ABSTRACT
Background:
Traditionally, total omentectomy is performed along with gastric resection and extended lymphadenectomy in gastric cancer (GC) surgery. However, solid evidences regarding its oncologic benefit is still scarce.
Aim:
To evaluate the incidence of metastatic omental lymph nodes (LN) in patients undergoing curative gastrectomy for GC, as well as its risk factors and patients’ outcomes.
Methods:
All consecutive patients submitted to D2/modified D2 gastrectomy due to gastric adenocarcinoma from March 2009 to April 2016 were retrospectively reviewed from a prospective collected database.
Results:
Of 284 patients included, five (1.8%) patients had metastatic omental LN (one: pT3N3bM0; two: pT4aN3bM0; one: pT4aN2M0 and one pT4bN3bM0). Four of them deceased and one was under palliative chemotherapy due relapse. LN metastases in the greater omentum significantly correlated with tumor’s size (p=0.018), N stage (p<0.001), clinical stage (p=0.022), venous invasion growth (p=0.003), recurrence (p=0.006), site of recurrence (peritoneum: p=0.008; liver: p=0.023; ovary: p=0.035) and death (p=0.008).
Conclusion:
The incidence of metastatic omental LN of patients undergoing radical gastrectomy due to GC is extremely low. Total omentectomy may be avoided in tumors smaller than 5.25 cm and T1/T2 tumors. However, the presence of lymph node metastases in the greater omentum is associated with recurrence in the peritoneum, liver, ovary and death.
HEADINGS: Gastric neoplasm, Recurrenc, Lymph node excisio, Gastrectom, Adenocarcinom, Omentum
RESUMO
Racional:
Tradicionalmente a omentectomia total é realizada juntamente com a ressecção gástrica associada à linfadenectomia na cirurgia do câncer gástrico. No entanto, evidências sólidas em relação ao seu benefício oncológico são escassas .
Objetivo: Avaliar a incidência de metástases em linfonodos do omento maior em pacientes submetidos à gastrectomia potencialmente curativa por câncer gástrico, assim como, avaliar os fatores de risco para a ocorrência e a evolução dos pacientes.
Métodos:
Pacientes consecutivos submetidos à gastrectomia D2/D2 modificada devido ao adenocarcinoma gástrico foram analisados retrospectivamente a partir de um banco de dados.
Resultados:
Dos 284 pacientes, cinco (1,8%) tinham linfonodos metastáticos no omento maior (um pT3N3bM0; dois pT4aN3bM0; um pT4aN2M0 e um pT4bN3bM0). Quatro faleceram e um estava em tratamento paliativo com quimioterapia devido à recidiva da doença. Os linfonodos metastáticos no omento maior tiveram correlação significativa com o tamanho do tumor (p=0,018), estádio N (p<0,001), estádio clínico (p=0,022), invasão venosa (p=0,003), recorrência (p=0,006), local de recorrência (peritônio p=0,008; fígado p=0,023; ovário p=0,035) e óbito (p=0,008).
Conclusão:
A incidência de linfonodos metastático no omento maior de pacientes submetidos à gastrectomia radical por câncer gástrico é baixa. A omentectomia total pode ser evitada em tumores menores que 5,25 cm e estádios T1/T2. Entretanto, a presença de metástases linfonodais no omento maior está associada à recidiva no peritônio, fígado, ovário e óbito.
DESCRITORES: Neoplasias gástrica, Recidiv, Excisão de linfonod, Gastrectomi, Adenocarcinom, Omento
INTRODUCTION
The prognostic relevance of the multimodal treatment in gastric cancer (GC) has been well stablished 30 . However, surgery remains the only possibility of cure, especially when is associated with early diagnosis 18 , 32 . It is also known that the lymph node (LN) involvement is the main prognostic factor after potential curative resection (R0) 28 . Thus, systematic removal of LN is considered crucial in GC surgery and the number of harvested LN as a direct measure of the quality of surgery 3 ,38.
The greater omentum (including the omental sac) is removed in block among with LN in this type of operation. It is believed that total omentectomy (TO) is essential to ensure the elimination of cancer cells during advanced GC surgery 11 . However, the greater omentum plays an important role in the peritoneal primary defense. Therein are the milky spots (mesenchymal cells covered by a mesothelium layer containing macrophages (70%), B lymphocytes (10%), T lymphocytes (10%) and mast cells. The omentum reduces intestinal adhesions and prevents free peritonitis. Patients who undergo to TO are more vulnerable to peritoneal infections, which are often associated with worse clinical outcomes 4 , 26 . Further, it has been reported that long-term overall survival (OS) does not differ between patients undergoing total or partial omentectomy (PO), whereas the incidence of complications is higher in the TO patients 9 , 36 .
There is no consensus regarding the oncologic value of omentectomy in GC surgery between the European, American and Japanese guidelines. The European guideline provides no guidance on this subject 34 , whereas the American guidelines recommend resection of the greater and lesser omentum 1 . Alternatively, the Japanese Gastric Cancer Association (JGCA) guidelines recommend the preservation of the omentum 3 cm distal to gastroepiploic vessels in patients with T1/T2 stage tumors and TO in T3/T4 tumors 19 . Its pivotal role is not discussed when there is a suspicion or evident metastatic invasion of the greater omentum. However, the unnecessary impact of TO might be substantial.
