention
| Cognitive process | Test | Outcome | Studies that use this test |
|---|---|---|---|
| Orienting attention | Spatial cue test – one cue (CS+) of the task was a signal for an aversive white noise burst (UCS) and that another cue (CS−) signaled its nonoccurrence | Average time to the target, the test trials consisted of 75% valid trials and 25% invalid trials Acquisition phase: 72 CS+, 72 CS−, 12 catch, and 10 digit trials Extinction phase 2: 36 CS+, 36 CS−, 6 catch, and 5 digit trials | Koster et al. (2005);13 Van Damme et al (2006)15 |
| Spatial cue test – shifting attention toward the direction of an endogenous arrowhead once without pain stimulation, once with a warm sensation, and once under thermal heat pain conditions | Average time to the target, the test trials consisted of valid, invalid and neutral (a horizontal line), or absent Valid: 160 trials; invalid: 40 trials; neutral: 40 trials; absent: 40 trials | Moore et al (2012)10 | |
| Spatial cue test – one cue (CS+) of the task was a signal for an aversive white noise burst (UCS) and that another cue (CS−) signaled its nonoccurrence | Average time to the target, the test trials consisted of 96 valid trials, 48 invalid trials, 24 catch trials, and 12 digit trials | Van Damme et al (2004)14 | |
| Spatial cue test – visual cues were LEDs presented close to the left or right hand, or centrally between both hands LED cues were followed by a somatosensory stimulus to one of both wrists | Average time to the LED target Experiment 1:12 valid trials, 12 invalid trials, 12 baseline trials, and 12 catch trials Experiment 2: 36 valid trials, 12 invalid trials, 12 baseline trials, and 12 catch trials | Van Damme et al (2012)16 | |
| Alerting attention | Continuous performance test | The reaction time to auditory signals at random second intervals under mild and moderate pain. The results were summarized using 10th, 50th, and 90th percentiles, in which 10th represents the fastest and 90th the slowest values | Kurita et al (2015)17 |
| Continuous performance test | The reaction time to respond with a single key press when three consecutive odd or even digits were presented | Moore et al (2012)10 | |
| Executive attention | Go/no-go task | Average time to go trials under pain or nonpain condition | Babiloni et al (2004)19 |
| Stroop task – numerical interference task counts the number of digits within each box, which the numerical values of the digits did not coincide with the number of digits counted in each box | Average time under no-pain and pain blocks | Cheng et al (2017)18 |
Abbreviations: CS, conditioned stimulus; CS+, conditioned stimulus was presented; CS−, conditioned stimulus disappeared; UCS, unconditioned stimulus.
Table 4.
The comparisons for three components of attention
| Cognitive process | Paper | Studies | Comparisons |
|---|---|---|---|
| Orienting attention (valid/invalid cue) | Koster et al (2005)13 | Acquisition phase | Mean reaction data that used CS+/CS− signals |
| Extinction phase 2 | Mean reaction data that used CS+/CS− signals | ||
| Moore et al (2012)10 | Experiment 3 | Mean reaction data under pain and baseline conditions | |
| Van Damme et al (2004)14 | Experiment phase | Mean reaction data that used CS+/CS− signals | |
| Van Damme et al (2006)15 | Experiment 1 acquisition phase | Mean reaction data that used CS+/CS− signals | |
| Experiment 2 acquisition phase | Mean reaction data that used CS+/CS− signals | ||
| Van Damme et al (2012)16 | Experiment 1 | Mean reaction data under pain and baseline conditions | |
| Experiment 2 | Mean reaction data under pain and baseline conditions | ||
| Alerting attention | Kurita et al (2015)17 | Experiment 1 | 10th, 50th, and 90th mean reaction data under mild pain and baseline conditions |
| Experiment 2 | 10th, 50th, and 90th mean reaction data under moderate pain and baseline conditions | ||
| Moore et al (2012)10 | Experiment 1 | Mean reaction data under pain and baseline conditions | |
| Executive attention | Babiloni et al (2004)19 | Behavioral experiment | Mean reaction data under pain and nonpainful conditions |
| Cheng et al (2017)18 | Behavioral experiment (A-type participants) | Mean reaction data under pain and nonpainful conditions | |
| Behavioral experiment (P-type participants) | Mean reaction data under pain and nonpainful conditions |
Abbreviations: CS+, conditioned stimulus was presented; CS−, conditioned stimulus disappeared; A-type, attention dominates; P-type, pain dominates.
