Abstract
Sphingosine kinases phosphorylate sphingosine to sphingosine 1-phosphate (S1P), which functions as a signaling molecule. We have previously shown that sphingosine kinase 2 (Sphk2) is important for insulin secretion. To obtain a better understanding of the role of Sphk2 in glucose and lipid metabolism, we have characterized 20- and 52-week old Sphk2−/− mice using glucose and insulin tolerance tests and by analyzing metabolic gene expression in adipose tissue. A detailed metabolic characterization of these mice revealed that aging Sphk2−/− mice are protected from metabolic decline and obesity compared to WT mice. Specifically, we found that 52-week male Sphk2−/− mice had decreased weight and fat mass, and increased glucose tolerance and insulin sensitivity compared to control mice. Indirect calorimetry studies demonstrated an increased energy expenditure and food intake in 52-week old male Sphk2−/− versus control mice. Furthermore, expression of adiponectin gene in adipose tissue was increased and the plasma levels of adiponectin elevated in aged Sphk2−/− mice compared to WT. Analysis of lipid metabolic gene expression in adipose tissue showed increased expression of the Atgl gene, which was associated with increased Atgl protein levels. Atgl encodes for the adipocyte triglyceride lipase, which catalyzes the rate-limiting step of lipolysis. In summary, these data suggest that mice lacking the Sphk2 gene are protected from obesity and insulin resistance during aging. The beneficial metabolic effects observed in aged Sphk2−/− mice may be in part due to enhanced lipolysis by Atgl and increased levels of adiponectin, which has lipid- and glucose-lowering effects.
Keywords: sphingolipids, sphingosine kinase, obesity, diabetes, insulin resistance, Sphk2
1. Introduction
Sphingolipids are derived from ceramide, which is generated de novo from L-serine and palmitoyl-CoA catalyzed by serine palmitoyltransferases [1, 2]. Ceramidases convert ceramide to sphingosine by hydrolyzing the fatty acids from ceramide. Sphingosine is then phosphorylated by two sphingosine kinases (Sphk1/2) to sphingosine 1-phosphate (S1P) [3]. Sphk1 has pro-survival activity, while Sphk2 is pro-apoptotic [3–5]. They differ in their activity, regulation, and localization [3, 6]. Sphk1 and Sphk2 null mice are viable, but deletion of both genes results in embryonic lethality [7]. While the function of Sphk1 has been studied in detail in various tissues, the exact function of Sphk2 remains to be determined [8]. A recent study suggests that polymorphisms in the Sphk2 gene may contribute to the genetic predisposition to type 1 diabetes [9].
Sphingolipids, including ceramide and S1P serve as signaling molecules [10, 11]. S1P plays an important role in cell signaling, proliferation, cell survival and differentiation [11]. It has intracellular as well as extracellular signaling functions by serving as a ligand for five different G protein-coupled receptors (S1P1-S1P5) [11, 12]. In mammals, there is a strong correlation between ceramide accumulation and age-related diseases, including type 2 diabetes, cardiovascular disease, cancer, and neurodegeneration [13–15]. While increased levels of ceramide are associated with insulin resistance in mice and humans, the role of S1P in regulation of glucose metabolism remains unclear [14, 16–19]. However, recent data suggest that increased levels of S1P are also associated with obesity and insulin resistance [20]. Plasma S1P levels have been shown to be elevated in both obese humans and rodents [21]. Furthermore, palmitate-induced increases in S1P levels have been associated with insulin resistance in pancreatic beta cells as well as in hepatocytes via activation of the S1P receptor subtype 2 (S1P2) [22, 23]. Administration of JTE-013, an S1P2 antagonist prevented the insulin resistance mediated by palmitate [20, 22, 23].
Adiponectin is mainly produced in adipocytes and has glucose- and lipid-lowering effects [24]. Recent studies suggest that the adiponectin receptors AdipoR1/R2 have ceramidase activity and are capable of converting ceramide to sphingosine [25]. Binding of adiponectin to AdipoR1/R2 receptors stimulates their ceramidase activity, thereby leading to lowering of ceramide levels. Ablation of the adiponectin receptors in mice results in accumulation of ceramide, which is associated with insulin resistance and glucose intolerance [26]. These findings suggest that the beneficial pleiotropic effects of adiponectin on glucose and lipid metabolism are due to its ability to lower ceramide levels.
We have previously shown that Sphk2 is important for insulin secretion from pancreatic beta cells [27]. A detailed characterization of Sphk2−/− mice revealed that they are protected from age-related obesity and metabolic decline, which may be in part due to elevated adiponectin levels and enhanced lipolysis in adipose tissue of Sphk2−/− mice.
2. Materials and methods
2.1. Animals
All animals were housed in a specific pathogen-free animal facility at the University of Kentucky on a 14-hour light and 10-hour dark cycle and kept under standard humidity and temperature conditions with free access to water and rodent chow. All animal procedures were reviewed and approved by the University of Kentucky Institutional Animal Care and Use Committee (Protocol 2011-0806). Sphk2−/− mice on C57BL/6N background were obtained from the Jackson Laboratories (Stock No: 019140) and have been previously described in detail [7]. Sphk2 heterozygous mating pairs were used to generate Sphk2−/− and wild type litter mates. Animals were euthanized with carbon dioxide followed by cervical dislocation.
