Abstract
Background
West Nile Virus (WNV), a member of the genus Flavivirus, is one of the most widely distributed arboviruses in the world. Despite some evidence for circulation of WNV in countries summarized by the World Health Organization as the Eastern Mediterrian Regional Office (EMRO), comprehensive knowledge about its epidemiology remains largely unknown. This study aims to provide a concise review of the published literature on WNV infections in the Eastern Mediterranean Regional Office of WHO (EMRO).
Methodology/principal findings
A systematic review of WNV prevalence studies on humans, animals and vectors in the EMRO region was performed by searching: Web of Science, Science Direct, Scopus, PubMed, Embase and Google Scholar. Finally, 77 citations were included, comprising 35 seroprevalence studies on general population (24460 individuals), 15 prevalence studies among patients (3439 individuals), 22 seroprevalence studies among animals (10309 animals), and 9 studies on vectors (184242 vector species). Of the 22 countries in this region, five had no data on WNV infection among different populations. These countries include Kuwait, Bahrain, Oman, Syria and Somalia. On the other hand, among countries with available data, WNV-specific antibodies were detected in the general population of all investigated countries including Djibouti (0.3–60%), Egypt (1–61%), Iran (0–30%), Iraq (11.6–15.1%), Jordan (8%), Lebanon (0.5–1%), Libya (2.3%), Morocco (0–18.8%), Pakistan (0.6–65.0%), Sudan (2.2–47%), and Tunisia (4.3–31.1%). WNV RNA were also detected in patient populations of Iran (1.2%), Pakistan (33.3%), and Tunisia (5.3% –15.9%). WNV-specific antibodies were also detected in a wide range of animal species. The highest seropositivity rate was observed among equids (100% in Morocco) and dogs (96% in Morocco). The highest seroprevalence among birds was seen in Tunisia (23%). In addition, WNV infection was detected in mosquitoes (Culex, and Aedes) and ticks (Argas reflexus hermanni). The primary vector of WNV (Culex pipiens s.l.) was detected in Djibouti, Egypt, Iran and Tunisia, and in mosquitoes of all these countries, WNV was demonstrated.
Conclusions
This first systematic regional assessment of WNV prevalence provides evidence to support the circulation of WNV in the EMRO region as nearly all studies showed evidence of WNV infection in human as well as animal/vector populations. These findings highlight the need for continued prevention and control strategies and the collection of epidemiologic data for WNV epidemic status, especially in countries that lack reliable surveillance systems.
Author summary
West Nile Virus (WNV) is a mosquito-borne Flavivirus belonging to the Flaviviridae family, which is endemic in a vast geographical area, including the EMRO region. However, the epidemiology of WNV in the EMRO region remains poorly understood. To address this gap, we performed a systematic review on WNV prevalence studies conducted on human populations, animals and vectors across Eastern Mediterranean countries. Our review indicated the infection of most investigated human, animal and vector populations with WNV; however, the paucity of epidemiological data underline the need for integrated surveillance programs as well as continued deployment of prevention and control strategies.
Introduction
West Nile Virus (WNV) is one of the most widely distributed arboviruses in the world, and a pathogen of public health significance in both humans and animals [1]. This mosquito-borne virus has been classified in the genus Flavivirus within the family Flaviviridae [2]. In nature, WNV is maintained in a zoonotic transmission cycle between birds and mosquitos, principally the Culex species. Susceptibility to WNV infection has also been indicated for many other vertebrate hosts including mammals, birds, reptiles, and amphibians [3]. Equines and humans are incidental “dead-end” hosts who do not play a role in the transmission cycle of the virus. However, equines and humans may manifest sever disease or death as a consequence of infection [4]. Since the first discovery of the virus in 1937 in the West Nile district of Uganda [5], it has undergone a substantial geographical migration, and spread around the globe. Infection with WNV was first identified in an EMRO country (Sudan) in the 1940s. Since then, infection with the virus has been reported in Egypt (1950s), Iran (1970s), and subsequently in several other countries across the region [6]. The prevention and control efforts substantially rely on effective surveillance of the infection in birds, vectors, animals, and humans. Despite several studies on different aspects of WNV epidemiology in the EMRO region, there are still many unknowns about the circulation of the virus and the driving factors of outbreaks [6, 7]. Understanding the epidemiology of WNV in the EMRO faces a number of challenges including inadequate knowledge of physicians about the nature of the disease, misdiagnosis of other common infectious diseases due to similarity in clinical presentations, poor diagnostic infrastructures and the absence of confirmatory assays for serological tests, and lack of a comprehensive and progressive monitoring and surveillance system in majority of countries. The latter has resulted in a gap in knowledge regading the prevalence of WNV infection in the EMRO region. Therefore, we designed a systematic review to provide a clear and comprehensive presentation of the virus prevalence distribution among human and animal populations as well as infection rate in vectors of the region, based on available data.
Methods
Data sources and search strategy
Articles were screened and selected according to the PRISMA criteria [8]. The PRISMA checklist completed for this review is presented in S1 File. We made an electronic literature search through Web of Science, Scopus, PubMed, Google Scholar, and Index Medicus for the Eastern Mediterranean region database (IMEMR) using different combinations of the following keywords ‘West Nile virus, West Nile Fever, WNV’ and the name of the EMRO countries as: Afghanistan, Bahrain, Djibouti, Egypt, Iran, Iraq, Jordan, Kuwait, Lebanon, Libya, Morocco, Oman, Pakistan, Palestine, Qatar, Saudi Arabia, Somalia, Sudan, Syria, Tunisia, United Arab Emirates, and Yemen (S2 File). All databases were searched for English-language original articles published from database inception to January 30, 2018. Choosing multiple sources for article search we aimed to enhance our sensitivity in finding relevant articles. To find citations that were not indexed in our target databases, we reviewed the reference lists of relevant articles.
Review selection
Studies identified through electronic and manual searches were listed in EndNote software (EndNote X7, Thomson Reuters). After exclusion of duplicate citations, two authors (MF, FS) independently reviewed titles and abstracts according to the research question. Relevant studies were obtained in full, and assessed for eligibility and risk of bias as described below. All original articles from peer-reviewed scientific journals with a cross-sectional or survey design that estimated the prevalence of WNV infection in humans, animals, or infection rate in vectors were potentially eligible for inclusion in this review. Relevant studies whose abstract was available but their full-text was not (even after contacting the authors via e-mail), were kept in this review in order to present all available data. Studies from outside of the EMRO region were excluded. Any disagreements between the review team were resolved through discussion.
Risk of bias assessment
The risk of bias in primary studies was assessed following the Cochrane approach [9]. We also considered individual studies’ sample size (precision) as a criterion to assess risk of bias, as proposed by Humphre, et al. [10]. Therefore, we evaluated each WNV prevalence study in three domains: 1) sampling method, 2) response level (the proportion of subjects who accept to participate in the study), and 3) type of assay used for the detection of WNV. Each study was considered to have a low risk of bias if: 1) it used probability-base/random sampling methods, 2) maintained participants’ response level at ≥80% [11, 12], or 3) it employed viral neutralization testing (VNT) for a prevalence study on the general population or used biological tests including viral genome detection and virus isolation from infected individuals. Studies were classified as having unclear risk of bias for a given domain if they did not provide information for that specific domain. Use of probabilistic sampling methods was only evaluated for studies on the general population, because acute infection studies included individuals attending to healthcare facilities. For studies that were conducted on blood samples collected and stored from blood donors, response rate criteria were not evaluated. Studies on human subjects were considered to have high precision if their sample sizes were ≥ 100 [13]. Moreover, in the studies on WNV vectors, minimum infection rates (MIR), that were calculated for samples of ≥ 1000 specimens, were considered as a reliable representation of the true infection rate in the vector population [14, 15].
Data extraction
Data was extracted from the selected studies using a researcher-made and piloted data extraction form in excel. For studies on human and animal subjects we extracted data on: first author, year of publication, year of implementation, country, city/governorate, sample size, participants’ age and sex (for human subjects only), animal species (for studies on animals), assay type, and estimated assay-based WNV prevalence. For studies on vector populations, further data was extracted including vector species, number of species (vectors) tested, collection methods, number and size of the pools as well as the number of positive pools for each species. WNV minimum infection rate (MIR) for each species was calculated by dividing the number of positive pools by the total number of specimens tested for that specific species and multiplied by 1000. When data was available, assay-specific MIRs were calculated and reported.
Results
Search results
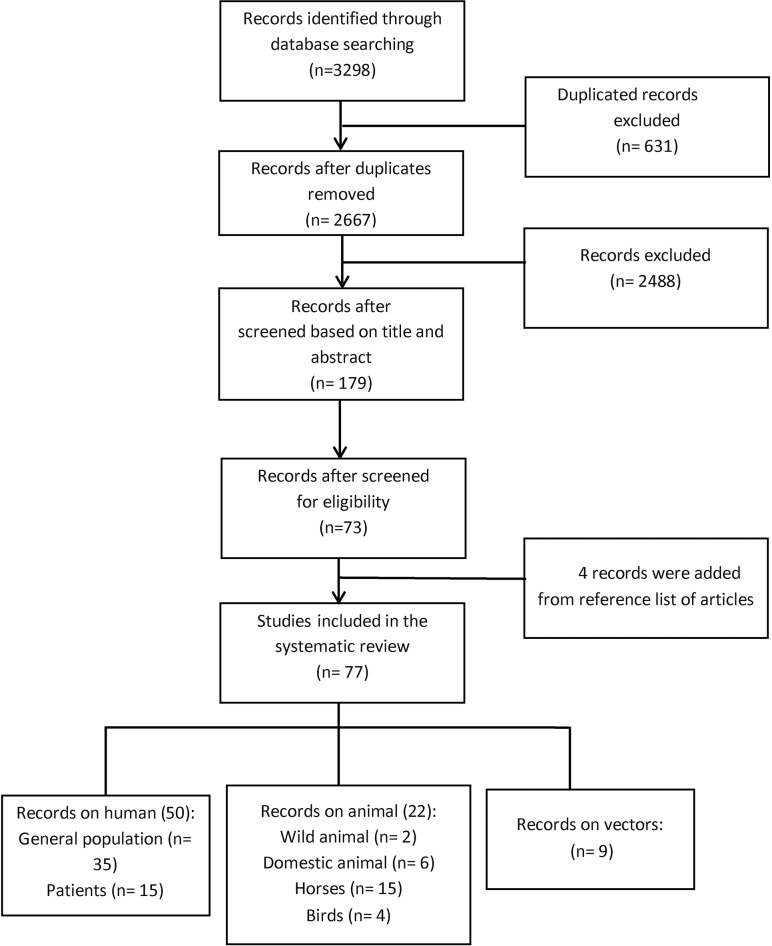
Database search resulted in 3298 records. After removal of duplicates, we initially screened the title and abstract of 2667 records, 2488 of which were excluded as they were irrelevant to this review. The remaining 179 papers were reviewed in full, of which 77 eligible reports on the prevalence/MIR of WNV covering 17 countries in the EMRO region were included in this systematic review. We identified two relevant citations by reviewing the reference list of these relevant studies [16, 17]. Fig 1 shows the literature search process. The full-text of five studies could not be obtained even after contacting the authors [18–22]. These studies were kept in this review to present all available data to the readers. All included studies on WNV entailed 27899 individuals (24460 general populations and 3439 patients), 10309 animals, and 184242 vector species.
Fig 1. Flow diagram of article selection for West Nile prevalence in human and animals, and infection rate in vectors of the EMRO region.
Four citations included data on more than one subject categories (i.e., humans, animal species, and vectors).
Risk of bias assessment results
A summary of the risk of bias assessment results is shown in Table 1. In brief, most human studies (28 out of 35) contained sample sizes of ≥100 participants, yielding a high precision in the reported prevalence measure. Thirty out of thirty-five studies on the general population reported their sampling strategy, fourteen of which utilized some forms of random sampling, and hence, had low risk of bias at this domain. In most studies on the general population (24 out of 35), risk of bias assessment was affected by unclear reporting in the ‘response rate’ domain. Six studies were performed on volunteers or on blood specimens stored in national reference laboratories or blood transfusion centers, and hence, were not subjected to risk of bias assessment in the ‘response rate’ domain. Viral neutralization test was performed in 40.0% and 13.3% of prevalence studies on the general and patient populations, respectively, which entails a low risk of bias for the assays used.