Orienting attention
Results are separated into valid and invalid cue conditions.
Valid cues
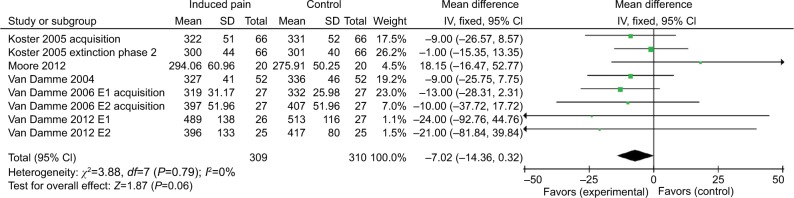
Valid cues are presented at the same location as the targets. Pooled results from eight comparisons across five studies indicated that induced-pain was associated with facilitated orienting under the valid cue condition. That is, there was a nearly significant effect estimate of −7.02 (95% CI =−14.36 to 0.32). Nonsignificant heterogeneity was detected (χ2=3.88, P=0.79, I2=0%). The results suggest that healthy individuals orient faster when they are in pain vs nonpain under the valid cue condition (Figure 2).
Figure 2.
Response time (ms) outcomes for valid cues.
Abbreviations: E1, experiment 1; E2, experiment 2.
Invalid cue
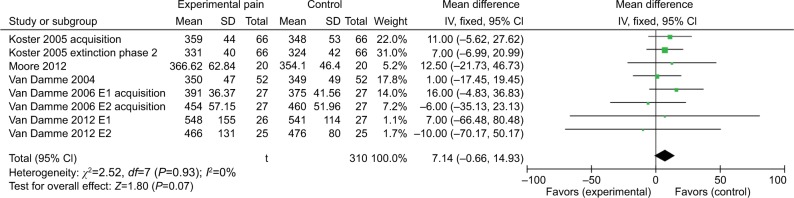
Invalid cues are presented at a different location than the target. Pooled results from eight comparisons across five studies indicated that induced pain was moderately associated with impaired orienting under the invalid cue condition. That is, there was a marginal significant effect estimate of 7.14 (95% CI =–0.66 to 14.93). Nonsignificant heterogeneity was detected for orientation under the invalid cue (χ2=2.52, P=0.93, I2=0%). The results suggest that the healthy individuals orient slower to the invalid cue when under the pain condition compared with no-pain condition (Figure 3).
Figure 3.
Response time (ms) outcomes for invalid cues.
Abbreviations: E1, experiment 1; E2, experiment 2.
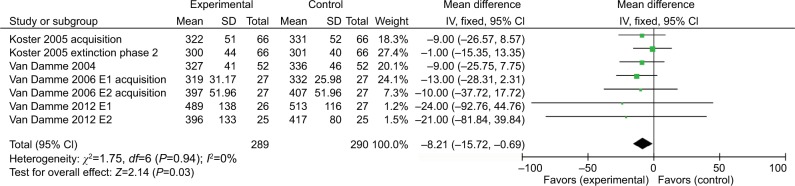
Attentional bias to pain
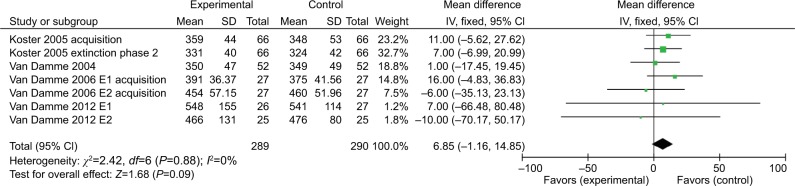
Four of five studies13–16 on orienting used a conditioned cue to indicate pain, while the other one9 did the experiment under pain condition. The four studies using the painful cue were analyzed in a separate meta-analysis. The results showed that induced pain under the painful valid cue showed a significant effect estimate of –8.21 (95%CI =−15.72 to –0.69); while induced pain under the painful invalid cue condition showed no significant estimate (6.85 [95%CI =–1.16to 14.85]) (Figures 4 and 5).