2.2. Metabolic Tests
All metabolic tests were carried out with male and female mice and followed the standard operating procedures as established previously [28]. The presented data were obtained with young mice (20-22 weeks of age) and old mice (50-54 weeks of age). For glucose tolerance tests (GTT), mice were fasted for 6 hours and i.p. injected with glucose (2g/kg). Mice were fasted for 4 hours before insulin tolerance tests (ITT) were carried out by i.p. administration of insulin (0.75 units/kg). In all tests, blood glucose was measured from tail vein at the indicated time points using the Nova Max Plus glucometer. Plasma insulin levels were measured using an insulin ELISA kit (Crystal Chem). Circulating levels of adiponectin and leptin were quantified using ELISA kits from Chrystal Chem. Triglyceride levels were measured using colorimetric kits from Abeam. Homeostasis model assessment of insulin resistance (HOMA-IR) was determined using the following formula: fasting glucose (mg/dL) × fasting insulin (μU/ml)/405.
2.3. Indirect Calorimetry and Body Composition Measurements
Indirect calorimetry to monitor food consumption, physical activity, O2 consumption, and CO2 production was measured using the TSE LabMaster system by the COBRE Metabolic Core at the University of Kentucky. Fat and lean body mass was determined on conscious mice using an EchoMRI-5000 whole-body composition analyzer (Echo Medical System) that uses magnetic resonance relaxometry.
2.4. Preparation of Protein Extracts and Western Blotting
Isolated tissues were rapidly frozen in liquid nitrogen and stored in −80°C until extract preparation. Total cellular extracts were prepared in lysis buffer (50mM Tris-HCI, pH 8.0, 20% glycerol, 140mM NaCI, 1% NP-40, 1mM EDTA, 1mM DTT and protease/phosphatase inhibitors) using a tissue homogenizer. After incubation for 30 min at 4°C, the lysates were centrifuged for 10 min at maximal speed and supernatants collected. Western blot analysis of whole cell lysates was conducted as previously described [29]. Sphk1 (sc-48825; rabbit polyclonal; dilution 1:1,000) and Sphk2 (sc-22704; goat polyclonal; 1:1,000) antibodies were from Santa Cruz Biotechnology, Inc and β-actin antibodies (A2228) were obtained from Sigma-Aldrich. CD36 (ab133625) and Atgl (#2439) antibodies were from Abeam and Cell Signaling, respectively. Western blots were visualized using secondary antibodies conjugated to HRP in conjunction with ECL reagents (Thermo Scientific).
2.5. Realtime (RT) PCR Analysis
Total RNA was isolated using Trizol (Life Technologies) and 1 μg of total RNA was used for cDNA synthesis with the qScript cDNA SuperMix kit (Quanta Biosciences). Quantitative RT-PCR (qRT-PCR) was performed on M×3005P real-time PCR instrument (Stratagene) using SYBR Green qPCR Master Mix (Applied Biosystems) as previously described [30]. All qRT-PCR data were normalized to β-actin mRNA levels. The genes and primers used for qRT-PCR quantification are listed in Table 1.
Table 1.
Sequences of primers used for RT-PCR
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Adipoq | GGAGAGAAAGGAGATGCAGGT | CTTTCCTGCCAGGGGTTC |
| Leptin | AAGACCATTGTCACCAGGATC | GAAGCCCAGGAATGAAGTCC |
| Acac | GGCCAGTGCTATGCTGAGAT | CCAGGTCGTTTGACATAATGGATG |
| CD36 | TGAGACTGGGACCATTGGTGAT | CCCAAGTAAGGCCATCTCTACCAT |
| Atgl | TGACCATCTGCCTTCCAGACT | TGTAGGTGGCGCAAGACAG |
| Cpt1 | GCTGGGCTACTCAGAGGATG | CACTGTAGCCTGGTGGGTTT |
| Dgat2 | AGGCCCTATTTGGCTACG TT | GATGCCTCCAGACATCAGGT |
| Sphk1 | TCCTGGAGGAGGCAGAGATA | GCTACACAGGGGTTTCTGGA |
| ActB | CGTGGGCCGCCCTAG | TTGGCCTTAGGGTTCAGGGG |
2.6. Statistics
Results are expressed as mean ± SEM. For comparison between groups a two-tailed unpaired Student’s t-test was used. A p value of less than 0.05 was considered as statistically significant. Statistical analysis was performed using SPSS. *p< 0.05; **p< 0.01; ***p< 0.001.
3. Results
3.1. Sphk2−/− mice have reduced weight and fat mass and are protected from age-dependent insulin resistance
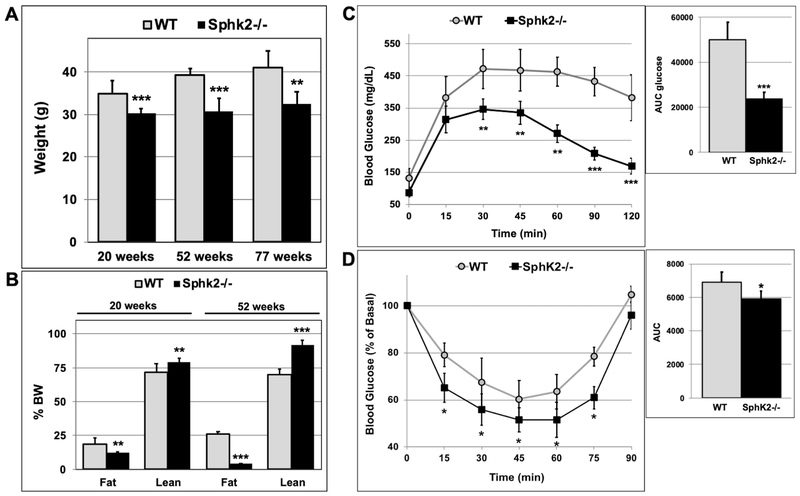
Aging is associated with weight gain and obesity. We measured the weight and fat mass of male Sphk2−/− and WT mice at 20 and 52 weeks of age (Fig 1A). At 20 weeks of age, the weight of male Sphk2−/− mice was about 10% less than of that of WT mice; however at 52-weeks Sphk2−/− mice displayed a 35% decrease in body weight compared to WT (Fig 1A). In fact, the weight of 52-week old male Sphk2−/− mice was similar to that of 20-week old mice. This suggests that while WT mice gained weight with age, the Sphk2−/− mice maintained a fairly constant body weight. The reduction in body weight in Sphk2−/− mice was first observed at 10 weeks of age (Fig S1A) and persisted in 77-week old male mice (Fig 1A). Echo-MRI analysis of lean and fat mass indicated a 36% decrease in fat and an 8% increase in lean mass (normalized to % BW) in male Sphk2−/− versus WT mice at 20 weeks of age (Fig 1B & S1B). At 52 weeks of age, the difference in fat and lean mass in Sphk2−/− versus WT was more pronounced with 85% decrease in fat and 22% increase in lean mass in male Sphk2−/− compared to WT mice (Fig 1B & S1B).
Fig 1. Male Sphk2−/− mice have reduced weight and fat mass and increased glucose tolerance and insulin sensitivity during aging.
(A) Weight of male WT and Sphk2−/− mice was measured at 20, 52, and 77 weeks of age (n=8-10). (B) Lean and fat mass in 20- and 52-week old male WT and Sphk2−/− mice was measured using Echo-MRI (n=5-7). (C) GTT was performed in 52-week old male Sphk2−/− and WT mice after a 6-hr fast; (n=5). (D) ITT was carried out in 54-week old male Sphk2−/− and WT mice after a 4-hr fast; (n=5). *p< 0.05; **p< 0.01; ***p< 0.001.
During a glucose tolerance test (GTT), 52-week old male WT mice were unable to normalize blood glucose levels after glucose administration within a 2-hour time frame (Fig 1C). In contrast, blood glucose levels in male Sphk2−/− mice were almost back to normal within 2 hours. The area under the curve (AUC) for glucose was significantly lower for Sphk2−/− versus WT mice (Fig 1C). Aged male Sphk2−/− mice were also more insulin sensitive compared to WT (Fig 1D). Consistent with these findings, the HOMA-IR index for 52-week old male Sphk2−/− mice (2.83 ± 0.77) was significantly lower compared to WT mice (11.38 ± 1.37), confirming that Sphk2−/− mice are protected from insulin resistance during aging. There was no difference in glucose tolerance and insulin sensitivity in 20-week old male WT versus Sphk2−/− mice (Fig S2A-B).
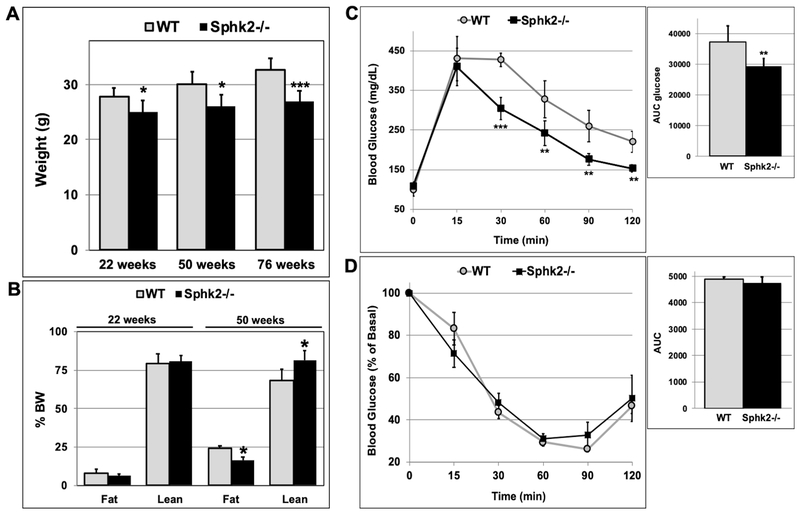
The decrease in weight gain was also observed in female Sphk2−/− mice at the age of 22, 50 and 76 weeks, although it was not as pronounced as in male Sphk2−/− mice (Fig 2A). While there was no significant difference in fat and lean mass in 22-week old female Sphk2−/− mice, 50-week old female Sphk2−/− mice had a 30% decrease in fat and 20% increase in lean mass compared to WT, when normalized to % BW (Fig 2B). 50-week old female Sphk2−/− mice displayed improved glucose tolerance compared to WT (Fig 2C), however insulin sensitivity in female Sphk2−/− versus WT mice was not significantly different (Fig 2D). These data suggest that ablation of Sphk2 in mice prevents age-related weight gain and obesity.
Fig 2. Female Sphk2−/− mice have decreased weight and fat mass, and increased glucose tolerance with aging.
(A) Weight of female Sphk2−/− and WT mice was measured at 22, 50, and 76 weeks of age (n=6-8). (B) Lean and fat mass in 22- and 50-week old female WT and Sphk2−/− mice was determined by Echo-MRI (n=5). (C) GTT and (D) ITT was performed in 50-week and 52-week old female Sphk2−/− and WT mice, respectively (n=5). *p< 0.05; **p< 0.01; ***p< 0.001.
3.2. Aged Sphk2−/− mice have lower fasting blood glucose levels and increased energy expenditure
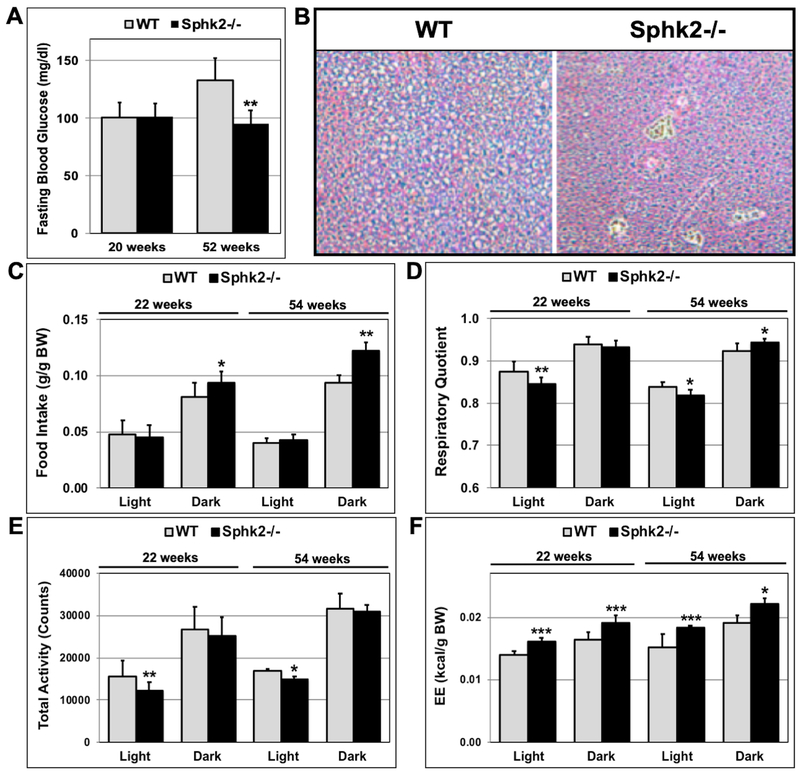
While random blood glucose levels between Sphk2−/− and WT mice at 20 and 52 weeks of age were similar, fasting blood glucose levels in 52-week old male Sphk2−/− mice were significantly lower (35% decrease) than in WT mice (Fig 3A). Moreover, 54-week old male WT mice showed signs of fatty liver compared to Sphk2−/− mice after H&E staining of paraffin-embedded liver sections (Fig 3B). There was no change in fasting blood glucose levels between male Sphk2−/− and WT mice at 20 weeks of age (Fig 3A). Fasting blood glucose levels in 50-week old female Sphk2−/− and WT were similar (Fig S2C). These data suggest that male Sphk2−/− mice are protected from age-dependent glucose intolerance and hepatic insulin resistance.
Fig 3. Male Sphk2−/− mice have lower fasting blood glucose levels and increased energy expenditure.
(A) Fasting blood glucose levels in 20- and 52-week old male Sphk2−/− and WT mice were measured after a 16hr-fast (n=5). (B) Paraffin-embedded liver sections of 54-week old male Sphk2−/− and WT mice were stained with H & E. Food intake (C), respiratory quotient (D), total activity (E) and energy expenditure (F) were determined using indirect calorimetry with 22- and 54-week old male mice (n=8-10). Food Intake and energy expenditure were normalized for body weight. *p< 0.05; **p< 0.01; ***p< 0.001.
Metabolic studies using indirect calorimetry with 22- or 54-week old male Sphk2−/− and WT mice suggested that food intake (corrected for body weight) was significantly increased during the dark cycle in 22- and 54-week old Sphk2−/− mice (Fig 3C). The respiratory quotient was decreased during the light cycle in both 22- and 54-week old Sphk2−/− mice, while it was increased during the dark cycle in 54-week old Sphk2−/− mice (Fig 3D). Although, total activity during the dark cycle was not significantly different between Sphk2−/− and WT mice, total activity during the light cycle was decreased in Sphk2−/− mice (Fig 3E). Energy expenditure (corrected for body weight) was increased during the light and dark cycle in both 22- and 54-week old male Sphk2−/− versus WT mice (Fig 3F). Energy expenditure corrected for lean body mass was also increased in Sphk2−/− male mice (Fig S4A).
Female Sphk2−/− mice displayed lower food intake during the light cycle, but higher food intake during the dark cycle (Fig. S3A). Respiratory quotient in female Sphk2−/− mice was decreased during light cycle similar to male Sphk2−/− mice (Fig. S3B). In contrast to male mice, total activity in female Sphk2−/− mice was reduced by about 50% during the light cycle compared to WT (Fig S3C). However, there was no significant difference in energy expenditure in female Sphk2−/− versus WT mice when normalized for body weight (Fig. S3D).
3.3. Sphk2−/− mice have increased expression and circulating levels of adiponectin during aging
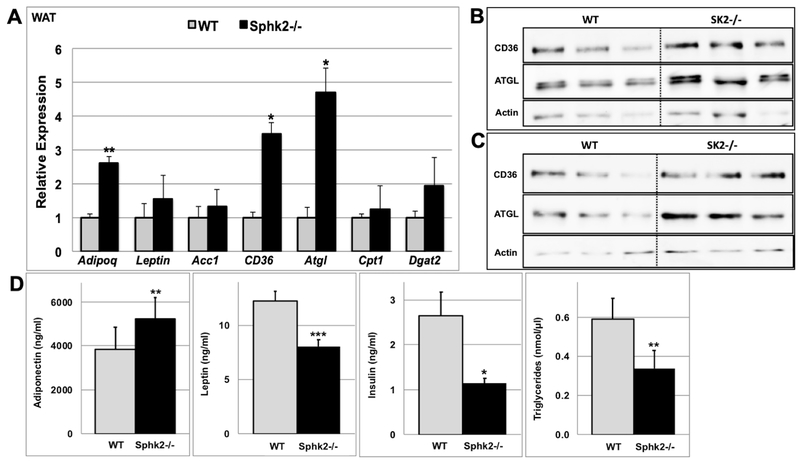
Aging Sphk2−/− mice weigh less and have decreased fat mass compared to WT mice (Fig. 1), suggesting that they have altered lipid metabolism. Expression analysis of adipokines as well as lipid metabolic genes in gonadal white adipose tissue (WAT) indicated that the expression of adiponectin (2.6-fold), CD36 (3.5-fold) and Atgl (4.7-fold) genes were significantly upregulated in 54-week old male Sphk2−/− versus WT mice (Fig 4A). However, there were no significant changes in expression of these genes in WAT of 22-week old Sphk2−/− versus WT mice (Fig. S4B). Consistent with the gene expression data, the levels of CD36 and Atgl protein were about 2-fold increased in WAT of Sphk2−/− mice compared to WT (Fig 4B-C). Atgl encodes for the adipocyte triglyceride lipase, which catalyzes the rate-limiting step in the hydrolysis of triglycerides [31, 32]. Upregulation of Atgl suggests increased lipolysis in WAT of Sphk2−/− mice. Increased expression of adiponectin in WAT of Sphk2−/− mice correlated with increased plasma levels of adiponectin (Fig 4D). Adiponectin displays glucose- and lipid-lowering effects [24]. Thus, it is possible that Sphk2−/− mice are protected from age-dependent obesity and insulin resistance in part due to increased levels of adiponectin. Plasma leptin, triglyceride and insulin levels were significantly lower in aged male Sphk2−/− versus WT mice (Fig 4D).
Fig 4. Aged male Sphk2−/− mice display increased expression and plasma levels of adiponectin.
The expression of various adipokines ((Adipoq (adiponectin) and leptin)), lipid metabolic genes (Acc1 (acetyl CoA carboxylase), CD36, Atgl (adipocyte triglyceride lipase), Cpt1 (carnitine palmitoyl transferase I), and Dgat2 (diglyceride acyltransferase)) was quantified in WAT (A) of 54-week old Sphk2−/− and WT male mice by qRT-PCR and normalized to beta-actin levels (n=4). CD36 and Atgl protein levels were measured in WAT of 54-week old Sphk2−/− and WT male mice under fed (B) or after 16hr fasting (C) conditions by immunoblotting with specific antibodies (n=3). (C) Plasma adiponectin, leptin, insulin, and triglyceride levels were measured in 54-week old male Sphk2−/− and WT male mice using ELISA (n=5). *p< 0.05; **p< 0.01; ***p< 0.001
4. Discussion
Sphingolipids have been implicated in many diseases, including obesity and diabetes. Two important sphingolipids that function as signaling molecules are ceramide and S1P. Increases in ceramide levels are associated with insulin resistance [14, 33]. S1P has been shown to counteract ceramide function [11]. We report here that deletion of sphingosine kinase 2, which produces S1P, is beneficial and protects from metabolic decline during aging. Lack of Sphk2 was associated with decreased weight and fat mass in both male and female mice, however the metabolic effects were more robust in male mice. Although aged female Sphk2−/− mice weighed less than WT, displayed decreased fat mass and had significantly improved glucose tolerance, there was no significant difference in fasting blood glucose levels, insulin sensitivity and energy expenditure between aged female Sphk2−/− and WT mice.
Aging is associated with weight gain and obesity. Age-related changes in adipose tissue composition and function lead to insulin resistance and metabolic dysfunction [34, 35]. Male Sphk2−/− weighed about 35% less than WT mice and displayed an 85% reduction in fat mass at 52 weeks of age, suggesting that they are protected from age-related changes in adipose tissue function and composition. Sphk2−/− male mice are more glucose tolerant and have lower fasting blood glucose levels during aging compared to WT. One of the hallmarks of insulin resistance is hyperinsulinemia that serves as a compensation mechanism. While aging WT mice were insulin resistant and displayed hyperinsulinemia, Sphk2−/− mice had decreased plasma insulin levels and were protected from hyperinsulinemia. Interestingly, plasma insulin levels were reduced by about 40% even in 20-week old Sphk2−/− mice (Fig S2D). This suggests that Sphk2−/− mice have chronically low levels of insulin, which is consistent with our previous observation that Sphk2 is required for insulin secretion [27].
Caloric intake as well as energy expenditure was significantly increased in male Sphk2−/− versus WT mice at 22- and 54-weeks of age. Despite the increased caloric intake, male Sphk2−/− mice have decreased body weight and fat mass, which is likely to be attributed to increased energy expenditure. Interestingly, energy expenditure in 50-week old female Sphk2−/− mice was not different from that of WT mice, suggesting the idea that the weight loss observed in aging female Sphk2−/− mice is not due to increased energy expenditure. This indicates that Sphk2 has different metabolic effects in male versus female mice.
