Abstract
Purpose:
The 8th edition of the TNM classification of malignant tumors for the first time includes an official staging system for thymic epithelial tumors (TETs) recognized by the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC). Staging is critical for the management of TETs, and determining stage accurately from imaging has the potential to improve clinical outcomes. We examine preoperative computed tomography (CT) characteristics of TETs associated with AJCC/UICC pathologic TNM stage.
Materials and Methods:
In this retrospective study, patients were included if they met all the following criteria: 1) diagnosis of TET, 2) had primary curative-intent surgery performed at our institution, and 3) had available preoperative CT imaging for review. Tumor pathology was staged according to the 8th edition TNM classification. Fifteen CT scan features were examined from each patient case according to the International Thymic Malignancy Interest Group (ITMIG) standard report terms in a blinded fashion. A Lasso regularized multivariate model was used to produce a weighted scoring system predictive of pathologic TNM stage.
Results:
Examining the 54 patients included, the following CT characteristics were associated with higher pathologic TNM stage when using the following scoring system: elevated hemidiaphragm (score of 6), vascular endoluminal invasion (score of 6), pleural nodule (score of 2), lobulated contour (score of 2), and heterogeneous internal density (score of 1). Area under the ROC curve was 0.76.
Conclusions:
TETs with clearly invasive or metastatic features seen on CT are associated with having higher AJCC/UICC pathologic TNM stage, as expected. However, features of lobulated contour and heterogeneous internal density are also associated with higher stage disease. These findings need to be validated in an independent cohort.
Keywords: IASLC/ITMIG, Masaoka-Koga stage, thymoma, thymic epithelial tumor, computed tomography
Introduction
Thymic epithelial tumors (TETs) are rare tumors of the prevascular (anterior) mediastinum [1]. For the first time, there is an official staging system for TETs recognized in the 2017 8th edition of the TNM Classification of Malignant Tumors by the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC) [2]. Historically, many staging systems have been used for TETs, including both non-TNM based and TNM based [3]. Many of these were based on small sample sizes at single institutions and have never been validated. The Masaoka-Koga staging system is still the most commonly used and the International Thymic Malignancy Interest Group (ITMIG) had established it as the unified staging system (Table 1) for use until publication of the 8th edition AJCC/UICC staging manuals [4]. There are limitations of Masaoka-Koga staging including lack of survival difference between stage I and II disease, stage III disease involving invasion of a large range of structures, and a predominantly pathologic staging system that is difficult to apply to clinical staging [5, 6].
Table 1:
IASLC/ITMIG TNM Staging and Masaoka-Koga Staging Definition
| IASLC/ITMIG TNM Stage[5] | ||
|---|---|---|
| Stage | TNM | Details |
| I | T1N0M0 | T1, tumor either limited to the thymus ± encapsulation or directly invading into mediastinal fat only or mediastinal pleura--T1a, no mediastinal pleural involvement --T1b, directly invading into mediastinal pleura |
| II | T2N0M0 | T2, tumor directly invading into pericardium |
| IIIA | T3N0M0 | T3, tumor directly invading into any of the following: lung, brachiocephalic vein, superior vena cava, phrenic nerve, chest wall, or extrapericardial pulmonary artery or vein |
| IIIB | T4N0M0 | T4, tumor directly invading into any of the following: aorta (ascending, arch, or descending), arch vessels, intrapericardial pulmonary artery, myocardium, trachea, esophagus |
| IVA | T any N1 M0 T any N0,1 M1a |
-N0, no nodal involvement -N1, anterior (perithymic) nodes -M0, no metastatic pleural, pericardial, or distant sites -M1a, separate pleural or pericardial nodule(s) |
| IVB | T any N2 M0,1a T any N any M1b |
-N2, deep intrathoracic or cervical nodes -M1b, pulmonary intraparenchymal nodule or distant organ metastasis |
| Masaoka-Koga Staging[4] | ||
| I | Grossly and microscopically completely encapsulated tumor | |
| IIA | Microscopic transcapsular invasion | |
| IIB | Macroscopic invasion into thymic or surrounding fatty tissue, or grossly adherent to but not breaking through mediastinal pleura or pericardium | |
| III | Macroscopic invasion into neighboring organs (i.e. great vessel, lung, pericardium) | |
| IVA | Pleural or pericardial metastases | |
| IVB | Lymphogenous or hematogenous metastasis | |
The IASLC (International Association for the Study of Lung Cancer)/ITMIG staging system, which has been included in the official 8th edition AJCC/UICC staging manuals, is based on TNM descriptors (Table 1). The T stage is derived from Masaoka-Koga staging and is defined by the highest level of invasion of one or more structures, with an overall decreased emphasis on tumor encapsulation [7]. The N stage (N1, N2) defines lymph node regions based on a lymph node map [8]. The M stage separates pleural or pericardial nodules (M1a) from pulmonary nodules or distant metastases (M1b) [9]. Stage groups I-IIIB are decided by the T component while stage IVA/B groups are decided by the N and/or M component [5].