Figure 4.
Response time (ms) outcomes for bias to pain stimulus.
Abbreviations: E1, experiment 1; E2, experiment 2.
Figure 5.
Response time (ms) outcomes for disengagement from pain stimulus.
Abbreviations: E1, experiment 1; E2, experiment 2.
Alerting attention
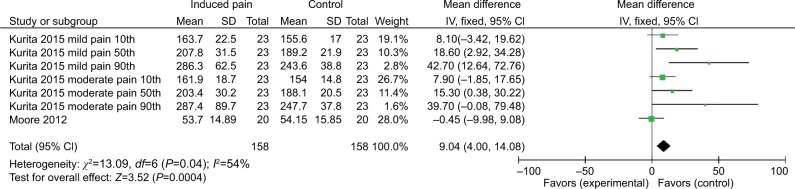
Pooled results from seven comparisons across two studies suggested that induced pain was significantly associated with impaired alerting. There was a significant effect estimate of 9.04 (95% CI =4 to 14.08). A moderately nonsignificant heterogeneity was detected for alerting attention (χ2=13.09, P=0.04, I2=54%) (Figure 6).
Figure 6.
Response time (ms) outcomes for alerting attention.
Executive attention
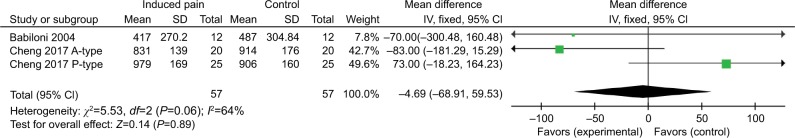
Pooled results from three comparisons across two studies suggested that induced pain has no significant effect on executive attention (effect estimate =−4.69 [95%CI =−68.91 to 59.53]). No significant heterogeneity was detected for executive attention (χ2=5.53, P=0.06, I2=64%) (Figure 7).
Figure 7.
Response time (ms) outcomes for executive attention.
Discussion
The present meta-analysis found that induced pain affected orienting and alerting but not executive attention.
Orienting
Valid cue condition
Participants with induced pain responded to valid cues faster (n=5 studies; n=309 subjects with induced pain, n=310 subjects with no induced pain) than those without pain. This finding is consistent with previous research.22,30 All the eight included comparisons used a version of exogenous spatial cue test and thus provided the most homogenous data set in the current meta-analysis. Under the condition of induced pain, four of five studies13–16 used pain as the cue, while the other one10 did the experiment under pain condition. Therefore, we may predict that the effect of pain on orienting attention would reveal attentional bias to pain. The result corroborated that participants showed significant bias to pain stimulation.
The valid cue task directs attention toward the correct location of the target and is used to predict the processing of the incoming target. Responses to the targets are therefore faster when subjects know in advance what type of movement they will make – a “motor set.” The “motor set” is a type of top-down attentional control. When individuals are stimulated by pain, a motor set in response to the stimulus site will be generated. Thus, when the target stimulus occurs at the same location with cue, the participants will respond more quickly. Schmidt et al demonstrated that attention was biased toward the location of pain stimulation by eye-tracking.31 Similarly, previous research has shown that a significant cue validity effect occurs when pain is used as a pre-cueing stimulus.8
Invalid cue condition
Participants experiencing induced pain responded to invalid cues slower than no-pain controls (n=5 studies; n=309 subjects with induced pain, n=310 subjects with no pain). Consistent with a previous study,32 these results provide evidence that participants with induced pain may experience difficulty in disengaging from pain sensation. The difference between the induced-pain group and the control group was marginally significant.