Table 1. Precision and risk of bias assessment for West Nile virus prevalence measures in the EMRO region.
| Author, Year | Country | Sampling Method¥ | Risk of bias | Precision | Ref. | ||
|---|---|---|---|---|---|---|---|
| In sampling¥ | In response rate | In assay selection | |||||
| General population | |||||||
| Andayi, 2014 | Djibouti | Random | Low | Unclear | Low | High | [23] |
| Faulde, 2012 | Djibouti | Conv. | Low | Low (100%) | High | Low | [24] |
| Youssef, 2017 | Egypt | Conv. | High | Unclear | High | High | [25] |
| Soliman, 2010 | Egypt | Random | Low | Unclear | Low | High | [26] |
| Darwish 1996 | Egypt | Unclear | Unclear | Unclear | High | High | [22] |
| Corwin, 1993 | Egypt | CS | Low | Low (93%) | High | High | [27] |
| Corwin, 1992 | Egypt | Random | Low | Low (78%) | High | High | [28] |
| Darwish, 1975 | Egypt | CS | Low | Unclear | High | High | [29] |
| Taylor, 1956 | Egypt | CS | Low | Unclear | Low | High | [30] |
| Aghaie, 2016 | Iran | Conv. | High | Unclear | High | High | [31] |
| Meshkat, 2015 | Iran | MSCS | Low | Unclear | High | High | [32] |
| Chinikar, 2013 | Iran | Unclear | Unclear | Unclear | High | Low | [33] |
| Sharifi, 2010 | Iran | Conv. | High | Low (100%)* | High | High | [34] |
| Saidi, 1976 | Iran | Random | Low | Unclear | Low | High | [17] |
| Saidi,1974 | Iran | Random | Low | Unclear | NA | High | [16] |
| Naficy, 1970 | Iran | Unclear | Unclear | Unclear | Low | High | [19] |
| Barakat, 2016 | Iraq | Conv. | High | Low (100%)** | Low | High | [35] |
| Batieha, 2000 | Jordan | Conv. | High | 56% | High | High | [36] |
| Gallian, 2010 | Lebanon | Conv. | High | Unclear | Low | High | [37] |
| Garabedian, 1971ǂ | Lebanon | Unclear | Unclear | Unclear | High | High | [18] |
| Shaibi, 2017 | Libya | Random | Low | Unclear | High | High | [38] |
| El Harrak 2016ǂ | Morocco | Conv. | High | Unclear | Low | High | [39] |
| El Rhaffouli, 2013 | Morocco | Conv. | High | Low (100%)* | Low | High | [40] |
| El Rhaffouli, 2012 | Morocco | Random | Low | Low (100%) | Low | High | [41] |
| Niazi 2017 | Pakistan | Random | Low | Unclear | High | High | [42] |
| Sugamata, 1989 | Pakistan | Unclear | Unclear | Unclear | Low | High | [43] |
| Sugamata, 1988 | Pakistan | Unclear | Unclear | Unclear | Low | Low | [44] |
| Darwish, 1983 | Pakistan | Conv. | High | Unclear | High | Low | [45] |
| Hayes, 1982 | Pakistan | Conv. | High | Low (100%)** | Low | High | [46] |
| Yousof 2017 | Sudan | Random | Low | Low (100%)* | High | Low | [47] |
| Farnon 2010 | Sudan | Conv. | High | Unclear | Low | Low | [48] |
| Salim, 1973 | Sudan | Conv. | High | Unclear | Low | High | [49] |
| Taylor, 1956 | Egypt | CS | Low | Unclear | Low | High | [30] |
| Smithbur, 1942 | Anglo- Egyptian Sudan |
CS | Low | Unclear | Low | High | [50] |
| Riabi, 2010 | Tunisia | Conv. | High | Low (100%)** | Low | High | [51] |
| Alfaresi, 2008 | UAE | Conv. | High | Unclear | High | Low | [52] |
| Patients | |||||||
| Elyan, 2014 | Afghanistan | NA | NA | Unclear | High | High | [53] |
| Darwish, 1987 | Egypt | NA | NA | Unclear | High | Low | [54] |
| Mohammed, 1970 | Egypt | NA | NA | Low (100%) | High | High | [55] |
| Abdel Wahab, 1970ǂ | Egypt | NA | NA | Unclear | NA | High | [20] |
| Chinikar, 2012 | Iran | NA | NA | Unclear | Low | High | [56] |
| Yaqub,2017 | Pakistan | NA | NA | Low (100%)€ | Low | High | [57] |
| Khan, 2016 | Pakistan | NA | NA | 100% | High | High | [58] |
| Bryan, 1996ǂ | Pakistan | NA | NA | Unclear | NA | High | [21] |
| Igarashi, 1994 | Pakistan | NA | NA | Unclear | Low | High | [59] |
| Depoortere, 2004 | Sudan | NA | NA | Low (100%)€ | High | Low | [60] |
| McCarthy, 1996 | Sudan | NA | NA | Unclear | High | High | [61] |
| Watts, 1994 | Sudan | NA | NA | Unclear | High | High | [62] |
| Riabi, 2014 | Tunisia | NA | NA | Unclear | Low | High | [63] |
| Feki, 2005 | Tunisia | NA | NA | Low (100%) | Low | Low | [64] |
| Qassem, 2014 | Yemen | NA | NA | Unclear | High | Low | [65] |
* On blood specimens stored in the blood transfusion center
** On volunteers
ǂ Studies were classified as having “Unclear” risk of bias for a given domain if they did not provide information for that specific domain. These studies were categorized as “Unclear” risk of bias.
¥ Use of probabilistic sampling methods was only evaluated for studies on the general population, because acute infection studies included individuals attending to healthcare facilities. So, risk of bias assessment for the “sampling” domain, was “Not Applicable” (NA) for patients.
€ On archived samples.
Abbreviations: Conv: Convenience sampling. CS: Cluster Sampling. MSCS: Multi-stage cluster sampling. NA: Not applicable to the field.
WNV prevalence among the general and patient populations of the EMRO region
A total of 50 human prevalence studies for WNV were identified, 35 of which estimated the seroprevalence in the general population. Furthermore, and 15 of them investigated the presence of WNV antibody or genetic material in patients suspected with WNV infection. Human studies covered 14 of 22 countries of the EMRO region, and were published from 1942 to 2017. The highest number of human studies were reported from Egypt (n = 10), Iran (n = 8), and Pakistan (n = 9), most of which targeted the general population. ELISAs were the most commonly used diagnostic method for the general and patient populations. Table 2 presents detailed data for these studies. The geographic distribution of human prevalence studies is also illustrated in Fig 2A and 2B.
Table 2. Summary of human prevalence studies for West Nile virus in the EMRO region (n = 50).
| Author, Pub. year | Study year | Country | City/governorate | SS | Participant characteristics | Assay | Prevalence (%) |
Ref |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male/Female | Age range (yrs) | IgG ELISA | IgM ELISA | NT | IF | HI | CF | RT-PCR | ||||||||
| General population | ||||||||||||||||
| Andayi, 2014 | 2010–2011 | Djibouti | Djibouti | 893§ | NA | NA | ELISA, NT | 0.6 | - | 0.3 | - | - | - | - | [23] | |
| Faulde, 2012 | 2010–2012 | Djibouti | Djibouti | 10 | NA | NA | IF | - | - | - | 60.0 | - | - | - | [24] | |
| Youssef, 2017 | 2013–2014 | Egypt | NA | 160 | 124/ 36 | 18–55 | ELISA, RT-PCR | 55.0 | - | - | - | - | - | 0 | [25] | |
| Soliman, 2010 | 1999–2000 | Egypt | Total | 5965 | NA | NA | ELISA, NT | 24.0 | - | 24.0 | - | - | - | - | [26] | |
| Fayoum | 1593 | 27.3 | - | 27.3 | - | - | - | - | ||||||||
| Sharqiya | 1292 | 13.8 | - | 13.8 | - | - | - | - | ||||||||
| Al Arish | 202 | 1.0 | - | 1.0 | - | - | - | - | ||||||||
| Nuweiba | 675 | 6.7 | - | 6.7 | - | - | - | - | ||||||||
| Qena | 2203 | 35.0 | - | 35.0 | - | - | - | - | ||||||||
| Darwish, 1996ǂ | NA | Egypt | Minufiya | 178 | NA | NA | ELISA, HI, IF | 45.0 | - | - | 26.4 | 37.6 | - | - | [22] | |
| Corwin, 1993 | 1991 | Egypt | Nile | 915 | 356/559 | <1–80 | ELISA | 20.0 | - | - | - | - | - | - | [27] | |
| Corwin, 1992 | 1989 | Egypt | Nile | 437 | 215/222 | 8–14 | ELISA | 3.0 | - | - | - | - | - | - | [28] | |
| Darwish, 1975 | 1969 | Egypt | Cairo | 1133 | NA | NA | HI | - | - | - | - | 50.0 | - | - | [29] | |
| Taylor, 1956 | NA | Egypt | Egyptian Nile delta | 1168 | NA | NA | NT | 61 | - | 61 | - | - | - | - | [30] | |
| Aghaie, 2016 | NA | Iran | Chabahar | 540 | 514/26 | 17–65 | ELISA,IF | 18.0 | - | - | 1.5 | - | - | - | [31] | |
| Meshkat, 2015 | 2011–2012 | Iran | Mashhad | 182 | 46/136 | 15–65 | ELISA | 11.0 | - | - | - | - | - | - |
[32] |
|
| Chinikar, 2013 | 2010–2011 | Iran | Total | 300 | NA | NA | ELISA | 1.3 | - | - | - | - | - | - | [33] | |
| Golestan | 90 | 2.2 | - | - | - | - | - | - | ||||||||
| Gilan | 70 | 1.4 | - | - | - | - | - | - | ||||||||
| Mazandaran | 71 | 0.0 | - | - | - | - | - | - | ||||||||
| Qom | 69 | 1.4 | - | - | - | - | - | - | ||||||||
| Sharifi, 2010 | 2005 | Iran | Tehran | 500 | 490/10 | 17–65 | ELISA, RT-PCR | 5.0 | 0 | - | - | - | - | 0 | [34] | |