Expression as well as circulating levels of adiponectin are increased Sphk2−/− mice compared to WT. Thus, it is possible that the improved metabolic effects seen in Sphk2−/− mice could be in part due to increased adiponectin levels. Adiponectin is produced from the white adipose tissue and its expression is decreased during obesity. Adiponectin has lipid- and glucoselowering effects and improves glucose metabolism [24]. Interestingly, there is a strong connection between adiponectin and ceramide levels. Adiponectin receptors AdipoR1 and AdipoR2 have been previously shown to function as ceramidases [25]. Thus, binding of adiponectin to its receptors decreases ceramide levels, which may be responsible for the lipid- and glucose-lowering effects of adiponectin. However, it is possible that the increased adiponectin levels in Sphk2−/− mice may be due to decreased obesity in these mice. As reported previously, we confirmed that deletion of Sphk2 leads to upregulation of plasma S1P by two-fold. Since S1P functions as a ligand for five different S1P receptors, increases in plasma S1P levels may lead to increased S1P signaling and thereby result in positive metabolic outcomes in Sphk2−/− mice.
Analysis of metabolic gene expression in WAT suggest that Sphk2−/− mice have increased expression of Atgl encoding for the adipose triglyceride lipase, which catalyzes the rate-limiting step of lipolysis [36]. This indicates that Sphk2−/− mice may be protected from obesity during aging in part due to increased lipolysis. The levels of the lipid transporter CD36 were also significantly increased in WAT of Sphk2−/− mice [31, 37], suggesting that lipid uptake into adipocytes of Sphk2 null mice is increased. Since Sphk2 is mainly localized to the nucleus, it is likely that is regulates the expression of lipid metabolic genes directly. Previous data suggest that Sphk2 and S1P interact with histone deacetylases HDAC1 and HDAC2 in the nucleus to inhibit their activity [38]. Consistent with the gene expression data, the levels of Atgl and CD36 protein were also increased about 2-fold in WAT of Sphk2−/− mice compared to WT. Since enzymes involved in the sphingolipid biosynthesis pathway have been shown to function as lipid sensors [39, 40], it is possible that Sphk2 has a similar function. Sphk2 could sense the rate of sphingolipid biosynthesis by binding to sphingosine. During increased flux, Sphk2 could mediate the suppression of processes that increase intracellular fatty acid levels, such as lipolysis (Atgl) and lipid uptake (CD36) during obesity. Loss of Sphk2 would lead to increased lipolysis and lipid uptake. Consistent with the idea, recent findings suggest that inhibition of fatty acid synthase (Fasn) and thereby downregulation of fatty acid synthesis decreases the flux through the sphingolipid biosynthesis pathway in colorectal cancer cells [41].
In agreement with our findings, recently published data suggest that Sphk2 and/or S1P are associated with negative health outcomes. Sphk2 has been shown to promote kidney fibrosis via phosphorylation of Fyn to activate STAT3 and AKT, which could not be rescued by addition of extracellular S1P [42]. Increased S1P levels have been recently associated with type 2 diabetes and obesity [21, 22]. Palmitate-induced increases in S1P levels in pancreatic beta cells have been demonstrated to antagonize insulin-stimulated cell growth and survival via activation of the S1P receptor subtype 2 (S1P2). Administration of JTE-013, an S1P2 antagonist rescued the beta-cell damage attributed to the enhanced S1P-S1P2 axis [22]. Similar data were also obtained in hepatocytes. An increase in hepatic S1P levels induced by palmitate caused insulin resistance via the S1P-S1P2 axis, which could be reversed using the S1P2 antagonist JTE-013 [23].
Sphk1 and Sphk2 both produce S1P, but they differ in their regulation, tissue-distribution, and localization. There were no changes in Sphk1 expression and protein levels in livers of Sphk2−/− mice (Fig S4C-D), suggesting that there is no compensation by overexpression of Sphk1 in Sphk2−/− mice. This is in agreement with the original publication on Sphk2−/− mice, where it was reported that Sphk1 expression was not altered in various tissue of Sphk2−/− mice [7]. However, several reports indicate that inhibition of Sphk2 results in upregulation of Sphk1 expression. It is possible that Sphk2 inhibitors increase Sphk1 levels by partially inhibiting Sphk1 activity. Although, there are no studies on Sphk1 function during aging, previously published data suggest that Sphk1 null mice are protected from HFD-induced insulin resistance [43]. Interestingly, Sphk1 null mice on a HFD gain as much weight as WT mice, but display improved glucose tolerance and insulin sensitivity [43]. Since Sphk2−/− mice do not gain weight during aging, this suggests that Sphk1 and Sphk2 have overlapping as well as unique functions with respect to obesity and insulin resistance.
In conclusion, mice lacking Sphk2 are protected from obesity and insulin resistance during aging. This indicates that increased Sphk2 levels or activity, and/or increased S1P levels during aging may contribute to obesity and metabolic dysfunction. Therefore, Sphk2 may be a potential drug target for treatment of age-related obesity and insulin resistance.
Supplementary Material
Highlights.
Deletion of Sphk2 in mice protects from age–related obesity and insulin resistance
Aged Sphk2−/− mice have decreased fat mass but increased lean mass
Sphk2−/− mice display increased energy expenditure compared to WT
Protection of Sphk2−/− mice from obesity and insulin resistance may be in part due to enhanced lipolysis and increased adiponectin levels
Acknowledgements
The authors thank Kara L. Larson for managing the initial Sphk2−/− animal colony and Dr. Carole Moncman for help with microscopy. We also thank Drs. Lisa Cassis and Wendy Katz from the COBRE Metabolic Core at the University of Kentucky for their assistance with the Indirect Calorimetry studies and EchoMRI measurements.
Funding
This work was supported by AHA grant 14GRNT20380383 (to SO) and by Bridge Funding from the University of Kentucky (to SO). The COBRE Metabolic Core was supported by grants P20GM103527 and P20RR021954 from NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
References
- [1].Merrill AH Jr., Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics, Chemical reviews, 111 (2011) 6387–6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tidhar R, Futerman AH, The complexity of sphingolipid biosynthesis in the endoplasmic reticulum, Biochimica et biophysica acta, 1833 (2013) 2511–2518. [DOI] [PubMed] [Google Scholar]
- [3].Maceyka M, Sankala H, Hait NC, Le Stunff H, Liu H, Toman R, Collier C, Zhang M, Satin LS, Merrill AH Jr., Milstien S, Spiegel S, SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism, The Journal of biological chemistry, 280 (2005) 37118–37129. [DOI] [PubMed] [Google Scholar]
- [4].Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer S, Nakamura S, Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis, The Journal of biological chemistry, 278 (2003) 46832–46839. [DOI] [PubMed] [Google Scholar]
- [5].Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, Payne SG, Bektas M, Ishii I, Chun J, Milstien S, Spiegel S, Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis, The Journal of biological chemistry, 278 (2003) 40330–40336. [DOI] [PubMed] [Google Scholar]
- [6].Pyne S, Lee SC, Long J, Pyne NJ, Role of sphingosine kinases and lipid phosphate phosphatases in regulating spatial sphingosine 1-phosphate signalling in health and disease, Cellular signalling, 21 (2009) 14–21. [DOI] [PubMed] [Google Scholar]
- [7].Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Praia RL, Essential role for sphingosine kinases in neural and vascular development, Molecular and cellular biology, 25 (2005) 11113–11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hatoum D, Haddadi N, Lin Y, Nassif NT, McGowan EM, Mammalian sphingosine kinase (SphK) isoenzymes and isoform expression: challenges for SphK as an oncotarget, Oncotarget, 8 (2017) 36898–36929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Holm LJ, Krogvold L, Hasselby JP, Kaur S, Claessens LA, Russell MA, Mathews CE, Hanssen KF, Morgan NG, Koeleman BPC, Roep BO, Gerling IC, Pociot F, Dahl-Jorgensen K, Buschard K, Abnormal islet sphingolipid metabolism in type 1 diabetes, Diabetologia, 61 (2018) 1650–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cowart LA, Sphingolipids: players in the pathology of metabolic disease, Trends in endocrinology and metabolism: TEM, 20 (2009) 34–42. [DOI] [PubMed] [Google Scholar]
- [11].Maceyka M, Harikumar KB, Milstien S, Spiegel S, Sphingosine-1-phosphate signaling and its role in disease, Trends in cell biology, 22 (2012) 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kihara Y, Maceyka M, Spiegel S, Chun J, Lysophospholipid receptor nomenclature review: IUPHAR Review 8, Br J Pharmacol, 171 (2014) 3575–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sacket SJ, Chung HY, Okajima F, Im DS, Increase in sphingolipid catabolic enzyme activity during aging, Acta pharmacologica Sinica, 30 (2009) 1454–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Summers SA, Sphingolipids and insulin resistance: the five Ws, Current opinion in lipidology, 21 (2010) 128–135. [DOI] [PubMed] [Google Scholar]
- [15].Trayssac M, Hannun YA, Obeid LM, Role of sphingolipids in senescence: implication in aging and age-related diseases, The Journal of clinical investigation, 128 (2018) 2702–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA, Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance, Cell Metab, 5 (2007) 167–179. [DOI] [PubMed] [Google Scholar]
- [17].Holland WL, Knotts TA, Chavez JA, Wang LP, Hoehn KL, Summers SA, Lipid mediators of insulin resistance, Nutrition reviews, 65 (2007) S39–46. [DOI] [PubMed] [Google Scholar]
- [18].Boden G, Ceramide: a contributor to insulin resistance or an innocent bystander?, Diabetologia, 51 (2008) 1095–1096. [DOI] [PubMed] [Google Scholar]
- [19].Zhao H, Przybylska M, Wu IH, Zhang J, Siegel C, Komarnitsky S, Yew NS, Cheng SH, Inhibiting glycosphingolipid synthesis improves glycemic control and insulin sensitivity in animal models of type 2 diabetes, Diabetes, 56 (2007) 1210–1218. [DOI] [PubMed] [Google Scholar]
- [20].Fayyaz S, Japtok L, Kleuser B, Divergent role of sphingosine 1-phosphate on insulin resistance, Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology, 34 (2014) 134–147. [DOI] [PubMed] [Google Scholar]
- [21].Kowalski GM, Carey AL, Selathurai A, Kingwell BA, Bruce CR, Plasma sphingosine-1-phosphate is elevated in obesity, PloS one, 8 (2013) e72449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Japtok L, Schmitz E.l., Fayyaz S, Kramer S, Hsu LJ, Kleuser B, Sphingosine 1-phosphate counteracts insulin signaling in pancreatic beta-cells via the sphingosine 1-phosphate receptor subtype 2, FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 29 (2015) 3357–3369. [DOI] [PubMed] [Google Scholar]
- [23].Fayyaz S, Henkel J, Japtok L, Kramer S, Damm G, Seehofer D, Puschel GP, Kleuser B, Involvement of sphingosine 1-phosphate in palmitate-induced insulin resistance of hepatocytes via the S1P2 receptor subtype, Diabetologia, 57 (2014) 373–382. [DOI] [PubMed] [Google Scholar]
- [24].Xia JY, Morley TS, PE. Scherer, The adipokine/ceramide axis: key aspects of insulin sensitization, Biochimie, 96 (2014) 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo MS, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE, Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin, Nat Med, 17 (2011) 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-lwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T, Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions, Nat Med, 13 (2007) 332–339. [DOI] [PubMed] [Google Scholar]
- [27].Cantrell Stanford J, Morris AJ, Sunkara M, Popa GJ, Larson KL, Ozcan S, Sphingosine 1-phosphate (S1P) regulates glucose-stimulated insulin secretion in pancreatic beta cells, The Journal of biological chemistry, 287 (2012) 13457–13464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman G.l., Wasserman DH, McGuinness OP, N.I.H.M.M.P.C. Consortium, Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice, Disease models & mechanisms, 3 (2010) 525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Vanderford NL, Andrali SS, Ozcan S, Glucose induces MafA expression in pancreatic beta cell lines via the hexosamine biosynthetic pathway, The Journal of biological chemistry, 282 (2007) 1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mosley AL, Corbett JA, Ozcan S, Glucose regulation of insulin gene expression requires the recruitment of p300 by the beta-cell-specific transcription factor Pdx-1, Molecular endocrinology, 18 (2004) 2279–2290. [DOI] [PubMed] [Google Scholar]
- [31].Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R, Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase, Science, 306 (2004) 1383–1386. [DOI] [PubMed] [Google Scholar]
- [32].Villena JA, Roy S, Sarkadi-Nagy E, Kim KH, Sul HS, Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis, The Journal of biological chemistry, 279 (2004) 47066–47075. [DOI] [PubMed] [Google Scholar]
- [33].Straczkowski M, Kowalska I, Baranowski M, Nikolajuk A, Otziomek E, Zabielski P, Adamska A, Blachnio A, Gorski J, Gorska M, Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes, Diabetologia, 50 (2007) 2366–2373. [DOI] [PubMed] [Google Scholar]
- [34].Guo SS, Zeller C, Chumlea WC, Siervogel RM, Aging, body composition, and lifestyle: the Fels Longitudinal Study, Am J Clin Nutr, 70 (1999) 405–411. [DOI] [PubMed] [Google Scholar]
- [35].Karakelides H, Irving BA, Short KR, O’Brien P, Nair KS, Age, obesity, and sex effects on insulin sensitivity and skeletal muscle mitochondrial function, Diabetes, 59 (2010) 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kim JY, Tillison K, Lee JH, Rearick DA, Smas CM, The adipose tissue triglyceride lipase ATGL/PNPLA2 is downregulated by insulin and TNF-alpha in 3T3-L1 adipocytes and is a target for transactivation by PPARgamma, Am J Physiol Endocrinol Metab, 291 (2006) E115–127. [DOI] [PubMed] [Google Scholar]
- [37].Glatz JF, Luiken JJ, From fat to FAT (CD36/SR-B2): Understanding the regulation of cellular fatty acid uptake, Biochimie, 136 (2017) 21–26. [DOI] [PubMed] [Google Scholar]
- [38].Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, Spiegel S, Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate, Science, 325 (2009) 1254–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sociale M, Wulf AL, Breiden B, Klee K, Thielisch M, Eckardt F, Sellin J, Bulow MH, Lobbert S, Weinstock N, Voelzmann A, Schultze J, Sandhoff K, Bauer R, Ceramide Synthase Schlank Is a Transcriptional Regulator Adapting Gene Expression to Energy Requirements, Cell Rep, 22 (2018) 967–978. [DOI] [PubMed] [Google Scholar]
- [40].Chaurasia B, Holland WL, Summers SA, Does This Schlank Make Me Look Fat?, Trends in endocrinology and metabolism: TEM, 29 (2018) 597–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jafari N, Drury J, Morris AJ, Onono FO, Stevens PD, Gao T, Liu J, Wang C, Lee EY, Weiss HL, Evers BM, Zaytseva YY, De Novo Fatty Acid Synthesis-Driven Sphingolipid Metabolism Promotes Metastatic Potential of Colorectal Cancer, Mol Cancer Res, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhu X, Shi D, Cao K, Ru D, Ren J, Rao Z, Chen Y, You Q, Dai C, Liu L, Zhou H, Sphingosine kinase 2 cooperating with Fyn promotes kidney fibroblast activation and fibrosis via STAT3 and AKT, Biochim Biophys Acta Mol Basis Dis, 1864 (2018) 3824–3836. [DOI] [PubMed] [Google Scholar]
- [43].Wang J, Badeanlou L, Bielawski J, Ciaraldi TP, Samad F, Sphingosine kinase 1 regulates adipose proinflammatory responses and insulin resistance, Am J Physiol Endocrinol Metab, 306 (2014) E756–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.