Under the invalid cue condition, it is necessary to disengage attention from the cued location and reorient attention to the opposite location. As shown in a previous study,32 the biological warning of pain demands attention, leading to disengage from the cue. These results provided evidence for the “motor set” hypothesis: RTs are longer under induced pain compared to no pain when an invalid cue is presented due to a conflict between the location of the target and the “motor set.”
The effects of pain on orienting attention differed under valid and invalid conditions. Pain facilitated processing when the cue was presented in the same location as the target, while pain inhibited processing when the cue was presented in the opposite location from the target. A study by Mangun and Hillyard provided evidence that spatial priming leads to changes in the sensory-perceptual processing of targets.33 It may be that the experience of pain leads to a disruption in the perceptual processing of reorientation which is necessary under the invalid cue condition. Further studies are needed to investigate this hypothesis.
Alerting attention
The current meta-analysis found that induced pain leads to a significant impairment in alerting attention (n=2 studies; n=158 subjects with induced pain, n=158 subjects with no pain). Seven comparisons used the CPT providing a reliable homogenous data set, suggesting high confidence in group differences.
Alerting attention is defined as the ability to maintain a mental arousal and preparedness for the processing of information with required effort.34 Prior studies have shown that pain interferes with mental processes and disrupts ongoing activities.12 One possible explanation for the impairment observed under conditions of pain is that while the CPT requires attentional coherence, pain may inhibit or distract the mental processes which results in decreasing capacity to maintain a state of alert arousal. It is not clear whether it is distraction or inhibition that leads to impairment. Future research should focus on separating the two mechanisms. Another possible explanation is that ongoing pain induces fatigue. Fatigue is one of the most common symptoms associated with various pains.35 Fatigue associated with ongoing pain may result in depleted energy necessary for attentional tasks.
Executive attention
Surprisingly, the current meta-analysis found no evidence (n=2 studies; n=57 subjects with induced pain, n=57 subjects with no induced pain) for an effect of induced pain on executive attention. Executive attention is required for the execution of goal-directed behaviors, including the planning of actions, expectations, responding, initiating, and maintaining deliberate behavior, monitoring the results of behavior, and interrupting or modifying behavior.34 Executive attention is necessary for the regulation of cognition and inhibition of responses. Inhibition is important for the successful attentional control of pain. Bjekić et al found that cognitive inhibition measured by the Stroop task is correlated with pain perception.36 In addition, a previous meta-analysis found an impairment in response inhibition in people with chronic pain.21 One possible explanation for the inconsistent results between the current study and the previous research in executive attention is the degree of variance between studies. The current meta-analysis included only two studies with three comparisons using different designs.
One study, using the Stroop test, found that pain significantly reduced task speed in P-type individuals but boosted performance in A-type individuals.18 Another study using the go/no-go test revealed that pain significantly affected performance.19 The Stroop test involves the ability to suppress cognitive conflict while the go/no-go test involves the ability to suppress behavioral conflict, leading to variation between studies. In addition, previous studies exclusively investigated the effects of chronic pain,6 while the current study analyzed the effects of induced pain on response inhibition. Future studies are needed to better understand the relationship between executive attention and laboratory-induced pain.
Strengths and limitations of the current study
The current meta-analysis used a rigorous search procedure to ensure that relevant studies were included, while excluding irrelevant and low-quality studies. Based on Posner’s attention theory, we selectively studied the influence of laboratory-induced pain on the three components of attention: orienting, alerting, and executive. Studies using the spatial cue test, CPT, Stroop test, and go-no/go test were included.