| Saidi, 1976 | 1971–1975 | Iran | NA | 698 | NA | NA | NT | - | - | 26.6 | - | - | - | - | [17] | |
| Saidi,1974 | NA | Iran | NA | 100 | NA | NA | NA | 10.0 | - | - | - | - | - | - | [16] | |
| Naficy, 1970ǂ | NA | Iran | NA | 2975 | NA | NA | NT, HI | 30.0 | - | - | - | - | - | - | [19] | |
| Barakat, 2016 | 2012–2013 | Iraq | Nasiriyah | 397 | NA | 10–82 | NT, IF, HI | - | - | 11.6 | 14.9 | 15.1 | - | - | [35] | |
| Batieha, 2000 | 1998 | Jordan | Hashimia | 261 | 75/186 | ≥ 5 | ELISA | 8.0 | - | - | - | - | - | - | [36] | |
| Gallian, 2010 | 2006 | Lebanon | Total | 627 | 74/553 | 18–59 | ELISA,NT | 1.0 | - | 0.5 | - | - | - | - | [37] | |
| Central Lebanon | 500 | |||||||||||||||
| Bekaa | 36 | |||||||||||||||
| Northern Lebanon | 46 | |||||||||||||||
| Southern Lebanon | 45 | |||||||||||||||
| Garabedian, 1971ǂ | NA | Lebanon | NA | 215 | NA | NA | HA, CF | NA | - | - | - | 0 | 0 | - | [18] | |
| Shaibi, 2017 | 2013 | Libya | Tripoli | 400 | 277/123 | 15–78 | ELISA | 2.3 | - | - | - | - | - | - | [38] | |
| El Harrak, 2016 | 2013 | Morocco | NA | 622 | NA | NA | ELISA, NT | - | 0 | 5.6 | - | - | - | - | [39] | |
| El Rhaffouli, 2013 | 2012 | Morocco | Wad-ad-Dahab | 250 | 147/103 | <1–80 | NT | - | - | 5.2 | - | - | - | - | [40] | |
| El Rhaffouli, 2012 | 2011 | Morocco | Total | 499 | 186/313 | 31–65 | NT | - | - | 11.8 | - | - | - | - | [41] | |
| Meknes | 150 | 46/104 | 37–67 | - | - | 4.7 | - | - | - | - | ||||||
| Rabat | 200 | 76/124 | 37–61 | - | - | 12.0 | - | - | - | - | ||||||
| Kenitra | 149 | 64/85 | 31–65 | - | - | 18.8 | - | - | - | - | ||||||
| Niazi, 2017 | NA | Pakistan | NA | 1860 | 1847/13 | 18–57 | RT-PCR | - | - | - | - | - | - | 0.21 | [42] | |
| Sugamata, 1989 | 1985 | Pakistan | Karachi | 150 | NA | 6–65 | NT | - | - | 53.3 | - | - | - | - | [43] | |
| Sugamata, 1988 | 1983 | Pakistan | Karachi | July | 33 | 14/19 | NA | HI | - | - | - | - | 55.0 | - | - | [44] |
| September | 48 | 29/15 | NA | HI | - | - | - | - | 65.0 | - | - | |||||
| 1985 | Karachi | July | 156 | 122/34 | NA | NT, HI | - | - | 50.0 | - | 53.0 | - | - | |||
| October | 156 | 122/34 | NA | NT, HI | - | - | 54.0 | - | 59.0 | - | - | |||||
| Darwish, 1983 | NA | Pakistan | Karachi, Sind, Punjab | 43 | NA | NA | CF | - | - | - | - | - | 11.6 | - | [45] | |
| Hayes, 1982 | 1978–1979 | Pakistan | Chiniot | 192 | NA | 1->61 | NT | - | - | 32.8 | - | - | - | - | [46] | |
| Change Manga national forest | 239 | NA | 1->61 | NT, HI | - | - | 38.5 | - | 33.1 | - | - | |||||
| Yousof, 2017 | 2016 | Sudan | Khartoum | 90 | NA | NA | ELISA | 44.4 | 2.2 | - | - | - | - | - | [47] | |
| Farnon, 2010 | 2005 | Sudan | Kortalla | 87 | 37/50 | 5–44< | NT | - | - | 39.1 | - | - | - | - | [48] | |
| Salim, 1973 | NA | Sudan | Sennar | 17 | NA | <1–40 | NT | - | - | 47.0 | - | - | - | - | [49] | |
| Taylor, 1956 | NA | Sudan | Southern Sudan | 350 | NA | NA | NT | 40 | - | 40 | - | - | - | - | [30] | |
| Smithburn, 1942 | 1939–1940 | Anglo-Egyptian Sudan | Total | 270 | NA | 4–75 | NT | - | - | 28.9 | - | - | - | - | [50] | |
| Red Sea coast | 23 | 5–55 | - | - | 13.0 | - | - | - | - | |||||||
| Eastern border | 75 | 4–60 | - | - | 33.3 | - | - | - | - | |||||||
| White Nile | 56 | 6–75 | - | - | 46.4 | - | - | - | - | |||||||
| Kordofan | 89 | 4–68 | - | - | 20.8 | - | - | - | - | |||||||
| Southwestern | 27 | 7–40 | - | - | 18.6 | - | - | - | - | |||||||
| Riabi, 2010 | 2003 | Tunisia | Monastir | 742 | 497/245 | 18–54 | ELISA,NT | 15.6 | - | 4.3 | - | - | - | - | [51] | |
| Mahdia | 102 | 68/34 | 19–45 | 31.1 | - | 13.7 | - | - | - | - | ||||||
| Alfaresi, 2008 | 2005 | UAE | UAE | 500 | - | - | RT-PCR | - | - | - | - | - | - | 0 | [52] | |
| Patients | ||||||||||||||||
| Elyan, 2014 | 2008–2010 | Afghanistan | Uruzgon, Helmand, Kandahar, Kabul | 913 | 493/420 | 20–59 | ELISA, NT | 30.4 | 0.5 | 2.6 | - | - | - | - | [53] | |
| Mohammed, 1970 | 1968 | Egypt | Alexandria | Acute sample | 120 | 60/60 | 3–13 | CF, HI | - | - | - | - | 4.3 | 0 | - | [55] |
| Convalescent sample | 48 | 24/24 | NA | HI | - | - | - | - | 14.6 | - | - | |||||
| Darwish, 1987 | 1985 | Egypt | Cairo | Prior infection | 55 | 32/23 | >10 | HI | - | - | - | - | 58.0 | - | - | [54] |
| Acute infection | - | - | - | - | 1.8 | - | - | |||||||||
| Abdel Wahab,1970ǂ | NA | Egypt | NA | 133 | NA | NA | NA | 3.7 | - | - | - | - | - | - | [20] | |
| Chinikar, 2012 | 2008–2009 | Iran | Isfahan | 249 | 126/123 | 10–81 | ELISA, RT-PCR | 2.4 | 0 | - | - | - | - | 1.2 | [56] | |
| Yaquba, 2017 | 2014–2015 | Pakistan | Rawalpindi/Islamabad, Lahore, and Faisalabad | 480 | NA | NA | ELISA, RT-PCR | 1.3 | - | - | - | - | 3.1 | - | [57] | |
| Khan, 2016 | 2015 | Pakistan | Karachi, Hyderabad, Mirpurkhas, Sukkur | 241 | NA | 10–50 | ELISA | - | 6.6 | - | - | - | - | - | [58] | |
| Bryan, 1996ǂ | NA | Pakistan | NA | 570 | NA | NA | NA | 33–41 | - | - | - | - | - | - | [21] | |
| Igarashi, 1994 | 1992 | Pakistan | Karachi | 24 | NA | NA | ELISA,RT-PCR | - | 0 | - | - | - | - | 33.3 | [59] | |
| Depoortere, 2004 | 2002 | Sudan | Ngorban, South Kordophan | Neurological sequelae | 8 | 7/6 | 6–84 | ELISA, NT | 62.5 | 87.5 | 6.6 | - | - | - | - | [60] |
| Convalescent | 5 | 0 | 20.0 | 0.1 | - | - | - | - | ||||||||
| McCarthy, 1996 | 1988 | Sudan | Khartoum | 196 | NA | 1–89 | ELISA | 60.0 | 0.3 | - | - | - | - | - | [61] | |
| Watts, 1994 | 1989 | Sudan | Karima | 185 | NA | 11–70 | ELISA | 59.0 | - | - | - | - | - | - | [62] | |
| Riabi, 2014 | 2003 | Tunisia | Monastir | 113 | NA | NA | ELISA, RT-PCR | 33.6 | - | - | - | - | 15.9 | [63] | ||
| Feki, 2005 | 1997 | Tunisia | Sfax | 57 | 50/7 | NA | ELISA, RT-PCR | 52.6 | - | - | - | - | 5.3 | [64] | ||
| Qassem, 2014 | 2013 | Yemen | NA | 42 | NA | All | ELISA | - | 14.3 | - | - | - | - | - | [65] | |
§ These individuals are selected from 324 households.
ǂ The data for these studies is driven from articles’ abstract, as their full-text could not be obtained.
Abbreviations: SS: Sample Size, ELISA: Enzyme-linked Immunosorbent Assay, NT: Neutralization Test, IF: Immunofluorescence Assay, HI: Hemagglutination Inhibition Assay, CF: Complement Fixation, RT-PCR: Reverse Transcriptase-Polymerase Chain Reaction, NA: Data was not available, UAE: United Arab Emirates.
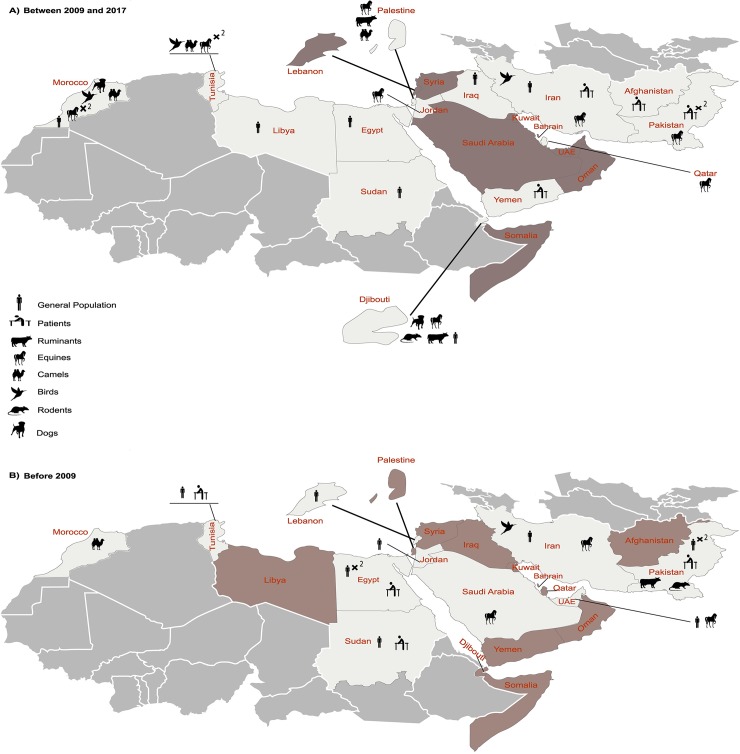
Fig 2. WNV infection reported among human and animal populations of the EMRO countries.
A) studies conducted between 2009 and 2017; B) studies conducted before 2009. Black symbols indicate samples with evidence of WNV infection. The exact sampling location and the prevalence values are provided in the Tables 2 and 3. Countries with no qualified study on human or animal populations are colored in gray. ×2: Where more than one study/sampling effort have been done on a particular species in the same geographic area. UAE: United Arab Emirates.
Regarding the general population, WNV antibodies were detected in 11 countries including Djibouti (n = 2, 0.3–60%), Egypt (n = 7, 1–61%), Iran (n = 6, 0–30%), Iraq (n = 1, 11.6–15.1%), Jordan (n = 1, 8%), Lebanon (n = 2, 0–1%), Libya (n = 1, 2.3%), Morocco (n = 3, 0–18.8%), Pakistan (n = 5, 0.2–65%), Sudan (n = 5, 2.2–47%), and Tunisia (n = 1, 4.3–31.1%). Since 2010, seroprevalence of WNV among the general population has been investigated in Djibouti, Egypt, Iran, Iraq, Libya, Morocco, and Sudan among which the lowest and highest median prevalence was found in Iran (median prevalence = 1.4, range: 0–18%; total SS = 1322; 2010–2012), and Egypt (median prevalence = 55%; total SS = 160, 2013–2014), respectively (Table 2).
In addition, the presence of WNV antibody or genetic material in patients was investigated in 15 human prevalence studies. In this regard, seven studies assessed WNV IgM, five of which detected the antibodies in patients’ sera. These studies were from Afghanistan (n = 1, 0.5%), Pakistan (n = 1, 6.6%), Sudan (n = 2, 0.3–87.5%), and Yemen (n = 1, 14.3%). Four studies [56, 59, 63, 64] used both serological and molecular assays to detect WNV IgM as well as WNV RNA in patients’ sera (Table 2).
