Abstract
BACKGROUND:
In 2010, there were approximately 2.2 million emergency room visits associated with traumatic brain injury (TBI), with 80 percent diagnosed as mild TBI or concussion. In addition, there are a large number of TBIs, especially mild TBIs, which go either unreported by patients or initially undiagnosed by clinicians. Our team has previously identified a panel of immune-related genes that can diagnose ischemic stroke at triage, and due to shared pathophysiological mechanisms of TBI and stroke, we hypothesized that this panel of genes may also be utilized for the diagnosis of TBI.
OBJECTIVES:
The primary aims of this pilot study were to: (1) characterize changes in a panel of immune-related genes in TBI; (2) identify immune-related biomarkers that may be used to diagnose TBI and (3) describe the peripheral immune response following TBI.
METHODS:
Blood was drawn from TBI patients no later than 24h of injury onset and matched control subjects. Real-time PCR was used to measure gene expression, and a white blood cell differential was performed to obtain neutrophil and lymphocyte percentages.
RESULTS:
Relative mRNA expression of ARG1, LY96, MMP9, s100a12 was significantly increased and CCR7 was significantly decreased in peripheral blood of TBI patients within 24 hours of injury compared to control subjects. We also observed a different pattern of leukocyte dynamics following TBI between mild and severe TBI.
CONCLUSIONS:
We have described a panel of immune-related genes that can accurately predict/diagnose TBI with higher sensitivity and specificity of other biomarkers to date.
Keywords: Traumatic brain injury, inflammation, neutrophil-lymphocyte ratio
1. Introduction
Traumatic brain injury (TBI) is defined as an injury that results in alteration of brain function or structure, caused by an external force (Souter et al., 2015). According to the Centers for Disease Control (CDC), in 2010, there were approximately 2.2 million emergency room visits associated with TBI (CDC, 2010), with80%beingdiagnosedasmildconcussion(Laker, 2011). TBI incidence is likely underestimated, given that the diagnosis of TBI is subjective and many individuals either do not seek medical care at all or only after chronic symptoms do not dissipate. Even mild TBI (mTBI) or concussion can result in chronic brain tissue injury, with detrimental physical, psychological, and social effects that result in loss of work, decreased quality of life, and significant healthcare expenses (Croall et al., 2014).
Aside from advanced neuroimaging that has shown promise in acute identification of suspected mTBI/concussion, there remains no quick unbiased test to rule in or rule out suspected mTBI/concussion in the emergency setting. Efforts to develop non-subjective, fiscally sustainable methods of triaging patients with suspected mTBI/concussion are still needed to ensure appropriate follow up care and evaluation of fitness to return to work/play/duty. The primary need for accurate identification of suspected mTBI/concussion is to ensure appropriate clinical follow up.
Our team has previously identified a panel of immune-related genes that can accurately triage suspected stroke from non-stroke patients in the emergency setting (Barr et al., 2010; Petrone et al., 2016; Tang et al., 2006). This five-gene panel consists of arginase 1 (ARG1), lymphocyte antigen 96 (LY96), matrix metalloproteinase 9 (MMP9), s100 calcium binding protein a12 (s100a12), and c-c chemokine-receptor type 7 (CCR7), and are highly correlated with peripheral blood immune system function [neutrophil/lymphocyte ratio (NLR)]. (Barr et al., 2010; Petrone et al., 2016). Due to shared post-injury pathophysiological mechanisms of TBI and stroke, we hypothesized that this panel of genes and their relationship with the NLR may also be useful for the triage of suspected mTBI/concussion. The primary aims of this pilot study were to: (1) characterize changes in blood gene expression of ARG1, LY96, MMP9, s100a12, and CCR7 in differing severities of TBI; (2) identify immune-related biomarkers that may be used in combination with peripheral blood gene expression for suspected mTBI/concussion triage, and (3) describe the peripheral blood immune system response following TBI. Our data serves as proof of principle that measuring peripheral blood gene expression and immune system function following suspected mTBI/concussion is a viable clinical option for aiding emergency mTBI/concussion triage.