Norman and Shallice37 proposed that the attentional system is susceptible to interruption by the presentation of a new superordinate goal in order to protect an organism from danger or harm. Pain is a biological mechanism that can interrupt ongoing attention with the aim of self-protection.32 The current study suggests induced-pain effects orienting attention and altering attention. However, due to the risk of biases, further studies are needed to confirm the relevance of our findings.
There are limitations that must be considered when interpreting the results of the current meta-analysis. First, attention is a dynamic process that is difficult to measure. Eye tracking techniques are often used to examine the time-course of overt visual attention in humans. Many prior studies have investigated attentional bias to pain-related information,38,39 but few have examined attentional bias to actual pain stimulation.31 Future research using eye-tracking to investigate the effects of actual pain stimulation on attention would be helpful to further our understanding of the effects of pain on attention performance. Second, we limited the meta-analysis to three components of attention. Other components of attention such as divided attention, selective attention, and working memory were not investigated. Previous research has found that chronic pain decreases performance on tests of these components of attention.40,41 Further studies are needed to explore the effects of induced pain on these components of attention. Finally, the behavioral paradigms included in the current meta-analysis may not be sensitive to acute pain and, therefore, the effects reported may be underestimated.
Conclusion
Experimentally induced pain affects some components of attention. Interestingly, the effect of pain can be both facilitative and disruptive. For example, pain disrupts orienting under an invalid painful cue condition while facilitating orienting under a valid painful cue condition. Pain is disruptive to alerting attention, while there is no obvious effect of pain on executive attention. Further studies are necessary to confirm the results of the current meta-analysis.
Footnotes
Disclosure
This work was funded by National Natural Science Foundation of China grant (31471061) to FL. The authors report no other conflicts of interests in this work.
References
- 1.Posner MI, Rothbart MK. Research on attention networks as a model for the integration of psychological science. Annu Rev Psychol. 2007;58(1):1–23. doi: 10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- 2.Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26(2):471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Crottaz-Herbette S, Menon V. Where and when the anterior cingulate cortex modulates attentional response: combined fMRI and Erp evidence. J Cogn Neurosci. 2006;18(5):766–780. doi: 10.1162/jocn.2006.18.5.766. [DOI] [PubMed] [Google Scholar]
- 4.Vervoort T, Trost Z, van Ryckeghem DM. Children’s selective attention to pain and avoidance behaviour: the role of child and parental catastrophizing about pain. Pain. 2013;154(10):1979–1988. doi: 10.1016/j.pain.2013.05.052. [DOI] [PubMed] [Google Scholar]
- 5.Crombez G, van Ryckeghem DM, Eccleston C, Van Damme S. Attentional bias to pain-related information: a meta-analysis. Pain. 2013;154(4):497–510. doi: 10.1016/j.pain.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Eccleston C. Chronic pain and attention: a cognitive approach. Br J Clin Psychol. 1994;33(4):535–547. doi: 10.1111/j.2044-8260.1994.tb01150.x. [DOI] [PubMed] [Google Scholar]
- 7.Attridge N, Keogh E, Eccleston C. The effect of pain on task switching: pain reduces accuracy and increases reaction times across multiple switching paradigms. Pain. 2016;157(10):2179–2193. doi: 10.1097/j.pain.0000000000000627. [DOI] [PubMed] [Google Scholar]