WNV prevalence in wild and domestic animals in the EMRO region
A total of 22 studies investigated the WNV seroprevalence in animals (Fig 2A and 2B). WNV antibodies were detected in 10 countries, including, Djibouti (n = 1), Iran (n = 4), Jordan (n = 1), Morocco (n = 4), Pakistan (n = 2), Palestine (n = 1), Qatar (n = 2), Saudi Arabia (n = 1), Tunisia (n = 5) and the United Arab Emirates (UAE; n = 1). In these studies, serological evidence of WNV infection was detected in a wide range of domestic and wild animals, including Buffalos (Pakistan, total SS = 33, prevalence = 15.1%), Camels (Morocco, total SS = 2775, prevalence = 8–23%; Palestine, total SS = 35, Prevalence = 40%; Tunisia, total SS = 120, Prevalence = 0–25.8%), Cows (Pakistan, total SS = 45, prevalence = 2.2%), Goats and sheep (Pakistan, total SS = 94, prevalence = 23.9%; Palestine, total SS = 95, prevalence = 14.7%), Dogs (Djibouti, total SS = 91, prevalence = 56.5%; Morocco, total SS = 231, prevalence = 54–96%), Ruminants (Djibouti, total SS = 11, prevalence = 25.3%), Equids (Iran, total SS = 1839, prevalence = 0.8–70.3%; Jordan, total SS = 253, prevalence = 24.9%; Morocco, total SS = 1189, prevalence = 25–100%; Pakistan, total SS = 449, prevalence = 65%; Palestine, total SS = 585, prevalence = 75%; Qatar, total SS = 421, prevalence: 0–27%; Saudi Arabia, total SS = 63, prevalence = 33.5%; Tunisia, total SS = 1473, prevalence = 28.0–45.2%; the UAE, total SS = 750, prevalence = 5.4–28.6%), and different types of wild and domestic birds (Iran, total SS = 519, prevalence = 15%; Morocco, total SS = 346, prevalence = 3.5%; Tunisia, total SS = 434, prevalence = 0.7–23%). Table 3 provides further details on these studies.
Table 3. Summary of animal prevalence studies for West Nile virus in the EMRO region (n = 22).
| Author, Pub. year | Study year | Country | City/governorate | Species | SS | Prevalence (%) | Ref | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ELISA | NT | IF | HI | CF | ||||||||||
| IgM | IgG | cELISA | ||||||||||||
| Domestic animals | ||||||||||||||
| Marié, 2016 | 2012–2016 | Djibouti | Djibouti | Dog, Ruminant | 91 | - | 56.5 | - | - | - | - | - | [66] | |
| Durand, 2016 | 2012 | Morocco | Total | Dog | 231 | - | - | 62.0 | 80.0 * | - | - | - | [67] | |
| Benslimane | - | - | 54.0 | - | - | - | ||||||||
| Kenitra | - | - | 96.0 | - | - | - | ||||||||
| Khenifra | - | - | 75.0 | - | - | - | ||||||||
| Sidi Slimane | - | - | 94.5 | - | - | - | ||||||||
| Touil, 2012 | 2003 | Morocco | Total | Camel | 556 | - | - | - | 10.4 | - | - | - | [68] | |
| Guelmim (Atlantic littoral) | 73 | - | - | - | 23.0 | - | - | - | ||||||
| Smara (Sahara) | 85 | - | - | - | 8.0 | - | - | - | ||||||
| Aousserd (Sahara) | 389 | - | - | - | 9.0 | - | - | - | ||||||
| 2009 | Total | 836 | - | - | - | 13.6 | - | - | - | |||||
| Oued Draa | 157 | - | - | - | 12.0 | - | - | - | ||||||
| Laayoune (Atlantic littoral) | 397 | - | - | - | 15.0 | - | - | - | ||||||
| Smara (Sahara) | 282 | - | - | - | 12.0 | - | - | - | ||||||
| Darwish, 1983 | 1983 | Pakistan | Karachi, Sind, Punjab | Total | 172 | - | - | - | - | - | - | 9.9 | [45] | |
| Cow | 45 | - | - | - | - | - | - | 2.2 | ||||||
| Buffalo | 33 | - | - | - | - | - | - | 15.1 | ||||||
| Sheep | 46 | - | - | - | - | - | - | 23.9 | ||||||
| Goat | 48 | - | - | - | - | - | - | 0 | ||||||
| Azmi, 2017 | 2014 | Palestine |
Nablus, Jericho, and Jenin | Goat, Sheep | 95 | - | - | 14.7 | - | - | - | - | [69] | |
| Camel | 35 | - | - | 40.0 | - | - | - | - | ||||||
| Hassine, 2017 | 2016 | Tunisia | Medenine | Camel | 87 | - | - | 0.0 | - | - | - | - | [70] | |
| Kebili | 31 | - | - | 25.8 | - | - | - | - | ||||||
| Wild animals | ||||||||||||||
| Marié, 2016 | 2016 | Djibouti | Djibouti | Ruminants | 11 | - | 25.3 | - | - | - | - | - | [66] | |
| Darwish, 1983 | 1983 | Pakistan | Karachi | Rodents | 157 | - | - | - | - | - | - | 4.5 | [45] | |
| Horses | ||||||||||||||
| Marié, 2016 | 2016 | Djibouti | Djibouti | Equids | 10 | - | 90.0 | - | - | - | - | - | [66] | |
| Pourmahdi, 2013 | 2011–2012 | Iran | Khuzestan Province | Horse | 155 | - | - | 70.3 | - | - | - | - | [71] | |
| Chinikar, 2013 | 2010–2012 | Iran | Total | Equine | 315 | - | 2.8 | - | - | - | - | - | [33] | |
| Golestan | 65 | - | 6.1 | - | - | - | - | - | ||||||
| Gilan | 98 | - | 2.0 | - | - | - | - | - | ||||||
| Isfahan | 152 | - | 1.9 | - | - | - | - | - | ||||||
| Ahmadnejad, 2011 | 2008–2009 | Iran | 27 provinces of Iran | Equine | 1054 | 0.8 | - | - | 23.6 | - | - | - | [72] | |
| Abutarbush, 2014 | 2012 | Jordan | Irbid, Ajlun and Jerash | Horse | 253 |
- |
- | - | 24.9 | 0 | - | - | [73] | |
| Amman and Madaba | ||||||||||||||
| Ma’an, Karak, Tafelah and Aqaba | ||||||||||||||
| Mafraq and Zarka | ||||||||||||||
| Jordan Valley and Balqa | ||||||||||||||
| Benjelloun, 2017 | 2011 | Morocco | 4 zones | Horse | 840 | - | - | 31.0 | - | - | - | - | [74] | |
| Durand, 2016 | 2012 | Morocco | Total | Horse | 349 | - | 60.0 | - | 74.0 | - | - | - | [67] | |
| Agadir | - | 65.0 | - | - | - | - | ||||||||
| Benslimane | - | 25.0 | - | - | - | - | ||||||||
| Casablanca | - | 75.0 | - | - | - | - | ||||||||
| Kenitra | - | 82.0 | - | - | - | - | ||||||||
| Khenifra | - | 29.0 | - | - | - | - | ||||||||
| Marrakech | - | 32.0 | - | - | - | - | ||||||||
| Meknes | - | 34.0 | - | - | - | - | ||||||||
| Salé | - | 50.0 | - | - | - | - | ||||||||
| Sidi Slimane | - | 100 | - | - | - | - | ||||||||
| Temara | - | 94.0 | - | - | - | - | ||||||||
| Zohaib, 2015 | 2012–2013 | Pakistan | Punjab, Khyber Pakhtunkhwa |
Equine | 449 | - | 65·0 | - | 55·4 | - | - | - | [75] | |
| Azmi, 2017 | 2014 | Palestine | NA | Equids | 585 | - | - | 75.0 | - | - | - | - | [69] | |
| DeCarlo, 2017 | NA | Qatar | Throughout the country | Horse | 161 | - | - | 27.0 | - | - | - | - | [76] | |
| Haroun, 2017 | 2006–2014 | Qatar | Qatar | Horse | 260 | 0 | - | 23.5 | - | - | - | - | [77] | |
| Al-Ghamdi, 2014 | 2007 | Saudi Arabia | Al-Ahsa | Horse | 63 | - | 33.3 | - | - | - | - | [78] | ||
| Bargaoui, 2015 | 2009 | Tunisia | Jendouba, Monastir, Chott El Jerid, Chott el Gharsa | Equine | 1189 | - | 28.0 | - | - | - | - | - | [79] | |
| Ben Hassine, 2014 | 2012 | Tunisia | Kebili | Equine | 284 | - | 45.2 | - | 42.3 | - | - | - | [80] | |
| Wernery, 2007 | NA | UAE | Total | Equine | 750 | - | - | 19.2 | - | - | - | - | [81] | |
| Al Fujairah | - | 11.5 | ||||||||||||
| Ras Al Khaimah | - | 5.4 | ||||||||||||
| Ajman | - | 7.1 | ||||||||||||
| Sharjah | - | 8.2 | ||||||||||||
| Dubai | - | 10.0 | ||||||||||||
| Al Ain | - | 12.0 | ||||||||||||
| Abu Dhabi | - | 28.6 | ||||||||||||
| Birds | ||||||||||||||
| Fereidouni, 2011 | 2003–2007 | Iran | Mazandaran, Gilan, West Azerbaijan, Tehran, Fars, Khuzestan | 27 species | NT+IF = 519; RT-PCR = 400 |
- | - | - | 15.0 | - | 0 | [82] | ||
| Figuerola, 2009 | 2008 | Morocco | Sidi Allal Tazi, Sidi Kacem | Wild birds | 346 | - | - | - | 3.5 | - | - | - | [83] | |
| Hammouda, 2015 | 2012–2015 | Tunisia | Gabès | Wild Sparrow | 154 | - | 0.7 | - | 0.7 | - | - | - | ||
| Kébili oases | 54 | - | 1.9 | - | 1.9 | - | - | - | [84] | |||||
| Ayadi, 2017 | 2015 | Tunisia | Total | Laughing doves | 226 | - | - | 17.0 | 10 | - | - | - | [85] | |
| Kettana | 102 | - | - | 23.0 | 15 | - | - | - | ||||||
| Gafsa | 53 | - | - | 13.0 | 6.0 | - | - | - | ||||||
| Degache | 26 | - | - | 4.0 | 4.0 | - | - | - | ||||||
| Oum-Errous | 45 | - | - | 16.0 | 7.0 | - | - | - | ||||||
* 80% of ELISA positive samples were positive by NT.
Abbreviations: SS: Sample Size, ELISA: Enzyme-linked Immunosorbent Assay, cELISA: Competitive ELISA, NT: Neutralization Test, IF: Immunofluorescence Assay, HI: Hemagglutination Inhibition Assay, CF: Complement Fixation, RT-PCR: Reverse Transcriptase-Polymerase Chain Reaction, NA: Data was not available, UAE: United Arab Emirates.
Infection rate of vectors with WNV in the EMRO region
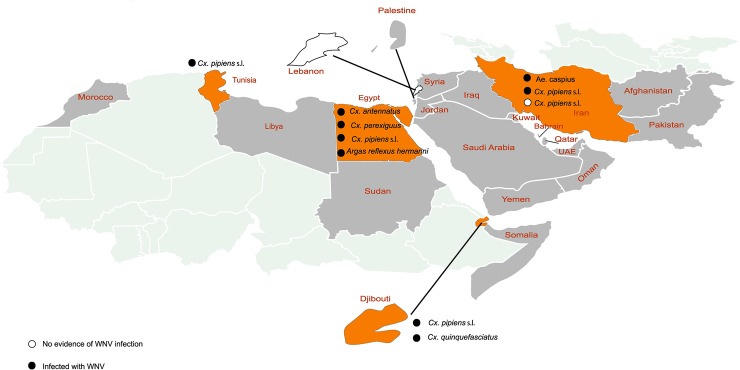
Nine studies investigated arthropods in order to analyze the WNV infection rate among vectors. These reports were from Djibouti (n = 2), Egypt (n = 2), Iran (n = 2), Lebanon (n = 1), Pakistan (n = 1), and Tunisia (n = 1). The primary vector of WNV, i.e., Cx. pipiens s.l. [2], was detected in Djibouti, Egypt, Iran, and Tunisia, and in all theses countries WNV infection in Cx.pipiens s.l. was identified. WNV infection was also detected in a wide range of other vector species, including Cx. quinquefasciatus (Djibouti), Ae. caspius (Iran), Cx. antennatus (Egypt), Cx. perexiguus (Egypt), and Argas reflexus hermannii (Egypt). Details for studies on WNV infection vectors are provided in Table 4 and Fig 3.