Staging is critical for the management of TETs [10, 11]. Masaoka-Koga stage I-II TETs are generally managed with upfront surgery, stage III TETs frequently require a multi-modality approach, and most stage IVA/B TETs are managed with systemic chemotherapy. The imaging modality of choice for the workup of TETs is computed tomography (CT) scan of the thorax with intravenous contrast. CT is important in differentiating TETs from other malignant and nonmalignant conditions, identifying need for pretreatment biopsy, and assessing surgical resectability. The ability to determine stage based on CT characteristics could improve outcomes by identifying patients with advanced disease (i.e. Masaoka-Koga stage III or higher) who would benefit from neoadjuvant therapy. There have been retrospective studies examining CT characteristics and their association with Masaoka and Masaoka-Koga staging [12] along with completeness of resection [13] and World Health Organization (WHO) histology subtype [14]. However, to our knowledge, CT characteristics have never been studied in relation to the now official IASLC/ITMIG staging for TETs. Therefore, we performed a retrospective review with a primary objective to analyze CT characteristics associated with IASLC/ITMIG pathologic stage.
Materials and Methods
Inclusion Criteria
This study was performed under an IRB approved protocol, including with an approval for waiver of consent for retrospective chart reviews. All investigators were in Health Insurance Portability and Accountability Act (HIPPA) compliance. We used our Cancer Institute Research Database to retrospectively identify thymic malignancies by ICD-9 diagnostic codes and pathology reports. Patients were included who fit all the following criteria: 1) had a diagnosis of TET [thymoma, thymic carcinoma, or neuroendocrine tumor of thymus (NETT)], 2) had their primary curative-intent surgery performed at our institution, and 3) had available preoperative CT imaging for review. For patients with more than one preoperative CT, the most recent was used. For patients who received neoadjuvant chemotherapy, the re-staging imaging was reviewed to account for the possibility of “downstaging.”
Standard Report Terms
There was no standard protocol for CT imaging, as 43% of studies were provided by outside institutions. The majority of studies were assessed with intravenous contrast (n=47; 87%). CT studies were reconstructed at variable section thickness: 1–2.5 millimeter (mm), n=33; 3–5 mm, n=20; and 7 mm, n=1. A chest radiologist reviewed the CT and was blinded to clinico-pathologic data. Images were analyzed and reported per ITMIG defined standard report terms for chest CT reports of anterior mediastinal masses suspicious for thymoma [15]. The characteristics evaluated included size (longest dimension on axial slice), contour (smooth versus lobulated), internal density (homogenous versus heterogeneous), and presence of calcification, infiltration of mediastinal fat, abutment of ≥50% of mediastinal vessels, vascular endoluminal invasion, abutment of adjacent mediastinal structures, invasion of adjacent mediastinal structures, elevated hemidiaphragm, pleural effusion, pleural nodule, pulmonary nodule, mediastinal lymph node enlargement (>1 cm in short axis diameter), and suspected extrathoracic metastases.
Clinical Characteristics, Staging, Histology, and Resection Status
Clinical characteristics were collected along with diagnosis and treatment parameters. Pathologic staging was assessed by the IASLC/ITMIG staging system (referred to as TNM hereafter) [5, 7, 9] as well as by the Masaoka-Koga staging system with ITMIG clarifications [4] (Table 1). Histology was classified by the 2004 WHO classification[16]. We performed retrospective chart review of pathology reports and operative reports to examine staging and WHO subtype. In cases where it was not clear, a blinded pathologist reviewed. Of note, lymph node dissections were not routinely performed and only 43% patients had at least one evaluable lymph node. Therefore, the majority of cases were classified as N0 unless there was definitive nodal involvement. Resection status was classified as R0 (complete), R1 (microscopic incomplete), and R2 (macroscopic incomplete).
Statistical Analysis
All CT variables except pulmonary nodules (all considered nonspecific) and extrathoracic metastases (none identified in the cohort) were included in the analyses. Standard tests for comparing variables were used: Wilcoxon for continuous variables and Fisher’s exact test for binary or ordinal variables. A proportional odds regression model was used to determine univariate associations of CT characteristics with ordered categories of TNM stage, Masaoka-Koga stage, WHO histology, and resection status. A Lasso regularized multivariate model including all CT variables was used to determine a scoring system associated with stage in our cohort. The model adds weights to each feature depending how much it contributes to the prediction of TNM stage (I vs. II–IV), T stage (T1 vs. T2–T4), and Masaoka-Koga stage (I–II vs. III–IV). Kaplan-Meier method was used to determine event-free survival (time of pathologic diagnosis to death or recurrence, whichever comes earlier) and overall survival (time of pathologic diagnosis to death). A two-sided P<0.05 was considered statistically significant. All the statistical analyses were implemented in R 3.2.2 (R Foundation for Statistical Computing).
Results
Cohort Characteristics
With a data cutoff of March 2015, 56 patients identified met inclusion criteria. Many patients were excluded because outside CT imaging was not routinely uploaded until 2008. Two patients were excluded because they had a pathologic complete response with neoadjuvant therapy for a final sample of 54 patients. The cases spanned 14.4 years (September 2000-February 2015). The median time between CT imaging and surgery was 27 days. The cohort had predominantly early pathologic stage disease as defined by both TNM and Masaoka-Koga stage (Table 2). However, there was stage migration to earlier stage disease with the newly defined TNM staging system. The most common WHO subtypes were AB and B2. Some CT characteristics were poorly represented (<10%) including mediastinal lymph node enlargement, elevated hemidiaphragm, and pleural effusion (see Table 1, Supplemental Digital Content 1). Patients with myasthenia gravis had smaller tumors at diagnosis (see Table 2, Supplemental Digital Content 1). More invasive CT features (i.e. vascular endoluminal invasion and invasion of mediastinal structures) along with higher pathologic TNM and Masaoka-Koga stage were significantly associated with receiving a pretreatment biopsy, neoadjuvant chemotherapy, and adjuvant therapy (see Tables 2+3 Supplemental Digital Content 1).
Table 2:
Cohort Characteristics
| Characteristic | N=54 (%) |
|---|---|
| Age, median years (range) | 59 (31–84) |
| Sex | |
| Male | 27 (50%) |
| Female | 27 (50%) |
| Zubrod Performance Status | |
| 0 | 23 (43%) |
| 1 | 31 (57%) |
| Race | |
| Asian | 20 (37%) |
| Caucasian | 27 (50%) |
| Other/Not reported | 7 (13%) |
| Autoimmune/Paraneoplastic Syndrome | |
| Myasthenia Gravis | 15 (28%) |
| Othera | 4 (7%) |
| None | 35 (65%) |
| Prior Malignancy | 8 (15%) |
| Pretreatment Biopsy | 28 (52%) |
| Neoadjuvant Chemotherapy | 15 (28%) |
| Time between imaging and surgery, median days (range) | 27 (1–110) |
| Median Sternotomy Approachb | 42 (78%) |
| Thymectomy | |
| Complete | 45 (83%) |
| Partial | 9 (17%) |
| Adjuvant Radiationc | 19 (35%) |
| Resection Statusd | |
| R0 | 41 (76%) |
| R1 | 7 (13%) |
| R2 | 6 (11%) |
| WHO Histologye | |
| A | 5 (9%) |
| AB | 14 (26%) |
| B1 | 7 (13%) |
| B2 | 15 (28%) |
| B3 | 5 (9%) |
| Thymic Carcinoma/NETT | 8 (15%) |
| IASLC/ITMIG TNM Stage | |
| I | 36 (67%) |
| II | 1 (2%) |
| IIIA | 8 (15%) |
| IIIB | 2 (4%) |
| IVA (N1 or M1a)f | 5 (9%) |
| IVB (N2 or M1b)f | 2 (4%) |
| T Stage | |
| T1 | 38 (70%) |
| T2 | 1 (2%) |
| T3 | 12 (22%) |
| T4 | 3 (6%) |
| Masaoka-Koga Stage | |
| I | 30 (56%) |
| IIA | 5 (9%) |
| IIB | 1 (2%) |
| III | 11 (20%) |
| IVA | 3 (6%) |
| IVB | 4 (7%) |
Other includes 2 pure red cell aplasia and 1 each of acromegaly and hypogammaglobulinemia.
Other includes 7 thoracoscopies and 1 each of partial median sternotomy, thoracotomy, cervical approach, partial median sternotomy and thoracotomy, and thoracoscopic and cervical approach.
Two received chemoradiation.
All incomplete resections occurred in TNM or Masaoka-Koga ≥ stage III disease.
WHO type A also includes 2 micronodular thymomas. Two B3 thymomas were mixed histology. One thymic carcinoma had a minor component of B3. Only 1 NETT.
Of the 5 IVA, 2 had N1 and 3 had M1a. Of the 2 IVB, both had N2 disease.
Table 3:
Univariate Associations of Preoperative CT Characteristics with Stage, Resection Status, and WHO Histology
| CT Characteristic Presenta | IASLC/ITMIG TNM Stage OR (95% CI) | T Stage OR (95% CI) | Masaoka-Koga Stage OR (95% CI) | Resection Status OR (95% CI) | WHO Histology OR (95% CI) |
|---|---|---|---|---|---|
| Size | 1.23 (0.98–1.54) p=0.08 |
NS | 1.21 (0.98–1.50) p=0.08 |
NS | NS |
| Lobulated contour | INFb (1.75-INF) p=0.005 |
INFb (1.43-INF) p=0.01 |
6.71 (1.33–33.78) p=0.02 |
INFb (INF-1.03) p=0.05 |
NS |
| Heterogeneous internal density | NS | NS | NS | NS | NS |
| Calcification | NS | 2.93 (0.86–10.05) p=0.09 |
NS | NS | NS |
| Infiltration of mediastinal fat | 5.04 (1.39–18.24) p=0.014 |
9.97b (2.30–43.18) p=0.002 |
3.74 (1.05–13.31) p=0.04 |
8.76 (2.17–35.30) P=0.002 |
2.91 (0.85–9.96) p=0.09 |
| Abutment of ≥50% of mediastinal vessels | NS | 6.16 (1.49–25.53) p=0.01 |
NS | NS | 3.15 (0.95–10.53) p=0.06 |
| Vascular endoluminal invasion | INFb (2.92-INF) p=0.0007 |
INFb (3.60-INF) p=0.0003 |
INFb (2.92-INF) p=0.0007 |
INFb (5.21-INF) p<0.0001 |
INFb (1.22-INF) p=0.02 |
| Abutment of adjacent mediastinal structures | NS | NS | NS | NS | NS |
| Invasion of adjacent mediastinal structures | 6.53 (1.89–22.58) p=0.003 |
6.04 (1.66–22.00) P=0.006 |
5.97 (1.72–20.76) p=0.005 |
4.84 (1.26–18.65) p=0.02 |
NS |
| Elevated hemidiaphragm | INFb (2.29-INF) p=0.002 |
11.67b (1.03–620.72) p=0.02 |
INFb (2.29-INF) p=0.002 |
NS | NS |
| Pleural effusion | NS | NS | NS | NS | NS |
| Pleural nodule | 3.78 (0.82–17.30) p=0.09 |
NS | NS | 4.28 (0.78–23.43) p=0.09 |
NS |
| Mediastinal lymph node enlargement | NS | NS | NS | NS | NS |
Abbreviations: OR=odds ratio, CI=confidence interval, cm=centimeter, NS=not significant, INF=infinity
Proportional odds regression model used to determine likelihood of more advanced stage, incomplete resection status, and more aggressive WHO histology. Odds ratios that are significant (p<0.05) or trended for significance are reported (≤0.05p<0.10).
Fisher’s exact test used if subgroup <6 to provide more accurate point estimate.
CT Features associated with Pathologic TNM Stage and Masaoka-Koga Stage
There was an almost perfect correlation between pathologic TNM and Masaoka-Koga stage in this cohort (correlation coefficient 0.95). The CT features associated with higher pathologic TNM and Masaoka-Koga stage (p<0.05) included lobulated contour, infiltration of mediastinal fat, invasion of adjacent mediastinal structures, vascular endoluminal invasion, and elevated hemidiaphragm (Table 3). In a multivariate analysis, a predictive score using the following characteristics was associated with higher TNM stage (from highest to lowest weight): elevated hemidiaphragm=vascular endoluminal invasion, pleural nodule, lobulated contour, and heterogeneous internal density (Table 4). A predictive score associated with higher Masaoka-Koga stage included (from highest to lowest weight): elevated hemidiaphragm, vascular endoluminal invasion, and lobulated contour (Table 4). The area under the receiver operating characteristic (ROC) curve for these predictive scores ranged 0.76–0.79 but the curves were unstable due to small patient numbers and a threshold with reliable sensitivity and specificity cannot be reported. Figure 1 is an example of CT features of an early stage (A) and advanced stage (B) TET. CT characteristics were also examined in association with T stage of TNM staging (Tables 3+4).
Table 4:
CT Imaging Predictive Score for Stage
| IASLC/ITMIG TNM Stage a | |
| CT Characteristic | Score |
| Elevated hemidiaphragm | 6 |
| Vascular endoluminal invasion | 6 |
| Pleural nodule | 2 |
| Lobulated contour | 2 |
| Heterogeneous internal density | 1 |
| T Stage of IASLC/ITMIG TNM Stageb | |
| CT Characteristic | Score |
| Vascular endoluminal invasion | 6 |
| Elevated hemidiaphragm | 3 |
| Infiltration of mediastinal fat | 2 |
| Lobulated contour | 1 |
| Masaoka-Koga Stagec | |
| CT Characteristic | Score |
| Elevated hemidiaphragm | 6 |
| Vascular endoluminal invasion | 5 |
| Lobulated contour | 3 |
AUC of the ROC 0.76
AUC of the ROC 0.72
AUC of the ROC 0.79
Figure 1 (A-D):
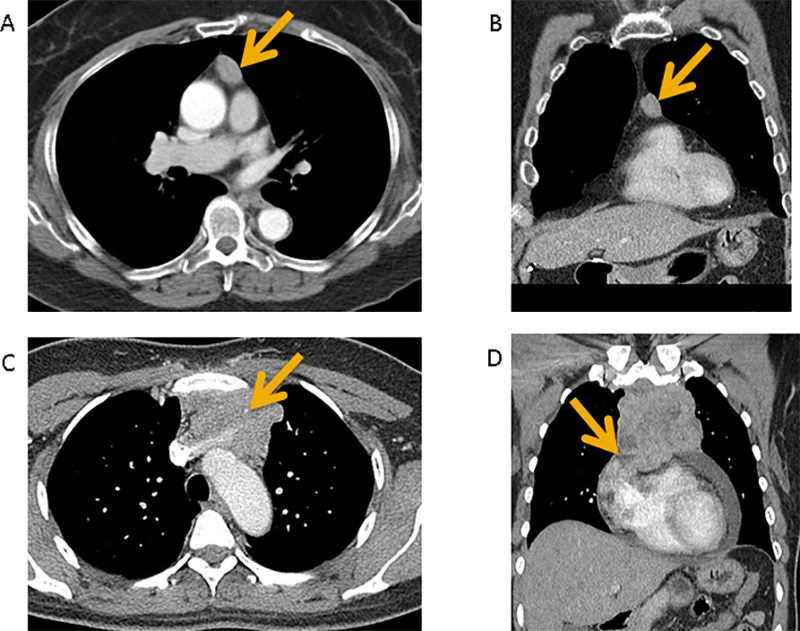
Contrast-enhanced CT of a pathologic IASLC/ITMIG TNM stage I (Masaoka-Koga stage IIA) type B2 thymoma demonstrates a prevascular mediastinal mass with smooth contours and homogeneous internal density in axial plane (A) and coronal plane (B). No invasive features are seen. Contrast-enhanced CT of a pathologic IASLC/ITMIG TNM stage IIIA (Masaoka-Koga stage III) type B3 thymoma demonstrates a prevascular mediastinal mass with lobulated contours and heterogeneous internal density in axial plane (C) and coronal plane (D). There is vascular endoluminal invasion of the innominate vein (C, arrow). There is abutment and also findings suggestive of invasion of adjacent mediastinal structures such as the pericardium (D, arrow).
Event-Free Survival and Overall Survival
When classifying stage by invasive versus non-invasive disease, the survival curves of TNM stage I versus II-IV and Masaoka-Koga stage I-II versus III-IV perfectly overlapped, as expected. Although there were only 14 total events total, the event-free survival was significantly shorter in the higher stage group (HR 9.49, 95% CI 2.11–42.67, p=0.0003) (Figure 2A). For overall survival, deaths only occurred in the higher stage group (Figure 2B). We were unable to correlate CT characteristics with clinical outcomes due to the low number of survival events.
Figure 2 (A-B):
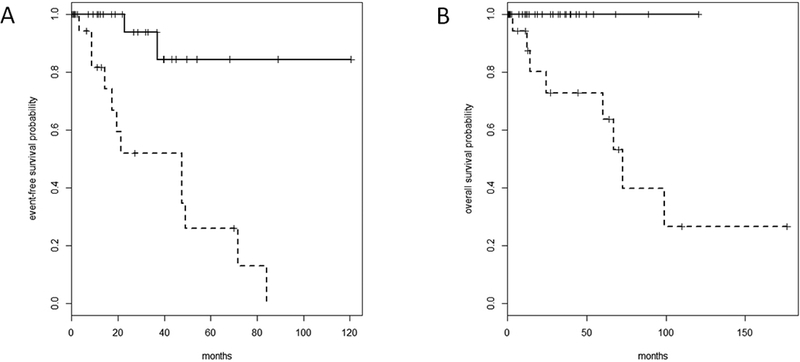
Event-free survival (EFS) (A) and Overall survival (OS) (B) by stage. Solid Line represents stage I tumor by IASLC/ITMIG stage (I-II by Masaoka-Koga stage). Dotted Line represents stage II-IV tumor by IASLC/ITMIG stage (III-IV by Masaoka-Koga stage). High rate of censoring: 40 of 54 censored for EFS and 46 of 54 censored for OS. (A) EFS is significantly shorter in the higher stage group (HR 9.49, 95% CI 2.11–42.67, p=0.0003; 47.5 months [17.6-not reached (NR)] versus NR). (B) For OS, the deaths only occurred in the higher stage group.
CT Features associated with WHO Histology and Resection Status
In addition, there was a positive correlation with stage and other prognostic factors in our cohort including resection status [correlation coefficient 0.74 (TNM) and 0.70 (Masaoka-Koga)] and WHO histology [0.64 (TNM) and 0.66 (Masaoka-Koga)]. The CT features associated with these factors are reported in Table 3, and WHO subgroup survival curves are reported in Figure 1, Supplemental Digital Content 2.
Discussion
Computed tomography (CT) is the gold standard diagnostic for patients with prevascular mediastinal masses suspicious for TETs [10, 11]. Despite the improved sensitivity of CT, detecting invasion can be challenging. Therefore, we retrospectively examined our institution’s cohort of TETs using ITMIG standard report terms to identify preoperative CT characteristics associated with pathologic staging, both the official IASLC/ITMIG TNM (TNM) staging system, which has been incorporated into the 2017 8th edition AJCC/UICC staging manuals, and the Masaoka-Koga staging system [15]. Masaoka-Koga stage is a strong independent prognostic factor for TETs, and the TNM staging system will continue to be validated as a prognostic factor with future use [6, 17]. The need to determine stage preoperatively is evident in our cohort, as pathologic stage was associated with important preoperative management decisions including performance of pretreatment biopsy and administration of neoadjuvant chemotherapy (see Table 3, Supplemental Digital Content 1). Although the rate of pre-treatment biopsy was relatively high in our cohort, with many being performed at outside institutions prior to referral, a significantly higher percentage were performed in those with pathologically invasive disease.
There was an almost perfect correlation between TNM stage and Masaoka-Koga stage in our cohort, which is expected given TNM staging is derived from Masaoka-Koga staging (Table 1) [5]. We performed two analyses including a proportional odds regression model to assess the association of each individual CT characteristic with hierarchical stage (Table 3) and also a Lasso regularized multivariate model incorporating all CT characteristics to create a predictive score for stage that adds weights to each characteristic depending on its importance in prediction (Table 4). The predictive score for TNM stage was heavily weighted for clearly invasive or metastatic features of vascular endoluminal invasion, elevated hemidiaphragm, and pleural nodule but also included features of lobulated contour and heterogeneous internal density. The predictive score for Masaoka-Koga stage was also heavily weighted for clearly invasive features of elevated hemidiaphragm and vascular endoluminal invasion but also included the feature of lobulated contour. Due to the small sample size of our cohort, these scores had unstable receiver operating characteristics and need to be validated in a larger independent cohort.
It is not surprising that clearly invasive CT features of vascular endoluminal invasion [13, 18] and elevated hemidiaphragm were associated with higher stage (both TNM and Masaoka-Koga). In addition, we confirmed prior reports in the literature of CT features that are not clearly invasive that are associated with higher stage including lobulated contour (both TNM and Masaoka-Koga) [12, 13, 18–21], heterogeneous internal density (TNM) [12, 20–22]; and infiltration of mediastinal fat (T-stage) [12, 13, 18]. We were unable to confirm prior studies that have found larger size [12, 13, 19–22] and calcification [12, 18, 21, 22] to be associated with higher stage. In a similar study by Marom et al. of 99 patients with thymoma, CT characteristics of both the primary tumor and distant disease independently associated with Masaoka stage III-IV disease in a multivariate model included pleural nodularity and tumor size (≥7 cm) [12].
Individual CT characteristics have limitations in regards to detecting early invasion. Qu et al. reported discrepancies between CT stage formulated based on imaging and pathologic Masaoka-Koga staging when there was tumor involvement of fat and blood vessels [20]. Other studies have reported that for determining invasion, there is limited utility in examining CT for loss or obliteration of fat plane [22] or for infiltration of mediastinal structures along with poor visualization of infiltration into the pleura and lung [21]. Therefore, a predictive score of several CT features, including those that are not clearly invasive, may be more useful in determining pathologic stage. For example, in a study by Tomiyama et al. of 50 patients with thymoma, the combination of three CT characteristics distinguished noninvasive from invasive thymoma: lobulated contour, cystic or necrotic portion, and multifocal calcification [21]. Despite active study, there are currently no preoperative imaging scores used in standard practice to determine staging of any tumor type [23, 24].
We also examined preoperative CT characteristics and their association with other prognostic factors of resection status and WHO histology (Table 3) but the small sample size limited a multivariate analysis.
We have the expected limitations of a retrospective study. First, regarding staging, (i) mediastinal pleural involvement is difficult to identify retrospectively and was rarely reported in pathology reports and (ii) lymph node sampling is not routinely performed for early stage TETs so assumptions for nodal status were necessary. However, we can expect an almost perfect inter-observer agreement between pathologists for Masaoka and TNM staging from the literature [25]. There is also selection bias in our cohort, since we only included patients who underwent a surgical resection. Second, we had a heterogeneous cohort, including thymomas and more aggressive thymic carcinomas. Although all thymic carcinomas in our cohort had invasive disease, there were also 10 representative thymomas with invasive disease. We also included patients who received neoadjuvant chemotherapy, but accounted for this by reviewing the re-staging CT scan. Third, we had a relatively small sample size. Therefore, the CT imaging based predictive score was essentially limited to distinguishing non-invasive versus invasive disease. Arguably, this may be the most important distinction for preoperative management of these tumors. However, we were unable to specifically discern Masaoka-Koga stage III disease and TNM stage II-IIIB disease where CT findings may be subtler. Such a predictive scoring system may also be useful in determining when to pursue more sensitive imaging for invasion such as magnetic resonance imaging (MRI) when invasion is not seen or equivocal on CT [26].
Finally, we only had one chest radiologist evaluate the CT imaging, although he was blinded to clinico-pathologic data. In previous studies with two or more radiologists, decision-making for discrepancies was frequently based on consensus [13]. Only Tomiyama et al. reported that there was relatively high agreement for two radiologists (83–96%) for specific CT features [27], and in another Tomiyama et al. study, the kappa statistic for agreement was good (0.77) when radiologists felt confident in their findings [21]. Due to a high percentage of outside imaging studies, there was no standard CT protocol but slice thickness was reasonable [18, 28] and contrast was administered in the majority of cases. In the 13% of cases where contrast was not administered, we were limited in our ability to evaluate the heterogeneity of the tumor. Some patients in this cohort also had alternative imaging studies, including MRI and positron emission tomography (PET) scan, which may have affected the decision to proceed with surgical resection.
In conclusion, to our knowledge, we are the first to identify a CT imaging based predictive score for the official IASLC/ITMIG TNM stage incorporated into the 8th edition AJCC/UICC staging manuals for TETs. The score is clearly exploratory given the limitations of our cohort, but highlights a potentially useful approach in diseases such as TETs where CT imaging is critical in management of the disease. The ability of this score in ranking patients according to their risk for higher stage disease needs to be validated in a larger independent cohort.
Supplementary Material
Acknowledgments
Conflicts of Interest and Source of Funding: Authors declare no relevant conflicts of interest. This work was supported by the TL1 Clinical Research Training Program of the Stanford Clinical and Translational Science Award to Spectrum [National Institute of Health (NIH) TL1 TR 001084] (SKP); Stanford Cancer Institute Fellowship Award, Stanford, CA (SKP); National Cancer Institute Cancer Center Support Grant 5P30CA124435 and Stanford NIH/NCRR CTSA Award Number UL1 RR025744 (Stanford Cancer Institute Research Database).
References:
- 1.Carter BW, Tomiyama N, Bhora FY, Rosado de Christenson ML, Nakajima J, Boiselle PM, et al. A modern definition of mediastinal compartments. J Thorac Oncol, 2014; 9(9 Suppl 2):S97–101. [DOI] [PubMed] [Google Scholar]
- 2.Carter BW, Benveniste MF, Madan R, Godoy MC, Groot PM, Truong MT, et al. IASLC/ITMIG Staging System and Lymph Node Map for Thymic Epithelial Neoplasms. Radiographics, 2017; 37(3):758–776. [DOI] [PubMed] [Google Scholar]
- 3.Filosso PL, Ruffini E, Lausi PO, Lucchi M, Oliaro A, & Detterbeck F. Historical perspectives: The evolution of the thymic epithelial tumors staging system. Lung Cancer, 2014; 83(2):126–132. [DOI] [PubMed] [Google Scholar]
- 4.Detterbeck FC, Nicholson AG, Kondo K, Van Schil P, & Moran C. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. J Thorac Oncol, 2011; 6(7 Suppl 3):S1710–1716. [DOI] [PubMed] [Google Scholar]
- 5.Detterbeck FC, Stratton K, Giroux D, Asamura H, Crowley J, Falkson C, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol, 2014; 9(9 Suppl 2):S65–72. [DOI] [PubMed] [Google Scholar]
- 6.Detterbeck F MS16.2 - Towards a TNM-Based Prognostic Classification for Thymic Tumours. 15th World Conference on Lung Cancer, Sydney, Australia, 2013. [Google Scholar]
- 7.Nicholson AG, Detterbeck FC, Marino M, Kim J, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposals for the T Component for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol, 2014; 9(9 Suppl 2):S73–80. [DOI] [PubMed] [Google Scholar]
- 8.Bhora FY, Chen DJ, Detterbeck FC, Asamura H, Falkson C, Filosso PL, et al. The ITMIG/IASLC Thymic Epithelial Tumors Staging Project: A Proposed Lymph Node Map for Thymic Epithelial Tumors in the Forthcoming 8th Edition of the TNM Classification of Malignant Tumors. J Thorac Oncol, 2014; 9(9 Suppl 2):S88–96. [DOI] [PubMed] [Google Scholar]
- 9.Kondo K, Van Schil P, Detterbeck FC, Okumura M, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposals for the N and M components for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol, 2014; 9(9 Suppl 2):S81–87. [DOI] [PubMed] [Google Scholar]
- 10.Anonymous. Thymomas and Thymic Carcinomas National Comprehensive Cancer Network, NCCN Practice Guidelines in Oncology, 2016; version 1.2016. [DOI] [PubMed] [Google Scholar]
- 11.Girard N, Ruffini E, Marx A, Faivre-Finn C, & Peters S. Thymic Epithelial Tumours: ESMO Clinical Practice Guidelines. Ann Oncol, 2015; 26 (suppl 5): v40–v55. [DOI] [PubMed] [Google Scholar]
- 12.Marom EM, Milito MA, Moran CA, Liu P, Correa AM, Kim ES, et al. Computed tomography findings predicting invasiveness of thymoma. J Thorac Oncol, 2011; 6(7):1274–1281. [DOI] [PubMed] [Google Scholar]
- 13.Hayes SA, Huang J, Plodkowski AJ, Katzen J, Zheng J, Moskowitz CS, et al. Preoperative computed tomography findings predict surgical resectability of thymoma. J Thorac Oncol, 2014; 9(7):1023–1030. [DOI] [PubMed] [Google Scholar]
- 14.Yanagawa M & Tomiyama N. Prediction of thymoma histology and stage by radiographic criteria. Thorac Surg Clin, 2011; 21(1):1–12. [DOI] [PubMed] [Google Scholar]
- 15.Marom EM, Rosado-de-Christenson ML, Bruzzi JF, Hara M, Sonett JR, & Ketai L. Standard report terms for chest computed tomography reports of anterior mediastinal masses suspicious for thymoma. J Thorac Oncol, 2011; 6(7 Suppl 3):S1717–1723. [DOI] [PubMed] [Google Scholar]
- 16.Travis WD, Brambilla E, Burke AP, Marx A, & Nicholson AG. Pathology and Genetics: Tumours of the Lung, Pleura, Thymus and Heart. (IARC, Lyon), 2004. [DOI] [PubMed] [Google Scholar]
- 17.Chalabreysse L WHO Classification and IASLC/ITMIG Staging Proposal in Thymic Tumors: Real-Life Assessment. 7th International Thymic Malignancy Interest Group, San Francisco, CA, 2016. [Google Scholar]
- 18.Zhao Y, Chen H, Shi J, Fan L, Hu D, & Zhao H. The correlation of morphological features of chest computed tomographic scans with clinical characteristics of thymoma. Eur J Cardiothorac Surg, 2015; 48(5):698–704. [DOI] [PubMed] [Google Scholar]
- 19.Ozawa Y, Hara M, Shimohira M, Sakurai K, Nakagawa M, & Shibamoto Y. Associations between computed tomography features of thymomas and their pathological classification. Acta Radiol, 2015; 10.1177/0284185115590288. [DOI] [PubMed] [Google Scholar]
- 20.Qu YJ, Liu GB, Shi HS, Liao MY, Yang GF, & Tian ZX. Preoperative CT findings of thymoma are correlated with postoperative Masaoka clinical stage. Acad Radiol, 2013; 20(1):66–72. [DOI] [PubMed] [Google Scholar]
- 21.Tomiyama N, Muller NL, Ellis SJ, Cleverley JR, Okumura M, Miyoshi S, et al. Invasive and noninvasive thymoma: distinctive CT features. J Comput Assist Tomogr, 2001; 25(3):388–393. [DOI] [PubMed] [Google Scholar]
- 22.Priola AM, Priola SM, Di Franco M, Cataldi A, Durando S, & Fava C. Computed tomography and thymoma: distinctive findings in invasive and noninvasive thymoma and predictive features of recurrence. Radiol Med, 2010; 115(1):1–21. [DOI] [PubMed] [Google Scholar]
- 23.Suidan RS, Ramirez PT, Sarasohn DM, Teitcher JB, Mironov S, Iyer RB, et al. A multicenter prospective trial evaluating the ability of preoperative computed tomography scan and serum CA-125 to predict suboptimal cytoreduction at primary debulking surgery for advanced ovarian, fallopian tube, and peritoneal cancer. Gynecol Oncol, 2014; 134(3):455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y & Mou L. A risk score system to preoperatively predict TNM stages in gastric cancer. Am J Clin Oncol, 2011; 34(2):130–134. [DOI] [PubMed] [Google Scholar]
- 25.Roden AC, Yi ES, Jenkins SM, Edwards KK, Donovan JL, Lewis JE, et al. Reproducibility of 3 histologic classifications and 3 staging systems for thymic epithelial neoplasms and its effect on prognosis. Am J Surg Pathol, 2015; 39(4):427–441. [DOI] [PubMed] [Google Scholar]
- 26.Ried M, Hnevkovsky S, Neu R, von Susskind-Schwendi M, Gotz A, Hamer OW, et al. Impact of Surgical Evaluation of Additional Cine Magnetic Resonance Imaging for Advanced Thymoma with Infiltration of Adjacent Structures: The Thoracic Surgeon’s View. Thorac Cardiovasc Surg, 2016; 10.1055/s-0036-1583765. [DOI] [PubMed] [Google Scholar]
- 27.Tomiyama N, Johkoh T, Mihara N, Honda O, Kozuka T, Koyama M, et al. Using the World Health Organization Classification of thymic epithelial neoplasms to describe CT findings. AJR Am J Roentgenol, 2002; 179(4):881–886. [DOI] [PubMed] [Google Scholar]
- 28.Priola AM & Priola SM. Considerations about the ability of computed tomography to predict the clinical stage of thymoma. Eur J Cardiothorac Surg, 2016; 10.1093/ejcts/ezw080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