- 8.Van Damme S, Crombez G, Lorenz J. Pain draws visual attention to its location: experimental evidence for a threat-related bias. J Pain. 2007;8(12):976–982. doi: 10.1016/j.jpain.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Vancleef LM, Peters ML. The interruptive effect of pain on attention. J Pain. 2006;7(1):21–22. doi: 10.1016/j.jpain.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Moore DJ, Keogh E, Eccleston C. The interruptive effect of pain on attention. Q J Exp Psychol. 2012;65(3):565–586. doi: 10.1080/17470218.2011.626865. [DOI] [PubMed] [Google Scholar]
- 11.Crombez G, Eccleston C, Baeyens F, Eelen P. The disruptive nature of pain: an experimental investigation. Behav Res Ther. 1996;34(11-12):911–918. doi: 10.1016/s0005-7967(96)00058-7. [DOI] [PubMed] [Google Scholar]
- 12.Vlaeyen JW, Morley S, Crombez G. The experimental analysis of the interruptive, interfering, and identity-distorting effects of chronic pain. Behav Res Ther. 2016;86:23–34. doi: 10.1016/j.brat.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Koster E, Crombez G, Van Damme S, Verschuere B, De Houwer J. Signals for threat modulate attentional capture and holding: Fear-conditioning and extinction during the exogenous cueing task. Cogn Emot. 2005;19(5):771–780. [Google Scholar]
- 14.Van Damme S, Lorenz J, Eccleston C, Koster EHW, De Clercq A, Crombez G. Fear-conditioned cues of impending pain facilitate attentional engagement. Neurophysiol Clin. 2004;34(1):33–39. doi: 10.1016/j.neucli.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Van Damme S, Crombez G, Eccleston C, Koster EH. Hyper-vigilance to learned pain signals: a componential analysis. J Pain. 2006;7(5):346–357. doi: 10.1016/j.jpain.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Van Damme S, Legrain V. How efficient is the orienting of spatial attention to pain? An experimental investigation. Pain. 2012;153(6):1226–1231. doi: 10.1016/j.pain.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 17.Kurita GP, Malver LP, Andresen T, et al. Does mutual compensation of the cognitive effects induced by pain and opioids exist? An experimental study. Psychopharmacology. 2015;232(8):1373–1381. doi: 10.1007/s00213-014-3768-y. [DOI] [PubMed] [Google Scholar]
- 18.Cheng JC, Bosma RL, Hemington KS, Kucyi A, Lindquist MA, Davis KD. Slow-5 dynamic functional connectivity reflects the capacity to sustain cognitive performance during pain. Neuroimage. 2017;157:61–68. doi: 10.1016/j.neuroimage.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Babiloni C, Brancucci A, Arendt-Nielsen L, et al. Cortical sensorimotor interactions during the expectancy of a go/no-go task: effects of painful stimuli. Behav Neurosci. 2004;118(5):925–935. doi: 10.1037/0735-7044.118.5.925. [DOI] [PubMed] [Google Scholar]
- 20.Vossel S, Thiel CM, Fink GR. Cue validity modulates the neural correlates of covert endogenous orienting of attention in parietal and frontal cortex. Neuroimage. 2006;32(3):1257–1264. doi: 10.1016/j.neuroimage.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 21.Berryman C, Stanton TR, Bowering KJ, Tabor A, McFarlane A, Moseley GL. Do people with chronic pain have impaired executive function? A meta-analytical review. Clin Psychol Rev. 2014;34(7):563–579. doi: 10.1016/j.cpr.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Buffington AL, Hanlon CA, McKeown MJ. Acute and persistent pain modulation of attention-related anterior cingulate fMRI activations. Pain. 2005;113(1):172–184. doi: 10.1016/j.pain.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Filbrich L, Alamia A, Burns S, Legrain V. Erratum to: orienting attention in visual space by nociceptive stimuli: investigation with a temporal order judgment task based on the adaptive psi method. Exp Brain Res. 2017;235(9):2901. doi: 10.1007/s00221-017-5020-6. [DOI] [PubMed] [Google Scholar]