Table 4. Summary of studies on the West Nile virus infection rate in vectors of the EMRO region (n = 9).
| Author, Pub. year | Study year | Country | City/governorate | Species | SS | Collection Method | Pools | Test | MIR* | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| (n) | (n/1000) | |||||||||
| Faulde, 2012 | 2010–2012 | Djibouti | Djibouti | Cx. quinquefasciatus | 19069 | CDC-light Traps | NA | RT-PCR | 0.9 | [24] |
| Cx. pipiens s.l. | 686 | 2.9 | ||||||||
| Faulde, 2010 | 2008–2009 | Djibouti | Djibouti | Culex spp. | 600 | CDC- light Traps | NA | RT-PCR | 0 | [86] |
| Turell, 2002§ | 1993 | Egypt | Aswan city (3 villages: NagÕ El Hagar, Sabil AbuEl Magd, ElRaghama, NagÕ El Ghuneimiya, El Naghaghra) |
Total | 36024 | NA | 32 | VI+IF | 0.8 | [87] |
| An. multicolor | 5 | 0 | ||||||||
| An. pharoensis | 145 | 0 | ||||||||
| An. tenebrosus | 245 | 0 | ||||||||
| Cx. antennatus | 2691 | 1.9 | ||||||||
| Cx. perexiguus | 9011 | 2.6 | ||||||||
| Cx. pipiens s.l. | 6982 | 0.3 | ||||||||
| Cx. poicilipes | 26 | 0 | ||||||||
| Ae. caspius | 16889 | 0 | ||||||||
| Uranotaenia unguiculata | 30 | 0 | ||||||||
| Sand flies | 676 | 0 | ||||||||
| Culicoides spp. | 200 | 0 | ||||||||
| Hard ticks | 78 | 0 | ||||||||
| Schmidt 1964 | 1960 | Egypt | Sheikh | Argas reflexus hermanni | 1400 | NA | 28 | VI | 4.3 | [88] |
| Bagheri, 2015 | 2015 | Iran | West Azerbaijan | An. maculipennis | 368 | Dipping | 45 | RT-PCR | 0 | [15] |
| Cx. longiareolata | 130 | 0 | ||||||||
| Cx. hortensis | 1 | 0 | ||||||||
| Cx. pipiens s.l. | 354 | 0 | ||||||||
| Cx. theileri | 618 | 0 | ||||||||
| Ae. caspius | 672 | 33.3 | ||||||||
| Shahhosseini, 2017 | 2015–2016 | Iran | Gilan, Mazandaran, Golestan, East Azerbaijan, Lorestan | Cx. pipiens s.l. | 21060 | Biogents Sentinel Traps | 1222 | RT-PCR | 0 | [14] |
| Cx. Sitiens | 10995 | 0 | ||||||||
| Cx. theileri | 3856 | 0 | ||||||||
| Cx. perexiguus | 486 | 0 | ||||||||
| Cx. pipiens s.l. | 326 | 3.1 | ||||||||
| An. hyrcanus | 180 | 0 | ||||||||
| An. maculipennis s.l. | 117 | 0 | ||||||||
| An. superpictus | 109 | 0 | ||||||||
| Cx. tritaeniorhynchus | 42 | 0 | ||||||||
| An. stephensi | 16 | 0 | ||||||||
| An. claviger | 15 | 0 | ||||||||
| Cx. pipiens form pipiens x molestus | 15 | 0 | ||||||||
| Culiseta longiareolata | 15 | 0 | ||||||||
| Cx. mimeticus | 14 | 0 | ||||||||
| Cx. hortensis | 11 | 0 | ||||||||
| Ae. caspius | 8 | 0 | ||||||||
| An. pseudopictus | 8 | 0 | ||||||||
| Cx. pipiens cf. quinquefasciatus | 8 | 0 | ||||||||
| An. dthali | 6 | 0 | ||||||||
| An. fluviatilis s.l. | 6 | 0 | ||||||||
| Cx. pipiens pipiens form molestus | 5 | 0 | ||||||||
| An. apoci | 4 | 0 | ||||||||
| An. marteri | 4 | 0 | ||||||||
| An. plumbeus | 4 | 0 | ||||||||
| Ae. vexans | 2 | 0 | ||||||||
| Garabedian, 1971ǂ | 1962–1963 | Lebanon | NA | Aedes spp. (mostly) | 5131 | NA | NA | VI | 0 | [18] |
| Reisen, 1982 | 1978–1979 | Pakistan | Punjab |
Cx. tritaeniorhynchus, Cx.quinquefasciatus, Cx. pseudovishnui |
44797 | Dipping, Biting, Resting outdoors & indoors | NA | VI | 0 | [89] |
| Wasfi, 2016 | 2014 | Tunisia | El Felta, Saddaguia | Cx. pipiens s.l. | 102 | CDC-light Traps | 21 | RT-PCR | 68.6 | [90] |
* MIR was calculated by dividing the number of positive pools by the total number of specimens tested and multiplied by 1000. Where the number of tested specimens is below 1000, the MIR may not accurately represent the true infection rate in the population, and should be interpreted with caution.
§ Mosquitoes were sorted to species, pooled, and processed for virus isolation both by intracerebral inoculation into suckling mice and by inoculation into cell culture. A total of 33 virus isolates was made from 36,024 mosquitoes. Virus identification was performed using indirect fluorescent antibody testing.
ǂ The data for this studiy is driven from articles’ abstract, as their full-text could not be obtained.
Abbreviations: SS: Sample Sizes, MIR: Minimum Infection Rate, NA: Data was not available, RT-PCR: Reverse Transcriptase-Polymerase Chain Reaction, VI: Virus Isolation, IF: Immunofluorescence Assay.
Fig 3. Vector infection with the WNV in the EMRO region.
From each study, only the names of vector species with evidence of WNV infection are written on the map. Countries, from which the main vector for WNV (i.e., Cx. pipiens s.l.) was detected, are colored in orange. These countries include Djibouti, Egypt, Iran, and Tunisia, all of which showed evidence of infection in the vector Cx. pipiens s.l.. Countries with no data on study on vectors are colored in gray. Among countries with available data, only Lebanon had zero infection rates for all studied vector species.
Discussion
Seroprevalence of WNV has been investigated in 14 of 22 countries in the EMRO region. Since 1942, WNV antibodies have been detected in the general population in 11 countries with available data, including: Djibouti, Egypt, Iran, Iraq, Jordan, Lebanon, Libya, Morocco, Pakistan, Sudan, and Tunisia. Our results also suggested that the overall seroprevalence of WNV has been lower in reports from more recent years (since 2010) compared to reports compiled between 1942 and 2009.
Although the presence of WNV infection remains unknown in countries without data in the EMRO region (n = 14), it can be implied that the virus may probably circulate within these countries as well. Existing evidence suggests cross-country dispersion of a number of viruses such as human immunodeficiency virus (HIV) [91] and hepatitis B virus (HBV) [92]. These observations can imply the hypothesis in which WNV also have dispersed across countries in the region, affecting localities (countries) adjacent to infected areas. The argument is further strengthened if we consider the transmission routes of HIV, HBV, and WNV. The transmission of HIV and HBV depends on effective human-to-human contacts, which acts as a barrier for virus dispersion over large geographic distances. However, similar to other arboviruses like Dengue and Crimean-Congo Hemorrhagic Fever [93, 94], the cross-country spread of WNV can be much easier and fast as it can be transmitted through a broad range of vectors and reservoirs.
Most of the seroepidemiological studies included in this review used ELISA for the detection of anti-WNV antibodies. Although this assay is simple, sensitive, and commercially available, it suffers from cross-reactivity with antibodies raised against other flaviviruses. So, using the ELISA method for testing individuals with a history of vaccination against, or infection with related flaviviruses can yield false positive results [95]. To achieve a more specific measurement, positive ELISA test results should be confirmed by the plaque reduction neutralization test (PRNT), which is considered as the gold standard method for WNV serological testing. However, PRNT can detect antibodies at levels that neutralize the virus; therefore, it has low sensitivity for seroepidemiological studies in weakly-exposed populations [95].
Approximately, one-fifth of WNV infected individuals demonstrate symptomatic infection [96]. Clinical symptoms are also non-specific to the disease and include fever, malaise, headache, back pain, myalgia, and anorexia. Therefore, WNV infected individuals can be misdiagnosed with other febrile infections. In areas with evidence of WNV circulation, WNV infection should be considered as a differential diagnosis for patients demonstrating non-differential febrile syndroms.
Non-specific sympotoms of the WNV infection also highlights the need for laboratory testing of suspected human cases. While WNV IgM is the most common target for confirmation of the infection, viral RNA testing can also be performed. Combining IgM detection and viral RNA testing can enhance the possibility of diagnosis in patints with West Nile fever, as indicated by Tilley et al. [97]. However, among 15 studies on patient populations, only four used a combination of serological and molecular assays for the diagnosis of WNV infection.
In this review, we have highlighted serological evidence of WNV infection from 22 independent studies conducted on animal populations in the region. These studies were carried out in 10 countries including, Djibouti, Iran, Jordan, Morocco, Pakistan, Palestine, Qatar, Saudi Arabia, Tunisia, and the UAE. Most studies, have investigated evidence of WNV infection among domestic animals. Since 2010, the highest prevalence of WNV among domestic animals, has been reported among dogs of Morocco and equids of Morocco, Pakistan, Palestine and Iran. The high rates of animal seropositivity and geographic distribution of animal infection reflect the favorable conditions for the circulation of WNV in these countries. In these areas, stronger preventive measures should be considered to reduce the risk of WNV transmission to humans and horses. High seropositivity among dogs and equids also suggests that these animals can be useful sentinels for WNV surveillance, as discussed by previous studies [98–100]. Resnick, et al. (2008) reported that WNV seroconversion in dogs happened six weeks prior to the infection in exposed human cases [100].
Only two studies from Pakistan (on rodents) [45] and Djibouti (on wild ruminants) [66]) have investigated wild animals’ infection with WNV. The paucity of published studies on the prevalence of WNV infection in wild animals of the EMRO region underlines a gap in current knowledge about the issue. Knowledge about the reservoirs’ infection and virus circulation among wild animals has important implications for forecasting the emergence or re-emergence of WNV epidemics[95]. So, it is recommended future seroprevalence studies include representative samples from wild animals to further illuminate the state of the infection among these hosts.
Four studies investigated the infection among birds from Iran, Morocco, and Tunisia, from which only two studies were recently performed (i.e., Tunisia, 2015 and 2017). These observations also highlight a gap in current knowledge, this time, on the extend of the infection among birds of the EMRO region. Birds play a critical role in the maintenance and spread of the virus. Prolonged high levels of viremia have been demonstrated in several bird species [101, 102]. The virus has also been isolated from several migratory birds. Thus, surveillance of WNV infection among birds would be of great importance, especially in areas with favorable ecological conditions for birds and mosquitoes. In this regards, a better understanding of birds migration routes would be helpful in selecting the most probable sites for tracking the virus [102], and subsequently making judgments on what areas might be focal points for the emergence of WNV outbreaks.
Mosquitoes and birds are currently considered to have the key role in the life cycle of the virus [2]. However, there are more than 30 other vertebrates such as lemurs, frogs, hamsters, squirrels, rabbits, and chipmunks that have been reported as possible reservoirs for the virus, since they can provide viremia levels that are sufficient to infect mosquito vectors [103]. The role of these reservoirs in the WNV life cycle and epidemic has been less regarded till now, and is an open area for future research.
Despite the critical role of the vector in the life cycle and the epidemic of WNV, only nine studies have investigated vector infection in the region. These studies have been conducted in Djibouti, Egypt, Iran, Pakistan, and Tunisia. The primary vector of WNV, i.e., Cx. pipiens s.l. [2] was detected in all investigared countries except Pakistan. Although WNV infection has been detected in more than 60 mosquito species, detection of viral infection in a mosquito alone does not indicate that the mosquito is a competent vector for the virus. In addition to Culex species, WNV has also been detected in Aedes and Mansonia mosquitoes. Additional studies are necessary to further clarify the potential role these species in the maintenance and transmission of WNV. Interestingly, WNV infection was observed in ticks Argas reflexus hermannii. Previous studies from other regions of WHO also detected WNV RNA in ticks R. turanicus and mites D. gallinae and O. sylvarum. However, their competency as vectors is less clear [104]. Reducing virus transmission from a vector is one of the main strategies of controlling arboviral diseases. Therefore, more efforts to identify the main vectors and understand virus–vector interaction in burdened countries would benefit disease control strategies [105].