In this context, over the past few years, laparoscopic gastrectomy has become an alternative procedure for early GC patient 19 . Studies are underway to confirm its efficacy in more advanced forms 16 , 17 . In open surgery, the dissection of the greater omentum through the avascular plane of the transverse colon can lead to TO quickly and satisfactorily. However, the complete resection of the greater omentum during laparoscopic gastrectomy is associated with longer operative time and higher risks of adjacent organs injury, without interfering in the recurrence or in the disease specific survival 21 .
There is a possibility that the LN in the greater omentum may be spared of metastatic involvement in GC patients. Therefore, this part of the procedure could be omitted, mainly in minimally invasive surgery, reducing surgical time and avoiding complications.
This study aimed to evaluate the incidence of metastatic LN in the greater omentum of patients who underwent radical gastrectomy for GC, as well as its risk factors and patients’ outcomes.
METHODS
The study was approved by the hospital ethics committee (NP898/2016) and registered at “Plataforma Brasil” (CAAE: 56307516.9.0000.0068) that collects all research projects that involve human beings in country.
Patients
We retrospectively reviewed all patients submitted to R0 gastrectomy due to gastric adenocarcinoma from March 2009 to April 2016 from a prospective collected database. Patients with gastric stump neoplasia, histological type different from adenocarcinoma, macroscopic metastatic involvement of the greater omentum (carcinomatosis), distant metastasis and emergency surgeries were excluded from the analysis.
Patients were staged in the preoperatively through abdominal/pelvis computed tomography, endoscopy and laboratory tests. Extension of gastric resection (total x subtotal) was based on the location of the tumor to obtain free proximal margin 37 . TNM staging was performed according to the TNM 7th edition 8 .
All cases were operated in a high-volume center by surgeons with extensive experience in the surgical management of GC. The surgical technique, extension of resection and dissection of LN chains followed the recommendations of the JGCA guidelines 19 . Total omentectomy and D2 lymphadenectomy was performed in all patients. Digestive tract was reconstructed through Roux-en-Y anastomosis. The survival status was assessed during follow-up. Patients without medical consultations for over one year were considered as the loss of follow-up. The type of recurrence was classified as peritoneal, local (lymph node or anastomosis) and distant (liver, ovary, lung, bone and others).
Pathological analysis
Routinely, at the end of each operation, the surgical specimen was prepared by a member of the surgical team. The omentum was divided from the specimen distally to the gastroepiploic vessels (LN station number 4, Figure 1). The LN stations and the omentum were sent in separate flasks for histopathological analysis. The materials were fixed in 10% formaldehyde for 24-48 h, and then analyzed. The tumor was described in order to characterize its size, location (antrum, body, proximal or diffuse), the Borrmann classification and the resection margins. All material was meticulously evaluated macroscopically, particularly to seek for implants. Through the visualization, palpation and section, the LN stations and the greater omentum were dissected to search for LN. When they were found, were described in terms of number, size and external macroscopic appearance. The LN larger than 0.6 cm were sliced and shown alone in a paraffin block. The other LN were represented together in a paraffin block. If suspected implant area was visualized, this area was described and represented. If no change was seen, about 3-5 cuts were performed.
FIGURE 1. Surgical specimen of subtotal gastrectomy with the greater omentum and lymph node chains dissected and sent in separate for histopathological analysis.
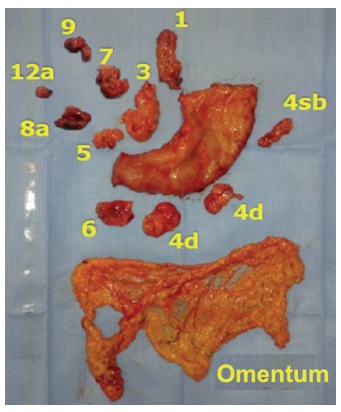
The slides were stained by the H&E method and evaluated by the pathologist in a conventional optical microscope.
Statistical analysis
The numerical variables were described by mean and standard deviation (SD) or median and quartiles, minimum and maximum values. The categorical variables were described by absolute and relative frequencies. The association between the LN involvement in the greater omentum with categorical variables was investigated by Fisher’s exact test or chi-square test and numerical variables by Wilcoxon-Mann-Whitney tests. The ROC (Receiver Operating Characteristic) curve was constructed to determine the cut-off of tumor’s size. The analysis was performed by SPSS® software version 18, adopting the significance level of 5%.
RESULTS
Of the total of 346 patients, 62 (17.9%) were excluded, who did not meet the inclusion/exclusion criteria of the study and were: 26 (41.9%) because they underwent degastrectomy; 12 (19.7%) had no adenocarcinoma (histopathological analysis revealed GIST and neuroendocrine tumor); eight (13.1%) had less than 15 LN harvested in the operation; seven (11.4%) had no tumor found in anatomopathological analysis (post-margins compromised in endoscopic resections); five (8.2%) had direct invasion of the omentum, three (4.9%) had distant metastasis (M1) and one (1.6%) had a synchronous tumor (colon neoplasia). After the exclusions, the study sample consisted of 284 patients.
TABLE 2. Surgery with multivisceral resection in the sample (n=284).
| Mesocolon - anterior sheet | n (%) |
| No | 90 (31.6) |
| Incomplete | 29 (10.2) |
| Yes | 165 (58.1) |
| Pancreatic capsule | |
| No | 96 (33.8) |
| Incomplete | 37 (13) |
| Yes | 151 (53.1) |
| Oncologic splenectomy | |
| No | 270 (95.1) |
| Yes | 14 (4.9) |
| Tactical splenectomy | |
| No | 282 (99.2) |
| Yes | 2 (0.8) |
| Hepatectomy | |
| No | 281 (98.9) |
| Yes | 3 (1.1%) |
| Colectomy | |
| No | 278 (97.8) |
| Yes | 6 (2.2) |
| Pancreatectomy | |
| No | 274 (96.4) |
| Yes | 10 (3.6) |
| Resection of the diaphragm | |
| No | 283 (99.6) |
| Yes | 1 (0.4) |
There was a male preponderance of 159 (55.9%) patients, with a mean age of 61.8 years (±11.9; 25-86). The mean body mass index (BMI) was 24.6 kg/m2 (±4.7; 14-46.5). Subtotal gastrectomy was performed in 182 (64.1%). The tumor was located at the antrum in 185 (65.2%). Forty (14.1%) patients received neoadjuvant chemotherapy. Open surgery was performed in 253 (89.1%). Thirty-day mortality was 3.1% (nine patients). The median follow-up period for all patients was 27.6 months (1-89.5). The median follow-up time of the disease-free patients was 34.3 months (1-89.5). The baseline characteristics of patients and the perioperative results are shown in Table 1. The pathological analysis is summarized in Table 3. Peritoneal washing was negative in all patients. The average number of LN resected was 41.2 (±17; 15-114). The average number of positive LN was 4.69 (±8.12; 0-53). The Lymph Node Ratio (LNR) was 0.113 (±0.447; 0-0.96). The intestinal histological type of Lauren occurred in 146 (51.4%) patients. Poorly differentiated tumors were also 146 (51.4%). Sixty-six (23.2%) had at least one LN in the greater omentum. Metastatic LN were found in five (1.8%) patients (one: pT3N3bM0; two: pT4aN3bM0; one: pT4aN2M0 and one pT4bN3bM0). The mean size of tumors of patients without metastatic omental LN was 4.8 cm (±2.96; 0.5-14.5), while the mean size of tumors of patients with metastatic LN was 8.06 cm (±2.75; 2.75-9, p=0.018). The cut-off point was 5.25 cm (area under the curve: 0.8072; IC95%: 0.6645-0.9498, Figure 2). Metastatic LN in the greater omentum was significantly correlated with N stage (p<0.001), clinical stage (p=0.022) and venous invasion growth (p=0.003).
TABLE 1. Baseline characteristics and perioperative results (n=284).
| Gender | n (%) |
| Male | 159 (55.9) |
| Female | 125 (44.1) |
| BMI kg/m2 (min-max) | 24.6 (14 - 46.5) |
| Resection Subtotal | 182 (64.1) |
| Total | 102 (35.9) |
| Approach | |
| Conventional | 253 (89.1) |
| Laparoscopic | 16 (5.6) |
| Robotic | 7 (2.5) |
| Hybrid | 8 (2.8) |
| Site of tumor | |
| Diffuse | 6 (2.1) |
| Antrum | 185 (65.2) |
| Body | 55 (19.3) |
| Proximal | 38 (13.3) |
| Borrmann classification * I | 28 (9.8) |
| II | 55 (19.3) |
| III | 135 (47.5) |
| IV | 56 (19.7) |
| Neoadjuvant chemotherapy Yes | 40 (14.1) |
| No | 244 (85.9) |
| Multivisceral resection # | |
| Yes | 36 (12.6) |
| No | 248 (87.4) |
| Mortality | |
| 30-day | 9 (3.1) |
| 90-day | 16 (5.6) |
(%); *10 patients not classified; #: details are shown in Table 2
TABLE 3. Pathological analysis according to lymph node involvement in the greater omentum.
| Metastatic omental LN | ||||
| Total (n=284) | No (n=279) | Yes (n=5) | ||
| pT stage | ||||
| T1/T2 | 116 | 116 (41.6) | 0 (0) | p1=0.081 |
| T3/T4 | 168 | 163 (58.4) | 5 (100) | |
| pN stage | ||||
| N0 / N1 | 162 | 162 (57.8) | 0 (0) | p1<0.001 |
| N2 | 60 | 59 (21.1) | 1 (20) | |
| N3a | 36 | 36 (12.9) | 0 (0) | |
| N3b | 27 | 23 (8.2) | 4 (80) | |
| Clinical stage | ||||
| I / II / IIIA | 205 | 205 (73.5) | 0 (0) | p1=0.022 |
| IIIB | 41 | 39 (13.9) | 2 (40) | |
| IIIC | 38 | 35 (12.5) | 3 (60) | |
| Tumor size (cm) & | 4.3 | 4.8 | 8.06 | p1= 0.018 |
| Lauren classification | ||||
| Intestinal | 146 | 145 (52) | 1 (20) | |
| Diffuse | 108 | 105 (37.6) | 3 (60) | p1=0.290 |
| Mixed | 30 | 29 (10.4) | 1 (20) | |
| Differentiation grade | ||||
| Well differentiated | 22 | 21 (7.5) | 1 (20) | |