- 24.Fechir M, Schlereth T, Kritzmann S, et al. Stress and thermoregulation: different sympathetic responses and different effects on experimental pain. Eur J Pain. 2009;13(9):935–941. doi: 10.1016/j.ejpain.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Brown CA, Jones AK. A role for midcingulate cortex in the inter-ruptive effects of pain anticipation on attention. Clin Neurophysiol. 2008;119(10):2370–2379. doi: 10.1016/j.clinph.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Valet M, Sprenger T, Boecker H, et al. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain-an fMRI analysis. Pain. 2004;109(3):399–408. doi: 10.1016/j.pain.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 27.Dowman R, Darcey T, Barkan H, Thadani V, Roberts D. Human intracranially-recorded cortical responses evoked by painful electrical stimulation of the sural nerve. Neuroimage. 2007;34(2):743–763. doi: 10.1016/j.neuroimage.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 28.Favril L, Mouraux A, Sambo CF, Legrain V. Shifting attention between the space of the body and external space: electrophysiological correlates of visual-nociceptive crossmodal spatial attention. Psychophysiology. 2014;51(5):464–477. doi: 10.1111/psyp.12157. [DOI] [PubMed] [Google Scholar]
- 29.Strigo IA, Simmons AN, Matthews SC, Craig AD. The relationship between amygdala activation and passive exposure time to an aversive cue during a continuous performance task. PLoS One. 2010;5(11):e15093. doi: 10.1371/journal.pone.0015093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun ZK, Wang JY, Luo F. Experimental pain induces attentional bias that is modified by enhanced motivation: an eye tracking study. Eur J Pain. 2016;20(8):1266–1277. doi: 10.1002/ejp.851. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt K, Gamer M, Forkmann K, Bingel U. Pain affects visual orientation: an Eye-Tracking study. J Pain. 2018;19(2):135–145. doi: 10.1016/j.jpain.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Eccleston C, Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol Bull. 1999;125(3):356–366. doi: 10.1037/0033-2909.125.3.356. [DOI] [PubMed] [Google Scholar]
- 33.Mangun GR, Hillyard SA. Modulations of sensory-evoked brain potentials indicate changes in perceptual processing during visual-spatial priming. J Exp Psychol Hum Percept Perform. 1991;17(4):1057–1074. doi: 10.1037//0096-1523.17.4.1057. [DOI] [PubMed] [Google Scholar]
- 34.Mezzacappa E. Alerting, orienting, and executive attention: developmental properties and sociodemographic correlates in an epidemiological sample of young, urban children. Child Dev. 2004;75(5):1373–1386. doi: 10.1111/j.1467-8624.2004.00746.x. [DOI] [PubMed] [Google Scholar]
- 35.Theobald DE. Cancer pain, fatigue, distress, and insomnia in cancer patients. Clin Cornerstone. 2004;6(1):S15–S21. doi: 10.1016/s1098-3597(05)80003-1. [DOI] [PubMed] [Google Scholar]
- 36.Bjekić J, Živanović M, Purić D, Oosterman JM, Filipović SR. Pain and executive functions: a unique relationship between Stroop task and experimentally induced pain. Psychol Res. 2018;82(3):580–589. doi: 10.1007/s00426-016-0838-2. [DOI] [PubMed] [Google Scholar]
- 37.Norman DA, Shallice T. Consciousness and self-regulation: Advances in research and theory. 4. New York: Plenum; 1986. Attention to action: Willed and automatic control of behavior; pp. 1–18. [Google Scholar]
- 38.Schoth DE, Ma Y, Liossi C. Exploring attentional bias for real-world, pain-related information in chronic musculoskeletal pain using a novel change detection paradigm. Clin J Pain. 2015;31(8):680–688. doi: 10.1097/AJP.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 39.Heathcote LC, Vervoort T, Eccleston C, et al. The relationship between adolescents’ pain catastrophizing and attention bias to pain faces is moderated by attention control. Pain. 2015;156(7):1334–1341. doi: 10.1097/j.pain.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 40.Oosterman JM, Derksen LC, van Wijck AJ, Veldhuijzen DS, Kessels RP. Memory functions in chronic pain: examining contributions of attention and age to test performance. Clin J Pain. 2011;27(1):70–75. doi: 10.1097/AJP.0b013e3181f15cf5. [DOI] [PubMed] [Google Scholar]
- 41.Grisart JM, Plaghki LH. Impaired selective attention in chronic pain patients. Eur J Pain. 1999;3(4):325–333. doi: 10.1053/eujp.1999.0138. [DOI] [PubMed] [Google Scholar]