The main limitations of this systematic review relate to the data. First, there is a paucity of prevalence studies in the EMRO region, and the quality of data reported by studies varied. For instance, many available studies on human populations were focused on adults, or did not report age and gender for the study sample. The remaining studies included a broad range of age groups (including infant, children, and adults), most of which did not report age and gender specific prevalence. Prevalence data on healthy infants and young children alone was particularly sparse. Therefore, the state of the epidemic among different age and sex groups remains unknown in this region and requires further study with representative samples. Although current data provides a good basis for an overall judgment about the presence of current/past WNV exposure in most investigated samples, they can hardly be used to infer the actual prevalence and state of the epidemic in most investigated countries. For example, only in four countries with available data on the ‘general population’ (i.e., Egypt, Iran, Lebanon, and Pakistan), the total number of tested individuals was reasonably representative of the target population (i.e., more than 1000). These ‘powerful’ studies, however, were not totally flawless. One of the main limitations of these studies was that some of them had used convenience (non-random) sampling methods. In convenience sampling, individuals have unequal and unknown probability of being selected [106]. Hence, the resulting seroprevalence estimates should be generalized to the target population with caution. Few studies available from animal populations in the region also suffered from the abovementioned shortcomings; i.e., non-random sampling and small sample sizes. For example, the seroprevalence of WNV has been investigated in Morocco, Palestine, and Tunisia, but only the study in Morocco has provided the estimate based on a fairly representative sample of 556 and 836 camels for the years 2003 and 2009, respectively. The case was even worse for the seroprevalence studies on dogs, cows, sheep, goats, buffalos, and birds as none of the available studies were well-powered enough (i.e., had small sample sizes). The situation was more satisfactory for the population of horses, where a number of studies with large sample sizes were available from different parts of Iran, Morocco, Pakistan, Palestine, Tunisia, and the UAE. Second, the relative dearth of recent seroprevalence studies, particularly from burdened areas for WNV infection and high-risk population groups is a serious limitation. As the face of WNV disease and its geographic range changes rapidly, WNV prevalence estimated by older studies may not properly reflect the current status of WNV circulation. Less accurate serological tests used by older studies also affect the validity and reliability of the prevalence estimates in these studies. Standardized seroprevalence studies at national levels are critical to best appraise the epidemic status, the impact of interventions and the potentials for future outbreaks. Third, substantial within-country heterogeneity in the prevalence of WNV was noted. This might be due to diversity in the geographical areas, target groups, and the reported sample sizes of studies. Local prevalence estimates, hence, might not be representative of national level prevalence, particularly in large countries with much geographic and ethnic disparities. Finally, our review is limited to reports written in English.
Conclusions
This review provides estimates of the scale of the WNV epidemic at country and regional levels in order to inform efforts for developing and implementing effective future responses. Our results suggested the circulation of WNV in humns, animals, or vectors of most investigated countries in the region. However, there is paucity of data about WNV infection, especially with respect to the burden of the infection in most countries across the region. Hence, further epidemiological studies that take into account the human, reservoir and vector dimension/aspect of the occurrence and distribution of the virus should be conducted particularly in high-prevalent countries. Such research effort will generate robust knowledge and a detailed understanding of the epidemiology of the infection in local populations, and foster in-depth investigations about transmission patterns of the virus. Identification of the geographic distribution of primary reservoirs of the virus and their infection status can also enhance targeted prevention and elimination efforts and aid forecasting attempts. Moreover, surveillance capacities in EMRO countries ought to be established or expanded for better monitoring of WNV infection at national and regional levels.
Supporting information
(DOC)
(DOCX)
Acknowledgments
The authors would like to thank Dr. Ehsan Mostafavi and Dr. Glory Atilola for the critical review of the manuscript.
Data Availability
All relevant data are within the manuscript.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Shomaker TS, Green EM, Yandow SM. Perspective: One Health: a compelling convergence. Acad Med. 2013;88(1):49–55. 10.1097/ACM.0b013e31827651b1 . [DOI] [PubMed] [Google Scholar]
- 2.Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ. West Nile virus. Lancet Infect Dis. 2002;2(9):519–29. Epub 2002/09/07. . [DOI] [PubMed] [Google Scholar]
- 3.McLean RG, Ubico SR, Bourne D, Komar N. West Nile virus in livestock and wildlife. Curr Top Microbiol Immunol. 2002;267:271–308. . [DOI] [PubMed] [Google Scholar]
- 4.van der Meulen KM, Pensaert MB, Nauwynck HJ. West Nile virus in the vertebrate world. Arch Virol. 2005;150(4):637–57. 10.1007/s00705-004-0463-z . [DOI] [PubMed] [Google Scholar]
- 5.Smithburn K, Hughes T, Burke A, Paul J. A Neurotropic Virus Isolated from the Blood of a Native of Uganda1. Am J Trop Med Hyg. 1940;1(4):471–92. [Google Scholar]
- 6.Sayed-Ahmed M. Incidence History of West Nile Virus in Africa and Middle East, With an Emphasis on Egypt: A Review. J Dairy Vet Anim Res. 2016;3(3):00080. [Google Scholar]
- 7.Gray TJ, Webb CE. A review of the epidemiological and clinical aspects of West Nile virus. Int J Gen Med. 2014;7:193–203. 10.2147/IJGM.S59902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–94. . [DOI] [PubMed] [Google Scholar]
- 9.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Wiley Online Library; 2008.
- 10.Humphrey JM, Cleton NB, Reusken CB, Glesby MJ, Koopmans MP, Abu-Raddad LJ. Dengue in the Middle East and North Africa: A Systematic Review. PLoS Negl Trop Dis. 2016;10(12):e0005194 10.1371/journal.pntd.0005194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fincham JE. Response rates and responsiveness for surveys, standards, and the Journal. Am J Pharm Educ. 2008;72(2):43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelley K, Clark B, Brown V, Sitzia J. Good practice in the conduct and reporting of survey research. Int J Qual Health Care. 2003;15(3):261–6. . [DOI] [PubMed] [Google Scholar]
- 13.Hatcher EL, Zhdanov SA, Bao Y, Blinkova O, Nawrocki EP, Ostapchuck Y, et al. Virus Variation Resource-improved response to emergent viral outbreaks. Nucleic Acids Res. 2017;45(D1):D482–D90. 10.1093/nar/gkw1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shahhosseini N, Chinikar S, Moosa-Kazemi SH, Sedaghat MM, Kayedi MH, Luhken R, et al. West Nile Virus lineage-2 in Culex specimens from Iran. Trop Med Int Health. 2017;22(10):1343–9. 10.1111/tmi.12935 PubMed PMID: WOS:000412120000014. [DOI] [PubMed] [Google Scholar]
- 15.Bagheri M, Terenius O, Oshaghi MA, Motazakker M, Asgari S, Dabiri F, et al. West Nile Virus in Mosquitoes of Iranian Wetlands. Vector-Borne Zoonotic Dis. 2015;15(12):750–4. 10.1089/vbz.2015.1778 PubMed PMID: WOS:000366723800006. [DOI] [PubMed] [Google Scholar]
- 16.S. S. Viral antibodies in preschool children from the Caspian area, Iran. Iran J Publ Hlth. 1974;3:83–91. [Google Scholar]
- 17.Saidi S, Tesh R, Javadian E, Nadim A. The prevalence of human infection with West Nile virus in Iran. Iran J Publ Hlth. 1976;5(1):8–13. [Google Scholar]
- 18.Garabedian GA, Matossian RM, Musalli MN. Serologic evidence of arbovirus infection in Lebanon. J Med Liban. 1971;24(4):339–50. Epub 1971/01/01. . [PubMed] [Google Scholar]
- 19.Naficy K, Saidi S. Serological survey on viral antibodies in Iran. Trop Geogr Med. 1970;22(2):183–8. Epub 1970/06/01. . [PubMed] [Google Scholar]
- 20.Abdel Wahab KS. Arboviruses and central nervous system disorders in Egypt. Acta Virol. 1970;14(6):501–6. Epub 1970/11/01. . [PubMed] [Google Scholar]
- 21.Bryan JP, Iqbal M, Ksiazek TG, Ahmed A, Duncan JF, Awan B, et al. Prevalence of Sand Fly fever, West Nile, Crimean-Congo hemorrhagic fever, and leptospirosis antibodies in Pakistani Military Personnel. Milit Med. 1996;161(3):149–53. PubMed PMID: WOS:A1996UA23600006. [PubMed] [Google Scholar]
- 22.Darwish M, el-Khashaab TH, Mostafa A, Hamid TA, Shope R. A comparative study of serological techniques for detection of West Nile virus antibodies. J Egypt Public Health Assoc. 1996;71(3–4):201–11. Epub 1996/01/01. . [PubMed] [Google Scholar]
- 23.Andayi F, Charrel RN, Kieffer A, Richet H, Pastorino B, Leparc-Goffart I, et al. A Sero-epidemiological Study of Arboviral Fevers in Djibouti, Horn of Africa. Plos Neglect Trop Dis. 2014;8(12):13 10.1371/journal.pntd.0003299 PubMed PMID: WOS:000346701000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faulde MK, Spiesberger M, Abbas B. Sentinel site-enhanced near-real time surveillance documenting West Nile virus circulation in two Culex mosquito species indicating different transmission characteristics, Djibouti City, Djibouti. J Egypt Soc Parasitol. 2012;42(2):461–74. Epub 2012/12/12. . [DOI] [PubMed] [Google Scholar]
- 25.Youssef SR, Eissa DG, Abo-Shady RA, Aly Fouad NT, Kattab DK, Fathey H, et al. Seroprevalence of anti-WNV IgG antibodies and WNV-RNA in Egyptian blood donors. J Med Virol. 2017;89(8):1323–9. Epub 2016/09/08. 10.1002/jmv.24682 . [DOI] [PubMed] [Google Scholar]
- 26.Soliman A, Mohareb E, Salman D, Saad M, Salama S, Fayez C, et al. Studies on West Nile virus infection in Egypt. J Infect Public Health. 2010;3(2):54–9. Epub 2010/08/13. 10.1016/j.jiph.2009.11.002 . [DOI] [PubMed] [Google Scholar]