2. Materials and methods
2.1. Research protocol approval and informed consent
This study received approval for human subject research from the institutional review boards of West Virginia University and Ruby Memorial Hospital (Morgantown, WV). Written informed consent was obtained from all subjects or their authorized representatives prior to performing study procedures.
2.2. Subject recruitment
TBI patients and control subjects were recruited from Ruby Memorial Hospital (Morgantown, WV). Male and female TBI patients were eligible for recruitment if the following inclusion criteria were met: (1) age ≥18 years, (2) arrived to emergency department within 24 hours of injury, and (3) diagnosis of mild, moderate, or severe TBI based on Glascow Coma Score, (4) no history of brain injury or other overt central nervous system disease, and (5) no history of recent hospitalization (within 30 days). Control subjects were eligible for recruitment if the following inclusion criteria were met: (1) age ≥18 years; (2) no history of brain injury or other overt central nervous system disease; and (3) no history of recent hospitalization (within 30 days). Control subjects were visitors of Ruby Memorial Hospital with no acute medical problems. Medical history was obtained directly from control subjects; however, complete access to medical records of control subjects was not available.
2.3. Neutrophil-lymphocyte ratio
For TBI patients, the neutrophil-lymphocyte ratio (NLR) was calculated from a white blood cell differential performed as a standard of care at several time points after injury: baseline (0–6 hours), 24 hours, 48 hours, and on the date of hospital discharge. NLR was not calculated for control subjects, as no white blood cell differential was performed.
2.4. RNA extraction
Peripheral venous whole blood was drawn from TBI subjects no later than 24h of injury onset. Blood was collected into PAXgene® Blood RNA tubes (Becton-Dickinson). Immediately after blood collection, tubes were inverted 8–10 times and stored at −80°C until analysis which was less than one year from collection. Whole blood was collected in serum separator tubes, centrifuged at 4000 g, aliquoted into microcentrifuge tubes within 1h of collection, and stored at −80°C until analysis.
PAXgene® Blood RNA tubes were thawed at room temperature overnight (16–20h) prior to RNA extraction. The PAXgene Blood RNA kit (Pre-Analytix) was used to purify/extract intracellular RNA, per manufacturer’s instructions. RNA concentration and quality was determined by absorbance using a Take3 Trio Microplate (BioTek®) read on a Syntek Hybrid Plate Reader and analyzed using Gen5 (BioTek) software. A260/A280 values between 1.8 and 2.2 were considered acceptable RNA quality.
2.5. Gene expression analysis
RNA was converted to complementary DNA (cDNA) using the High-Capacity Reverse Transcription Kit (Applied Biosystems). cDNA (10ng) was used for quantitative real-time principal component regression (PCR) amplification using SYBR Green chemistry using the Rotor-Gene Q real-time PCR cycler (Qiagen). The following Quantitect primers (Qiagen) were used: ARG1 (NM_000045, NM_001244438), LY96 (NM_015364), MMP9 (NM_004994), s100a12 (NM_005621), and CCR7 (NM_001838). Gene expression was normalized using both PPIB (NM_000942) and B2M (NM_004048). Fold change differences were calculated by the ΔΔCT method.
2.6. Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics (Version 24). Statistical significance was taken at the 5% alpha level (p<0.05). Fisher’s exact test to compare control and TBI for categorical variables, such as age, sex, and medical history. A Mann-Whitney U test was used to compare median fold change values for ARG1, LY96, MMP9, s100a1, and CCR7 between control and TBI. Spearman’s Rank Correlation was used to measure the strength of association between the genes. A receiver operating characteristic (ROC) curve analysis was performed to determine if any of the individual genes could predict TBI. Coordinate points for ROC analysis were used to determine the maximum sensitivity and specificity for each gene predictor. We then used the individual gene predictors in a k-means clustering analysis (KCA). This set of genes was used to group cases together into clusters based on the pattern of expression of these genes.
3. Results
3.1. Clinical characteristics
Twenty-eight subjects (19 control and 9 TBI) were included in this pilot study. Demographic and medical information for subjects is summarized in Table 1. Smoking was significantly higher in TBI patients compared to controls (p = 0.01); however, there were no other significant differences in any recorded demographic or medical information between TBI and control.
Table 1.
Baseline demographic and physical characteristics of study participants (N = 28)
| Characteristic | TBI (N = 9) | CONTROL (N = 19) | Difference statistic |
|---|---|---|---|
| Demographic | |||
| Age (mean ± SD years) | 54 ± 29 | 53 ± 10 | t = 0.015, p = 0.98 |
| Sex (% female) | 78% | 79% | χ2 = 0.005, p = 0.99 |
| Medical, n (%) | |||
| Length of Stay (days) | 4.6 | 5.3 | t = −0.346, p = 0.740 |
| Prior TBI | 0 | 0 | - |
| Prior Stroke/TIA | 0 | 0 | - |
| Hypertension | 5 (56) | 9 (47) | χ2= 0.444, p = 0.51 |
| Hyperlipidemia | 1(11) | 8 (42) | χ2= 1.981, p = 0.16 |
| Diabetes | 3 (33) | 2(11) | χ2= 2.715, p = 0.09 |
| Smoking | 3 (33) | 0 | χ2=7.093, p = 0.01* |
3.2. Changes in ARG1, LY96, MMP9, s100a12, and CCR7 following TBI
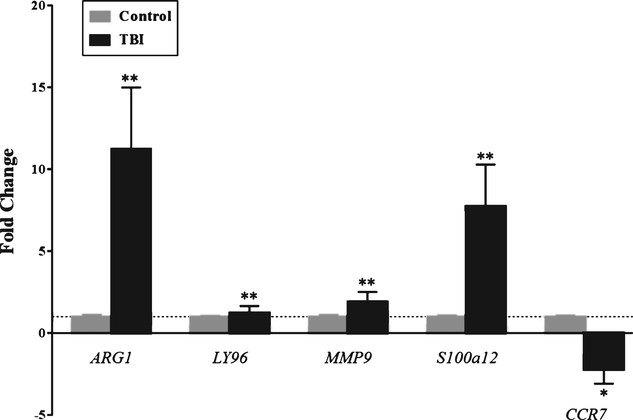
Relative mRNA expression of ARG1, LY96, MMP9, s100a12 was significantly increased in peripheral blood of TBI patients within 24 hours of injury compared to control subjects (Fig. 1). Specifically, there was an 11 fold increase in ARG1, a 1.2 fold increase in LY96, 1.9 fold increase in MMP9, and a 7.8 fold increase in s100a12 mRNA expression (Fig. 1). Further, relative mRNA expression of CCR7 was significantly decreased 2.6 fold in peripheral blood of TBI patients within 24 hours of injury compared to control subjects (Fig. 1). We examined the correlation among the five genes. ARG1 was positively correlated with LY96, MMP9, and s100a12, and negatively correlated with CCR7 (Table 3). The correlation matrix in Table 2 summarizes the strength of the relationships between each of the five genes.
Fig. 1.
Relative mRNA Expression of ARG1, LY96, MMP9, s100a12, and CCR7 in TBI Compared to Control. Relative mRNA expression of ARG1, LY96, MMP9, s100a12 was significantly increased in peripheral blood of TBI patients within 24 hours of injury compared to control subjects. The mean fold changes for ARG1, LY96, MMP9, s100a12 were 11, 1.2, 1.9, and 7.8, respectively. There was a 2.6 fold decreased in relative CCR7 mRNA expression in TBI compared to control. Gray bars represent control subjects and black bars represent TBI patients. Data is expressed as fold change±SD, and the dotted line at Fold Change = 1 is a reference to the control group. ** represents significance at p < 0.001, * represents significance at p < 0.05.
Table 3.
Final cluster centers of K-means cluster analysis (KCA)
| Gene | Cluster 1 TBI | Cluster 2 Control |
|---|---|---|
| (1/dCT) | (1/dCT) | |
| ARG1 | 0.25 | 0.13 |
| LY96 | 0.28 | 0.19 |
| MMP9 | 0.33 | 0.20 |
Table 2.
Spearman rank correlation matrix for potential markers of traumatic brain injury (N = 28)
| ARG1 | LY96 | MMP9 | S100a12 | CCR7 | |
|---|---|---|---|---|---|
| ARG1 | 0.770** | 0.724** | −0.587** | −0.388* | |
| LY96 | 0.770** | 0.748** | −0.545** | −0.248 | |
| MMP9 | 0.724** | 0.748** | −0.531** | −0.184 | |
| S100a12 | −0.587** | −0.545** | −0.531** | 0.251 | |
| CCR7 | −0.388* | −0.248 | −0.184 | 0.251 |
p <0.05
p <0.001.
3.3. Identification of immune-related gene profile for TBI diagnosis
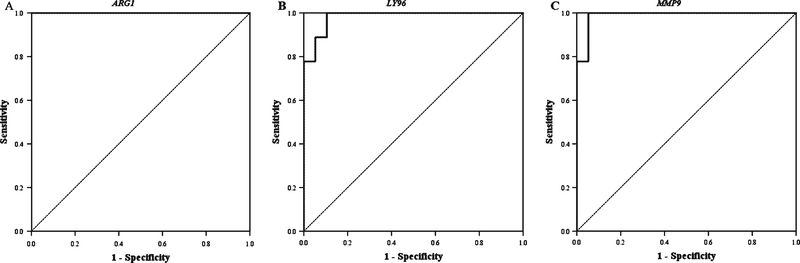
Next, we sought to determine if this panel of genes could be used to accurately predict TBI in this population when compared to the final clinical diagnosis. First, we performed ROC analysis to determine if each individual gene could predict TBI. ARG1, LY96, and MMP9 were all significant predictors of TBI, with a high level of sensitivity and specificity. In ROC analysis, ARG1 had an AUC= 1 and ARG1 expression (1/dCT) ≥0.16 predicted TBI with 100 percent sensitivity and 100 percent specificity (Fig. 2A), LY96 had an AUC= 0.982 and LY96 expression (1/dCT) ≥0.22 predicted TBI with 100 percent sensitivity and 91 percent specificity (Fig. 2B), and MMP9 had an AUC= 0.988 and MMP9 expression (1/dCT) ≥0.24 predicted TBI with 100 percent sensitivity and 95 percent specificity (Fig. 2C). In contrast, both s100a12 and CCR7 alone were identified as poor predictors of TBI in ROC analysis (not shown).
Fig. 2.
Receiver Operating Characteristic (ROC) Analysis for Prediction of TBI. (A) ARG1 had an AUC=1 and ARG1 expression (1/dCT) ≥ 0.16 predicted TBI with 100 percent sensitivity and 100 percent specificity. (B) LY96 had an AUC=0.982 and LY96 expression (1/dCT) ≥ 0.22 predicted TBI with 100 percent sensitivity and 91 percent specificity. (C) MMP9 expression (1/dCT) ≥ 0.24 predicted TBI with 100 percent sensitivity and 95 percent specificity.
Following ROC analysis, we performed k-means clustering analysis (KCA) to classify TBI and control, based on a pattern or cluster of gene expression. Because only ARG1, LY96, and MMP9 were significant predictors of TBI in ROC analysis, we used only these three genes to generate clusters in KCA. Using two clusters, the patterns extracted by KCA are shown in Table 3. 100 percent of TBI patients were classified into cluster 1, and 100 percent of control patients were classified into cluster 2. The cluster centers for each cluster are shown in Table 3. The TBI cluster, cluster 1, was defined by higher levels of ARG1, LY96, and MMP9 than in the Control cluster 2 (Table 3).
3.4. Neutrophil and lymphocyte dynamics following TBI
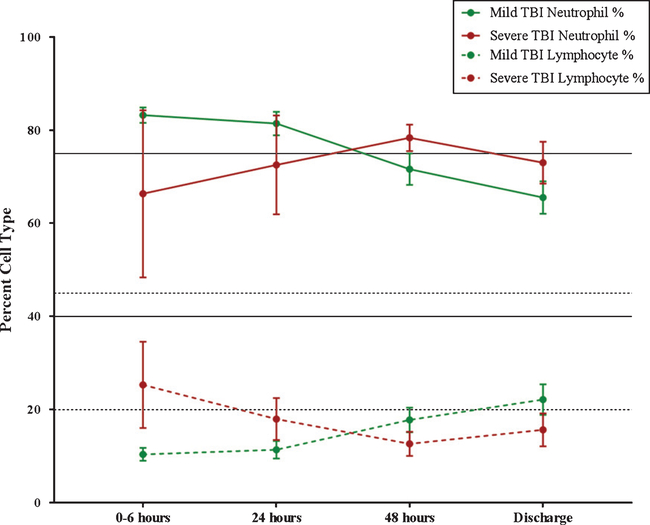
Next, we aimed to characterize changes in peripheral neutrophil and lymphocyte counts following TBI. We compared neutrophil and lymphocyte percent (percent of total white blood cell) between mild and severe TBI at four time points after injury: 0–6 hours, 24 hours, 48 hours, and at discharge.
3.5. Neutrophil dynamics
From 0–6 hours post-TBI, mild patients had a mean neutrophil percent, above normal range, at 83.2 percent, whereas severe TBI had a mean neutrophil percent within normal range, at 66.3 percent (Fig. 3). At 24 hours post-TBI, mild TBI patients still had a higher neutrophil percentage than severe TBI, with a mild TBI neutrophil percentage of 81.4 percent compared to 72.5 percent in severe TBI (Fig. 3). At 48 hours following TBI, mild TBI neutrophil percentage decreased to percentage within normal range at 71.7 percent, whereas the percentage of neutrophils increased above normal range to 78.3 percent from 24–48 hours in the severe TBI group (Fig. 3). Lastly, neutrophil percent continues to decrease in both groups from 48 hours to discharge; however, the mean neutrophil percent remains higher in the severe TBI group compared to mild (Fig. 3).
Fig. 3.
Neutrophil and Lymphocyte Dynamics Following TBI. Neutrophil percentage is graphed as a solid line: solid green = mild TBI, solid red = severe TBI. Solid black lines on the graph indicate the normal range of neutrophil percentage (range = 40–75 percent). Lymphocyte percentage is graphed as a dashed line: dashed green = mild TBI, dashed red = severe TBI. Dashed black lines on the graph indicate the normal range of lymphocyte percentage (range = 20–45 percent).
3.6. Lymphocyte dynamics
From 0–6 hours post-TBI, mild patients had a mean lymphocyte percent, below normal range, at 10.4 percent, whereas severe TBI had a mean lymphocyte percent within normal range, at 25.3 percent (Fig. 3). At 24 hours post-TBI, mild TBI patients still had a lower lymphocyte percentage than severe TBI, with a mild TBI lymphocyte percentage of 11.4 percent compared to 18 percent in severe TBI (Fig.3). At 48 hours following TBI, mild TBI lymphocyte percentage increased to 17.8 percent, whereas the percentage of lymphocytes decreased further below normal range to 12.7 percent in the severe TBI group (Fig. 3). Lastly, lymphocyte percent continues to increase in both groups from 48 hours to discharge; however, while the mean lymphocyte percent increases to within normal range, at 22.2 percent in the mild TBI group, lymphocyte percentage at discharge remains below normal range at 15.7 percent in severe TBI (Fig. 3).
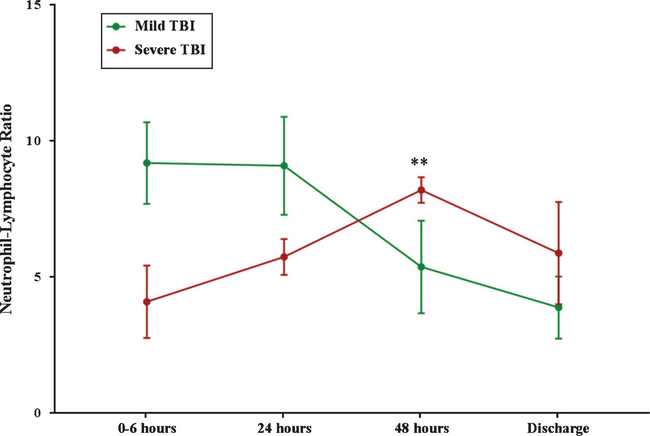
3.7. Neutrophil-lymphocyte ratio (NLR) following TBI
At 0–6 hours and 24 hours post-injury, the mean NLR was higher in mild TBI compared to severe TBI at 9.1 and 4.1 compared to 9.1 and 5.7, respectively (Fig. 4). At 48 hours, the mean NLR for mild TBI decreased to 5.4, whereas the mean NLR increased to 8.2 in the severe group (Fig. 4). Mean NLR decreased from 48 hours to discharge in both mild and severe TBI; however, at discharge, severe TBI patients had a mean NLR of 5.9 and mild TBI patients had a substantially lower mean NLR of 3.89 (Fig. 4).
Fig. 4.
Neutrophil-Lymphocyte Ratio Following TBI. The change in NLR is graphed as a solid line: solid green = mild TBI, solid red = severe TBI. * represents a significant difference at p < 0.05 between mild and severe TBI.
4. Discussion
One of the many physiological responses to TBI is an activation of the peripheral immune system (Kelso & Gendelman, 2014; Lozano et al., 2015) triggered by mechanical injury and brain tissue damage. The peripheral blood immune response is well characterized and critical to controlling local brain damage and facilitating repair. Generally speaking, the immune system consists of two arms: the innate and adaptive immune systems. The cells of the innate immune system, predominantly neutrophils, are rapidly activated following brain injury and migrate from peripheral lymphoid organs into the brain over the course of minutes and hours to facilitate acute inflammation. The cells of the adaptive immune response, predominantly T lymphocytes, become activated over time and t migrate from the peripheral blood into the brain hours to days following injury to control innate inflammatory mechanism and facilitate repair. Both arms of the immune system are crucial in clearing damaged brain tissue in the acute phase following brain injury and promoting brain and vascular repair in the chronic phase of acute ischemic stroke (AIS); however, an excessive or imbalanced immune response following TBI likely contributes to increased brain tissue injury and poor outcome (Kelso & Gendelman, 2014; Lozano et al., 2015). While the exact details of what constitutes a healthy immune response versus a detrimental response are debatable and likely different for each individual, it is widely accepted that an excessive innate response in combination with a poor adaptive immune response is detrimental. The neutrophil-lymphocyte ratio (NLR) is a simple biomarker that provides a quantitative measure of the balance between innate and adaptive peripheral immune responses (Petrone et al., 2016). An elevated NLR indicates an excessive innate immune cell response, a lack or poor adaptive immune cell response, or both, and an elevated NLR is associated with increased severity of injury and poor prognosis in a variety of conditions, including trauma (Dilektasli et al., 2016). Our study is one of the first to confirm a relationship between elevated NLR and severity of TBI. Although we do not collect outcome data, we hypothesize that the changes in the NLR may also predict recovery since; a recent publication supports this hypothesis and illustrates that elevated NLR is associated with a poorer 3-month outcome in patients with acute intracerebral hemorrhage (Lattanzi, Cagnetti, Provinciali, & Silvestrini, 2016).
The sensitivity and specificity for predicting TBI using a pattern of expression of associated immune related genes (ARG1, LY96, and MMP9) is much higher than what is currently accepted in clinical practice (100%/91% vs 94%/59%, (Donnelly et al., 2011)). We find a similar enhancement of diagnostic ability using a pattern recognition approach for acute stroke triage. The search for diagnostic blood biomarkers has been difficult for all types of brain injury, particularly for mild and ambiguous cases. Most scientists and clinicians have searched for brain specific targets in the peripheral blood, and when dealing with mild brain injuries this can be extremely difficult to find using current technologies. For example, the most extensively studied blood biomarkers for TBI include S100β, neuron-specific enolase, glial fibrillary acidic protein, and Tau (Kawata et al., 2016), all of which are brain specific. Our approach does not rely on finding brain specific biomarkers. Rather we propose to employ machine learning to the identification of different immune response patterns in the peripheral blood following various types of neuro-related brain insults. Relying on pattern recognition and ratios of biomarkers, rather than specific biomarkers, allows for optimization and learning over time and ensures that the diagnostic panels can be generalized to various populations of patients. The overall list of biomarkers becomes less important and the pattern of expression becomes the diagnostic. Because the immune response has two separate approaches that balance one another, the overall response and how that response changes over time in each individual gives the most information when identifying acute brain injuries. Contrary to popular scientific belief, the link between the peripheral and central immune systems is undeniable (Louveau et al., 2015), and monitoring the peripheral blood immune response provides an extreme amount of information concerning the brain health of the patient and their overall ability to return to work/play/duty activities. Although there is still a lot to learn regarding the relationships between the peripheral blood immune system response and mTBI/concussion, seminal studies in this area support our findings and contribute to a strong foundation for the development of a clinical point of care (POC) blood test based on patterns of immune responses for triaging suspected mTBI/concussion (Al Nimer et al., 2011; Guardado et al., 2016; Tobin et al., 2014).
Our analyses suggest that the peripheral blood immune response continues to evolve around or near 48 hours post injury. Following the specific patterns of neutrophils and lymphocytes can aid clinical decisions regarding extending therapeutic treatments, as well as return to work/play/duty activities. Severe TBI patients had NLR measures greater than 5.0, 48 hours post injury, which is expected and implies significant imbalance between innate and adaptive immune system responses. This information is helpful, when considering the use of NLR in mTBI/concussion. We may be able to extrapolate the high NLR average in severe TBI patients to suggest an ongoing chronic inflammation that has yet to be resolved primarily because of the extent of tissue injury. An NLR pattern that looks similar to that of a severe TBI patient in an mTBI/concussion patient may signify the presence of brain injury and could be used to rule in or rule out an mTBI/concussion diagnosis as well as guide sub-acute clinical follow up and long term care. Although our study is based on a very small sample size, the data is intriguing and supports continued development and additional clinical studies to determine thresholds of immune related genes and the NLR for clinical use.
5. Conclusions
The peripheral blood immune response is a powerful predictor of acute brain injury and likely clinically useful for triaging suspected mTBI/concussion. Our study is one of the first to confirm a relationship between the NLR and a specific pattern of immune related genes in the peripheral blood predictive of TBI with high sensitivity and specificity. Our approach relies on the power of machine learning to identify specific patterns of immune response in the peripheral blood associated with various types of brain injury. There is much potential in this approach, the challenge is to further develop and refine analytical techniques for use at the patient bedside.
Acknowledgments
The data in this manuscript was partially funded by a Robert Wood Johnson Foundation Nurse Faculty Scholar award to T. Barr ID: 70319, an Intramural NINR/NIH Contract ID: HHSN263201100872P to T. Barr and a WVU Stroke COBRE Award Sub-Project 2P20 GM109098 to T. Barr.
Footnotes
Conflict of interest
T. Barr, V. Gionis, and R. Giersch receive salary support from Valtari Bio™ Inc. T. Barr and R. Giersch are co-inventors on two provisional patent applications for the use of multi-omic biomarkers to identify stroke and brain injury (PCT/US16/41585 and PCT/US16/41585).
References
- Al Nimer F, Beyeen AD, Lindblom R, Strom M, Aeinehband S, Lidman O, & Piehl F (2011). Both MHC and non-MHC genes regulate inflammation and T-cell response after traumatic brain injury. Brain Behav Immun, 25(5), 981–990. doi: 10.1016/j.bbi.2010.10.017 [DOI] [PubMed] [Google Scholar]
- Barr TL, Conley Y, Ding J, Dillman A, Warach S, Singleton A, & Matarin M (2010). Genomic biomarkers and cellular pathways of ischemic stroke by RNA gene expression profiling. Neurology, 75(11), 1009–1014. doi: 10.1212/WNL.0b013e3181f2b37f [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (2010). Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. Atlanta, GA: Center for Disease Contol and Prevention. [Google Scholar]
- Croall ID, Cowie CJ, He J, Peel A, Wood J, Aribisala BS, . . . & Blamire AM (2014). White matter correlates of cognitive dysfunction after mild traumatic brain injury. Neurology, 83(6), 494–501. doi: 10.1212/wnl.0000000000000666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilektasli E, Inaba K, Haltmeier T, Wong MD, Clark D, Benjamin ER, . . . & Demetriades D (2016). The prognostic value of neutrophil to lymphocyte ratio on mortality in critically Ill trauma patients. J Trauma Acute Care Surg. doi: 10.1097/ta.0000000000000980 [DOI] [PubMed] [Google Scholar]
- Donnelly KT, Donnelly JP, Dunnam M, Warner GC, Kittleson CJ, Constance JE, . . . & Alt M (2011). Reliability, sensitivity, and specificity of the VA traumatic brain injury screening tool. J Head Trauma Rehabil, 26(6), 439–453. doi: 10.1097/HTR.0b013e3182005de3 [DOI] [PubMed] [Google Scholar]
- Guardado P, Olivera A, Rusch HL, Roy M, Martin C, Lejbman N, . . . & Gill JM (2016). Altered gene expression of the innate immune, neuroendocrine, and nuclear factor-kappa B (NF-kappaB) systems is associated with posttraumatic stress disorder in military personnel. J Anxiety Disord, 38, 9–20. doi: 10.1016/j.janxdis.2015.12.004 [DOI] [PubMed] [Google Scholar]
- Kawata K, Liu CY, Merkel SF, Ramirez SH, Tierney RT, & Langford D (2016). Blood biomarkers for brain injury: What are we measuring? Neurosci Biobehav Rev, 68, 460–473. doi: 10.1016/j.neubiorev.2016.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso ML, & Gendelman HE (2014). Bridge between neuroimmunity and traumatic brain injury. Curr Pharm Des, 20(26), 4284–4298. [PMC free article] [PubMed] [Google Scholar]
- Laker SR (2011). Epidemiology of concussion and mild traumatic brain injury. Pm r, 3(10 Suppl 2), S354–358. doi: 10.1016/j.pmrj.2011.07.017 [DOI] [PubMed] [Google Scholar]
- Lattanzi S, Cagnetti C, Provinciali L, & Silvestrini M (2016). Neutrophil-to-lymphocyte ratio predicts the outcome of acute intracerebral hemorrhage. Stroke, 47(6), 1654–1657. doi: 10.1161/strokeaha.116.013627 [DOI] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, . . . & Kipnis J (2015). Structural and functional features of central nervous system lymphatic vessels. Nature, 523(7560), 337–341. doi: 10.1038/nature14432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano D, Gonzales-Portillo GS, Acosta S, de la Pena I, Tajiri N, Kaneko Y,&Borlongan CV (2015). Neuroinflammatory responses to traumatic brain injury: Etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr Dis Treat, 11, 97–106. doi: 10.2147/ndt.s65815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrone AB, O’Connell GC, Regier MD, Chantler PD, Simpkins JW,&Barr TL (2016). The Role of Arginase 1 in Post-Stroke Immunosuppression and Ischemic Stroke Severity. Transl Stroke Res, 7(2), 103–110. doi: 10.1007/s12975-015-0431-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souter MJ, Blissitt PA, Blosser S, Bonomo J, Greer D, Jichici D, . . . & Yeager S (2015). Recommendations for the critical care management of devastating brain injury: Prognostication, psychosocial, and ethical management : A position statement for healthcare professionals from the neurocritical care society. Neurocrit Care, 23(1), 4–13. doi: 10.1007/s12028-015-0137-6 [DOI] [PubMed] [Google Scholar]
- Tang Y, Xu H, Du X, Lit L, Walker W, Lu A, . . . & Sharp FR (2006). Gene expression in blood changes rapidly in neutrophils and monocytes after ischemic stroke in humans: A microarray study. J Cereb Blood Flow Metab, 26(8), 1089–1102. doi: 10.1038/sj.jcbfm.9600264 [DOI] [PubMed] [Google Scholar]
- Tobin RP, Mukherjee S, Kain JM, Rogers SK, Henderson SK, Motal HL, . . . & Shapiro LA (2014). Traumatic brain injury causes selective, CD74-dependent peripheral lymphocyte activation that exacerbates neurodegeneration. Acta Neuropathol Commun, 2, 143. doi: 10.1186/s40478-014-0143-5 [DOI] [PMC free article] [PubMed] [Google Scholar]