Abstract
Purpose
To report the presentation and management outcomes of endophthalmitis with endoscopic vitrectomy.
Methods
This is a retrospective interventional case series conducted at a tertiary eye care center in south India. Thirty three eyes of 33 patients were included. The medical records of the patients who underwent endoscopic vitrectomy for endophthalmitis from April 2014 to March 2018 were reviewed. Data with regard to age, gender, etiology of endophthalmitis, corneal and retinal examination, type of intervention, final anatomic and visual outcome, and the total follow-up were collected. The main outcome measures were the final visual acuity and evisceration rates.
Results
The mean age at presentation was 46.84±19.89 years, with a median age of 50 years. Based on etiology, 13 eyes (39.4%) were post-trauma endophthalmitis, eleven eyes (33.33%) were post-cataract surgery, three eyes (9.09%) were endogenous, three eyes (9.09%) were post-perforated corneal ulcer, two eyes (6.06%) were post-retinal surgery, and one eye (3.03%) was post-combined cataract and corneal surgery. Twenty-four eyes (72.72%) had a favorable anatomic outcome at the last visit, and five eyes (15.15%) had a favorable visual outcome. Of those with unfavorable visual outcome, ten eyes had further visual potential. Sixteen eyes (48.48%) showed a positive culture on microbiologic evaluation. The predominant organism isolated was Pseudomonas aeruginosa. Evisceration was required only in one eye (3.03%).
Conclusion
Endoscopic vitrectomy allows early management of endophthalmitis in spite of hazy media. This ensures a reasonable visual outcome, controls the infection, and reduces the incidence of evisceration in these eyes.
Keywords: evisceration, trauma, vitrectomy
Introduction
Endophthalmitis is defined as inflammation of the inner layers of the eye with exudation in the vitreous cavity resulting from intraocular colonization by microorganisms.1 Unless diagnosed and treated in time, it can lead to severe vision loss. On clinical presentation, endophthalmitis is characterized by varied degree of corneal edema, anterior chamber fibrin, cataract in phakic individuals, and vitritis. The current knowledge suggests that early vitrectomy is indicated in cases of severe endophthalmitis presenting with less than hand motions vision.2,3 But in the acute setting, often the surgical view is compromised due to corneal edema, inflammatory membranes, and hemorrhages (in case of trauma). This precludes the possibility of achieving a satisfactory vitrectomy.
An ophthalmic endoscope helps circumvent this restriction and allows visibility into the vitreous cavity bypassing the hazy anterior segment media.4–6 Though the use of endoscopy in ophthalmology has been described in a few isolated series earlier, a detailed description of its usage in managing endophthalmitis is sparse. In the current communication, we describe our experience of treating a series of cases with endophthalmitis using endoscopic visualization.
Methods
Study design
This is a retrospective, non-comparative, consecutive case series conducted at a tertiary eye care center in south India. The study was approved by the institutional ethics committee (the LV Prasad Eye Institute Ethics Committee). The data of all the patients were handled confidentially. As this was a retrospective study and only previous patient records were analyzed, requirement of a patient consent for the same was waived by the ethics committee. It conformed to the tenets of the Declaration of Helsinki. Clinical and microbiologic records of all patients with endophthalmitis who underwent endoscopic vitrectomy between April 2014 and March 2018 were reviewed and analyzed.
Methodology
All demographic and clinical information was collected from the patient’s records.
Clinical presentation of patients decided the course of management by the treating physician. Cases either underwent primary endoscopy or underwent endoscopic procedure after initial primary management based on the clinical decision. All patients underwent vitreous biopsy initially, and empirical intravitreal vancomycin (1 mg/0.1 mL) and ceftazidime (2.25 mg/0.1 mL) were given. Based on culture reports further interventions were planned. Vitreous samples were subjected to basic microbiological testing (calcofluor white, Gram, and Giemsa stains) and culture (aerobic and anaerobic). All patients received topical antibiotics like ciprofloxacin 0.3%, cycloplegics and topical steroids, and oral ciprofloxacin 750 mg twice a day after initial intervention. The outcome at the last visit was evaluated in terms of anatomic and functional outcome. A favorable anatomic outcome was defined as preservation of the globe, absence of hypotony, attached retina, and absence of active inflammation at the last visit. A functional success was defined as a vision of ≥20/400 at the last visit. Evisceration was performed in cases that developed a painful blind eye, had prolapse of intraocular contents due to a corneal perforation, or showed progression to panophthalmitis.
Endoscopy technique
Endoscopy was done using the 20/23G endoscope (E2 Laser and Endoscopy System; EndoOptiks, Inc, Little Silver, NJ, USA) with light and video dual function. The E2 Ophthalmic Laser Endoscopy System console uniquely combines endoscopic imaging and laser treatment capability. The console houses a high-resolution video camera, 175 or 300 W xenon light source and an 810 nm diode laser. The endoscope presents a wide-field image allowing a panoramic intraocular view of the entire retina or a close-up (down to 0.75 mm) and highly magnified view of pathology. The in-built video adapter offers optimum zoom and manual focus of the endoscopic image. The resolution of the 20G camerra is 10,000 pixels while that of the 23G camera is 6,000 pixels. The surgical steps included sterile draping of the eye followed by creating two superior sclerotomies as per the surgeon’s preference for a standard three-port vitrectomy surgery. The endoscope was then maneuvered to the mid-pupillary retrolental location and position was confirmed on the TV monitor. The vitrector was then moved toward the endoscope to position it in the vitreous cavity. Vitrectomy was then performed under endoscopic visualization. A thorough vitrectomy, to the extent possible, was attempted. The end point of surgery was taken as visibility of the disc and the retina. Wherever possible an attempt was made to induce posterior vitreous detachment (PVD). If a strong adherence was noted on the induction, PVD induction was avoided.
The cases presented with associated retinal detachment, retinal breaks, or foreign bodies, and were treated appropriately (Figures 1–4). In cases with associated retinal detachment, after a thorough anterior and core vitrectomy, a PVD was induced and the vitrectomy was completed. The retina was flattened either with a fluid air exchange tamponade or a perfluorocarbon liquid (PFCL) tamponade. After flattening, all breaks were lasered and the air/PFCL was exchanged with silicone oil. In cases with associated foreign bodies, after completing the vitrectomy, the foreign bodies were approached using an intraocular magnet, and if magnetic, were removed using the same. Non-magnetic foreign bodies were removed using an intraocular forceps.
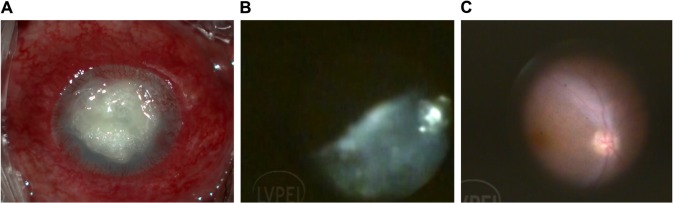
Figure 1.
Panel shows endophthalmitis with a post-glue application perforated corneal ulcer with endophthalmitis.
Notes: Endophthalmitis with corneal infiltrates precluding fundus view (A). Intraoperative view showing vitreous cutter clearing the exudates (B). Clear fundus view after removing the exudates showing relatively healthy retina (C). The endoscopic view shows vitreous being removed and finally presenting a healthy looking disc and macula.
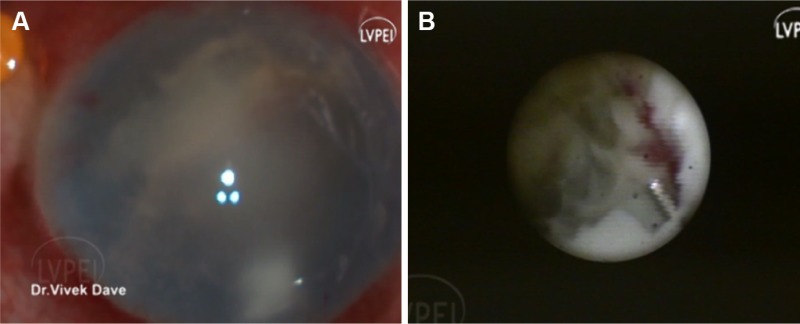
Figure 2.
Panel shows endophthalmitis with corneal edema and endoexudates (A) and endoscopic view shows necrotic retina with poor visual prognosis (B).
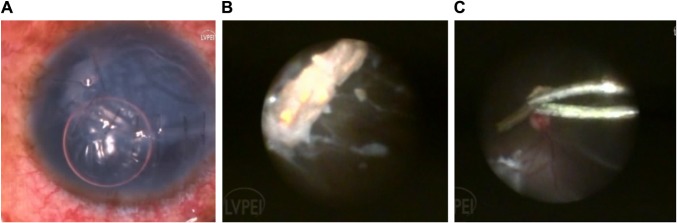
Figure 3.
Panel shows a repaired corneal tear with vitreous exudates (A). A wooden foreign body on endoscopic view which is being removed with foreign body forceps (B). Wooden foreign body being removed with a forceps with concurrent retinal detachment (C).
Note: Background shows concurrent retinal detachment (C).
Figure 4.
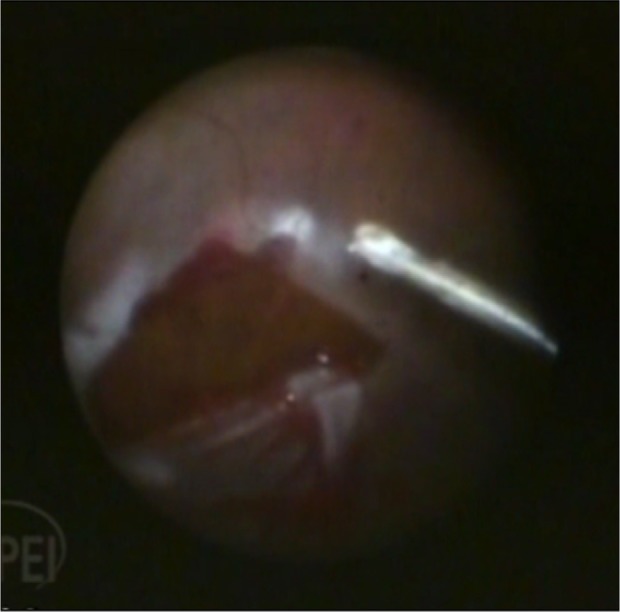
Figure shows retina flattened under perfluorcarbon liquid and a large retinal break.
Results
In the described time period, 33 eyes diagnosed as having endophthalmitis were managed with endoscopic vitrectomy. The study included 25 males (75.75%) (P<0.0001). The mean age at presentation was 46.84±19.89 years, with a median age of 50 years. Based on the etiology of the disease, 13 eyes (39.4%) were post-trauma endophthalmitis, eleven eyes (33.33%) were post-cataract surgery, three eyes (9.09%) were endogenous, three eyes (9.09%) were post-perforated corneal ulcer, two eyes (6.06%) as post-retinal surgery, and one eye (3.03%) was post-combined cataract and corneal surgery (Table 1).
Table 1.
Demographic and clinical data of endophthalmitis cases undergoing endoscopic vitrectomy
| Case no | Gender | Age (years) | Presenting vision | Setting of endophthalmitis | Anterior chamber findings | Interval between start of symptoms and presentation | Cause of poor visualization | Need for endoscopy | Procedure performed | Follow-up in months | Final visual acuity | Final anatomic outcome | Final visual outcome | Cause of low final vision | Further visual potential |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 15 | PL | Trauma | Hypopyon | 2 days | Corneal edema | Non-resolving vitritis | PPV + EL + SOI | 20 | 20/320 | F | F | Macular scar | N |
| 2 | M | 46 | PL | Trauma | Hypopyon and exudates | 4 days | Repaired corneal tear | RIOFB | PPV + EL + IOFB removal | 9 | No PL | UF | UF | Phthisis | N |
| 3 | F | 74 | PL | Post-cataract | No view | 6 months | Vascularized cornea | Chronic vitreous exudates | PPV | 8 | CF 1 m | F | UF | Optic atrophy | N |
| 4 | M | 24 | PL | Trauma | Hypopyon | 2 days | Corneal edema | RIOFB | PPV + EL + IOFB removal | 15 | PL | UF | UF | RD | N |
| 5 | M | 25 | PL | Trauma | No view | 30 days | Corneal edema | Non-resolving vitritis with RD | PPV + EL + SOI | 3 | No PL | UF | UF | Phthisis | N |
| 6 | M | 51 | HM | Trauma | Hypopyon | 2 days | Corneal edema and infiltrates | Vitritis with RIOFB | PPV + EL + SOI + IOFB removal | 5 | 20/400 | F | F | Graft failure | Y |
| 7 | M | 73 | HM | Buckle infection | Endoexudates | 1 day | Corneal edema | Vitritis | PPV + AC wash | 2 | HM | F | UF | Corneal scar | Y |
| 8 | M | 25 | PL | Trauma | Hypopyon | 2 days | Repaired laceration | RIOFB and vitritis | PPV + EL + PPL + IOFB removal | 7 | 20/125 | F | F | Corneal scar | Y |
| 9 | M | 4 | PL | Trauma | Hyphema | 3 days | Repaired corneal tear | Vitritis | PPV + SOI | 10 | No PL | UF | UF | Phthisis | N |
| 10 | M | 60 | PL | Endogenous | No view | 1 month | Corneal edema | Vitritis | PPV + SOI | 6 | PL | F | UF | Optic atrophy | N |
| 11 | M | 60 | PL | Endogenous | No view | 1 month | Corneal edema | Vitritis | PPV + SOI | 6 | PL | F | UF | Optic atrophy | N |
| 12 | M | 50 | PL | Post-corneal ulcer | Hypopyon | 10 days | Stromal infiltrate | Vitritis | PPV | 4 | HM | F | UF | Corneal scar | Y |
| 13 | F | 72 | HM | Post-corneal ulcer | Hypopyon | 1 month | Infiltrate with hypopyon | Vitritis | PPV | 2 | HM | F | UF | Corneal scar | Y |
| 14 | F | 60 | CFCF | Post-cataract surgery | Hyphema and exudates | 1 month | Blood stained and endothelial exudates | Vitritis | PPV + FAE | 3 | CFCF | F | UF | Corneal scarring | Y |
| 15 | M | 30 | PL | Post-trauma | Cells and fibrin | 1 week | Corneal ring infiltrate and edema | RIOFB and vitritis | PPV + IOFB removal | 5 | HM | UF | UF | Corneal scarring and RD | N |
| 16 | M | 26 | PL | Post-trauma | Hazy view | 1 day | Microcystic edema | Vitritis + RIOFB | PPV + IOFB removal | 3 | No PL | UF | UF | Eviscerated | N |
| 17 | M | 71 | CFCF | Post-cataract surgery | Hazy view | 2 months | Decompensated cornea | Vitritis + RD | PPV + EL + SOI | 6 | HM | UF | UF | Hypotony with ciliary atrophy | N |
| 18 | M | 58 | HM | Post-cataract surgery | Hypopyon and iris exudates | 2 weeks | Microcystic edema | Persisting vitritis | PPV | 3 | PL | UF | UF | Hypotony | N |
| 19 | F | 61 | HM | Post-cataract surgery | Hypopyon | 4 days | Stromal edema | Persisting vitritis | PPV | 2 | HM | F | UF | Corneal scar | Y |
| 20 | M | 51 | CFCF | Post-DSEK + cataract surgery | Hazy view | 3 days | Microcystic edema | Persisting vitritis | PPV | 2 | CFCF | F | UF | Resolving corneal infiltrate | Y |
| 21 | M | 81 | PL | Post-cataract surgery | Hypopyon and hyphema | 1 month | Corneal epithelial defect with infiltrate | Persisting vitritis | PPV + SOI | 2 | HM | F | UF | Necrotic retina | N |
| 22 | M | 28 | 20/320 | Endogenous | Hazy view | 1 month | Corneal scar due to resolved keratitis | Perisisting vitritis | PPV | 3 | No PL | UF | UF | Phthisis | N |
| 23 | M | 35 | HM | Post-retinal surgery | Hazy view | 3 days | Stromal infiltrate | Persisting vitritis | PPV + MP + SOI | 5 | 20/800 | F | UF | Coloboma | N |
| 24 | F | 36 | PL | Post-trauma | Hypopyon | 6 days | Stromal infiltrate | Persisting vitritis | PPV | 4 | 20/400 | F | F | Irregular astigmatism | Y |
| 25 | F | 55 | PL | Post-cataract surgery | Hypopyon | 1 day | Stromal infiltrate | Persisting vitritis | PPV | 1 | PL | F | UF | Corneal scarring | Y |
| 26 | F | 70 | HM | Post-cataract surgery | Fibrin | 1 day | Stromal infiltrate | Persisting vitritis | PPV + SOI | 1 | PL | F | UF | Corneal scarring | Y |
| 27 | M | 65 | PL | Post-cataract surgery | Hazy view | 1 day | Stromal infiltrate | Persisting vitritis | PPV + SOI | 1 | PL | F | UF | Necrotic retina | N |
| 28 | M | 50 | HM | Perforated corneal ulcer | Hazy view | 6 weeks | Stromal infiltrate and iris prolapse | Vitritis | PPV+ Glue BCL | 1 | 20/320 | F | F | Graft with sutures in situ | Y |
| 29 | F | 60 | PL | Post-cataract surgery | Hazy view | 1 day | Stromal infiltrate | Vitritis | PPV + EL + SOI | 1 | HM | F | UF | Persistent epithelial defect | Y |
| 30 | M | 44 | HM | Post-trauma | Streaky hypopyon | 1 day | Corneal tear with iris prolapse | Persistent vitritis | PPV + PPL | 2 | PL | F | UF | Necrotic retina | N |
| 31 | M | 24 | HM | Post-trauma | AC exudates | 1 day | Corneal tear with infiltrate | Persistent vitritis | PPV | 2 | PL | F | UF | Necrotic retina | N |
| 32 | M | 22 | PL | Post-trauma | Plaque over the iris | 1 day | Corneal edema | Vitritis | PPV | 1 | PL | F | UF | Necrotic retina | N |
| 33 | M | 40 | PL | Post-cataract surgery | Hyphema and hypopyon | 1 day | Corneal infiltrate | Vitritis | PPV + SOI | 1 | PL | F | UF | Corneal scar | Y |
Abbreviations: CFCF, counting fingers close to face; EL, endolalser; F, favorable; HM, hand motions vision; IOFB, intraocular foreign body; N, no; PL, perception of light; PPV, pars plana vitrectomy; SOI, silicone oil injection; UF, unfavorable; Y, yes; AC, anterior chamber; DSEK, descemet stripping endothelial keratoplasty; RIOFB, retained intraocular foreign body; RD, retinal detachment; BCL, bandage contact lens; CF, counting fingers; PPL, pars plana lensectomy; FAE, fluid air exchange.
Nineteen eyes (57.57%) had only perception of light (PL) vision, whereas 14 eyes (42.42%) had at least hand motions close to face at presentation. Twenty four eyes (72.72%) had a favorable anatomic outcome at the last visit, whereas only five eyes (15.15%) had a favorable visual outcome at the last visit. Among the remaining 28 eyes which had an unfavorable visual outcome at the last visit, ten eyes had further vision potential after a second corneal procedure was planned as assessed by the viability of the retina visualized intraoperatively on endoscopy. Evisceration was required only in one eye. Samples from 16 eyes (48.48%) showed a positive culture on microbiological evaluation. The predominant organism isolated was Pseudomonas aeruginosa followed by Streptococcus pneumoniae (Table 2).
Table 2.
Microbiological outcome of the cases in the current study
| Case number | Cultured organism |
|---|---|
| 1 | No growth |
| 2 | No growth |
| 3 | No growth |
| 4 | No growth, Gram-negative bacilli on smear |
| 5 | No growth |
| 6 | Streptococcus pseudoporcinus |
| 7 | Streptococcus pneumoniae |
| 8 | No growth, Gram-positive cocci in pairs and chains on smear |
| 9 | S. pneumoniae |
| 10 | No growth |
| 11 | No growth |
| 12 | Fusarium solani |
| 13 | No growth |
| 14 | No growth |
| 15 | No growth |
| 16 | No growth |
| 17 | No growth |
| 18 | Candida famata |
| 19 | Pseudomonas aeruginosa |
| 20 | P. aeruginosa and Exserohilum rostratum |
| 21 | Unidentified fungus |
| 22 | No growth, Gram-positive cocci in pairs and chains |
| 23 | No growth |
| 24 | P. aeruginosa |
| 25 | P. aeruginosa |
| 26 | P. aeruginosa |
| 27 | P. aeruginosa |
| 28 | No growth |
| 29 | P. aeruginosa |
| 30 | Klebsiella oxytoca |
| 31 | S. pneumoniae |
| 32 | No growth |
| 33 | P. aeruginosa |
Discussion
The current study reports the management of cases of endophthalmitis with endoscopic vitrectomy. The ophthalmic endoscope was first introduced by Thorpe in 1934 for the extraction of non-metallic foreign bodies.7 Ever since, there have been reports of its usage for various retina- and glaucoma-related pathologies.8–10 Our group recently reported the sensitivity of diagnostic endoscopy in prognosticating the posterior segment before conducting a definitive vision-restoring anterior segment procedure.11 In conditions like endophthalmitis where acute management is paramount, but is often obstructed by limited anterior segment visualization, the application of endoscopy is ideal. The other alternative to managing such cases is performing a temporary keratoprosthesis placement, vitrectomy, and then followed by an immediate penetrating keratoplasty.12 Though a viable option, this process increases the surgical time and the complexity of the surgery, and in case the eye is deemed inoperable on table, time and energy investment would become void as keratoplasty would still be obligatory. Conversely, it has been shown that both temporary keratoprosthesis and endoscopy have comparable anatomic and visual outcomes in managing cases with ocular trauma and opaque cornea.13 The study also suggested that endoscopy allows earlier diagnoses and fewer surgical interventions when compared to keratoprosthesis.
Ben-nun et al described seven cases of corneal edema with posterior segment pathologies managed with endoscopic vitrectomy.6 Both the presenting visual acuity and the final visual outcome were better in their series than the current series. This is explained by the fact that only one case in that series had endophthalmitis. One large series of endophthalmitis managed with endoscopy was described by De Smet et al.2 They described 15 eyes managed with endoscopy. Post-cataract surgery was the commonest etiology in their series (7/15 eyes [46.67%]). In their series, overall eight patients (53.33%) retained useful vision. In contrast, in the current study, only five eyes (15.15%) had a favorable visual outcome at the last visit. This relatively poorer visual improvement could be explained by the fact that in our series, the predisposing cause was predominantly open globe injury, which itself is an independent risk factor for poor visual outcome. Ren et al14 have also described a series of endophthalmitis cases that were managed with endoscopic vitrectomy. In their series, akin ours, 16/21 eyes (76.19%) were post-trauma. Useful vision was achieved in 3/21 eyes (14.28%). They too had predominantly post-trauma endophthalmitis (76.19%). Table 3 describes the comparative results of the current study with previous large studies.
Table 3.
Comparative outcome of previous studies on endoscopy in endophthalmitis with the current study
| Parameters | De Smet et al2 | Ren et al14 | Current series |
|---|---|---|---|
| Total eyes | 15 | 21 | 33 |
| Mean age (years) | 64 | 34 | 47 |
| Male gender (%) | 8 (53.33%) | 15 (71.42%) | 25 (75.75%) |
| Vision at presentation not more than PL | 9 (60%) | 12 (57.14%) | 19 (57.57%) |
| Final vision of at least CFCF | 8 (53.33%) | 7 (33.33%) | 9 (27.27%) |
| Post-cataract surgery etiology | 7 (46.66%) | 4 (19.04%) | 11 (33.33%) |
| Post-trauma etiology | 2 (13.33%) | 16 (76.19%) | 13 (39.4%) |
| Mean follow-up (months) | 6a | 18–36b | 4.42±4.21 |
| Culture positivity rate | 14 (93.33%) | 11 (52.38%) | 16 (48.48%) |
| Evisceration/enucleation rate | 4 (26.66%) | 2 (9.52%) | 1 (3.03%) |
Notes:
SD is not reported;
mean and SD are not reported. Data presented as n (%) unless stated otherwise.
Abbreviations: PL, perception of light; CFCF, counting fingers close to face.
In the current study, though the final visual outcome was poor, ten eyes among those with poor visual outcome had a further scope of vision improvement by a future corneal procedure. This was determined by noting fairly healthy retinae and discs in those cases during the endoscopic procedure. It is well known that involvement of cornea in cases of endophthalmitis predisposes the eye to risk of evisceration.15–18 In these studies, the evisceration rates varied from 14% to 43%. These studies suggest that an etiology of trauma, poorer presenting visual acuity, and delayed intervention increase the risk of evisceration. In the current study, where the cases were managed relatively early in spite of poor visualization by endoscopy, the evisceration rate noted was 3.03% (1/33 eyes). This suggests that early intervention by endoscopy causes reduction of the organism load and may lead to containment of the infection. The containment of infection avoids the progression of endophthalmitis to panophthalmitis. This progression, if occurs, reduces the possibility of any visual gain drastically.19 The need to eviscerate an eye also has a psychological impact and can reduce the quality of life.20
This study has its inherent limitations. Due to retrospective nature of the study, we could not assess various confounding factors. Small sample size was a limiting factor to reach a statistical significance of the impact of these factors. Most of the cases in this study were post-trauma endophthalmitis. Trauma itself is a confounding factor for a final poor visual outcome. It would be difficult to differentiate the reason for poor visual recovery in post-trauma endophthalmitis group, as it may be due to trauma or due to subsequent endophthalmitis. We propose that this technique leads to a reduction in eviseration rates. Though this is not proven directly due to lack of a control arm in the current study, comparison with literature citing evisceration rates in conventional management of endophthalmitis does suggest so.15–18 As described earlier, a few cases are likely to benefit from further corneal intervention as the posterior segment was deemed viable on endoscopic examination. The final visual acuity obtained in these cases could not be reported, as definitive corneal interventions in these patients are pending as of current writing.
Conclusion
Endoscopic vitrectomy is a unique tool that allows for early detailed surgical intervention in cases of endophthalmitis where conventional approach is precluded due to poor media clarity. This may lead to better final visual and anatomic outcomes and obviate the need for evisceration significantly.
Acute endophthalmitis necessitates prompt treatment, often requiring early vitrectomy. This is precluded due to media opacity in most cases. The current study describes the application of endoscopic vitrectomy in such situations, as it helps to operate circumventing the compromised media clarity.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mamalis N. Endophthalmitis. J Cataract Refract Surg. 2002;28(5):729–730. doi: 10.1016/s0886-3350(02)01350-0. [DOI] [PubMed] [Google Scholar]
- 2.De Smet M, Ghyczy-Carlborg EAE, Mura M. Endoscopy for the management of severe endophthalmitis. Acta Ophthalmol Scand. 2006;84(Supplement 239):96–98. [Google Scholar]
- 3.Endophthalmitis Vitrectomy Study Group A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol. 1995;113:1479–1496. [PubMed] [Google Scholar]
- 4.Boscher C, Lebuisson DA, Lean JS, Nguyen-Khoa JL. Vitrectomy with endoscopy for management of retained lens fragments and/or posteriorly dislocated intraocular lens. Graefes Arch Clin Exp Ophthalmol. 1998;236(2):115–121. doi: 10.1007/s004170050051. [DOI] [PubMed] [Google Scholar]
- 5.Valmaggia C, De Smet M. Endoscopic management of neo-vascular glaucoma. Klin Monatsbl Augenheilkd. 2004;221:343–346. doi: 10.1055/s-2004-812870. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Nun J. Cornea sparing by endoscopically guided vitreoretinal surgery. Ophthalmology. 2001;108(8):1465–1470. doi: 10.1016/s0161-6420(01)00642-x. [DOI] [PubMed] [Google Scholar]
- 7.Thorpe HE. Ocular endoscopy: an instrument for removal of intravitreous nonmagnetic foreign bodies. Trans Am Acad Ophthalmol. 1934;39:422–424. [Google Scholar]
- 8.Norris JL, Cleasby GW, Nakanishi AS, Martin LJ. Intraocular endoscopic surgery. Am J Ophthalmol. 1981;91(5):603–606. doi: 10.1016/0002-9394(81)90058-1. [DOI] [PubMed] [Google Scholar]
- 9.Uram M. Ophthalmic laser microendoscope ciliary process ablation in the management of neovascular glaucoma. Ophthalmology. 1992;99(12):1823–1828. doi: 10.1016/s0161-6420(92)31718-x. [DOI] [PubMed] [Google Scholar]
- 10.Uram M. Ophthalmic laser microendoscope endophotocoagulation. Ophthalmology. 1992;99(12):1829–1832. doi: 10.1016/s0161-6420(92)31717-8. [DOI] [PubMed] [Google Scholar]
- 11.Reddy Pappuru RR, Tyagi M, Paulose RM, et al. Role of diagnostic endoscopy in posterior segment evaluation for definitive prognostication in eyes with corneal opacification. Am J Ophthalmol. 2017;176:9–14. doi: 10.1016/j.ajo.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Ozimek M, Nowomiejska K, Forlini C, Rejdak R. Posttraumatic endophthalmitis due to tobacco drying wire treated with vitrectomy, temporary keratoprosthesis, and keratoplasty. Ophthalmol J. 2016;1(1):36–39. [Google Scholar]
- 13.Chun DW, Colyer MH, Wroblewski KJ. Visual and anatomic outcomes of vitrectomy with temporary keratoprosthesis or endoscopy in ocular trauma with opaque cornea. Ophthalmic Surg Lasers Imaging. 2012;43(4):302–310. doi: 10.3928/15428877-20120618-09. [DOI] [PubMed] [Google Scholar]
- 14.Ren H, Jiang R, Xu G, et al. Endoscopy-assisted vitrectomy for treatment of severe endophthalmitis with retinal detachment. Graefes Arch Clin Exp Ophthalmol. 2013;251(7):1797–1800. doi: 10.1007/s00417-013-2297-4. [DOI] [PubMed] [Google Scholar]
- 15.O’Neill EC, Yeoh J, Fabinyi DC, et al. Risk factors, microbial profiles and prognosis of microbial keratitis-associated endophthalmitis in high-risk eyes. Graefes Arch Clin Exp Ophthalmol. 2014;252(9):1457–1462. doi: 10.1007/s00417-014-2732-1. [DOI] [PubMed] [Google Scholar]
- 16.Scott IU, Flynn HW, Feuer W, et al. Endophthalmitis associated with microbial keratitis. Ophthalmology. 1996;103(11):1864–1870. doi: 10.1016/s0161-6420(96)30415-6. [DOI] [PubMed] [Google Scholar]
- 17.Tsai YY, Tseng SH. Risk factors in endophthalmitis leading to evisceration or enucleation. Ophthalmic Surg Lasers. 2001;32(3):208–212. [PubMed] [Google Scholar]
- 18.Xuehui L, Ds N, Zheng K. Risk factors for endophthalmitis requiring evisceration or enucleation. Sci Rep. 2016;6:28100. doi: 10.1038/srep28100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pappuru RR, Dave VP, Pathengay A. Endophthalmitis progressing to panophthalmitis: Clinical features, demographic profile and factors predicting outcome. Semin Ophthalmol. 2017;19:1–4. doi: 10.1080/08820538.2017.1416411. [DOI] [PubMed] [Google Scholar]
- 20.Ahn JM, Lee SY, Yoon JS. Health-related quality of life and emotional status of anophthalmic patients in Korea. Am J Ophthalmol. 2010;149(6):1005–1011. doi: 10.1016/j.ajo.2009.12.036. [DOI] [PubMed] [Google Scholar]