- 27.Corwin A, Habib M, Watts D, Darwish M, Olson J, Botros B, et al. Community-based prevalence profile of arboviral, rickettsial, and hantaan- like viral antibody in the Nile River Delta of Egypt. Am J Trop Med Hyg. 1993;48(6):776–83. [DOI] [PubMed] [Google Scholar]
- 28.Corwin A, Habib M, Olson J, Scott D, Ksiazek T, Watts DM. The prevalence of arboviral, rickettsial, and Hantaan-like viral antibody among schoolchildren in the Nile river delta of Egypt. Trans R Soc Trop Med Hyg. 1992;86(6):677–9. Epub 1992/11/01. . [DOI] [PubMed] [Google Scholar]
- 29.Darwish MA, Ibrahim AH. Prevalence of antibodies to arboviruses in Egypt. Results of a serologic survey among 1,113 university students. Am J Trop Med Hyg. 1975;24(6 Pt 1):981–5. Epub 1975/11/01. . [DOI] [PubMed] [Google Scholar]
- 30.Taylor R, Work T, Hurlbut H, Rizk F. A Study of the Ecology of West Nile Virus in Egypt1. Am J Trop Med Hyg. 1956;5(4):579–620. [DOI] [PubMed] [Google Scholar]
- 31.Aghaie A, Aaskov J, Chinikar S, Niedrig M, Banazadeh S, Mohammadpour HK. Frequency of West Nile Virus Infection in Iranian Blood Donors. Indian J Hematol Blood Transfus. 2016;32(3):343–6. 10.1007/s12288-015-0567-5 PubMed PMID: WOS:000378994200015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meshkat Z, Chinikar S, Shakeri M, Manavifar L, Moradi M, Mirshahabi H, et al. Prevalence of West Nile virus in Mashhad, Iran: A population—based study. Asian Pac J Trop Med. 2015;8(3):203–5. 10.1016/S1995-7645(14)60315-1 PubMed PMID: WOS:000351891700006. [DOI] [PubMed] [Google Scholar]
- 33.Chinikar S, Shah-Hosseini N, Mostafavi E, Moradi M, Khakifirouz S, Jalali T, et al. Seroprevalence of West Nile Virus in Iran. Vector-Borne Zoonotic Dis. 2013;13(8):586–9. 10.1089/vbz.2012.1207 PubMed PMID: WOS:000322967800008. [DOI] [PubMed] [Google Scholar]
- 34.Sharifi Z, Talebian A, Shooshtari MM. A study of West Nile Virus (WNV) infection in iranian blood donors. Vox Sang. 2009;97:123–. PubMed PMID: WOS:000270833500262. [Google Scholar]
- 35.Barakat AM, Smura T, Kuivanen S, Huhtamo E, Kurkela S, Putkuri N, et al. The Presence and Seroprevalence of Arthropod-Borne Viruses in Nasiriyah Governorate, Southern Iraq: A Cross-Sectional Study. Am J Trop Med Hyg. 2016;94(4):794–9. 10.4269/ajtmh.15-0622 PubMed PMID: WOS:000373242400016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Batieha A, Saliba EK, Graham R, Mohareb E, Hijazi Y, Wijeyaratne P. Seroprevalence of West Nile, Rift Valley, and sandfly arboviruses in Hashimiah, Jordan. Emerg Infect Dis. 2000;6(4):358–62. Epub 2000/07/25. 10.3201/eid0604.000405 ; PubMed Central PMCID: PMCPmc2640900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallian P, de Micco P, Ghorra P. Seroprevalence of West Nile virus in blood donors at Hotel Dieu de France, Beirut, Lebanon. Transfusion. 2010;50(5):1156–8. PubMed PMID: WOS:000277123900032. [DOI] [PubMed] [Google Scholar]
- 38.Shaibi T, Saadawi WK, Aghila H, Annajar BB. Prevalence of IgG antibodies for the West Nile virus in human population in Tripoli, Libya. J Vector Borne Dis. 2017;54(2):183–6. Epub 2017/07/28. . [PubMed] [Google Scholar]
- 39.El Harrak Y, Kasouati J, Loutfi C, Benjelloun A, El Harrak M, Hadef R, et al. Anticipating West Nile virus transmission risk from the Moroccan blood donation: Yassine El Harrak. Eur J Public Health. 2016;26(suppl_1). [Google Scholar]
- 40.El Rhaffouli H, Lahlou-Amine I, Lotfi C, Laraqui A, Bajjou T, Fassi-Fihri O, et al. Serological evidence of West Nile Virus infection among humans in the southern Provinces of Morocco. J Infect Dev Ctries. 2013;7(12):999–1002. 10.3855/jidc.3399 PubMed PMID: WOS:000339853100015. [DOI] [PubMed] [Google Scholar]
- 41.El Rhaffouli H, El Harrak M, Lotfi C, El Boukhrissi F, Bajjou T, Laraqui A, et al. Serologic Evidence of West Nile Virus Infection among Humans, Morocco. Emerg Infect Dis. 2012;18(5):880–1. 10.3201/eid1805.110826 PubMed PMID: WOS:000303556800032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niazi SK, Alam M, Yazdani MS, Ghani E, Rathore MA. Nucleic Acid Amplification Test For Detection Of West Nile Virus Infection In Pakistani Blood Donors. JAMC. 2017;29(4):547–50. Epub 2018/01/14. . [PubMed] [Google Scholar]
- 43.Sugamata M, Miura T. Effects of the season of birth on the levels of antibodies against west nile virus in the pakistani population. driscoll d, box eo, lieth h, editors. the hague: s p b academic publ; 1989. p 303–8. [Google Scholar]
- 44.Sugamata M, Ahmed A, Miura T, Takasu T, Kono R, Ogata T, et al. Seroepidemiological study of infection with west nile virus in karachi, pakistan, in 1983 and 1985. J Med Virol. 1988;26(3):243–7. 10.1002/jmv.1890260304 PubMed PMID: WOS:A1988Q744500003. [DOI] [PubMed] [Google Scholar]
- 45.Darwish MA, Hoogstraal H, Roberts TJ, Ahmed IP, Omar F. A sero-epidemiological survey for certain arboviruses (Togaviridae) in Pakistan. Trans R Soc Trop Med Hyg. 1983;77(4):442–5. 10.1016/0035-9203(83)90106-2. [DOI] [PubMed] [Google Scholar]
- 46.Hayes CG, Baqar S, Ahmed T, Chowdhry MA, Reisen WK. West Nile virus in Pakistan. 1. Sero-epidemiological studies in Punjab Province. Trans R Soc Trop Med Hyg. 1982;76(4):431–6. Epub 1982/01/01. . [DOI] [PubMed] [Google Scholar]
- 47.Yousof SY, Ahmed SE, MH N, Nour MM, Saleh MS, Garbi MI, et al. Seroprevalence of West Nile Virus among Blood Donors at Central Blood Bank, Khartoum State, Sudan. Annals Med Biomed Sci. 2018;4 (1):8–10. [Google Scholar]
- 48.Farnon EC, Gould LH, Griffith KS, Osman MS, Kholy AE, Brair ME, et al. Household-based sero-epidemiologic survey after a yellow fever epidemic, Sudan, 2005. Am J Trop Med Hyg. 2010;82(6):1146–52. Epub 2010/06/04. 10.4269/ajtmh.2010.09-0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salim AR, Porterfield JS. A serological survey on arbovirus antibodies in the Sudan. Trans R Soc Trop Med Hyg. 1973;67(2):206–10. Epub 1973/01/01. . [DOI] [PubMed] [Google Scholar]
- 50.SMITHBURN KC, JACOBS HR. Neutralization-Tests against Neurotropic Viruses with Sera Collected in Central Africa. J Immunol. 1942;43(5):9–23. [Google Scholar]
- 51.Riabi S, Gallian P, Gaaloul I, Simon S, Harrath R, Hassine M, et al. Prevalence of IgG antibodies against West Nile virus in blood donors during the 2003 outbreak in Tunisia. Trans R Soc Trop Med Hyg. 2010;104(7):507–9. 10.1016/j.trstmh.2010.03.001 PubMed PMID: WOS:000279427100010. [DOI] [PubMed] [Google Scholar]
- 52.Alfaresi M, Elkoush A. West Nile virus in the blood donors in UAE. Indian J Med Microbiol. 2008;26(1):92–3. Epub 2008/01/30. . [DOI] [PubMed] [Google Scholar]
- 53.Elyan DS, Moustafa L, Noormal B, Jacobs JS, Aziz MA, Hassan KS, et al. Serological evidence of Flaviviruses infection among acute febrile illness patients in Afghanistan. J Infect Dev Ctries. 2014;8(9):1176–80. 10.3855/jidc.4183 PubMed PMID: WOS:000343791000012. [DOI] [PubMed] [Google Scholar]
- 54.Darwish MA, Feinsod FM, Scott RM, Ksiazek TG, Botros BAM, Farrag IH, et al. Arboviral causes of non-specific fever and myalgia in a fever hospital patient population in Cairo, Egypt. Trans R Soc Trop Med Hyg. 1987;81(6):1001–3. 10.1016/0035-9203(87)90378-6 [DOI] [PubMed] [Google Scholar]
- 55.Mohammed YS, Gresikova M, Adamyova K, Ragib AH, el-Dawala K. Studies on arboviruses in Egypt: II. Contribution of arboviruses to the aetiology of undiagnosed fever among children. J Hyg. 1970;68(3):491–5. 10.1017/S002217240004239X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chinikar S, Javadi A, Ataei B, Shakeri H, Moradi M, Mostafavi E, et al. Detection of West Nile virus genome and specific antibodies in Iranian encephalitis patients. Epidemiol Infect. 2012;140(8):1525–9. 10.1017/S0950268811002056 PubMed PMID: WOS:000306936000021. [DOI] [PubMed] [Google Scholar]
- 57.Yaqub T, Shabbir MZ, Mukhtar N, Tahir Z, Abbas T, Amir E, et al. Detection of selected arboviral infections in patients with history of persistent fever in Pakistan. Acta tropica. 2017;176:34–8. Epub 2017/07/30. 10.1016/j.actatropica.2017.07.019 . [DOI] [PubMed] [Google Scholar]
- 58.Khan E, Farooqi JQ, Barr KL, Prakoso D, Nasir A, Kanji A, et al. Flaviviruses as a Cause of Undifferentiated Fever in Sindh Province, Pakistan: A Preliminary Report. Front Public Health. 2016;4:8 Epub 2016/02/26. 10.3389/fpubh.2016.00008 ; PubMed Central PMCID: PMCPmc4754388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Igarashi A, Tanaka M, Morita K, Takasu T, Ahmed A, Ahmed A, et al. Detection of west Nile and Japanese encephalitis viral genome sequences in cerebrospinal fluid from acute encephalitis cases in Karachi, Pakistan. Microbiol immunol. 1994;38(10):827–30. Epub 1994/01/01. . [DOI] [PubMed] [Google Scholar]
- 60.Depoortere E, Kavle J, Keus K, Zeller H, Murri S, Legros D. Outbreak of West Nile virus causing severe neurological involvement in children, Nuba Mountains, Sudan, 2002. Trop Med Int Health. 2004;9(6):730–6. 10.1111/j.1365-3156.2004.01253.x PubMed PMID: WOS:000222300500012. [DOI] [PubMed] [Google Scholar]
- 61.McCarthy MC, Haberberger RL, Salib AW, Soliman BA, El-Tigani A, Khalid IO, et al. Evaluation of arthropod-borne viruses and other infectious disease pathogens as the causes of febrile illnesses in the Khartoum Province of Sudan. J Med Virol. 1996;48(2):141–6. Epub 1996/02/01. . [DOI] [PubMed] [Google Scholar]
- 62.Watts DM, El-Tigani A, Botros BAM, Salib AW, Olson JG, McCarthy M, et al. Arthropod-borne viral infections associated with a fever outbreak in the Northern Province of Sudan. J Trop Med Hyg. 1994;97(4):228–30. [PubMed] [Google Scholar]
- 63.Riabi S, Gaaloul I, Mastouri M, Hassine M, Aouni M. An outbreak of West Nile Virus infection in the region of Monastir, Tunisia, 2003. Pathog Glob Health. 2014;108(3):148–57. 10.1179/2047773214Y.0000000137 PubMed PMID: WOS:000335443600007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feki I, Marrakchi C, Ben Hmida M, Belahsen F, Ben Jemaa M, Maaloul I, et al. Epidemic West Nile virus encephalitis in Tunisia. Neuroepidemiology. 2005;24(1–2):1–7. 10.1159/000081042 PubMed PMID: WOS:000226021500001. [DOI] [PubMed] [Google Scholar]
- 65.Qassem MAM, Jaawal AAT. Dengue fever or West Nile virus outbreak? Yemen 2013. Inter J Infect Dis. 2014;21:457–. 10.1016/j.ijid.2014.03.1364 PubMed PMID: WOS:000209704000945. [DOI] [Google Scholar]
- 66.Marié JL, Lafrance B, Maquart M, Mulot B, Leclerc A, Davoust B, et al. West Nile virus circulation in Djibouti. Inter J Infect Dis. 2016;53, Supplement:158 10.1016/j.ijid.2016.11.385. [DOI] [Google Scholar]