| Mod. differentiated | 116 | 116 (41.6) | 0 (0) | p1=0.098 |
| Poorly differentiated | 146 | 142 (50.9) | 4 (80) | |
| Lymphatic invasion growth * | ||||
| Yes | 143 | 138 (49.5) | 5 (100) | p1=0.060 |
| No | 140 | 140 (50.5) | 0 (0) | |
| Venous invasion growth * | ||||
| Yes | 48 | 44 (15.8) | 4 (80) | p1=0.003 |
| No | 235 | 234 (82.2) | 1 (20) | |
| Perineural invasion growth * | ||||
| Yes | 140 | 136 (48.7) | 4 (80) | p1=0.211 |
| No | 143 | 142 (51.3) | 1 (20) | |
| Intestinal metaplasia | ||||
| Yes | 133 | 130 (46.6) | 3 (60) | p3= 0.552 |
| No | 151 | 149 (53.4) | 2 (40) | |
| Tumor site | ||||
| Antrum | 28 | 182 (65.2) | 3 (60) | |
| Body | 55 | 55 (19.7) | 0 (0) | p1=0.279 |
| Proximal | 135 | 36 (12.9) | 2 (40) | |
| Diffuse | 56 | 6 (2.1) | 0 (0) | |
| Neoadjuvant therapy | ||||
| Yes | 40 | 39 (14) | 1 (20) | |
| No | 244 | 240 (86) | 4 (80) | |
| Gastrectomy | ||||
| Subtotal | 182 | 179 (64.1) | 3 (60) | p1>0.999 |
| Total | 102 | 100 (35.9) | 2 (40) | |
| Omental size (cm3) & | - | 720 (11.5-6,075) | 1,087 (595-3,81) | p2=0.331 |
| Harvested LN & | 41.2 (15-114) | 41.1 (15-114) | 47.2 (31-73) | p2=0.332 |
| Positive harvested LN & | 4.7 (0-53) | 4.4 (0-53) | 20.8 (4-31) | p2=0.001 |
| Patients with omental LN & | 66 (23) | 61 (22) | 5 (100) | |
| No. omental LN & | 0 (1-6) | 0 (1-6) | 0 (1-3) | |
| No. positive omental LN | 5 | 0 | 5 (100) | |
(%); p1=Fisher’s exact test; p2=Mann-Whitney’s test; p3=Qui-square test; *=one patient undetermined; &=values are mean(range)
FIGURE 2. The area under the ROC curve (0.8072 - (IC95%:0.6645 - 0.9498) shows that patients with metastatic LN in the omentum have larger tumors.
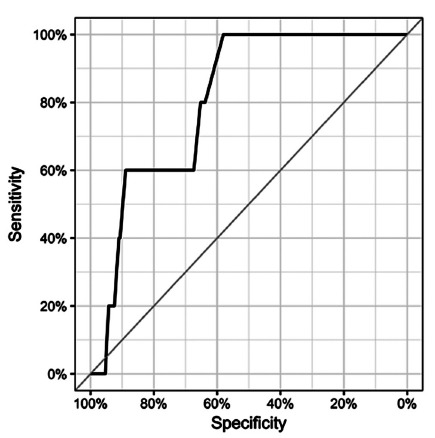
Cutt-off=5.25 cm; specificity=65.2%; sensitivity= 80%; negative predictive value=99.4%; positive predictive value=3.96%; accuracy=65.4%
During the follow-up period 163 (57.4%) were free of the disease. The cancer relapse was found in 65 (22.8%) patients and the most frequent site was in the peritoneum (46.2%). Four patients with metastatic omental LN died and the other one was under palliative chemotherapy due to the relapse on the liver and pleura. We found association between metastatic LN in the omentum with recurrence (p=0.006), site of recurrence (peritoneum: p=0.008; liver: p=0.023; ovary: p=0.035) and death (p=0.008, Table 4).
TABLE 4. Correlation between the presence of metastatic lymph nodes in the omentum and patient status.
| Metastatic omental LN | |||
| Patient status | No (n=224) | Yes (n=4)* | |
| Recurrence | |||
| No | 163 (72.8) | 0 (0) | p=0.006 |
| Yes | 59 (27.2) | 4 (100) | |
| Peritoneal | |||
| No | 197 (87.9) | 1 (25) | p=0.008 |
| Yes | 27 (12.1) | 3 (75) | |
| Local | |||
| No | 196 (87.5) | 4 (100) | p>0.999 |
| Yes | 28 (12.5) | 0 (0) | |
| Anastomosis | |||
| No | 222 (99.1) | 4 (100) | p>0.999 |
| Yes | 2 (0.9) | 0 (0) | |
| Lymph node | |||
| No | 199 (88.8) | 4 (100) | p>0.999 |
| Yes | 25 (11.2) | 0 (0) | |
| Distant | |||
| No | 205 (91.5) | 1 (25) | p=0.003 |
| Yes | 19 (8.5) | 3 (75) | |
| Liver | |||
| No | 211 (94.2) | 2 (50) | p=0.023 |
| Yes | 13 (5.8) | 2 (50) | |
| Lung | |||
| No | 222 (99.1) | 3 (75) | p=0.052 |
| Yes | 2 (0.9) | 1 (25) | |
| Ovary | |||
| No | 223 (99.5) | 3 (75) | p=0.035 |
| Yes | 1 (0.5) | 1 (25) | |
| Bone | |||
| No | 222 (99.1) | 4 (100) | p>0.999 |
| Yes | 2 (0.9) | 0 (0) | |
| Others # | |||
| No | 221 (98.7) | 3 (75) | p=0.069 |
| Yes | 3 (1.3) | 1 (25) | |
| Outcome | |||
| Free of disease | 163 (72.7) | 0(0) | |
| Relapse under treatment | 14 (6.3) | 1 (25) | |
| Death | 78 (34.8) | 4 (75) | p=0.008 |
| Loss of follow-up | 24 (10.7) | 0 (0) | |
p=Fisher’s exact test; (%); #=brain, pleura; *=the cause of death is unknown in one patient with positive lymph node in the omentum and therefore could not be attributed to disease relapse
Patients who did not relapse prior to loss of follow-up or in whom this data was unavailable were removed from the sample. For this analysis, 228 patients remained.
DISCUSSION
Despite the great advances of the multimodal therapy that have led to greater OS, free-margin gastrectomy associated with adequate lymphadenectomy remain crucial components in GC surgery with curative intent. Once questioned, extended lymphadenectomy (D2) provides better local control of the disease, allows accurate staging and avoids the stage migration phenomenon. In addition, surgery leads to better overall long-term survival. 6 , 7 , 31
Traditionally, TO is performed as a part of subtotal/total gastrectomy with lymphadenectomy. The complete removal of the greater omentum has been considered essential to ensure the elimination of micrometastasis 23 . However, there is no consensus regarding the real benefit of TO on survival improvement and relapse decrement.
Several experimental studies have reported that cancer cells sown in the peritoneal cavity preferentially grow in the omentum, specifically at the milky spots 12 , 27 . Besides, many researchers insist that the extra nodal expansion occurs in some metastatic LN, which means that cancer cells spread from the LN capsule to adjacent adipose tissue 24 .
Conversely, the greater omentum contains zones with high concentrations of immune system cells that can contribute to remove foreign bodies and bacteria 33 . Yet, it reduces the possibility of intestinal adhesions, not only by creating a mechanical barrier between the bowel and the abdominal wall, but also due to the production of fibrinolytic factors by the mesenchymal cells 5 . Other possible advantages of the omental preservation are the decrease in operative time (mainly in minimally invasive surgery), the blood loss and the reduction of complications such as abdominal abscess, ascites, anastomotic leakage, ileus, wound infection and iatrogenic lesions of the mesocolon and colon 10 , 14 , 22 . Moreover, several studies have demonstrated no difference in OS nor disease free survival (DFS) between total and partial omentectomy in GC surgery 10 , 13 , 14 , 21 , 22 . Kim et al. 21 compared TO with PO in 146 patients operated by laparoscopy for advanced GC. Propensity score match analysis (T and N parameters) showed no difference between the groups regarding DFS (TO versus PO: 83.3% vs. 90.5%, p=0.442).
Although that there are some studies that compared short/long-term outcomes, complications, relapse and survival between TO and PO in GC surgery, specific studies regarding the incidence of metastatic omental LN are lacking. Haverkamp et al. 15 prospectively evaluated the presence of omental LN and tumor deposits in 50 patients undergoing gastrectomy for GC. One (2%) had metastatic omental LN (stage IB) and four (8%) omental tumor deposits (stages IB, IIA, IIB and IIIA). Patients with tumor deposits had significantly reduced 1-year DFS compared to patients without tumor deposits (0 vs. 58.7%, p=0.003). However, no significant difference in 1-year OS of was found (25.0 vs. 67.4%, respectively, p=0.079). The authors did not find any predictive factors for omental metastasis 31 .
On the other hand, another prospective trial named OMEGA analyzed the presence of omental metastasis in 100 patients. Metastasis were detected in five (5%) patients (two with metastatic LN and three with tumor cell deposits). All of them were at least stage pT3 with macroscopically non-radical resection (p<0.001). Yet, omental metastasis was also significantly correlated with linitis plastica or location in the proximal third of the stomach (p=0.002), tumor diameter of 5 cm or larger, stage III-IV disease (p=0.010) and (y)pM1 category (p<0.001) 20 .
The present study corroborates the OMEGA trial results. It was found a significant correlation with tumors larger than 5.25 cm. Still, as well as the Japanese guidelines, all patients with omental LN metastasis were categorized as stage pT3/T4, which allows us to state that TO may be omitted in stage T1/T2 tumors. In fact, patients with positive omental LN had very advanced disease and there was a significant correlation with N stage (p<0.001) and clinical stage (p=0.022). Nevertheless, the vast majority of pT3/T4 tumors were free of metastatic omental LN (pT3: 1 positive/93 negative; pT4: 4 positive/75 negative), suggesting that TO may be also avoided in many T3/T4 tumors. The heart of the matter is how to identify these patients preoperatively, as only tumor’s size was the only risk factor associated with omental disease. Other significant parameters such as venous invasion growth (p=0.003) could only be assessed postoperatively. The rarity of the incidence also suggests that other conditions may be involved with omental LN disease.
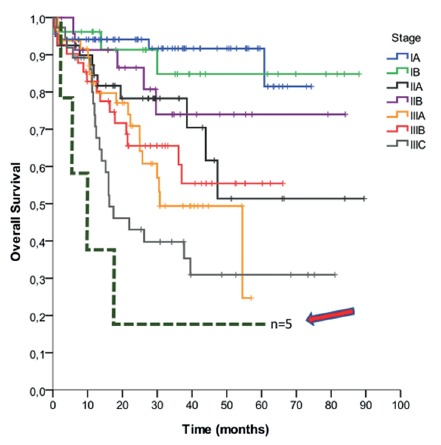
Another interesting aspect is the fact that omental LN metastasis was associated with recurrence (p=0.006), site of recurrence (peritoneum: p=0.008; liver: p=0.023; ovary: p=0.035) and death (p=0.008). Figure 3 represents OS curve of patients according to clinical stage and patients with omental LN metastasis. It may be alleged that, when there is omental LN involvement, systemic disease is in course, with very dismal prognosis. The purpose of performing TO is to remove micro metastasis and therefore to avoid mostly peritoneal recurrence. As in all patients in this cohort TO was performed, it can be speculated that removing the entire omentum during radical gastrectomy does not prevent relapse and, above all, death.
FIGURE 3. Kaplan-Meier curve of survival: each line represents the OS of patients within a single clinical stage (TNM 7th edition). The arrow indicates patients with omental metastatic LN.
This study has some limitations. Firstly, five patients (three: stage pT4aN3aM1; one: pT4bN3M0 and one: pT3N2M0) with tumor deposits in the greater omentum were not included. There was not enough information whether metastatic omental involvement was diagnosed during surgery or through pathological analysis. Obviously, apart from identifying metastatic LN, to identify tumor deposits is essential to achieve R0 resection. In any case, the staging of these patients confirms that the omental disease is associated with advanced disease. Secondly, the oncotic cytology of peritoneal lavage was negative in all patients, similar to OMEGA trial. Some studies indicate up to 10% incidence of positive cytology in patients without peritoneal metastases 25 , 29 . Peritoneal cytology could be obtained in diagnostic laparoscopy and, certainly, would influence the decision to proceed with TO and possibly offer more aggressive treatments such as hyperthermia intraperitoneal chemotherapy, which is now under evaluation 2 , 35 .
CONCLUSION
The incidence of metastatic omental LN of patients undergoing radical gastrectomy due to GC is extremely low. Total omentectomy may be avoided in tumors smaller than 5.25 cm and T1/T2 tumors. However, lymph node metastasis in the greater omentum is associated with recurrence in the peritoneum, liver, ovary and death.
ACKNOWLEDGMENTS
The authors thanks Marina Alessandra Pereira, Carol Viviana Serna González and Cynthia Chiaradia for data collection and statistical support.
Footnotes
Financial source: none
REFERENCES
- 1.Ajani JA, Barthel JS, Bekaii-Saab T, Bentrem DJ, D’Amico TA, Das P, et al. Gastric cancer. J Natl Compr Canc Netw. 2010;8(4):378–409. doi: 10.6004/jnccn.2010.0030. http://www.ncbi.nlm.nih.gov/pubmed/20410333 [DOI] [PubMed] [Google Scholar]
- 2.Badgwell B, Blum M, Das P, Estrella J, Wang X, Ho L, et al. Phase II Trial of Laparoscopic Hyperthermic Intraperitoneal Chemoperfusion for Peritoneal Carcinomatosis or Positive Peritoneal Cytology in Patients with Gastric Adenocarcinoma. Ann Surg Oncol. 2017;24(11):3338–3344. doi: 10.1245/s10434-017-6047-4. http://www.ncbi.nlm.nih.gov/pubmed/28799004 [DOI] [PubMed] [Google Scholar]
- 3.Barchi LC, Yagi OK, Jacob CE, Mucerino DR, Ribeiro U, Marrelli D, et al. Predicting recurrence after curative resection for gastric cancer. Eur J Surg Oncol. 2016;42(1):123–131. doi: 10.1016/j.ejso.2015.08.164. http://www.ncbi.nlm.nih.gov/pubmed/26365755 [DOI] [PubMed] [Google Scholar]
- 4.Beelen RH. Role of omental milky spots in the local immune response. Lancet (London, England) 1992;339(8794):689–689. doi: 10.1016/0140-6736(92)90857-y. http://www.ncbi.nlm.nih.gov/pubmed/1347391 [DOI] [PubMed] [Google Scholar]
- 5.Cerci C, Eroglu E, Sutcu R, Celikbas B, Kilbas A. Effects of omentectomy on the peritoneal fibrinolytic system. Surg Today. 2008;38(8):711–715. doi: 10.1007/s00595-007-3705-3. http://www.ncbi.nlm.nih.gov/pubmed/18668314 [DOI] [PubMed] [Google Scholar]
- 6.Chen T, Yan D, Zheng Z, Yang J, Dong XDE. Evolution in the surgical management of gastric cancer: is extended lymph node dissection back in vogue in the USA. World J Surg Oncol. 2017;15(1):135–135. doi: 10.1186/s12957-017-1204-6. http://www.ncbi.nlm.nih.gov/pubmed/28716043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Degiuli M, De Manzoni G, Di Leo A, D’Ugo D, Galasso E, Marrelli D, et al. Gastric cancer: Current status of lymph node dissection. World J Gastroenterol. 2016;22(10):2875–2893. doi: 10.3748/wjg.v22.i10.2875. http://www.ncbi.nlm.nih.gov/pubmed/26973384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edge S, Byrd D, Carducci M, Compton C. AJCC - Cancer Staging Manual [Internet] 7th . New York, NY: 2009. https://cancerstaging.org/references-tools/deskreferences/Pages/default.aspx [Google Scholar]
- 9.Fujita J, Tsukahara Y, Ikeda K, Akagi K, Kan K, Hata S, et al. Evaluation of Omentum Preserving Gastrectomy for Advanced Gastric Cancer. Japanese J Gastroenterol Surg. 2003 http://joi.jlc.jst.go.jp/JST.Journalarchive/jjgs1969/36.1151?from=CrossRef [Google Scholar]
- 10.Hagiwara A, Sawai K, Sakakura C, Shirasu M, Ohgaki M, Yamasaki J, et al. Complete omentectomy and extensive lymphadenectomy with gastrectomy improves the survival of gastric cancer patients with metastases in the adjacent peritoneum. Hepatogastroenterology. 2018;45(23):1922–1929. http://www.ncbi.nlm.nih.gov/pubmed/9840177 [PubMed] [Google Scholar]
- 11.Hagiwara A, Takahashi T, Sawai K, Taniguchi H, Shimotsuma M, Okano S, et al. Milky spots as the implantation site for malignant cells in peritoneal dissemination in mice. Cancer Res. 1993;53(3):687–692. http://www.ncbi.nlm.nih.gov/pubmed/8425204 [PubMed] [Google Scholar]
- 12.Hasegawa S, Kunisaki C, Ono H, Oshima T, Fujii S, Taguri M, et al. Omentum-preserving gastrectomy for advanced gastric cancer: a propensity-matched retrospective cohort study. Gastric Cancer. 2013;16(3):383–388. doi: 10.1007/s10120-012-0198-6. http://www.ncbi.nlm.nih.gov/pubmed/22983455 [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa S, Yamamoto Y, Taguri M, Morita S, Sato T, Yamada R, et al. A Randomized Phase II Trial of Omentum-preserving Gastrectomy for Advanced Gastric Cancer. Jpn J Clin Oncol. 2013;43(2):214–216. doi: 10.1093/jjco/hys208. http://www.ncbi.nlm.nih.gov/pubmed/23242583 [DOI] [PubMed] [Google Scholar]
- 14.Haverkamp L, Brenkman HJF, Ruurda JP, Ten Kate FJW, van Hillegersberg R. The Oncological Value of Omentectomy in Gastrectomy for Cancer. J Gastrointest Surg. 2016;20(5):885–890. doi: 10.1007/s11605-016-3092-4. http://www.ncbi.nlm.nih.gov/pubmed/26895951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hur H, Lee HY, Lee H-J, Kim MC, Hyung WJ, Park YK, et al. Efficacy of laparoscopic subtotal gastrectomy with D2 lymphadenectomy for locally advanced gastric cancer: the protocol of the KLASS-02 multicenter randomized controlled clinical trial. BMC Cancer. 2015;15:355–355. doi: 10.1186/s12885-015-1365-z. http://www.ncbi.nlm.nih.gov/pubmed/25939684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inaki N, Etoh T, Ohyama T, Uchiyama K, Katada N, Koeda K, et al. A Multi-institutional, Prospective, Phase II Feasibility Study of Laparoscopy-Assisted Distal Gastrectomy with D2 Lymph Node Dissection for Locally Advanced Gastric Cancer (JLSSG0901) World J Surg. 2015;39(11):2734–2741. doi: 10.1007/s00268-015-3160-z. http://www.ncbi.nlm.nih.gov/pubmed/26170158 [DOI] [PubMed] [Google Scholar]
- 17.Jacob C, Gama-Rodrigues J, Zilberstein B. Câncer gástrico precoce: complicações e mortalidade após gastrectomia e linfadenectomia regrada: experiência com 178 casos em uma única instituição. ABCD Arq Bras Cir Dig. 2006;19(4):146–152. [Google Scholar]
- 18.Japanese Gastric Cancer Association JGC Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2017;20(1):1–19. doi: 10.1007/s10120-016-0622-4. http://www.ncbi.nlm.nih.gov/pubmed/27342689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jongerius EJ, Boerma D, Seldenrijk KA, Meijer SL, Scheepers JJG, Smedts F, et al. Role of omentectomy as part of radical surgery for gastric cancer. Br J Surg. 2016;103(11):1497–1503. doi: 10.1002/bjs.10149. http://www.ncbi.nlm.nih.gov/pubmed/27550526 [DOI] [PubMed] [Google Scholar]
- 20.Kim D, Lee J, Kim W. A comparison of total versus partial omentectomy for advanced gastric cancer in laparoscopic gastrectomy. World J Surg Oncol. 2014;12(1):64–64. doi: 10.1186/1477-7819-12-64. http://www.ncbi.nlm.nih.gov/pubmed/24669875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim M-C, Kim K-H, Jung GJ, Rattner DW. Comparative study of complete and partial omentectomy in radical subtotal gastrectomy for early gastric cancer. Yonsei Med J. 2011;52(6):961–966. doi: 10.3349/ymj.2011.52.6.961. http://www.ncbi.nlm.nih.gov/pubmed/22028160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawrance RJ, Loizidou M, Cooper AJ, Alexander P, Taylor I. Importance of the omentum in the development of intra-abdominal metastases. Br J Surg. 1991;78(1):117–119. doi: 10.1002/bjs.1800780135. http://www.ncbi.nlm.nih.gov/pubmed/1998851 [DOI] [PubMed] [Google Scholar]
- 23.Lee I-S, Park Y-S, Ryu M-H, Song MJ, Yook J-H, Oh S-T, et al. Impact of extranodal extension on prognosis in lymph node-positive gastric cancer. Br J Surg. 2014;101(12):1576–1584. doi: 10.1002/bjs.9640. http://doi.wiley.com/10.1002/bjs.9640 [DOI] [PubMed] [Google Scholar]
- 24.Lee SD, Ryu KW, Eom BW, Lee JH, Kook MC, Kim Y-W. Prognostic significance of peritoneal washing cytology in patients with gastric cancer. Br J Surg. 2012;99(3):397–403. doi: 10.1002/bjs.7812. http://www.ncbi.nlm.nih.gov/pubmed/22101572 [DOI] [PubMed] [Google Scholar]
- 25.Meza-Perez S, Randall TD. Immunological Functions of the Omentum. Trends Immunol. 2017;38(7):526–536. doi: 10.1016/j.it.2017.03.002. http://www.ncbi.nlm.nih.gov/pubmed/28579319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oosterling SJ, van der Bij GJ, Bögels M, van der Sijp JRM, Beelen RHJ, Meijer S, et al. Insufficient ability of omental milky spots to prevent peritoneal tumor outgrowth supports omentectomy in minimal residual disease. Cancer Immunol Immunother. 2006;55(9):1043–1051. doi: 10.1007/s00262-005-0101-y. http://www.ncbi.nlm.nih.gov/pubmed/16311732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereira MA, Ramos MFKP, Dias AR, Yagi OK, Faraj SF, Zilberstein B, et al. Detection of occult lymph node tumor cells in node-negative gastric cancer patients. ABCD Arq Bras Cir Dig (São Paulo) 2017;30(1):30–34. doi: 10.1590/0102-6720201700010009. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0102-67202017000100030&lng=en&tlng=en [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ribeiro-JR U, Safatle AR, Zilbertein B, Mucerino D, Yagi O, C B, et al. Does the Intraoperative Peritoneal Lavage Cytology Add Prognostic Information in Patients With Potentially Curative Gastric Resection. J Gastrointest Surg. 2006;10(2):170–177. doi: 10.1016/j.gassur.2005.11.001. http://www.ncbi.nlm.nih.gov/pubmed/16455447 [DOI] [PubMed] [Google Scholar]
- 29.Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-Year Outcomes of a Randomized Phase III Trial Comparing Adjuvant Chemotherapy With S-1 Versus Surgery Alone in Stage II or III Gastric Cancer. J Clin Oncol. 2011;29(33):4387–4393. doi: 10.1200/JCO.2011.36.5908. http://www.ncbi.nlm.nih.gov/pubmed/22010012 [DOI] [PubMed] [Google Scholar]
- 30.Songun I, Putter H, Kranenbarg EM-K, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11(5):439–449. doi: 10.1016/S1470-2045(10)70070-X. http://www.ncbi.nlm.nih.gov/pubmed/20409751 [DOI] [PubMed] [Google Scholar]
- 31.Toneto MG, Viola L. Current status of the multidisciplinary treatment of gastric adenocarcinoma. ABCD Arq Bras Cir Dig (São Paulo) 2018;31(2):e1373. doi: 10.1590/0102-672020180001e1373. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0102-67202018000200504&lng=en&tlng=en [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uzunköy A, Ozbilge H, Horoz M. The influence of omentectomy on bacterial clearance: an experimental study. Ulus Travma Acil Cerrahi Derg. 2009;15(6):541–545. http://www.ncbi.nlm.nih.gov/pubmed/20037870 [PubMed] [Google Scholar]
- 33.Van Cutsem E, Dicato M, Geva R, Arber N, Bang Y, Benson A, et al. The diagnosis and management of gastric cancer: expert discussion and recommendations from the 12th ESMO/World Congress on Gastrointestinal Cancer, Barcelona, 2010. Ann Oncol. 2011;22:v1–v9. doi: 10.1093/annonc/mdr284. http://www.ncbi.nlm.nih.gov/pubmed/21633049 [DOI] [PubMed] [Google Scholar]
- 34.Van der Kaaij RT, Braam HJ, Boot H, Los M, Cats A, Grootscholten C, et al. Treatment of Peritoneal Dissemination in Stomach Cancer Patients With Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC): Rationale and Design of the PERISCOPE Study. JMIR Res Protoc. 2017;6(7):e136. doi: 10.2196/resprot.7790. http://www.ncbi.nlm.nih.gov/pubmed/28705789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watanabe N, Nashimoto A, Yabusaki H, Takii Y, Tsuchiya Y, Tanaka O. Evaluation of omento-bursectomy for t2 and t3 gastric cancers. Nihon Rinsho Geka Gakkai Zasshi (Journal Japan Surg Assoc. 2004;65(10):2570–2574. http://joi.jlc.jst.go.jp/JST.Journalarchive/ringe1998/65.2570?from=CrossRef [Google Scholar]
- 36.Zilberstein B, Malheiros C, Lourenço LG, Kassab P, Jacob CE, Weston AC, et al. Consenso brasileiro sobre câncer gástrico: diretrizes para o câncer gástrico no Brasil. ABCD Arq Bras Cir Dig (São Paulo) 2013;26(1):2–6. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0102-67202013000100002&lng=pt&tlng=pt [Google Scholar]
- 37.Zilberstein B, Mucerino DR, Yagi OK, Ribeiro-Junior U, Lopasso FP, Bresciani C, et al. Resultados da gastrectomia D2 para o câncer gástrico: dissecção da cadeia linfática ou ressecção linfonodal múltipla. ABCD Arq Bras Cir Dig (São Paulo) 2012;25(3):161–164. doi: 10.1590/s0102-67202012000300005. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0102-67202012000300005&lng=pt&tlng=pt [DOI] [PubMed] [Google Scholar]