- 67.Durand B, Haskouri H, Lowenski S, Vachiery N, Beck C, Lecollinet S. Seroprevalence of West Nile and Usutu viruses in military working horses and dogs, Morocco, 2012: dog as an alternative WNV sentinel species? Epidemiol Infect. 2016;144(9):1857–64. 10.1017/S095026881600011X PubMed PMID: WOS:000377820300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Touil N, Cherkaoui Z, Lmrabih Z, Loutfi C, Harif B, El Harrak M. Emerging Viral Diseases in Dromedary Camels in the Southern Morocco. Transbound Emerg Dis. 2012;59(2):177–82. 10.1111/j.1865-1682.2011.01282.x PubMed PMID: WOS:000301122700010. [DOI] [PubMed] [Google Scholar]
- 69.Azmi K, Tirosh-Levy S, Manasrah M, Mizrahi R, Nasereddin A, Al-Jawabreh A, et al. West Nile Virus: Seroprevalence in Animals in Palestine and Israel. Vector-Borne Zoonotic Dis (Larchmont, NY). 2017;17(8):558–66. Epub 2017/06/20. 10.1089/vbz.2016.2090 . [DOI] [PubMed] [Google Scholar]
- 70.Hassine TB, Amdouni J, Monaco F, Savini G, Sghaier S, Selimen IB, et al. Emerging vector-borne diseases in dromedaries in Tunisia: West Nile, bluetongue, epizootic haemorrhagic disease and Rift Valley fever. Onderstepoort J Vet Res. 2017;84(1):e1–e3. Epub 2017/04/12. 10.4102/ojvr.v84i1.1316 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pourmahdi M, Ghadrdan Mashadi A, Seifi Abad Shapouri M, Zeinvand M. Aserological survey on antibodies against West Nile virus in horses of Khuzestan province. Iran J Vet Med. 2013;7(3):185–91. [Google Scholar]
- 72.Ahmadnejad F, Otarod V, Fallah MH, Lowenski S, Sedighi-Moghaddam R, Zavareh A, et al. Spread of West Nile virus in Iran: a cross-sectional serosurvey in equines, 2008–2009. Epidemiol Infect. 2011;139(10):1587–93. 10.1017/S0950268811000173 PubMed PMID: WOS:000295592900018. [DOI] [PubMed] [Google Scholar]
- 73.Abutarbush SM, Al-Majali AM. West Nile Virus Infection in Horses in Jordan: Clinical Cases, Seroprevalence and Risk Factors. Transbound Emerg Dis. 2014;61:1–6. 10.1111/tbed.12191 PubMed PMID: WOS:000340537900001. [DOI] [PubMed] [Google Scholar]
- 74.Benjelloun A, El Harrak M, Calistri P, Loutfi C, Kabbaj H, Conte A, et al. Seroprevalence of West Nile virus in horses in different Moroccan regions. Vet Med Sci. 2017;3(4):198–207. Epub 2017/11/21. 10.1002/vms3.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zohaib A, Saqib M, Beck C, Hussain MH, Lowenski S, Lecollinet S, et al. High prevalence of West Nile virus in equines from the two provinces of Pakistan. Epidemiol Infect. 2015;143(9):1931–5. 10.1017/S0950268814002878 PubMed PMID: WOS:000355760600017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DeCarlo C, Omar AH, Haroun MI, Bigler L, Bin Rais MN, Abu J, et al. Potential Reservoir and Associated Factors for West Nile Virus in Three Distinct Climatological Zones. Vector-Borne Zoonotic Dis (Larchmont, NY). 2017;17(10):709–13. Epub 2017/09/06. 10.1089/vbz.2016.2098 . [DOI] [PubMed] [Google Scholar]
- 77.Haroun M, Siddiq AM, Farag EA, Dlissi E, El Hussein AM, Mohammed HO. Occurrence of Equine West Nile Virus among horses In Qatar: A Preliminary Investigation. Eur Sci J. 2017;SPECIAL/ edition. [Google Scholar]
- 78.Al-Ghamdi GM. Incidence of West nile virus in Al-Ahsa, Saudi Arabia. Inter J Virol. 2014;10 (2):163–7. [Google Scholar]
- 79.Bargaoui R, Lecollinet S, Lancelot R. Mapping the serological prevalence rate of West Nile fever in equids, Tunisia. Transbound Emerg Dis. 2015;62(1):55–66. Epub 2013/08/03. 10.1111/tbed.12077 . [DOI] [PubMed] [Google Scholar]
- 80.Ben Hassine T, De Massis F, Calistri P, Savini G, BelHaj Mohamed B, Ranen A, et al. First detection of co-circulation of West Nile and Usutu viruses in equids in the south-west of Tunisia. Transbound Emerg Dis. 2014;61(5):385–9. Epub 2014/07/30. 10.1111/tbed.12259 . [DOI] [PubMed] [Google Scholar]
- 81.Wernery U, Kettle T, Moussa M, Babiker H, Whiting J. West Nile Fever in the United Arab Emirates. Wild Life Middle East. 2007;2(3). [Google Scholar]
- 82.Fereidouni SR, Ziegler U, Linke S, Niedrig M, Modirrousta H, Hoffmann B, et al. West Nile Virus Monitoring in Migrating and Resident Water Birds in Iran: Are Common Coots the Main Reservoirs of the Virus in Wetlands? Vector-Borne Zoonotic Dis. 2011;11(10):1377–81. 10.1089/vbz.2010.0244 PubMed PMID: WOS:000295870800011. [DOI] [PubMed] [Google Scholar]
- 83.Figuerola J, Baouab RE, Soriguer R, Fassi-Fihri O, Llorente F, Jimenez-Clavero MA. West Nile Virus Antibodies in Wild Birds, Morocco, 2008. Emerg Infect Dis. 2009;15(10):1651–3. 10.3201/eid1510.090340 PubMed PMID: WOS:000270580600021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hammouda A, Lecollinet S, Hamza F, Nasri I, Neb A, Selmi S. Exposure of resident sparrows to West Nile virus evidenced in South Tunisia. Epidemiol Infect. 2015;143(16):3546–9. 10.1017/S0950268814003860 PubMed PMID: WOS:000365173600020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ayadi T, Hammouda A, Poux A, Boulinier T, Lecollinet S, Selmi S. Evidence of exposure of laughing doves (Spilopelia senegalensis) to West Nile and Usutu viruses in southern Tunisian oases. Epidemiol Infect. 2017;145(13):2808–16. Epub 2017/08/15. 10.1017/S0950268817001789 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Faulde MK, Ahmed AA. Haematophageous vector monitoring in Djibouti city from 2008 to 2009: first records of Culex pipiens ssp. torridus (IGLISCH), and Anopheles sergentii (theobald). J Egypt Soc Parasitol. 2010;40(2):281–94. Epub 2011/01/21. . [PubMed] [Google Scholar]
- 87.Turell MJ, Morrill JC, Rossi CA, Gad AM, Cope SE, Clements TL, et al. Isolation of west nile and sindbis viruses from mosquitoes collected in the Nile Valley of Egypt during an outbreak of Rift Valley fever. J Med Entomol. 2002;39(1):248–50. Epub 2002/04/05. . [DOI] [PubMed] [Google Scholar]
- 88.Schmidt JR, Said MI. Isolation of west nile virus from the african bird argasid, argas reflexus hermanni, in egypt. J Med Entomol.1964;1:83–6. Epub 1964/04/01. . [DOI] [PubMed] [Google Scholar]
- 89.Reisen WK, Hayes CG, Azra K, Niaz S, Mahmood F, Parveen T, et al. West Nile virus in Pakistan. II. Entomological studies at Changa Manga National Forest, Punjab Province. Trans R Soc Trop Med Hyg. 1982;76(4):437–48. Epub 1982/01/01. . [DOI] [PubMed] [Google Scholar]
- 90.Wasfi F, Dachraoui K, Cherni S, Bosworth A, Barhoumi W, Dowall S, et al. West Nile virus in Tunisia, 2014: First isolation from mosquitoes. Acta Trop. 2016;159:106–10. 10.1016/j.actatropica.2016.03.037 PubMed PMID: WOS:000377309400013. [DOI] [PubMed] [Google Scholar]
- 91.Eybpoosh S, Bahrampour A, Karamouzian M, Azadmanesh K, Jahanbakhsh F, Mostafavi E, et al. Spatio-temporal history of HIV-1 CRF35_AD in Afghanistan and Iran. PLoS One. 2016;11(6):e0156499 10.1371/journal.pone.0156499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zehender G, Ebranati E, Gabanelli E, Shkjezi R, Lai A, Sorrentino C, et al. Spatial and temporal dynamics of hepatitis B virus D genotype in Europe and the Mediterranean Basin. PLoS One. 2012;7(5):e37198 10.1371/journal.pone.0037198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mostafavi E, Holakouie-Naieni K, Salehi Vaziri M. Reply: Surveillance of Crimean-Congo Haemorrhagic Fever in Pakistan. J Med Microbiol Infect Dis. 2017;5(3):69–70. [Google Scholar]
- 94.Baniasadi V, Salehi-Vaziri M, Jalali T, Azad-Manjiri S, Mohammadi T, Khakifirouz S, et al. An Imported Case of Dengue Fever in Iran, 2015. Iran J Virol. 2016;10(1):31–4. [Google Scholar]
- 95.Beck C, Jimenez-Clavero MA, Leblond A, Durand B, Nowotny N, Leparc-Goffart I, et al. Flaviviruses in Europe: complex circulation patterns and their consequences for the diagnosis and control of West Nile disease. Int J Environ Res Public Health. 2013;10(11):6049–83. 10.3390/ijerph10116049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zou S, Foster GA, Dodd RY, Petersen LR, Stramer SL. West Nile fever characteristics among viremic persons identified through blood donor screening. J Infect Dis. 2010;202(9):1354–61. 10.1086/656602 . [DOI] [PubMed] [Google Scholar]
- 97.Tilley PA, Fox JD, Jayaraman GC, Preiksaitis JK. Nucleic acid testing for West Nile virus RNA in plasma enhances rapid diagnosis of acute infection in symptomatic patients. J Infect Dis. 2006;193(10):1361–4. 10.1086/503577 [DOI] [PubMed] [Google Scholar]
- 98.Davoust B, Maquart M, Roqueplo C, Gravier P, Sambou M, Mediannikov O, et al. Serological Survey of West Nile Virus in Domestic Animals from Northwest Senegal. Vector-Borne Zoonotic Dis. 2016;16(5):359–61. 10.1089/vbz.2015.1881 . [DOI] [PubMed] [Google Scholar]
- 99.Davoust B, Leparc-Goffart I, Demoncheaux JP, Tine R, Diarra M, Trombini G, et al. Serologic surveillance for West Nile virus in dogs, Africa. Emerg Infect Dis. 2014;20(8):1415–7. 10.3201/eid2008.130691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Resnick MP, Grunenwald P, Blackmar D, Hailey C, Bueno R, Murray KO. Juvenile dogs as potential sentinels for West Nile virus surveillance. Zoonoses Public Health. 2008;55(8–10):443–7. 10.1111/j.1863-2378.2008.01116.x . [DOI] [PubMed] [Google Scholar]
- 101.Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, et al. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9(3):311–22. 10.3201/eid0903.020628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Malkinson M, Banet C. The role of birds in the ecology of West Nile virus in Europe and Africa. Curr Top Microbiol Immunol. 2002;267:309–22. Epub 2002/06/27. . [DOI] [PubMed] [Google Scholar]
- 103.Chancey C, Grinev A, Volkova E, Rios M. The global ecology and epidemiology of West Nile virus. Biomed Res Int. 2015;2015:376230 Epub 2015/04/14. 10.1155/2015/376230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mumcuoglu KY, Banet-Noach C, Malkinson M, Shalom U, Galun R. Argasid ticks as possible vectors of West Nile virus in Israel. Vector-Borne Zoonotic Dis (Larchmont, NY). 2005;5(1):65–71. Epub 2005/04/09. 10.1089/vbz.2005.5.65 . [DOI] [PubMed] [Google Scholar]
- 105.Reisen W, Brault AC. West Nile virus in North America: perspectives on epidemiology and intervention. Pest Manag Sci. 2007;63(7):641–6. 10.1002/ps.1325 . [DOI] [PubMed] [Google Scholar]
- 106.Emerson RW. Convenience sampling, random sampling, and snowball sampling: How does sampling affect the validity of research? J Vis Impair Blind (Online). 2015;109(2):164. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript.